Abstract
Overexpression of the small heat shock protein Hsp27 has been shown by us to inhibit the in vitro proliferation rate and to delay tumor development of a human melanoma cell line (A375) in nude mice. We hypothesized that Hsp27 may influence the neoplastic phenotype. In the present study Hsp27 transfectants from this cell line were analyzed for various cellular aspects associated with the metastatic process. We found that Hsp27-overexpressing clones exhibited an altered cellular morphology as compared with control transfected cells. The Hsp27-positive cells tended to develop an epithelial-like phenotype growing in clusters and were characterized by a loss of transcytoplasmic stressfibers. In parallel, Hsp27-expressing cells lost the ability to form colonies in soft agar. The invasive potential was studied in vitro by the use of a reconstituted extracellular matrix–coated filter (Matrigel). Compared with controls, Hsp27-overexpressing cells showed decreased cell invasiveness through Matrigel. A correlation between invasion and activation of matrix metalloproteinases (MMPs) has been shown in several cell models. Secretion of MMPs (MMP-2 and MMP-9) was studied by gelatin-substrate zymogram analysis, as well as by a sensitive gelatinase activity assay. The Hsp27-transfected A375 melanoma cell line showed decreased secretion of MMP-2 and MMP-9 as compared with the control transfected cells. Integrins are adhesion receptors and function in cell invasion by mediating cell movement on matrix molecules and by regulating the expression of MMPs. Both fluorescence-activated cell sorter analysis and immunofluorescence analysis revealed a loss of αvβ3 integrin in Hsp27-transfected cell colonies. Our results demonstrate that Hsp27 overexpression has a profound impact on several parameters regulating the invasive and metastatic potential of melanoma cells in vitro.
INTRODUCTION
The heat shock proteins (Hsps), also called stress proteins, are ubiquitous molecules induced in cells exposed to sublethal heat shock, present in all living cells, and highly conserved during evolution (Hightower 1991). Their functions are to protect cells from environmental stress damage by binding to partially denatured proteins, to regulate the correct folding, and to cooperate in transporting newly synthesized polypeptides to the target organelles. These molecular chaperones are involved in normal and pathological processes, including cancer, revealing the changes in expression (Morimoto et al 1994).
The small Hsp Hsp27 acts as a molecular chaperone and has a variety of functions, including the roles in signal transduction, regulation of growth (Spector et al 1992, 1993; Knauf et al 1993; Kindas-Mügge et al 1996, 1998; Richards et al 1996), development (Pauli et al 1990; Gernold et al 1993; Michaud et al 1997; Jantschitsch et al 1998), differentiation (Shakoori et al 1992; Stahl et al 1992; Kindas-Mügge and Trautinger 1994; Spector et al 1995; Trautinger et al 1995), and tumorigenesis (Ciocca et al 1993). The Hsp27 has been shown to have functional properties that are typical for oncogenes and antioncogenes. Increased levels of Hsp27 have been detected in a number of malignancies, such as breast cancer (Thor et al 1991), endometrical cancer (Geisler et al 1999), and prostate cancer (Bonkhoff et al 2000), and are considered as a negative prognostic factor. In contrast, in patients with malignant fibrous histiocytoma (Tetu et al 1992) and neuroblastoma (Ungar et al 1994), high levels of Hsp27 have been correlated with good prognosis. An inverse relationship between Hsp27 and oncogenicity has been observed in adenovirus-transformed rat cells (Zantema et al 1989).
Tumor progression to the invasive and metastatic stage represents the most ominous development in tumorigenesis. Cell motility and the ability of invasive growth are considered to be of special importance in the metastatic cascade. Because we found (Kindas-Mügge et al 1996) that the overexpression of Hsp27 in a stably transfected melanoma cell line (A375) was accompanied by the inhibition of the growth rate in vitro and by a delay and a reduced rate of tumor appearance in athymic nude mice, we started to investigate whether Hsp27 overexpression has an influence on the metastatic tumor process. A375 cell clones stably transfected with Hsp27 were established (Kindas-Mügge et al 1996). Various cellular aspects associated with the metastatic phenotype, namely, cell motility, invasion, secretion of matrix metalloproteinases (MMPs), and expression of integrins, were analyzed.
MATERIALS AND METHODS
Transfection and cell culture
The melanoma cell line A375 was obtained from the American Type Culture Collection (Rockville, MD, USA). A375 cells were transfected with the human Hsp27 gene together with the plasmid pSG5neo conferring resistance to G418 by calcium phosphate precipitation. Stably transfected clones were selected as described previously (Kindas-Mügge et al 1996). The transfection vector carrying the human Hsp27 gene under the control of its own and the SV40 promoter (pSG2711) was a generous gift from Marja Jäättelä (Jäättelä et al 1992). Clones A375/2A and A375/11A express high levels of Hsp27. A375/neo is the mock transfected control cell line, and A375/wt designated the nontransfected (wild type) cells.
Cells were cultured in Dulbecco modified Eagle medium (DMEM, Life Technologies Inc, Paisley, Scotland, UK) supplemented with 10% fetal calf serum (FCS), penicillin (110 U/mL), and streptomycin (100 μl/mL) at 37°C in 7.5% CO2. Selective media used for the cultivation of transfected cell lines were further supplemented with G418 (0.4 mg/mL; Sigma, St Louis, MO, USA). Cells were subcultured weekly. All experiments were performed with cells grown less than 5 weeks after recovery from frozen stock.
Western blotting analysis of Hsp27 expression
Cells were rapidly trypsinized and lysed by repeated freeze-thawing cycles in distilled water containing 1 mM phenylmethylsulfonylfluoride (Sigma), aprotinin (1–2 μg/mL, Sigma), and leupeptin (1–2 μg/mL, Sigma). The lysates were centrifuged, and the supernatants were collected. The total protein content was measured (Bio-Rad Protein Assay, Bio-Rad Lab, Hercules, CA, USA), and 10 μg of the total protein was subjected to 14% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After electrophoresis and electrophoretic transfer onto nitrocellulose membranes, Hsp27 was visualized using a specific monoclonal antibody (MAb) (Neo Markers, Fremont, CA, USA) by incubating the blot overnight at 4°C. The bound antibody was detected by incubation with the peroxidase-conjugated rabbit anti-mouse antibody (Dako Diagnostics Ag, Vienna, Austria) and by chemoluminiscence, according to instructions of the manufacturer (ECL, Amersham Pharmacia Biotech, Buckinghamshire, UK).
Immunofluorescense analysis of actin
To determine the effect of Hsp27 on the actin filament organization, cells were grown in tissue culture chambers (Nunc Lab Tek, Naperville, FL, chamber slides). The cells were washed with phosphate-buffered saline (PBS) (10 mM NaH2PO4, 130 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, pH 7.5) and fixed for 15 minutes in methanol-acetone (1:1) at −20°C, followed by incubation with fluorescein-conjugated phalloidin (0.1 mg/mL), a probe specific for F-actin, for 45 minutes. The cells were washed in PBS and analyzed using indirect immunofluorescence confocal microscopy.
Colony formation
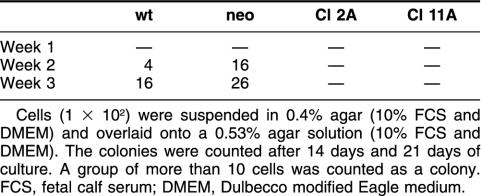
To examine the Hsp27 influences on anchorage-independent growth of A375, cells (1 × 102) were suspended in 0.4% agar (10% FCS-DMEM) and overlaid onto a 0.53% agar solution (10% FCS-DMEM). Cultures were maintained at 37°C, and the rate of colony growth was assessed by counting colonies after 14 days and 21 days. A group of more than 10 cells was counted as a colony.
In vitro invasion assay
To assess the invasive potential, the Matrigel-coated filter system (Becton Dickinson Labware, Bedford, MA, USA) was used. The Matrigel matrix is a reconstituted and solubilized basement membrane preparation. The Matrigel invasion chambers are characterized by an 8.0-μm pore size (1 × 105 pores/cm2), and because the Matrigel matrix occludes the pores of the polyethylene terephthalate (PET) membrane, noninvasive cells are blocked from migrating through the membrane. The chambers were rehydrated with 1 mL media for 2 hours at room temperature. After rehydration, the chambers were placed in the lower compartment, which was previously loaded with 500 μL of the media. Cell suspensions (4 × 105 cells per 900 μL medium) were added to the invasion chambers, and the cells were allowed to invade the Matrigel at 37°C in 10% CO2 for 24 hours. After incubation, noninvading cells were removed by gently washing the filters. The filters were stained by papanicolaou, and cells were counted on the lower surface of the membrane and expressed as a percentage of the sum of the control cells to wild type cells. Also, invaded cells in the lower well were stained and counted.
Zymography of MMPs
MMPs MMP-2 and MMP-9 have gelatinase activity and were analyzed by gelatin-substrate zymography.
Cell lines (1–2 × 107 cells) were grown to 90% confluency and incubated for 24 hours in a medium (10 mL) with phorbol-12-myristate-13-acetate (PMA, 100 ng/mL, Sigma) and without serum. The cultured medium was collected, separated from residual cells by centrifugation, and concentrated 50- to 100-fold by Biomax filter tubes (Millipore, Bedford, MA, USA). The samples were stored at −80°C until analysis.
Samples (10 μL) were run on 10% polyacrylamide gels copolymerized with 0.1% gelatin. After electrophoresis, the gels were washed twice with 2.5% Triton for 15 minutes and developed overnight in 50 mM Tris-HCL buffer, pH 7.5, containing 200 mM NaCl, 5 mM CaCl2, 1 μM ZnCl2, 0.07% NaN3, and 1% Triton at 37°C. Gels were fixed in methanol–acetic acid (1:1), stained with Coomassie blue, and destained in multiple changes of methanol–acetic acid (50:10%). Proteolysis was detected as clear bands in a dark field.
Gelatinase activity assay
The MMP activity was, in addition, measured by a gelatinase activity assay, using a convenient available kit as described by the manufacturer (Roche Diagnostics GmbH, Mannheim, Germany). Briefly, aliquots of the samples were activated with a 4-aminophenyl-mercury acetate solution (2.5 mM, pH 7.0–7.5) and incubated for 2 hours at 37°C. The samples were added to biotin-labeled gelatin, and the MMPs were allowed to digest the gelatin for a reaction time of 60 minutes at 37°C. After incubation, reaction products were transferred to a streptavidin-coated microtiter plate and were shaken for 30 minutes at room temperature. The biotin-labeled gelatin or a fragment thereof binds to the streptavidin-coated microtiter plate via its free biotin residues. The plate was washed and incubated for 60 minutes at room temperature with streptavidin-peroxidate (POD), which can bind to the remaining free biotin residues of the biotin-labeled gelatin. After washing, the color solution 2,2 Azino-bis-(3′-ethyl benzothiazoline)-6-sulfonic acid (ABTS) was added, and in 5-minute intervals the color formation was measured at 450 nm (reference wavelength 562 nm) with a microtiter plate photometer. For a correct blank the same volume of serum-free medium was concentrated and treated in the same way. In samples with low gelatinase activity, POD is bound by strepatavidin to the gelatinase-biotin complex and converts the added enzyme substrate to a green end product, measured at 450 nm. In samples with high gelatinase activity, small fragments that have no capacity to bind the POD conjugate occur, and no color reaction occurs. High gelatinase activity yields a low signal, and low activity causes high signals. MMP-9 (Alexis Biochemicals, Eubio, Vienna, Austria) was used as a positive control.
Expression of integrins αvβ3, αvβ5, and ICAM-1: fluorescence-activated cell sorter and immunofluorescence analyses
Cell suspensions (1 × 106 cells per 100 μL PBS) were incubated with appropriate dilutions of the anti-integrin antibody for 45 minutes at 4°C (1:25 αvβ3 (4 μg), 23C6, Santa Cruz Biotechnology Inc, Santa Cruz, CA; 1:50 αvβ5 (4 μg), MAB1961, Chemicon Int, Temecula, CA, USA; 1;rc10 ICAM-1 (1 μg), Biodesign, Saco, ME). The cells were washed and incubated with secondary phycoerythrin (PE)-labeled anti-IgG (20 μL, Becton Dickinson, San Jose, CA, USA) for 45 minutes at 4°C. After a final washing, the cells (500 μL PBS) were analyzed with a flow cytometer (FACS Calibur, Becton Dickinson; immunocytometry systems). For a negative control, the cells were incubated with isotype IgG11 antibody (Becton Dickinson).
For immunofluoresence staining of αvβ3, cytospin preparations incubated with anti-integrin antibody, followed by secondary PE-labeled anti-IgG, as indirect immunofluorescence confocal microscopy.
RESULTS
Expression of Hsp27 transfectants
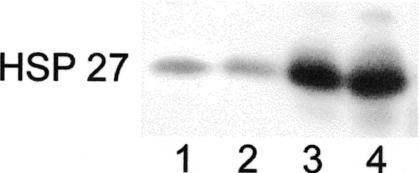
In order to assess the influence of Hsp27 on the metastatic process, Hsp27 transfectants from the A375 melanoma cell line were analyzed by Western blot analysis for Hsp27 expression (Fig 1). Cell lines transfected with the plasmids for neo and wt were used as controls.
Fig 1.
Western blot analysis of Hsp27-transfected and control cells (A375). An equal amount (10 μg) of cell lysates were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electrophoretically transferred onto the nitrocellulose membrane, and analyzed by using the anti-human Hsp27 antibody. Lanes 1–4: A375/wt, A375/neo, A375 Cl 2A, and A375 Cl 11A
Morphology of Hsp27 transfectants and localization of actin
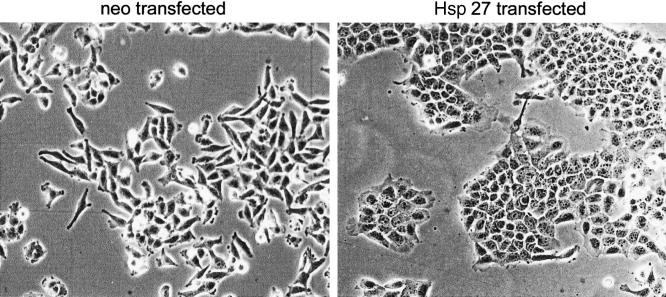
As observed by microscopy, Hsp27-overexpressing clones tended to exhibit an epithelial-like phenotype, growing in clusters, exhibiting enhanced plasma membrane ruffling and lamellipodia-like structures (Fig 2). Control transfected cells displayed a lowly differentiated phenotype with elongated structures and filopodia-like protrusions. This altered cell morphology indicates an influence of Hsp27 on the cytoskeletal organization.
Fig 2.
Morphology of neo- and Hsp27-transfected (clone 11A) cells (A375)
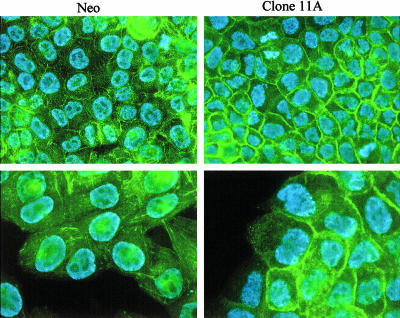
To study the intracellular distribution of actin in the cells, immunofluorescence microscopy was performed, using fluorescein isothiocyanate–conjugated phalloidin as the F-actin dye. In Hsp27 transfectants, increased concentrations of F-actin were found at the cell cortex (Fig 3). In contrast, control cells showed low cortical F-actin concentration and numerous cytoplasmic stress fibers.
Fig 3.
Localization of F-actin in neo- and Hsp27-transfected (clone 11A) cells (A375). Fluorescein isothiocyanate–conjugated phalloidin was used in conjunction with indirect immunofluorescence confocal microscopy to study the intercellular distribution of actin in neo- and Hsp27-transfected (clone 11A) cells. Two magnifications are shown (400×, 630×)
Colony-forming ability of Hsp27-transfected clonal cells and control cells
To clarify the relationship between Hsp27 and anchorage-independent growth, we investigated the effect of overexpression of Hsp27 on the growth potential of A375 cells in soft agar. The colony-forming ability of transfected and control cells was examined. As shown in Table 1, Hsp27-transfected cells had completely lost its colony-forming ability, characteristic for A375/wt and A375/neo.
Table 1.
Number of colonies in soft agar
Invasive potential of Hsp27-transfected and control cells
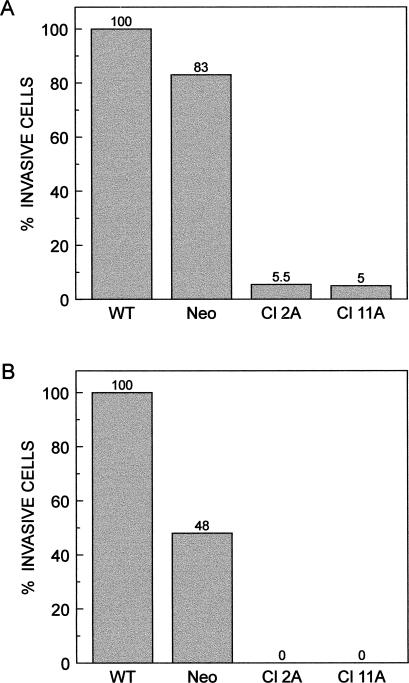
The invasiveness of the cells was studied by the use of Matrigel-coated filters. Cells suspended in the medium were allowed to invade the Matrigel for 24 hours and 72 hours. Cell migration was measured either as the proportion of cells that moved from the top to the bottom of the filters (24 hours) or as the proportion of cells that moved from the top to the bottom chambers (72 hours). The Hsp27-overexpressing clones showed much less (5% of control and wild type) invasive capacity than did the control cells within 24 hours (Fig 4A; Table 2). After 72 hours of incubation, the number of cells that passed through the filters into the lower compartment were counted. No Hsp27 transfectants were found to have penetrated the Matrigel filter (Fig 4B). According to these results, Hsp27-overproducing transfectants compared with control cells showed reduced cell invasiveness.
Fig 4.
(A) Invasive potential of Hsp27-transfected and control cells (A375). A total of 4 × 105 cells was used for the invasion assay, and they were allowed to invade the Matrigel for 24 hours. The filters were stained, and cells were counted on the lower surface of the membrane and expressed as a percentage of the sum of control cells to wild type cells. (B) After 3 days of incubation, the number of cells that passed through the filters into the lower well were counted and expressed as a percentage of the sum of control cells to wild type cells penetrating through
Table 2.
Invasive potential of Hsp27-transfected and control cells
Gelatin zymography
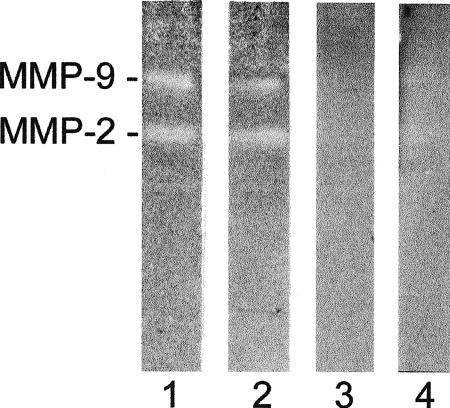
MMPs activity was analyzed by a traditional gelatin zymography assay. Serum-free conditioned media from Hsp27 transfectants and control cells incubated for 24 hours with PMA were analyzed by zymography (Fig 5). The wt as well as the neo-transfected cells released considerable amounts of MMP-2 and MMP-9. No enzyme activity was demonstrated by the Hsp27-transfected cells.
Fig 5.
Zymography analysis of Hsp27 transfectants and control cells (A375). Lanes 1–4: A375/wt, A375/neo, A375 Cl 2A, and A375 Cl 11A
Gelatinase activity assay
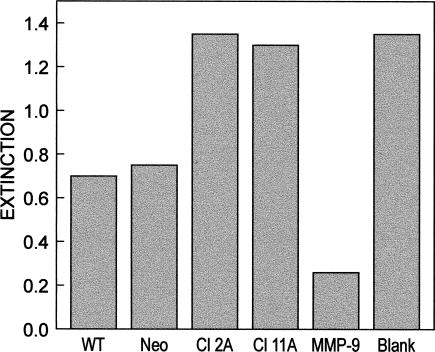
In order to confirm the results of the zymography, a sensitive evaluation system for gelatinase activity in a microplate format was used. In this assay system, a low extinction value indicated high activity and consequently high MMP release of the tested cell line. Conditioned media from Hsp27 transfectants and control cells were analyzed. Supernatants of Hsp27 clones reached distinctly higher absorbance, ie, lower gelatinase activity values as compared with control cell supernatants (Fig 6). This indicated a decreased secretion of MMP-2 and MMP-9, which is in agreement with our results from gelatin zymography (Fig 5).
Fig 6.
Gelatinase activity assay. Lanes 1–4: A375/wt, A375/neo, A375 Cl 2A, and A375 Cl 11A; lane 5: positive control, MMP-9 (2 ng), lane 6: negative control, control reaction with the serum-free medium without the enzyme
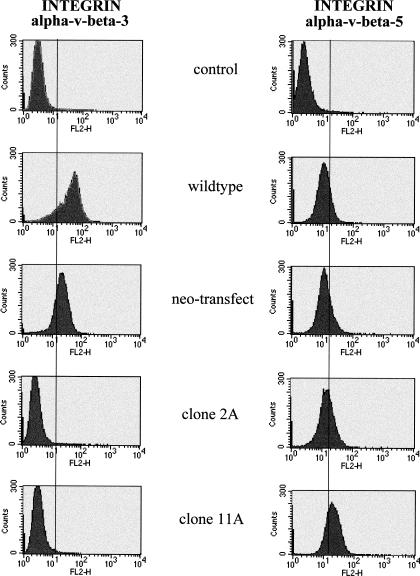
Fluorescence-activated cell sorter analysis of the expression of integrins αvβ3, αvβ5, and ICAM-1
Because it is known that αvβ3 integrin expression is upregulated in malignant melanoma as compared with the melanocytes (Albelda et al 1990), we investigated if Hsp27 overexpression may have an influence on the integrin expression pattern. The Hsp27-transfected cell lines, as well as the controls, were incubated with antibodies to the extracellular domain of the human αvβ3 integrin subunit and were then analyzed by fluorescence-activated cell sorter (FACS). Control cells expressed high levels of αvβ3, whereas values for Hsp27-transfected cells did not exceed background levels (Fig 7).
Fig 7.
FACS analysis of integrins αvβ3 and αvβ5 expression in Hsp27-transfected and control cells (A375). Cells were incubated with antibodies to the respective integrin subunit and analyzed by FACS. Control serum, subclass-matched immunoglobulin, was used as the negative control. FACS, fluorescence-activated cell sorter
We next determined whether the different cell lines express the αvβ5 complex, which has some ligand overlap with αvβ3 but appears functionally distinct. In both control cells as well as Hsp27 transfectants a comparable expression of αvβ5 was identified (Fig 7). Also, the expression of ICAM-1 was equal in all tested cell lines (data not shown).
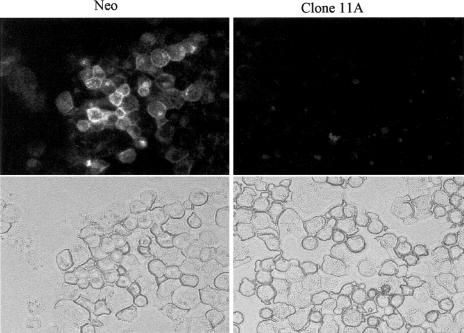
Immunofluorescence analysis of integrin αvβ3 expression
Because of the striking difference in αvβ3 expression between Hsp27 transfectants and control cells by FACS analysis, an immunofluorescence analysis was also performed. Cytospin preparations incubated with αvβ3 integrin antibodies were analyzed with indirect immunofluorescence confocal microscopy. The Hsp27 cells failed to display αvβ3 immunostaining in contrast to the controls (Fig 8). These data confirm the results of the FACS analysis of the expression of the αvβ3 integrin shown in Fig 7.
Fig 8.
Immunofluorescence analysis of integrin αvβ3 expression in neo- and Hsp27-transfected (11A) cells (A375). Cytospin preparations incubated with αvβ3 were analyzed with indirect immunofluorescence confocal microscopy
DISCUSSION
In the present study the role of the human Hsp Hsp27 in metastatic processes as defined by in vitro behavior, such as cell motility and invasion, secretion of MMPs, integrin expression, and anchorage-independent cell growth, was examined. Stable Hsp27-expressing transfectants of a melanoma cell line and cells transfected with the plasmid for neomycin as well as wild-type cells (A375) were analyzed (Kindas-Mügge et al 1996).
We observed that Hsp27 overexpression in this human melanoma cell line was associated with altered cell morphology. A less elongated structure with the tendency to grow in clusters compared with the control cells was demonstrated. This might be an effect on the organization of the cytoskeleton and is in accordance with Lemieux et al (1997), who found that the Hsp27-overexpressing human breast cancer cell line MDA-MB231 exhibited a disrupting effect on a more elongated structure (filopodia-like form). In addition, Mairesse et al (1996) have reported that inhibition of Hsp27, using MCF-7 breast cancer cells transfected with human Hsp27 complementary deoxyribonucleic acid in the antisense orientation, promoted the reorganization of the cytoskeleton.
Actin filaments (F-actin) participate in a number of motile events of a cell. We found an increased concentration of F-actin at the cell cortex in the Hsp27-overexpressing clone, indicating an epithelial-like phenotype. This suggests that Hsp27 might directly affect the cytoskeletal organization in this cell line. Modulation of the actin microfilaments dynamics caused by the overexpression of Hsp27 has been reported in fibroblastic cells by Lavoie et al (1993). One of the major roles of Hsp27 in vivo may be in the regulation of the structure and dynamics of actin filaments (Miron et al 1991; Benndorf et al 1994; Landry and Huot 1999).
Cell motility and the ability of invasive growth are considered to be of special importance for the metastatic behavior of cancer cells. To assess cell invasion in vitro, we used Matrigel-coated filters, an assay widely used for in vitro measurement of the invasive capacity of malignant cells. We found a decreased cell invasiveness in Hsp27-overexpressing cells as compared with the control cells. The disrupting effect of Hsp27 on the filopodia-like membrane structure and the F-actin reorganization into an epitheloid phenotype may explain the reduced cell invasiveness in the Matrigel assay. However, Lemieux et al (1997), who also found Hsp27-transfected cells (breast cancer cell line) to be less elongated than the control transfected cells, reported a decreased cell motility but an increased invasiveness in Matrigel. The difference observed in vitro between the cell lines might reflect the tissue specificity of the Hsp27 function.
Proteolytic enzymes such as MMPs are important factors for tissue invasion of malignant cells. A correlation between the activation of MMPs and the invasion through basement membrane like Matrigel barriers has been demonstrated (Martelli et al 1993). The secretion of gelatinases MMP-2 and MMP-9, both of which are often overexpressed in malignant cells, was investigated in Hsp27 transfectants and control cells. We found a reduced secretion of MMP-2 and MMP-9 in Hsp27-overexpressing cells, analyzed by zymograms as well as by a gelatinase activity assay. This corresponds well with the reduced rate of invasion of Hsp27-transfected cells through Matrigel.
Interaction with the extracellular matrix is mediated primarily through the integrin family of the cell adhesion molecules. These cell surface receptors are heterodimers and consist of α and β subunits. It has been observed that the αvβ3 expression is characteristic for melanoma cells (Albelda et al 1990), it is not expressed on normal melanocytes, and its appearance coincides with its progression to the invasive phase. The most striking difference noted between our cell lines was the marked contrast in the expression of the αvβ3 subunit. Flow cytometry analysis with anti-integrin–specific MAbs to αvβ3 integrin revealed a high expression level in control cells, whereas Hsp27-transfected cells failed to express this subunit. These findings were confirmed by immunofluorescence analysis, using labeled anti-integrin αvβ3 MAb. The Hsp27 transfectants did not react with this antibody.
Evidence that the integrin αvβ3 localizes with MMP-2 in a functionally active form on the cell surface has been demonstrated (Brooks et al 1996). The integrin αvβ3 is known to recognize arginine glycine aspartic acid (RGD) binding sites on gelatin (partly degraded collagen), which leads to the up-regulation of the MMP-2 expression. MMP-2 then completes the degradation of gelatin (Ivaska and Heino 2000). This suggests that the contrast in the expression of the αvβ3 subunit between transfectants and control cells might account for the low expression of MMP-2, as well as the low invasion potential. These findings might indicate that a single cell surface receptor can regulate both cell migration and matrix degradation, thereby facilitating invasion.
We and others have reported that the cell growth arrest of malignant cells is related to Hsp27 (Spector et al 1992, 1993; Knauf et al 1993; Kindas-Mügge et al 1996, 1998; Richards et al 1996). The influence of Hsp27 on the anchorage-independent growth potential was examined in A375 cells. The Hsp27 transfectants showed no colony formation as compared with control cells, which indicates a less aggressive growth behavior.
It is suggested that Hsp27 phosphorylation is closely linked to p38 stress-activated protein kinase (Landry and Huot 1999). The Hsp27 is a downstream target of p38, and the function of Hsp27 as an actin polymerization modulator has been demonstrated (Miron et al 1991). It has been shown that the p38 mitogen-activated protein kinase (MAPK) also plays an important role in the regulation of MMP expression (Westermarck et al 1998). Specially blocking the p38 MAPK pathway by specific chemical inhibitor (SB2035580) inhibits Hsp27 phosphorylation as well as the expression of some MMPs (MMP-1, MMP-9, and MMP-13) in transformed keratinocytes, melanoma, and squamous cell carcinoma cells (Simon et al 1998). In addition, the invasion of cells through a Matrigel basement membrane was reduced by 60% after the inhibition of the p38 MAPK (Denkert et al 2001). Whether there is a relationship between p38-Hsp27 and the p38-MMP pathways is not known. As a working hypothesis, one can propose that the importance of the p38-Hsp27 pathway is related to the concentration of Hsp27. In cells with a high level of Hsp27 expression, activation of the p38-Hsp27 pathway probably has a dominant influence on the organization of the cytoskeleton. In this situation Hsp27 could compete with other regulatory proteins—in this case, the regulation of the p38-MMPs pathway, leading to a reduction of MMP secretion and Matrigel invasion.
Our findings regarding Hsp27 might have potential implications for melanoma therapy. Metastatic melanoma is an example of a malignant disease that responds poorly to various treatments including chemotherapy. The goal of cancer gene therapy is to administer a vector expressing therapeutic genes to treat cancer cells in order to prevent tumor dissemination through the body. The main problem of gene therapy is “how to maximize the delivery of the genes of interest specifically to the tumor cells.”
Recently, Park et al (1999) have shown, by combining the lineage-specific human tyrosinase promoter with a copy of an upstream-acting melanocyte-specific enhancer element, a melanoma-specific gene expression, increasing the cytotoxicity of the purine nucleoside phosphorylase gene for prodrug activation in melanoma cells.
Summing up, our data show that Hsp27 has a distinct impact on several cellular characteristics involved in the process of malignant progression of the melanoma cells. The Hsp27-induced reversal of the malignant phenotype of A375 cells suggests this stress protein as an interesting target for melanoma gene therapy. Local regional application of specific agents or delivery of genes for the induction of Hsp27 may prove the concepts for the development of new strategies for the treatment of solid malignancies.
Acknowledgments
This work was supported by Hochschuljubiläumsstiftung der Stadt Wien, grant number H-223/99 and a research fellowship to S.A. from the Institute of Cancer Research, University of Vienna.
REFERENCES
- Albelda S, Mette S, Elder D, Stewert R, Damjanovich L, Herlyn M, Buck C. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- Benndorf R, Haye K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein hsp25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269(32):20780–20784. [PubMed] [Google Scholar]
- Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Estrogen receptor gene expression and its relation to the estrogen-inducible hsp27 heat shock protein in hormone refractory prostate cancer. Prostate. 2000;45(1):36–41. doi: 10.1002/1097-0045(20000915)45:1<36::aid-pros4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Brooks P, Strömblad S, Sanders L, Schalscha T, Aimes R, Stetler-Stevenson W, Quigley J, Cheresh D. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Ciocca D, Oesterreich S, Chamness G, McGuire W, Fugua A. Biological and clinical implications of heat shock protein 27 000(Hsp27). A review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- Denkert C, Siegert A, Leclere A, Turzynski A, and Hauptmann S 2001 P38 mitogen-activated protein kinase regulates MMP-2 expression and invasion of malignant melanoma cells. Proc Amer Assoc Cancer Res 41, BL3-04. [DOI] [PubMed] [Google Scholar]
- Geisler J, Geisler H, Tammela M, Miller G, Wiemann M, Zhou Z. A study of heat shock protein 27 in endometrial carcinoma. Gynecol Oncol. 1999;72:347–350. doi: 10.1006/gyno.1998.5283. [DOI] [PubMed] [Google Scholar]
- Gernold M, Knauf U, Gaestel M, Stahl J, Koetzel P. Development and tissue-specific distribution of mouse small heat shock protein hsp25. Dev Genet. 1993;14:103–111. doi: 10.1002/dvg.1020140204. [DOI] [PubMed] [Google Scholar]
- Hightower L. Heat shock proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Heino J. Adhesion receptors and cell invasion: mechanisms of integrin-guided degradation of extracellular matrix. Cell Mol Life Sci. 2000;57:16–24. doi: 10.1007/s000180050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäättelä M, Wissing D, Bauer P, Li G. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantschitsch CH, Kindas-Mügge I, Metze D, Amann G, Micksche M, Trautinger F. Expression of the small heat shock protein hsp27 in developing human skin. Br J Dermatol. 1998;139:247–253. doi: 10.1046/j.1365-2133.1998.02361.x. [DOI] [PubMed] [Google Scholar]
- Kindas-Mügge I, Herbacek I, Jantschitsch Ch, Micksche M, Trautinger F. Modification of growth and tumorigenicity in epidermal cell lines by DNA-mediated gene transfer of 27 000 heat shock protein (hsp27) Cell Growth Diff. 1996;7:1167–1174. [PubMed] [Google Scholar]
- Kindas-Mügge I, Micksche M, Trautinger F. Modification of growth in small heat shock (hsp27) gene transfected breast carcinoma. Anticancer Res. 1998;18:413–418. [PubMed] [Google Scholar]
- Kindas-Mügge I, Trautinger F. Increased expression of the 27 000 heat shock protein (hsp27) in in-vitro differentiated normal human keratinocytes. Cell Growth Differ. 1994;5:777–781. [PubMed] [Google Scholar]
- Knauf U, Bielka H, Gaestel M. Overexpression of the small heat shock protein hsp25 inhibits growth of Ehrlich ascites tumor cells. J Cell Physiol. 1993;156:619–625. [Google Scholar]
- Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat shock protein 27 kDa (hsp27) Biochem Soc Symp. 1999;64:79–89. [PubMed] [Google Scholar]
- Lavoie J, Hickey E, Weber L, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268(32):24210–24214. [PubMed] [Google Scholar]
- Lemieux P, Oesterreich S, Lawrence J, Steeg P, Hilsenbeck S, Harvey J, Fugua S. The small heat shock protein hsp27 increases invasiveness but decreases motility of breast cancer cells. Invasion Metastasis. 1997;17:113–123. [PubMed] [Google Scholar]
- Mairesse M, Horman S, Mosselmans R, Galand P. Antisense inhibition of the 27kDa heat shock protein production affects growth rate and cytoskeletal organization in MCF-7 cells. Cell Biol Int. 1996;20(3):205–212. doi: 10.1006/cbir.1996.0025. [DOI] [PubMed] [Google Scholar]
- Martelli M, Campana A, Bischof P. Secretion of matrix metalloproteinases by human endometrial cells in vitro. J Reprod Fertil. 1993;98:67–76. doi: 10.1530/jrf.0.0980067. [DOI] [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay R. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53(1):104–113. doi: 10.1007/PL00000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114(2):255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R, Tissieres A, and Georgopoulos C 1994 The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1–30. [Google Scholar]
- Park B, Brown C, Yun H, Alexander H, Horti J, Raje S, Figg W, Bartlett D. Augmentation of melanoma-specific gene expression using a tandem melanocyte-specific enhancer results in increased cytotoxicity of the purine nucleoside phosphorylase gene in melanoma. Hum Gene Ther. 1999;10:889–898. doi: 10.1089/10430349950018292. [DOI] [PubMed] [Google Scholar]
- Pauli D, Tonka CH, Tissieres A, Arrigo A. Tissue-specific expression of the heat shock protein hsp27 during Drosophila melanogaster development. J Cell Biol. 1990;111:817–828. doi: 10.1083/jcb.111.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E, Hickey E, Weber L, Master J. Effect of overexpression of the small heat shock protein hsp27 on the heart and drug sensitivities of human testis tumor cells. Cancer Res. 1996;56:2446–2451. [PubMed] [Google Scholar]
- Shakoori A, Obersdorf AM, Owen TA, Weber LA, Hickey E, Stein JL, Lian JB, Stein GS. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem. 1992;48:277–287. doi: 10.1002/jcb.240480308. [DOI] [PubMed] [Google Scholar]
- Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen activated protein kinase by SB 203580 blocks PMA induced Mr 92 000 IV collagenase secretion in in-vitro invasion. Cancer Res. 1998;58:1135–1139. [PubMed] [Google Scholar]
- Spector N, Hardy L, Ryan C, Wilson H, Miller WH Jr,, Humes J, Nadler L, Luedke E. 28-kDa mammalian heat shock protein, a novel substrate of a growth regulatory protease involved in differentiation of human leukemia cells. J Biol Chem. 1995;270(3):1003–1006. doi: 10.1074/jbc.270.3.1003. [DOI] [PubMed] [Google Scholar]
- Spector N, Ryan C, Levine H, Nadler L, Arrigo A. Heat shock protein is a unique marker of growth arrest during differentiation of HL-60 cells. J Cell Physiol. 1993;156:619–625. doi: 10.1002/jcp.1041560322. [DOI] [PubMed] [Google Scholar]
- Spector N, Samson W, Ryan C, Gribben J, Urba W, Welch W, Nadler L. Growth arrest of human B lymphocytes is accompanied by induction of the low molecular weight mammalian heat shock protein (hsp28) J Immunol. 1992;148(6):1668–1673. [PubMed] [Google Scholar]
- Stahl J, Wobus A, Ihrig S, Lutsch G, Bielka H. The small heat shock protein hsp25 is accumulated in P19 embryonal carcinoma cells and embryonic stem cells of line BLC6 during differentiation. Differentiation. 1992;51:33–37. doi: 10.1111/j.1432-0436.1992.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Tetu B, Lacasse B, Bouchard H, Lagace R, Huot J, Landry J. Prognostic influence of hsp27 expression in malignant fibrous histiocytoma: a clinicopathological and immunohistochemical study. Cancer Res. 1992;52:2325–2328. [PubMed] [Google Scholar]
- Thor A, Benz C, and Moore D. et al. 1991 Stress response protein (srp-27) determination in primary human breast carcinomas: clinical, histological and prognostic correlations. J Natl Cancer Inst. 83:170–178. [DOI] [PubMed] [Google Scholar]
- Trautinger F, Kindas-Mügge I, Dekrout B, Knobler R, Metze D. Expression of the 27 kDa heat shock protein in human epidermis and in epidermal neoplasms: an immuno-histological study. Br J Dermatol. 1995;133:194–202. doi: 10.1111/j.1365-2133.1995.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Ungar D, Hailat N, Strahler J, Kuick R, Brodeur G, Seeger R, Reynolds P, Hanash S. Hsp27 expression in neuroblastoma: correlation with disease stage. J Natl Cancer Inst. 1994;86:780–784. doi: 10.1093/jnci/86.10.780. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Holmström T, Ahonen M, Eriksson J, Kähäri V. Enhancement of fibroblast collagenase-1 (MMP-1) gene expression by tumor promotor okadaic acid is mediated by stress-activated protein kinases Jun N-terminal kinase and p38. Matrix Biol. 1998;17:547–557. doi: 10.1016/s0945-053x(98)90107-x. [DOI] [PubMed] [Google Scholar]
- Zantema A, de Jong E, Lardenoije R, van der Eb AJ. The expression of heat shock protein hsp27 and a complexed 22-kilodalton protein is inversely correlated with oncogenicity of adenovirus-transformed cells. J Virol. 1989;63:3368–3375. doi: 10.1128/jvi.63.8.3368-3375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]