Abstract
Following heat stress, the mammalian intestinal epithelial cells respond by producing heat shock proteins that confer protection under stressful conditions, which would otherwise lead to cell damage or death. Some of the noxious processes against which the heat shock response protects cells include heat stress, infection, and inflammation. The mechanisms of heat shock response–induced cytoprotection involve inhibition of proinflammatory cytokine production and induction of cellular proliferation for restitution of the damaged epithelium. This can mean selective interference of pathways, such as nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK), that mediate cytokine production and growth responses. Insight into elucidating the exact protective mechanisms could have therapeutic significance in treating intestinal inflammations and in aiding maintenance of intestinal integrity. Herein we review findings on heat shock response–induced intestinal epithelial protection involving regulation of NF-κB and MAPK cytokine production.
INTRODUCTION
The intestinal epithelium is exposed to an array of injurious agents ranging from pathogens like viruses or bacteria to their products, xenobiotics, chemicals, immune and inflammatory cytokines, and thermal and related stress stimuli. To some extent, it serves as a protective barrier between these agents and the sterile host environment. Exposure to such noxious stimuli may lead to a complex, but well-coordinated, signal transduction process to maintain intestinal integrity and function. The well-coordinated mechanisms result in increased proliferation of crypt cells, secretion of enzymes, and synthesis and secretion of immune and inflammatory cytokines and heat shock proteins (Hsps).
Following inflammation-inducing stimuli, such as pathogens or proinflammatory cytokines, a transcriptional activator of several genes, nuclear factor kappa B (NF-κB), is activated (Rogler et al 1998). Concurrently, the mitogen-activated protein kinase (MAPK) pathway can be activated. The activation leads to the expression of cytokine receptors, cell adhesion molecules, viral genes, and various inflammatory cytokines, including neutrophil chemoattractants that attract leukocytes to the respective sites to induce inflammation (Baldwin 1996; Hobbie et al 1997; Awane et al 1999; Martin et al 1999; Cario et al 2000; Yue and Mulder 2000). In addition, MAPK is activated by stress and growth factors that modulate the transcription of genes coding for protective and growth proteins leading to cellular proliferation and migration that are vital for restitution of the damaged epithelium. NF-κB is a critical regulator of the early pathogen response and an activator of the immune mediators. On the other hand, thermal stress induces the production of the putative Hsps through activation of the heat shock transcription factor (HSF). The Hsps produced protect cells against further injury by rescuing intracellular proteins from irreversible denaturation; hence the term “chaperones” (Wu 1995). Two groups of proteins, Hsps and proinflammatory cytokines, seem to operate antagonistically. Interestingly, anti-inflammatory cytokines that oppose the proinflammatory cytokines seem to work in favor of the Hsps for cytoprotection. Accumulating evidence reveals that Hsps suppress inflammatory gene expression and thereby inhibit the synthesis of inflammatory cytokines to curb inflammation. Blockade of NF-κB or MAPK-mediated inflammatory responses by Hsps or other agents can be of therapeutic significance. However, the actual mechanisms by which Hsps may act to suppress inflammatory cytokine production through these pathways are incompletely understood.
Because of the emerging significance of cytoprotection by intracellular mediators, we decided to review the possible roles of Hsps in regulating inflammatory pathways that may be significant for intestinal protection.
PROINFLAMMATORY CYTOKINE PRODUCTION
The NF-κB pathway
Although production of inflammatory cytokines in the intestinal mucosa is mainly a function of specialized cells of the immune system, such as the intraepithelial lymphocytes and other monocytes, the intestinal epithelial cells (IEC) are also involved in intestinal defense. They are known to produce an array of inflammatory cytokines either constitutively or after stimulation by pathogens such as viruses and bacteria, proinflammatory cytokines, ionization radiation, and chemicals such as phorbol myristate acetate (PMA) (Thanos and Maniatis 1995; Elewaut et al 1999). Most, if not all, of the produced inflammatory cytokines are mediated by the transcriptional activator NF-κB through the NF-κB pathway (Baeuerle and Henkel 1994) (Fig 1). The NF-κB is a p50-p65 Rel family protein heterodimer that transcribes various genes. The Rel family proteins are composed of 2 groups. One group consists of p50 (NF-κB1) and p52 (NF-κB2). This group has deoxyribonucleic acid (DNA)-binding and dimerization domains and a nuclear localization signal. The second group consists of p65 (Rel A), Rel (c-Rel), and Rel B. In addition to DNA-binding and dimerization domains, the second group is composed of transcriptional activation domains (Thanos and Maniatis 1995). The NF-κB normally occurs in its inactive form bound to the inhibitory kappa B (IκB) family proteins (IκB-α, IκB-β, IκB-γ, and BcI-3) in the cytoplasm. Activation of the transcriptional activity of the NF-κB requires the phosphorylation of IκB proteins and their subsequent degradation to generate the p50-p65 that translocates into the nucleus and activates the respective genes (Thanos and Maniatis 1995).
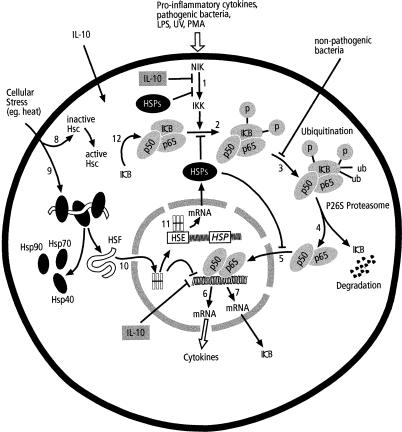
Fig 1.
Cytokine regulation by NFκB pathway and the interaction with stress response. Inflammatory agents activate NIK that in turn activates IKK (1) to phosphorylate IκB (2). Phosphorylated IκB is ubiquitinated (3) prior to proteasome degradation of IκB (4) that releases free p50-p65. The heterodimer p50-p65 translocates into the nucleus (5) for transcriptional activation of inflammatory cytokines (6) and IκB (7). Stress stimuli acts on constitutive HSPs (8) and activates cytoplasmic bound inactive monomeric HSF (9) by freeing binding Hsps. HSF then translocates into the nucleus (10), trimerises and activates HSE to produce HSPs (11). Both inducible and activated constitutive HSPs may suppress cytokine production by inhibiting activation of IKK (1), stabilizing NFκB/IκB complex (2), or maintaining p65 in the cytoplasm (5). The HSF may repress expression of inflammatory cytokines (6). Anti-inflammatory cytokines may inhibit IKK activation (1) or inflammatory gene expression (6). Over-expression of IκB stabilizes the NFκB/I&kappa:B complex (12) to inhibit degradation. Non-pathogenic bacteria abrogate ubiquitination (3).
From the NF-κB–dependent cytokine production pathway, it can be deduced that blockade of this pathway at any point to inhibit its transcriptional activation reduces or arrests the production of the inflammatory cytokines and hence inflammation. Anti-inflammatory cytokines such as interleukin (IL)-10 and IL-4, nonvirulent bacteria such as Salmonella spp, intestinal bacterial fermentation products like short-chain fatty acids, and the heat shock response repress inflammatory cytokine production by abrogation of some steps in the NF-κB pathway (Schottelius et al 1999; Wu et al 1999; Neish et al 2000; Berin et al 2001).
The MAPK pathways
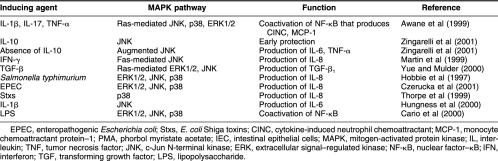
Some cytokine secretions are signaled through the MAPK pathways that are known to transduce the extracellular stress signals. These pathways consist of extracellular signal–regulated kinases (ERK) 1/2, c-Jun N-terminal kinases (JNK) (also known as stress-activated protein kinases), and p38. Their activation is through cascades of MAPK, MAPK kinases, and MAPK kinase kinases that are in turn activated by the various extracellular stimuli (Fig 2). Inflammatory cytokines, such as IL-1β, IL-17, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, pathogenic bacteria, such as Salmonella typhimurium and Escherichia coli, lipopolysaccharides (LPS), thermal and oxidative stresses, PMA, and growth responses can all activate the MAPK-signaling cascades in the IEC, leading to the activation of genes coding for inflammatory cytokines (Hobbie et al 1997; Awane et al 1999; Martin et al 1999; Cario et al 2000; Yue and Mulder 2000; Czerucka et al 2001). Some of the cytokines mediated through MAPK in IEC are listed in Table 1.
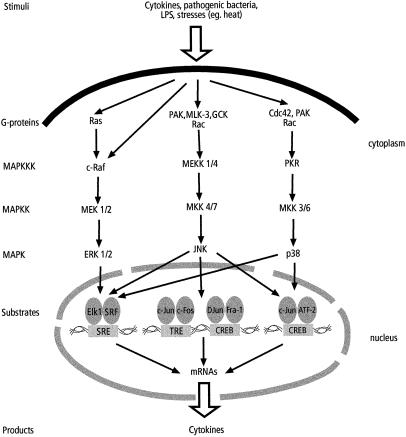
Fig 2.
Cytokine production by MAPK pathways. Various inflammatory agents and stress stimuli activate MAPK pathways and their subsequent substrates leading to transcriptional activation of genes coding for cytokines. This activation is followed by production of an array of inflammatory cytokines. See text for details and Table 1 for summarized specific pathway stimulation and respective cytokine production
Table 1.
Cytokine production by IEC in association with activated MAPK pathways
THE ROLE OF THE HEAT SHOCK RESPONSE IN CYTOKINE REGULATION
The heat shock response and its protection of the IEC
Most cells and organisms react to heat and a variety of stressors by rapid synthesis of a group of evolutionary conserved proteins, ranging in size from 8 kDa to 150 kDa (Wu 1995; Ovelgönne et al 2000), termed Hsps. They are classified into several families according to their molecular weights. The major Hsp families include HSP150, HSP110, HSP90, HSP70, HSP60, HSP40, HSP20, and HSP8.5 (Table 2).
Table 2.
Major heat shock proteins (Hsps) known in mammalian cells
The heat shock response is induced by various stimuli, including thermal stress, heavy metals such as sodium arsenite and zinc ions, bacteria, and bacterial exo- and endotoxins, viral infections, ischemia, nutritional deficiency, ionizing radiation, oxidants, some IFN inducers, and cytokines in different cells including the IEC (Wu 1995). Following heat shock or other stresses, Hsps are produced after transactivation of the genes by a family of DNA-binding proteins called the HSFs (HSF1–4, of which the best known is HSF1). In unstressed cells the inactive HSF is bound to the cytoplasmic Hsp40 (Hdj-1), Hsp70, and Hsp90 in a monomeric form without the DNA-binding activity. In response to stress, HSF is released and translocated into the nucleus, where it assembles into a trimer and binds to a specific consensus heat shock regulatory element (HSE) in the heat shock gene promoter to exert the transcriptional activation (Santoro 2000; Han et al 2001) (Fig 3). Heat shock response also activates constitutive Hsps that, together with the induced Hsps, affect cytoprotection.
Fig 3.
Regulation of HSPs. Stress stimuli activates inactive form of constitutive HSPs into active form (1). The inactive monomer non-DNA binding cytoplasmic HSF that resides bound to HSPs in unstressed cells is activated by stress stimuli and dissociates into HSPs and DNA-binding HSF (2). The active HSF translocates into the nucleus (3), trimerises and binds to HSPs gene promoter prior to undergoing phosphorylation at serine residues (4). Phosphorylated HSF attaches to the HSE located upstream of the HSPs genes (5) followed by transcription activation that results into production of HSPs and HSF (6). The HSF produced maintains the circle while HSPs are released into cytoplasm (7). High levels of cytoplasmic HSPs causes nuclear localization of HSPs (8) that in turn, bind to HSF to repress HSPs transcriptional activation
The various Hsp families are differentially expressed in the IEC and may relate to the type or intensity of the epithelial damage, or location of the IEC. Hsp60, Hsp72, and Hsp90 are expressed in the colonic mucosa after hyperthermia. In response to acetic acid–induced intestinal lesions, Hsp72 and Hsp90 inductions are protective. Their inductions precede that of Hsp60, which has no protective effect (Otani et al 1997). Although Hsp72 is induced by cellular injury, intestinal motility may be enough to induce Hsp60. Kuwabara et al (1994) and Sasahara et al (1998) demonstrated that water immersion stress that causes functional diarrhea without histopathological changes induces Hsp60 but not Hsp72 nor Hsp90 in both colon and small IEC. In these cases Hsp60 was observed to have no protective role against acetic acid–induced intestinal lesions. Hsp72 and Hsp73 have also been reported to have no protective function against small IEC indomethacin-induced injuries (Jin et al 1997). The chaperone function for a particular Hsp may, therefore, be specific to certain intestinal injuries or type and location of the IEC along the alimentary tract.
Cytokines involved in heat shock induction imply an interrelationship among these mediators. Because of their protective role, Hsps can be expected to down-regulate inflammatory cytokines to overcome inflammation. This suggests a mechanism of modulating cytokine production by both NF-κB and MAPK pathways. Several studies have shown that the heat shock response does, in fact, inhibit some cytokine production mediated by NF-κB and modulates MAPK-dependent cytokine production in a specific manner.
Hsps inhibit the NF-κB inflammatory cytokine production by IEC
There is accumulating evidence to suggest that Hsp induction abrogates the activation of the NF-κB inflammatory pathway and thereby inhibits proinflammatory gene expression (Cahill et al 1996; Chu et al 1997). Hsps appear to inhibit NF-κB transcriptional activation either by inhibiting IκB degradation or by directly repressing the NF-κB transcriptional activity (Chu et al 1997; Jobin et al 1999; Yoo et al 2000). Hsps are believed to prevent IκB degradation by inhibiting IκB kinase (IKK) activation, but how this occurs is unclear.
The normally occurring cytoplasmic IκB–NF-κB complex exists via an interaction between the IκB-α ankyrin domains and the nuclear localization sites. Human HSP70 has nuclear localization sites (Dang and Lee 1989). The presence of these nuclear localization sites raises the possibility that Hsps can specifically interact with the consensus IκB-α ankyrin domain and in turn hamper IκB–NF-κB phosphorylation and the subsequent IκB degradation (Yoo et al 2000).
There is strong evidence that Hsps enhance IκB production. In this case, elevated levels of IκB stabilize the IκB–NF-κB complex, resulting in hampered IκB degradation. Wong et al (1997) identified a 20-bp heat shock responsive segment in the human IκB-α that could be a functional heat shock responsive element in NF-κB transcriptional inhibition. In their study stress induced IκB-α messenger ribonucleic acid (mRNA) and protein expression, stabilizing the IκB–NF-κB association and suppressing the NF-κB transcriptional activation. Consistently, Pritts et al (2000) observed that heat stress was associated with the maintained IEC cytoplasmic IκB-α levels and the decreased endotoxin-induced NF-κB DNA-binding transcriptional activity. Both studies suggest dual mechanisms for NF-κB inhibition by heat shock response, increased expression of IκB-α and inhibition of the degradation of IκB-α.
Chaperones function by shielding the already synthesized proteins from degradation, mediating these stabilizing effects through protein-protein interaction. Stabilization of IκB-α against phosphorylation and degradation following stress responses could partly be through this mechanism as well.
Though the mechanism is enigmatic, HSF acts as a transcriptional repressor of the cytokine genes. Cahill et al (1996) in their study on human monocytes showed that HSF represses the IL-1β gene responding to LPS by binding to a specific HSE in the IL-1β promoter. They suggested transcriptional repressor mechanisms that were distinct from those involved in the activation. In turn, this could block other cytokines that are secreted in response to intracellular IL-1β. This offers another potential mechanism for the down-regulation of cytokine expression by Hsps in IEC.
Hsps modulate the MAPK pathway to confer IEC protection
The protective role of Hsps in the IEC occurs through the modulation of the MAPK pathways. Hsps may selectively influence ERK1/2, JNK, and p38 MAPK pathways in various stressful conditions (Hill and Treisman 1995; Tilly et al 1996; Gabai et al 1998; Ng and Bogoyevitch 2000). Though most Hsps signal through the Ras-Raf–independent ERK1/2 MAPK pathway, the activation of the downstream genes may be specific. Hsp90 mediates normal IEC growth signals via ERK1/2, thereby protecting cells against apoptosis (Hostein et al 2001). Likewise, sodium arsenite induces Hsp70 synthesis in IEC via ERK1/2 activation of the HSF (Chen et al 2001). This Hsp70 together with Hsc70 suppresses JNK signaling, leading to cellular protection against various stresses (Mosser et al 2000). The JNK pathway has a potential to down-regulate IL-10 production to favor intestinal inflammation. Hence, its suppression by Hsp70 and Hsc70 is vital for anti-inflammatory responses. Interestingly, Hsp27 and Hsp72 prevent both repression of IL-10 production and induction of apoptosis by modulating the activity of the JNK. Subsequently, the accumulated Hsps suppress JNK to protect cells against stresses (Tilly et al 1996; Gabai et al 1998).
IL-6 production by the IEC after AP-1 activation by stress response is mediated through the JNK pathway. The stimulated JNK pathway activates c-Jun and c-Fos heterodimer members of AP-1 leading to IL-6 production (Andoh et al 1999; Yeh et al 2000). Though IL-6 is a proinflammatory cytokine, its production after heat shock induction could be protective. Barton and Jackson (1993) demonstrated a protective role for IL-6 against death from septic shock in mice. In their study IL-6 was observed to confer a significant reduction of the LPS-induced septic shock mortality.
CROSS TALK BETWEEN HSFs, NF-κB AND MAPK SIGNAL TRANSDUCTION PATHWAYS
Cellular responses at the gene level are highly conserved. The HSFs responding to establish cytoprotection after the heat shock response, the NF-κB for inducing inflammation and the MAPK responding to both protection and inflammation, seem to be coordinated in a very specific way. In this coordination the activation of the HSFs antagonizes the inflammatory activities of MAPK and NF-κB while favoring the anti-inflammatory responses. Similarly, inflammatory responses mediated by both MAPK and NF-κB seem to complement, if not support, each other.
Inhibition of the activities of the inflammatory cytokine TNF-α in the IEC is associated with the inhibition of the NF-κB and MAPK inflammatory responses. Interestingly, this modulation favors the protective, proliferative MAPK responses, induced by the epidermal growth factor, that are important in epithelial restitution after injury (Kaiser et al 1999).
In other systems, an increase in the HSF DNA-binding activity and the subsequent Hsp production is associated with the enhanced protective influences of the ERK1/2 MAPK pathway and the suppressed JNK and p38 MAPK responses. Consistently, a decrease in the Hsp production is accompanied by an increase in the JNK MAPK activity that favors inflammatory responses. Absence of the protective ERK1/2 MAPK pathway blocks HSF transcription and Hsp up-regulation (Kim et al 1999; Tsuji et al 2000). Hsp protection of the IEC through the inhibition of JNK activation is also reported. Sodium arsenite induces Hsp70 in the IEC via the ERK1/2 MAPK pathway. This induction is associated with the HSF activation. Hsp70 together with Hsc70 produced through this pathway suppress the JNK pathway to confer cytoprotection (Mosser et al 2000).
The high constitutive levels of Hsp90 observed in unstressed cells (Buchner 1999) seem to play a vital role in modulating cellular protection through its interaction with other Hsps and various kinases. Recently, Hsp90 was found to be a repressor of the double-stranded ribonucleic acid–dependent protein kinase PKR, and its inhibition activates the kinase (Donze et al 2001). PKR is activated in response to viral infection, and it favors inflammation by interacting with IKK to catalyze NF-κB transcriptional activation (Zamanian-Daryoush et al 2000; Gil et al 2001). In addition, PKR activates MAPK p38 and contributes to LPS-induced IL-6 and IL-12 production (Goh et al 2000). These findings indicate a pivotal role of PKR linking NF-κB and MAPK that is controlled by Hsps. The phenomenon is such that the constitutive levels of Hsp90 suppress the PKR and thereby keep NF-κB inactive. Blockade of this Hsp not only activates PKR but also enhances the p38 activity and the ubiquitin-dependent proteasome degradation, a mechanism important for NF-κB activation (Schulte et al 1997).
The inhibition of Hsp production may also account for the decreased Hsp-augmented IEC production of IL-6 that is partly associated with the inhibition of the AP-1 activation (Hungness et al 2000). Inhibiting AP-1 activation suppresses inflammatory cytokine production even without inactivating NF-κB, indicating that blockade of either MAPK or NF-κB decreases inflammatory cytokine production. The production of both IL-8 and IL-6 depends on both pathways, and the inhibition of p38 MAPK and subsequent AP-1 activation abolish their production (Hobbie et al 1997; Hungness et al 2000). Interestingly, inhibiting NF-κB ubiquitin-dependent proteasome degradation by proteasome inhibitors activates HSF and AP-1 through p38 and JNK MAPK pathways (Tacchini et al 2001), resulting in a protective role. This implies a vital role for constitutive Hsps with regard to mediating signals to both MAPK and NF-κB pathways (Fig 4).
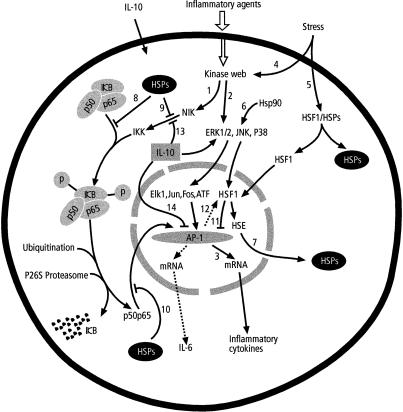
Fig 4.
Interaction of stress and inflammatory responses. Inflammatory agents activate NF-κB (1) and MAPK pathways (2) through several kinases resulting into activation of AP-1 and subsequent production of inflammatory cyokines (3). Stress factors activate MAPK pathway through a series of kinases (4) and HSF1 (5) that translocates into the nucleus. The MAPK pathway is also activated by constitutive Hsp90 (6). Activated HSF1 binds to HSE followed by HSPs production (7) that block NF-κB pathway (8, 9, 10). The HSF1 may inhibit AP-1 inflammatory cytokine transcription activation (11). AP-1 has potential to activate HSF1 transcriptional activation (12). IL-10 blocks both IKK activation (13) and AP-1 activation (14) to repress inflammatory cytokine production. Dotted arrows represent stress response AP-1 products that are not produced by NF-κB. For more details see text
These observations imply a tightly regulated communication network among various signal transduction pathways to elicit cellular and stimuli specific responses. HSF transcription and Hsp up-regulation is associated with protective MAPK activities mainly mediated through the ERK1/2 pathway. On the other hand, NF-κB inflammatory responses are connected with JNK and p38 MAPK pathways. More importantly, all 3 MAPK pathways can be activated at one time, but their responses are highly insulated from one another.
THERAPEUTIC SIGNIFICANCE
Selective blockade of the inflammatory NF-κB and MAPK pathways is important in reducing intestinal inflammations. Manipulations of these pathways are underway as an effective approach to inhibit proinflammatory gene expression. The principal mechanisms may be to activate HSF and MAPK protective responses while inhibiting inflammatory NF-κB and MAPK pathways.
A number of studies suggest a close reciprocal relationship between the activities of HSF and NF-κB. In these studies the inhibitors of NF-κB activation that effectively prevented IκB-α degradation were potent activators of HSF transcription. Prostaglandins A and J were found to activate HSF and induce the synthesis of Hsps that protected cells against hyperthermia and virus infection (Santoro 1997). These prostaglandins were potent inhibitors of NF-κB transcription activation (Rossi et al 1997). Though their dual effects may neither be dependent nor be linked to one another, these findings strengthen the approach that maneuvering either of the pathways could be of therapeutic significance. Javadpour et al (1998) observed that a tyrosine kinase inhibitor herbimycin-A protected ischemic animals by inhibiting neutrophil infiltration, a sequel of the NF-κB–dependent chemokine synthesis. They suggested that the effect was because of the increased expression of Hsps by activated HSF.
The observations that enhanced ERK1/2 and suppressed JNK and p38 in association with up-regulation of Hsps confer protection against stress seem to be the principle underlying the working of some drugs. In such cases the absence of ERK1/2 that subsequently abolishes HSF transcriptional activation may render the drugs ineffective (Kim et al 1999; Tsuji et al 2000). By contrast, the activation of ERK1/2 by enteropathogenic E. coli via phosphorylation of the upstream Hsp54 enhances bacterial internalization (Czerucka et al 2000). Under such circumstances, prevention of phosphorylation of such Hsps may have therapeutic importance.
Advances in the direct inhibition of the inflammatory NF-κB or MAPK pathway without the involvement of Hsps are well reported. Potent inflammatory bowel disease drugs, mesalamine (5-aminosalicylic acid) derivatives, inhibit both inflammatory pathways (Kaiser et al 1999). Cytokine-suppressive anti-inflammatory drugs block the production of proinflammatory cytokines at a posttranslational level by binding to p38 and subsequently inhibiting its kinase activity (Lee et al 1994). Working together with NF-κB activation for cytokine production, inhibition of p38 could overcome both NF-κB and MAPK inflammatory responses (Hobbie et al 1997). Similarly, intracolonic introduction of single-stranded molecules of 15–25 bp (antisense phosphorothioate oligonucleotides) that can hybridize to the p65 mRNA and inhibit its expression has been shown to decrease the transcriptional activation of NF-κB. This medication lowers the synthesis of the proinflammatory cytokines IL-1, IL-6, and TNF-α and improves intestinal inflammation (Murano et al 2000).
Although inhibition of proinflammatory cytokines by the blockade of the NF-κB or MAPK pathway aids curb inflammation, long-term suppression of these pathways in humans or animals may not be a good idea to advocate for treating intestinal inflammations. The reasons partly stem from the findings by Inan et al (2000) and Erdman et al (2001) who showed high cytokine expression by the IECs of knockout and heterozygote mouse strains with less NF-κB. This may imply that NF-κB plays an important role in maintaining intestinal homeostasis and that other transcription factors can play a major role in inflammatory gene expression. In other cell systems prolonged inhibition of NF-κB results in massive cell death by apoptosis (Iimuro et al 1998).
CONCLUSIONS
The maintenance of the normal intestinal function at all situations is complex and involves many factors. These factors are regulated at multiple levels and interact with one another in a highly organized manner. Although NF-κB and MAPK pathways are involved in the production of inflammatory cytokines and cellular proliferation, their modulation by the heat shock response is beneficial for diverse harmful stimuli. Generally, the resulting Hsps not only protect cells by acting as chaperones but also down-regulate proinflammatory cytokine production to curb noxious processes, like heat stress, infection, and inflammation. In reality, the resulting heat shock response–induced cytoprotection may culminate in the restoration of the intestinal epithelial function.
REFERENCES
- Andoh A, Fujiyama Y, Hata K, Araki Y, Takaya H, Shimada M, Bamba T. Counter-regulatory effect of sodium butyrate on tumor necrosis factor–alpha (TNF-α)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells. Clin Exp Immunol. 1999;118:23–29. doi: 10.1046/j.1365-2249.1999.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awane M, Andres PG, Li DJ, Reinecker HC. NF-κB–inducing kinase is a common mediator of IL-17, TNF-α–, and IL-1β–induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–5344. [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Baldwin A. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Barton BE, Jackson JV. Protective role of interleukin-6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993;61:1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berin MC, Dwineli MB, Eckmann L, and Kagnoff MF 2001 Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 280 G. 1217–G1226. [DOI] [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Waterman WR, Xie Y, Auron PE, Calderwood SK. Transcriptional repression of the proinflammatory 1β gene by heat shock factor 1. J Biol Chem. 1996;271:24874–24879. [PubMed] [Google Scholar]
- Cario E, Rosenberg M, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- Chen YC, Tsai SH, Shen SC, Lin JK, Lee WR. Alternative activation of extracellular signal–regulated protein kinases in curcumin and arsenite-induced Hsp70 gene expression in human colorectal carcinoma cells. Eur J Cell Biol. 2001;80:213–221. doi: 10.1078/0171-9335-00158. [DOI] [PubMed] [Google Scholar]
- Chu EK, Ribeiro SP, Slutsky AS. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25:1727–1732. doi: 10.1097/00003246-199710000-00025. [DOI] [PubMed] [Google Scholar]
- Czerucka D, Dahan S, Mograbi B, Rossi B, and Rampal P 2000 Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli–infected T84 cells. Infect Immun 68: 5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect Immun. 2001;69:1298–1305. doi: 10.1128/IAI.69.3.1298-1305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Lee WMF. Nuclear and nucleolar targeting sequences of c-erb-A, c-myb, N-myc, p53, Hsp70, and HIV tat proteins. J Biol Chem. 1989;254:18019–18023. [PubMed] [Google Scholar]
- Donze O, Abbas-Terki T, Picard D. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 2001;20:3771–3780. doi: 10.1093/emboj/20.14.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–1466. [PubMed] [Google Scholar]
- Erdman SE, Fox GJ, Dangler CA, Feldman D, Horwitz BH. Cutting edge: typhlocolitis in NF-κB–deficient mice. J Immunol. 2001;166:1443–1447. doi: 10.4049/jimmunol.166.3.1443. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Yaglom JA, Volloch VZ, Sherman MY. Role of HSP70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett. 1998;438:1–4. doi: 10.1016/s0014-5793(98)01242-3. [DOI] [PubMed] [Google Scholar]
- Gil J, Rullas J, Garcia MA, Alcami J, Esteban M. The catalytic activity of dsRNA-dependent protein kinase, PKR, is required for NF-kappaB activation. Oncogene. 2001;20:385–394. doi: 10.1038/sj.onc.1204109. [DOI] [PubMed] [Google Scholar]
- Goh KC, deVeer MJ, Williams BRG. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SI, Oh SY, Woo SH, Kim KH, Kim JH, Kim HD, Kang HS. Implication of a small GTPase Rac1 in the activation of c-Jun N-terminal kinase and heat shock factor in response to heat shock. J Biol Chem. 2001;276:1889–1895. doi: 10.1074/jbc.M006042200. [DOI] [PubMed] [Google Scholar]
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Hobbie S, Chen LM, Davis RJ, Galan JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003–4009. [PubMed] [Google Scholar]
- Hungness ES, Pritts TA, Luo G, Sun X, Penner CG, Hasselgren PO. The transcription factor activator protein-1 is activated and interleukin-6 production is increased in interleukin-1β–stimulated human enterocytes. Shock. 2000;14:386–391. doi: 10.1097/00024382-200014030-00025. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhovlen R, Grisham JW, Brenner DA. NF-kappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Investig. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan MS, Tolmacheva V, Wang QS, Rosenberg DW, and Giardina C 2000 Transcription factor NF-κB participates in regulation of epithelial cell turnover in the colon. Am J Physiol Gastrointest Liver Physiol. 279 G. 1282–G1291. [DOI] [PubMed] [Google Scholar]
- Javadpour M, Kelly CJ, Chen G, Bouchier-Hayes DJ. Herbimycin-A attenuates ischaemia-reperfusion induced pulmonary neutrophil infiltration. Eur J Vasc Endovasc Surg. 1998;16:377–382. doi: 10.1016/s1078-5884(98)80003-8. [DOI] [PubMed] [Google Scholar]
- Jin M, Otaka M, and Otani S. et al. 1997 Effect of pre-induction of heat shock proteins on indomethacin-induced small intestinal lesion in rats. J Gastroenterol. 32:34–39. [DOI] [PubMed] [Google Scholar]
- Jobin C, Holt L, Bradham CA, Streetz K, Brenner DA, Sartor RB. TNF receptor-associated factor-2 is involved in both IL-1β and TNF-α signaling cascades leading to NF-κB activation and IL-8 expression in human intestinal epithelial cells. J Immunol. 1999;162:4447–4454. [PubMed] [Google Scholar]
- Kaiser GC, Yan F, Polk B. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor κB activation in mouse colonocytes. Gastroenterology. 1999;116:602–609. doi: 10.1016/s0016-5085(99)70182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim D, Jung GS, Um JH, Chung BS, Kang CD. Involvement of c-Jun NH2-terminal kinase pathway in different regulation of heat shock proteins by anticancer drugs. Biochem Biophys Res Commun. 1999;262:516–522. doi: 10.1006/bbrc.1999.1229. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Otaka M, Itoh H, Tashima Y, Zeniya A, Fujimori S, Masamune O. Regulation of 60-kDa heat shock protein expression by systemic stress and 5-hydroxytryptamine in rat colonic mucosa. J Gastroenterol. 1994;29:721–726. doi: 10.1007/BF02349277. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, and McDonnell PC. et al. 1994 A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 372:739–746. [DOI] [PubMed] [Google Scholar]
- Martin A, Maria T, Andrew A, Palladino AA, Faris M, Carramanzana NM, Nel AE, and Targan SR 1999 Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am J Physiol. 276(Gastrointest Liver Physiol 39). G599–G605. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of Hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano M, Maemura K, and Hirata I. et al. 2000 Therapeutic effect of intracolonically administered nuclear factor κB (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- Ng DCH, Bogoyevitch MA. The mechanism of heat shock activation of ERK mitogen-activated protein kinases in the interleukin 3–dependent pro-B cell line BaF3. J Biol Chem. 2000;275:40856–40866. doi: 10.1074/jbc.M004639200. [DOI] [PubMed] [Google Scholar]
- Otani S, Otaka M, and Jin M. et al. 1997 Effect of preinduction of heat shock proteins on acetic acid–induced colitis in rats. Dig Dis Sci. 42:833–846. [DOI] [PubMed] [Google Scholar]
- Ovelgönne JH, Koninkx JFJG, Pusztai A, Bardocz S, Kok W, Ewen SWB, Hendriks HGCJM, van Dijk JE. Decreased level of heat shock proteins in gut epithelial cells after exposure to plant lectins. Gut. 2000;46:679–687. doi: 10.1136/gut.46.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritts TA, Wang Q, Sun X, Moon R, Fischer DR, Fischer JE, Wong HR, Hasselgren PO. Induction of the stress response in vivo decreases nuclear factor-kappa B activity in jejunal mucosa of endotoxemic mice. Arch Surg. 2000;135:860–866. doi: 10.1001/archsurg.135.7.860. [DOI] [PubMed] [Google Scholar]
- Rogler G, Brand K, and Vogl D. et al. 1998 Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 115:357–369. [DOI] [PubMed] [Google Scholar]
- Rossi A, Elia G, Santoro MG. Inhibition of nuclear factor kappa B by prostaglandin A1: an effect associated with heat shock transcription factor activation. Proc Natl Acad Sci U S A. 1997;94:746–775. doi: 10.1073/pnas.94.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MG. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 1997;5:276–281. doi: 10.1016/S0966-842X(97)01066-4. [DOI] [PubMed] [Google Scholar]
- Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59:55–63. doi: 10.1016/s0006-2952(99)00299-3. [DOI] [PubMed] [Google Scholar]
- Sasahara H, Otaka M, and Itoh S. et al. 1998 Effect of preinduction of heat-shock proteins on acetic acid–induced small intestinal lesions in rats. Dig Dis Sci. 43:2117–2130. [DOI] [PubMed] [Google Scholar]
- Schottelius AJG, Mayo MW, Sartor RB, Bardwin AS Jr. Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Won GA, Neckers LM. Geldanamycin-induced destabilization of Raf-1 involves the proteosome. Biochem Biophys Res Commun. 1997;239:655–659. doi: 10.1006/bbrc.1997.7527. [DOI] [PubMed] [Google Scholar]
- Tacchini L, Dansi P, Matteucci E, Bernelli-Zazzera A, Desiderio MA. Influence of proteasome and redox state on heat shock–induced activation of stress kinases, AP-1 and HSF. Biochim Biophys Acta. 2001;1538:76–89. doi: 10.1016/s0167-4889(00)00141-5. [DOI] [PubMed] [Google Scholar]
- Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- Thorpe CM, Hurley BP, Lincicome LL, Jacewicz MS, Keusch GT, Acheson DW. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect Immun. 1999;67:5985–5993. doi: 10.1128/iai.67.11.5985-5993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly BC, Gaestel M, Engel K, Edixhoven MJ, de Jonge HR. Hypo-osmotic cell swelling activates the p38 MAP kinase signaling cascade. FEBS Lett. 1996;395:133–136. doi: 10.1016/0014-5793(96)01028-9. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Inanami O, Kuwabara M. Neuroprotective effect of α-phenyl-N-tert-butylnitrone in gerbil hippocampus is mediated by the mitogen-activated protein kinase pathway and heat shock proteins. Neurosci Lett. 2000;282:41–44. doi: 10.1016/s0304-3940(00)00844-2. [DOI] [PubMed] [Google Scholar]
- Wong HR, Ryan M, Wispé JR. Stress response decrease NF-κB nuclear translocation and increases I-κBα expression in A549 cells. J Clin Investig. 1997;99:2423–2428. doi: 10.1172/JCI119425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Wu GD, Huang N, Wen X, Keilbaugh SA, Yang HJ. High-level expression of IκB-β in the surface epithelium of the colon: in vitro evidence for an immunomodulatory role. J Leukoc Biol. 1999;66:1049–1056. doi: 10.1002/jlb.66.6.1049. [DOI] [PubMed] [Google Scholar]
- Yeh KY, Yeh M, Glass J, Granger DN. Rapid activation of NFκB and AP-1 and target gene expression in postischemic rat intestine. Gastroenterology. 2000;118:525–534. doi: 10.1016/s0016-5085(00)70258-7. [DOI] [PubMed] [Google Scholar]
- Yoo C, Lee S, Lee C, Kim YW, Han SK, Shim Y. Anti-inflammatory effect of heat shock protein induction is related to stabilization of IκBα through preventing IκB kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–5423. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor β1 production in a smad-dependent pathway. J Biol Chem. 2000;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. NF-kappaB activation by double-stranded–RNA-activated protein kinase (PKR) is mediated through NF-kappaB–inducing kinase and IkappaB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B, Yang Z, Hake PW, Denenberg A, Wong HR. Absence of endogenous interleukin 10 enhances early stress response during post-ischaemic injury in mice intestine. Gut. 2001;48:610–622. doi: 10.1136/gut.48.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]