Abstract
Current approaches to reducing restenosis do not balance the reduction of vascular smooth muscle cell proliferation with the increase in the healing of the endothelium. Building on our previous work, we present our study on the effects of Nitinol-based nanotubular coatings with different nanotube diameters on the reduction of restenosis. Here, we demonstrate that the nanotubular coatings reduced primary human aortic smooth muscle cell (HASMC) proliferation and increased the migration (by more than 4 times), collagen (by 2–3 times per cell) and elastin (by 5–8 times per cell) production of primary human aortic endothelial cells (HAEC). Furthermore, a significant increase in elastin and soluble collagen production of HAEC was observed with an increase in nanotube diameter. Our findings suggest that nanotubes-coated Nitinol may provide a surface conducive for HAEC reendothelialization while reducing the proliferation of HASMC.
Keywords: nanotube diameter, nitinol, restenosis, primary human aortic endothelial and smooth muscle cells, collagen and elastin production
Graphical abstract

INTRODUCTION
Percutaneous coronary interventions are a common medical procedure in the United States of America.1 About 75% of the patients who received a stent in these procedures were given a drug eluting stent (DES).1 DES have greatly decreased the rates of in-stent restenosis (ISR) but interventional centers have recorded increased incidences of late stent thrombosis (LST) as compared to bare metal stents (BMS).2 While the incidences of LST are rare, they are typically associated with high morbidity and mortality.2 The causes for LST in DES may be complicated and device-specific3 but the main underlying concern is the delayed endothelium healing that has been observed in patients with DES suffering from LST.2,4,5 Therefore, it is vital that any approach to reduce ISR has to not only target the proliferation of vascular smooth muscle cells but also improve the healing of the endothelium.
Consequently, there have been studies that describe alternative approaches to overcoming the problem of ISR without the use of drugs. Nanotopography presents a particularly interesting approach due to its ability to modulate cell functionality.6–11 Specifically, studies have shown that endothelial cell functions such as cell spreading, migration12 and proliferation are enhanced on vertically aligned nanotubular coatings7,10,13 while vascular smooth muscle cell functions such as proliferation and migration are decreased.7,12,14
However, in addition to investigating proliferation and migration, it is also essential to evaluate the production of collagen and elastin from vascular endothelial and smooth muscle cells. These two proteins are vital components of the extracellular matrix (ECM) in blood vessels and play significant roles in signaling and modulating the functions of vascular smooth muscle cells. Vascular smooth muscle cells are understood to produce excessive collagen in response to vascular injury, leading to neointimal hyperplasia (NIH) and ISR.15–17 However, polymerized collagen fibrils reduce vascular smooth muscle cell proliferation via the α2β1 signaling pathway.18,19 This may be one of multiple pathways with which the healing of the endothelium can help reduce NIH. Therefore, it is also important to understand the effect of the coating on vascular endothelial cell collagen production. We are also interested in the effects of the coating on elastin production. Besides playing an important role structurally, elastin also regulates the proliferation of vascular smooth muscle cells.20,21 When elastin was delivered as a therapeutic, it was shown to reduce vascular smooth muscle cell proliferation and migration.21 Furthermore, elastin content at the lesion site has been shown to have an inverse correlation to rates of restenosis.22
Recently, we have demonstrated that nitinol-based nanotubular coatings have the potential for reducing restenosis while improving endothelial healing via endothelial cell spreading and migration.12 Moreover, studies by other groups have also shown that the diameter of upright nanotubes has an effect on the functions of cells culture on them.8–10,23 Incorporating these findings and our previous work, we have designed an investigation into the effects of nitinol-based nanotubular coatings with controlled diameters of 110 and 70 nm on the functions of primary human aortic endothelial cells (HAEC) and human aortic smooth muscle cells (HASMC).
MATERIALS AND METHODS
Substrate Preparation
Nitinol foils (superelastic, pickled surface, Alfa Aesar, U.S.A.) were cut into 1 × 1 cm pieces and cleaned successively by ultrasonication in dilute micro-90 solution (Internationl Products Corporation), acetone and ethanol. These were dried under nitrogen and used as control Nitinol substrates. For the 110 and 70 nm experimental groups, they were synthesized as described in our previous study.12 Anodization was carried out in a Teflon container with the cleaned Nitinol foil as the working anode and a platinum foil (Alfa Aesar, 0.1 mm thick, 1.5 × 3 cm, 99.99%) as the counter electrode. The electrolyte for the 110 nm experimental group contained 1.48 g of NH4F (Sigma-Aldrich), 490 mL of ethylene glycol (Sigma-Aldrich) and 8.35 mL of Millipore water. The electrolyte for the 70 nm experimental group contained 1.4 g of NH4F (Sigma-Aldrich), 490 mL of ethylene glycol (Sigma-Aldrich) and 8.35 mL of Millipore water. The voltage and anodization duration were 85 V and 4 min respectively for both experimental groups. Each substrate was thoroughly rinsed in Millipore water and ethanol after anodization and stored dry in a Petri dish at room temperature. For all cell culture experiments, the nitinol substrates were sterilized with ethanol and rinsed twice in sterile PBS before use.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was conducted at the San Francisco State University with a Carl Zeiss Ultra 55 FE-SEM. Nitinol substrates were sputter-coated (Cressington-HR sputter coater) with iridium to a thickness of 3 nm.
Cell Growth Assay and Staining
Primary HAEC and HASMC (Lonza, U.S.A.) were maintained and cultured following manufacturer’s instructions and all cell culture experiments were conducted with cells between passages 3 and 6. Cell growth assays were conducted in 24-well tissue culture plates. Each well contained one 1 × 1 cm nitinol substrate with a seeding density of 12 000 HAEC per well and 10000 HASMC per well. For immunofluorescence staining, substrates were rinsed with PBS, fixed in 3.7% paraformaldehyde solution and blocked with a solution containing 2.5% bovine serum albumin, 0.1% Triton-X and PBS. Cells were F-actin-stained with TRITC-conjugated Phalloidin (Sigma-Aldrich) and nuclei-stained with Hoechst 33258 (Invitrogen). Microscopy imaging was conducted with a Nikon C1si spectral confocal microscope. Images were compiled and cells were counted in ImageJ.
HAEC Migration Assay
The migration assay was conducted as described in our previous study.12 First, we prepared a 4 mg/mL rat tail collagen (BD Biosciences, U.S.A.) gel in a 12-well tissue culture plate following manufacturer’s instructions. Each well contained 285 μL of collagen gel. HAEC were seeded at 18000 cells per well on top of each gel and cultured until they reached confluency. Nitinol substrates were then cautiously pushed into the gels over the cells, with the nanotubular-coated side facing up. Cell culture was maintained for 4 days before the substrates were carefully lifted and transferred into fresh 24-well tissue culture plates. Cell number was quantified using the CyQuant assay (Molecular Probes, U.S.A.) according to manufacturer’s instructions.
Collagen Assay
HAEC and HASMC were seeded onto the Nitinol substrates and cultured in 24-well tissue culture plastic for 7 days using the same protocols as aforementioned. On day 5, 1 mL of fresh media was replenished per well. This media was then collected on day 7 and the soluble collagen content was analyzed using the Sircol Collagen Assay (Bicolor, U.K.) following manufacturer’s instructions (Isolation & Concentration protocol). Each Nitinol substrate was subsequently transferred to fresh 24-well tissue culture plastic and gently rinsed with sterile PBS. ECM collagen extraction was carried out following manufacturer’s instructions with ice-cold 0.5 M acetic acid (Sigma-Aldrich) and 0.1 mg/mL pepsin (Sigma-Aldrich) solution. Collagen content was normalized per cell and to the control group, using average cell numbers obtained from the cell growth assays.
Elastin Assay
HAEC and HASMC were seeded onto the Nitinol substrates and cultured in 24-well tissue culture plastic for 7 days using the same protocols as mentioned above. On day 7, each Nitinol substrate was carefully transferred into fresh 24-well tissue culture plastic and gently rinsed in sterile PBS. The Fastin Elastin Assay (Bicolor, U.K.) was used to quantify the α-elastin content (soluble tropoelastins, lathyrogenic elastins and insoluble elastins) from the cells following manufacturer’s instructions. Elastin production was normalized by cell number and to Control.
Statistical Analysis
All data are presented as mean ± standard error of mean. One-way ANOVA (α = 0.05) and t test were run using StatPlus. ANOVA values are only described for F > Fcrit.
RESULTS AND DISCUSSION
Substrate and Cellular Imaging
We synthesized nanotubular coatings on nitinol with two different anodization conditions as described above. After obtaining SEM images of the coatings, we characterized nanotube diameters using ImageJ and obtained average nanotube diameters of 106 nm ± 4 and 72 nm ± 5 nm for the two anodization conditions. For the purposes of this study, the nanotube diameters will be referred to as 110 and 70 nm (Figure 1A, B respectively). This was accomplished by changing the amount of ammonium fluoride in the electrolyte solution while maintaining the anodization voltage and duration. These, along with the flat unmodified nitinol substrates as control, formed the experimental groups for our study (henceforth referred to as 110, 70, and Control).
Figure 1.

Scanning electron micrograph of the nanotubular coating on the Nitinol substrates, with an average nanotube diameter of (A) 110 and (B) 70 nm. The control surface is shown in C. Scale bars represent 300 nm.
To observe the effects of the coating on cell morphology of HAEC and HASMC, we seeded these cells and cultured them for up to a week. The cells were fixed, stained, and imaged on a spectral confocal microscope on day 7 (Figure 2). We observed that HAEC cultured on Control (Figure 2A) were more confluent than on 110 and 70 (Figure 2B, C). HAEC were also observed with greater cell spreading on 110 and 70 than on Control. There were no observable differences in morphology between HAEC on 110 and 70. HASMC were also observed to be more confluent on Control (Figure 2F). Furthermore, we observed that the cells have become well-aligned. Taken together, these observations suggest that cell morphology might be affected more greatly by the presence of a nanotubular coating but not by the change in nanotube diameter from 110 to 70 nm.
Figure 2.
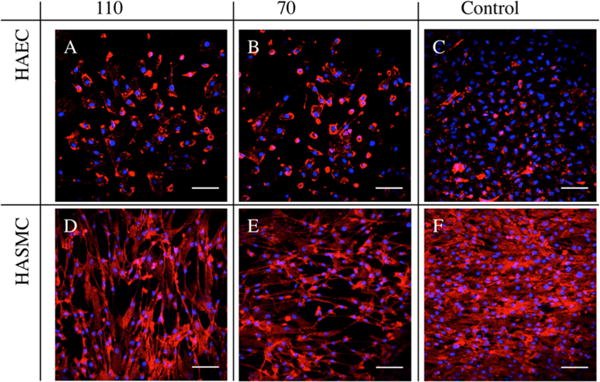
Immunofluorescent images of 7 day culture of (A–C) HAEC and (D–F) HASMC on 110, 70, and Control. Cells were nuclei-stained with DAPI and F-actin-stained with TRITC-Phalloidin (represented as blue and red, respectively). Scale bars are 50 μm. HAEC were observed to demonstrate increased cell spreading on (A) 110 and (B) 70 and were less confluent than Control (C). HASMC were well-aligned and observed to be more confluent on (F) Control than on (D) 110 and (E) 70.
HASMC Growth
The primary cause of restenosis is NIH, which has been associated with the excessive proliferation of vascular smooth muscle cells in response to the vascular injury sustained during angioplasty and stent deployment.15 As seen in Figure 3, the number of HASMC that adhered to 110 was significantly lower than that on Control after 1 day in culture. Furthermore, the number of HASMC on both 110 and 70 were significantly less than that on Control at day 7. This suggests that the nanotubular coatings have the potential to reduce the adhesion and proliferation of HASMC, which may prove useful in the context of restenosis. However, there was no significant difference between 110 and 70. This suggests that for the cellular functions of adhesion and proliferation, the HASMC respond similarly within the range of these size scales (110 and 70 nm) on nitinol substrates. This phenomenon was also observed in previous studies with other cell types on titania nanotubes of similar diameters.10,23
Figure 3.

HASMC numbers are shown after culture for 1 and 7 days. At day 1, there was a significant difference in the number of HASMC that were adhered to the 110 as compared to Control. By day 7, there was a significant difference in the number of HASMC on 110 and 70 as compared to Control. One-way ANOVA for Day 1 (F(2,12) = 7.719, p = 0.007). One-way ANOVA for Day 7 (F(2,12) = 10.121, p = 0.003). N = 5, * = p < 0.01.
HAEC Growth and Migration
We investigated the effects of two different nanotube diameters on HAEC growth. As seen in Figure 4, there was no significant difference in the number of HAEC that adhered to all three substrates after day 1. However, there was a significant difference in the number of HAEC on 110 and 70 as compared to Control on day 7. The proliferation rate of HAEC was lower on the nanotubes-coated nitinol substrates, with no significant difference seen between the two sizes. This data is also presented in terms of relative cell number between Day 7 and Day 1 for each experimental group in Table S1. This decrease in proliferation is similar to what we observed in our previous study12 and suggests that decreasing the nanotube diameter by approximately 35% did not affect the proliferation of HAEC on nitinol-based nanotubular coatings. It is important to note that the HAEC on the nanotubular coatings did proliferate and increase in number after 7 days.
Figure 4.

HAEC numbers are shown after 1 and 7 days of culture. There was no significant difference in the number of HAEC adhered after day 1. However, there was a significant difference in the number of HAEC on 110 and 70 as compared to Control after 7 days of culture. One-way ANOVA for day 7 (F(2,6) = 25.648, p = 0.001). N = 3, * = p < 0.01.
The proliferation of endothelial cells is not the only factor involved in the healing of the endothelium. Migration of endothelial cells is important for re-establishing reendothelialization of the injured endothelial layer after stent deployment. Therefore, we investigated the migration of HAEC onto the substrates. As shown in Figure 5, there was a significant increase in the number of HAEC that migrated onto the 110 and 70 as compared to Control. Both 110 and 70 nm diameters had a similar effect on HAEC migration. We noted that the fold increase in migration is greater than the decrease in proliferation. In the context of balloon angioplasty and stent deployment, there is typically severe denudation of the endothelium at the lesion site.17 For endothelium healing to occur, the migration of endothelial cells is required before they can proliferate and reform the endothelium. Therefore, the overall effect of the coatings on HAEC growth and migration combined may be greater than that of the control surface.
Figure 5.
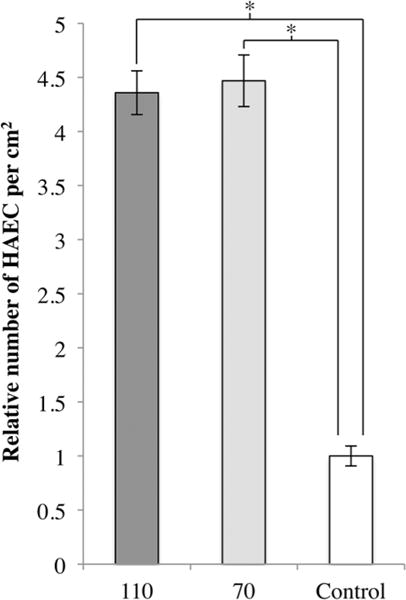
Relative number of HAEC that migrated onto each substrate after 4 days, normalized to Control. One-way ANOVA (F(2,9) = 109.557, p < 0.001). N = 4, * = p < 0.00001.
Collagen Production
The production of collagen is an important factor to consider for both HAEC and HASMC function and vascular healing. Although the total collagen production per substrate was not significantly different in Figure S1, there is a significant increase in the production of collagen per cell for HAEC cultured on 110 and 70 as compared to Control (Figure 6A, B). There was also a significant difference between the collagen released into the media per cell by HAEC cultured on 110 and 70. For endothelium healing, collagen production by endothelial cells is required to form the ECM upon which they grow. In particular, type IV collagen is a major component of the basement membrane.24 The formation of the basement membrane allows the endothelium to heal and presents a physical barrier that separates the vascular smooth muscle cells in the media layer from the intima.24 Another study has shown that fibrillar collagen decreases the proliferation of vascular smooth muscle cells through the α2β1 signaling pathway.18 In the context of preventing restenosis, the increase in the production of collagen per cell by HAEC either as soluble factors or for the formation of ECM has the potential to reduce the migration and proliferation of HASMC. The increase in HAEC collagen production by cells in contact with nanotube coatings suggests the possibility of a surface that is conducive for the recovery of denuded endothelium.
Figure 6.

Relative collagen production per HAEC and HASMC normalized to Control. (A) Collagen released into media between Days 5 and 7 of HAEC culture. One-way ANOVA (F(2,11) = 48.885, p < 0.001). N = 5. (B) Collagen laid down in ECM at the end of 7 days of HAEC culture. One-way ANOVA (F(2,6) = 36.943, p < 0.001). N = 3. (C) Collagen released into media between Days 5 and 7 of HASMC culture. N = 5. (D) Collagen laid down in ECM at the end of 7 days of HASMC culture. N = 3. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, *** = p < 0.0001.
For HASMC, the production of collagen is commonly associated with NIH and restenosis.15,17 The effect of the coating on HASMC collagen production is much more attenuated than that on HAEC. In fact, one-way ANOVA did not reveal a significant difference among 110, 70, and Control (Figure 6C, D). This was also observed for total collagen production (Figure S1). Although excessive proliferation and collagen production are typical characteristics of vascular smooth muscle cells with a “synthetic” phenotype, the results from our cell growth assay and collagen assay suggest that HASMC cultured on nitinol nanotubular coatings may not adopt this phenotype.
Elastin Production
Another protein of interest for the modulation of restenosis is elastin. In Figure 7A, we show that there is a significant difference in the elastin production of HAEC cultured on 110 and 70 nm nanotubes and Control. HAEC on 70 nm nanotubes demonstrated an increase of more than 4 times the amount of elastin produced as compared to control, while HAEC on 110 nm nanotubes showed an even greater increase of more than 7 times. This trend of increasing elastin production with increasing nanotube diameter is similar to that seen in Figure 6A for soluble collagen production in HAEC. In Figure 7B, although ANOVA did not reveal statistical significance, we observed more than twice the amount the elastin produced in HASMC on 110 and 70 nm nanotubes as compared to Control (t test between 110 and Control gave p < 0.05). Elastin plays significant roles in arterial morphogenesis, homeostasis, structural support and regulation of vascular smooth muscle cell function.20–22 A previous study has shown that delivering exogenous elastin in vivo to porcine vessels that were injured reduced NIH.21 The increase in elastin production of both HAEC and HASMC on the coatings (Figure 7) may contribute toward the reduction of NIH by regulating HASMC proliferation and migration, and guide them away from the “synthetic” phenotype.
Figure 7.
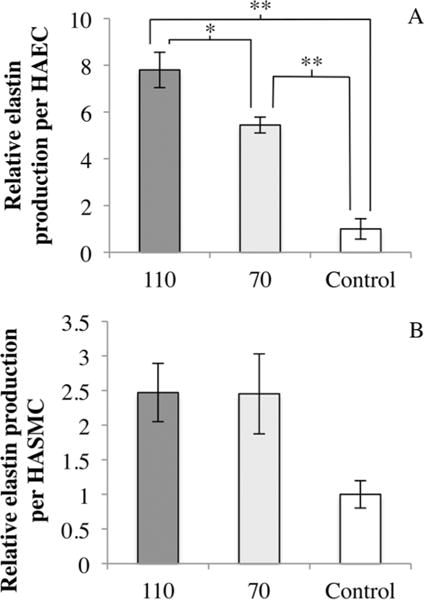
(A) Relative elastin production per HAEC normalized to Control. Elastin production was significantly different between 110, 70, and Control. One-way ANOVA (F(2,6) = 40.470, p < 0.001). (B) Relative elastin production per HASMC normalized to Control. N = 3, * = p < 0.05, ** = p < 0.01.
CONCLUSION
Summarizing the findings from this study, we have shown that nanotubular coatings on nitinol have the potential to reduce restenosis by reducing HASMC adhesion and proliferation while increasing HAEC migration and collagen and elastin production. In particular, decreasing nanotube diameter significantly reduced the production of elastin and soluble collagen in HAEC. Future studies can be conducted on the effects of the variation of other properties of the coating, such as the distance between adjacent nanotubes, on these cells. Overall, the effect of increased migration and collagen and elastin production demonstrated the potential of the coatings for improving reendothelialization. Thus, nanotubular coatings on nitinol have the potential to provide a more balanced approach toward reducing restenosis, by not just decreasing vascular smooth muscle cell proliferation but also improving the healing of the endothelium.
Supplementary Material
Acknowledgments
Funding for this work was provided by The Alf red E. Mann Institute for Biomedical Engineering. We gratefully acknowledge use of the Carl Zeiss Ultra 55 FE-SEM and supporting equipment at SF State. The FE-SEM and supporting facilities were obtained under NSF-MRI award #0821619 and NSF-EAR award #0949176, respectively. Spectral confocal microscopy was conducted at the Nikon Imaging center, UCSF.
ABBREVIATIONS
- HAEC
human aortic endothelial cells
- HASMC
human aortic smooth muscle cells
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomater-ials.5b00553.
Relative cell numbers (Day 7/Day 1) for HAEC and HASMC on 110, 70 and Control Total collagen production for HAEC and HASMC (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart Disease and Stroke Statistics–2014 Update: A Report From the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lüscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa G. Stent thrombosis of drug eluting stent: pathological perspective. J Cardiol. 2011;58(2):84–91. doi: 10.1016/j.jjcc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler, Thromb, Vasc Biol. 2007;27(7):1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 6.Brammer K, Oh S, Gallagher J. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008;8(3):786–793. doi: 10.1021/nl072572o. [DOI] [PubMed] [Google Scholar]
- 7.Peng L, Eltgroth ML, LaTempa TJ, Grimes CA, Desai TA. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials. 2009;30(7):1268–1272. doi: 10.1016/j.biomaterials.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Oh S, Brammer KS, Li YSJ, Teng D, Engler AJ, Chien S, Jin S. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7(6):1686–1691. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 10.Park J, Bauer S, Schmuki P, von der Mark K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009;9(9):3157–3164. doi: 10.1021/nl9013502. [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Li H, Lü W, Li J, Wang J, Zhang Z, Liu Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials. 2011;32(29):6900–6911. doi: 10.1016/j.biomaterials.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Lee PP, Cerchiari A, Desai TA. Nitinol-Based Nanotubular Coatings for the Modulation of Human Vascular Cell Function. Nano Lett. 2014;14:5021–5028. doi: 10.1021/nl501523v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan CC, Sreerekha PR, Divyarani VV, Nair S, Chennazhi K, Menon D. Influence of titania nanotopography on human vascular cell functionality and its proliferation in vitro. J Mater Chem. 2012;22(4):1326. [Google Scholar]
- 14.Peng L, Barczak AJ, Barbeau RA, Xiao Y, LaTempa TJ, Grimes CA, Desai TA. Whole genome expression analysis reveals differential effects of TiO2 nanotubes on vascular cells. Nano Lett. 2010;10(1):143–148. doi: 10.1021/nl903043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopal V, Rockson SG. Coronary restenosis: a review of mechanisms and management. Am J Med. 2003;115(7):547–553. doi: 10.1016/s0002-9343(03)00477-7. [DOI] [PubMed] [Google Scholar]
- 16.Lafont A, Durand E, Samuel JL, Besse B, Addad F, Lévy BI, Desnos M, Guérot C, Boulanger CM. Endothelial dysfunction and collagen accumulation: two independent factors for restenosis and constrictive remodeling after experimental angioplasty. Circulation. 1999;100(10):1109–1115. doi: 10.1161/01.cir.100.10.1109. [DOI] [PubMed] [Google Scholar]
- 17.Chan JM, Rhee J-W, Drum CL, Bronson RT, Golomb G, Langer R, Farokhzad OC. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc Natl Acad Sci U S A. 2011;108(48):19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87(6):1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 19.Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: Structure versus signal. Trends Cell Biol. 2003;13(1):51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 20.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393(May):276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 21.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 22.Brooke BS, Bayes-Genis A, Li DY. New insights into elastin and vascular disease. Trends Cardiovasc Med. 2003;13(5):176–181. doi: 10.1016/s1050-1738(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 23.Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S. Improved bone-forming functionality on diameter-controlled TiO(2) nanotube surface. Acta Biomater. 2009;5(8):3215–3223. doi: 10.1016/j.actbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Pauly RR, Passaniti a, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband Ya, Smith L, Weinstein C, Lakatta EG. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994;75(1):41–54. doi: 10.1161/01.res.75.1.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


