Abstract
The recent elucidation of the mammalian unfolded protein response pathway has revealed a unique and transcriptionally complex signal transduction pathway that protects cells from a variety of physical and biochemical stresses that can occur during normal development and in disease states. Although the stress conditions are monitored in the endoplasmic reticulum, the beneficial effects of this pathway are extended to other cellular organelles and to the organism itself.
THE ENDOPLASMIC RETICULUM IS A MAJOR SITE OF PROTEIN SYNTHESIS
In eucaryotic cells, proteins destined for the nucleus, cytosol, mitochondria, and peroxisomes are synthesized on free polysomes, whereas resident proteins of the endocytic and exocytic organelles, as well as cell surface and secreted proteins, are translated on membrane polysomes and translocated across the membrane of the endoplasmic reticulum (ER). These secretory pathway proteins enter the ER through a proteinaceous channel, called the translocon, as extended polypeptide chains and find themselves in a unique oxidizing and Ca2+-rich environment where posttranslational modifications, such as the addition of N-linked glycans and the formation of intrachain disulfide bonds, occur (Gething and Sambrook 1992). Within this concentrated mixture of proteins in various stages of synthesis and modification, the nascent chain must fold and, in many cases, assemble with other nascent proteins into multimeric complexes. Thus, the constraints and complexities encountered during the folding of ER secretory pathway proteins are even greater than those encountered by proteins folding in the cytosol and may therefore be more sensitive to subtle changes in the extracellular environment, which in turn alter the intracellular environment. The maturation of nascent proteins in the ER is aided and monitored by resident ER molecular chaperones and folding enzymes that associate with the newly synthesized proteins to prevent their aggregation and help them fold and assemble correctly (Ellgaard et al 1999). Through a process called ER quality control, proteins that do not mature properly are retained in the ER and eventually targeted for ER-associated degradation (ERAD), a process that also relies on the action of chaperones (Brodsky et al 1999).
ALTERATIONS IN THE ER ENVIRONMENT AFFECT PROTEIN FOLDING AND ACTIVATE A PROTECTIVE RESPONSE
Changes in the normal physical environment of the cell (eg, decreases in pH, energy, oxygen, glucose, or other nutrients) can affect the normal biosynthesis of proteins in the ER (Lee 2001). Low-glucose conditions inhibit the N-linked glycosylation that is required for the normal maturation of most secretory pathway proteins, a situation that can be induced pharmacologically with tunicamycin or 2-deoxyglucose. The ER contains the cellular calcium stores, and alterations in ER Ca2+ levels have profound effects on protein folding in this compartment (Sambrook 1990). Depletion of ER calcium can be achieved chemically by treating cells with A23187, thapsigargin, ionomycin, or ethylene glycol-bis(aminoethylether)-tetra-acetic acid. Finally, the ER possesses an oxidizing environment, which favors the formation of disulfide bonds that act to stabilize protein folding and assembly of protein subunits. Changes in the oxidizing potential of the ER dramatically affect protein folding and can be achieved experimentally by using hypoxic chambers, cobalt chloride, dithiothreitol, or 2-β-mercaptoethanol. All of these changes result in the accumulation of unfolded proteins in the ER, thereby producing a stress signal that activates a signal transduction pathway termed the unfolded protein response (UPR) (Kozutsumi et al 1988). In addition, a number of physiological insults or pathological conditions that affect the environment of multiple organelles, and therefore have more global effects on protein folding in the cell, also activate the UPR. These include alcohol abuse, renal toxicity, alterations in cellular pH, disruption of proteasome function, viral infection, and cancer (Lee 2001). In fact, any chemical insult or condition that affects the amino acid side chain interactions of ER proteins would be expected to interfere with their proper folding and thus activate the UPR. The primary effects of UPR activation are designed to protect the ER from the accumulation of unfolded proteins, but in addition, they also serve to limit damage to other organelles and, in extreme cases, to ultimately protect the organism by providing a mechanism to eliminate cells that experience prolonged stress. The fact that protein folding in the ER is affected by environmental stresses that specifically alter ER homeostasis, as well as by some conditions that also disrupt cytosolic folding, may make UPR signaling a particularly sensitive indicator of adverse physiological conditions and explain why the downstream responses are so broad.
CHARACTERISTICS OF THE MAMMALIAN ER STRESS RESPONSE
The hallmark of the ER stress response, and perhaps the only component of the response that is truly ER specific, is the coordinate transcriptional up-regulation of at least 12 resident ER proteins that include molecular chaperones and folding enzymes (Ellgaard et al 1999; Lee 2001). Although these proteins are constitutively expressed to aid and monitor normal protein biosynthesis, their association with nascent chains is transient, and they can be reused many times (Knittler and Haas 1992; Ellgaard et al 1999). During ER stress, secretory pathway proteins are unable to fold, and the chaperones must remain associated with them to prevent their aggregation. Thus, the increased expression of chaperones ensures that there are sufficient quantities to bind all proteins that are unable to fold properly. Whereas some of the stress conditions only affect newly synthesized proteins (eg, inhibitors of glycosylation), others can actually cause completely folded proteins to unfold (eg, reducing agents) (Valetti and Sitia 1994), making the demands for chaperones more immediate and even higher. The coordinate up-regulation of folding enzymes both aids in keeping the unfolded proteins soluble and allows for rapid refolding when the stress subsides. All eucaryotic organisms share this part of the response.
A second characteristic of the UPR, and one unique to metazoans, is the inhibition of protein synthesis (Prostko et al 1992; Brostrom et al 1996). This occurs via phosphorylation of the α subunit of the eucaryotic translation initiation factor (eIF2α) at Ser51, which reduces the frequency of translation initiation and thereby inhibits new protein synthesis (Pathak et al 1988; Scheuner et al 2001). A number of proteins translated on free polyribosomes are also affected (Brewer et al 1999; Harding et al 2000), suggesting that translation inhibition is quite general and not restricted to proteins entering the ER. This part of the response clearly serves to limit the load of unfolded proteins in all organelles and, as described subsequently, provides unique opportunities for regulating gene expression as well.
In addition to diminishing protein synthesis during conditions that are adverse for proper folding and increasing the pools of chaperones to stabilize proteins that cannot fold, activation of the UPR allows eucaryotic cells to increase their degradative capacity in order to rid themselves of improperly folded proteins (Casagrande et al 2000; Friedlander et al 2000). Together, these 3 responses insure that insoluble protein aggregates do not form to damage the ER. The inhibition of protein synthesis and increased activity of the proteasome affect the accumulation of damaged proteins anywhere in the cells and thus also afford protection to other organelles during stress.
In addition to these cytoprotective responses, 2 other responses occur when the UPR is activated that might be viewed as more protective to the organism than to the cell itself. The first is the arrest of cells in the G1 phase of cell cycle to prevent the propagation of cells experiencing stress (Melero 1979; Carlberg and Larsson 1993). The signaling that leads to cell cycle arrest begins to occur fairly rapidly and is reversible if the stress condition is alleviated. The second response leads to the activation of apoptotic pathways, which occurs if the stress is not resolved after an appropriate period of time (Zinszner et al 1998; Nakagawa et al 2000). Both of these responses may have particular ramifications for virally infected cells or cells experiencing early neoplastic growth, as discussed later. It is not currently clear how the protective components of the UPR are balanced against those that contribute to cell death or if the timing for these 2 outcomes varies in different cell types or at different points in development.
COMPONENTS OF THE MAMMALIAN UPR SIGNALING PATHWAY
The UPR pathway was first delineated in yeast. The identification of an unfolded protein response element (UPRE) in the yeast Kar2 (BiP) promoter (Mori et al 1992) paved the way for genetic approaches to identify the components of the signaling pathway. Ire1-Ern1 was the first to be identified and is a transmembrane ER-localized kinase that possesses an N-terminal stress-sensing domain in the lumen of the ER and a cytoplasmic kinase domain (Cox et al 1993; Mori et al 1993). In response to ER stress, Ire1 dimerizes and is phosphorylated in trans, which serves to activate a unique endonuclease activity at its C-terminus (Sidrauski and Walter 1997). The target of this activity is a precursor messenger ribonucleic acid (mRNA) that encodes the Hac1 transcription factor, which is not translated in the absence of stress. After cleavage by Ire1p and religation by Rlg1, Hac1p is synthesized and regulates the expression of UPR target genes by binding to the UPRE in their promoter (Cox and Walter 1996). This single signaling cascade is responsible for activating the UPR in yeast.
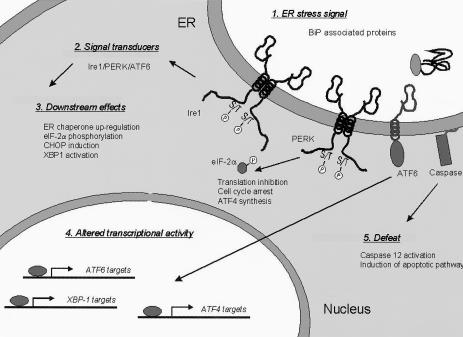
In higher eucaryotes the elements of this signaling pathway are conserved but greatly expanded (Fig 1). First, 2 Ire1 homologs exist in mammalian cells: Ire1α, which is ubiquitously expressed (Tirasophon et al 1998), and Ire1β, whose expression is restricted to gut epithelium (Wang et al 1998). Both proteins possess a luminal stress-sensing domain, a cytosolic kinase domain, and the unique endonuclease domain found in yeast Ire1p. Very recently, the mRNA of the mammalian transcription factor XBP-1 was shown to be a target of Ire1's endonuclease activity (Shen et al 2001; Yoshida et al 2001; Calfon et al 2002). The alternatively processed XBP-1 mRNA produces a transcription factor with a new C-terminus encoding a transactivation domain tethered to the unchanged DNA–binding domain in its N-terminus. The stress-altered XBP-1 protein binds to and transactivates the ER stress elements (ERSEs) present in mammalian ER chaperone promoters (Yoshida et al 1998; Yoshida et al 2001).
Fig. 1.
Aspects of the mammalian unfolded protein response (UPR) and the component of the pathway that regulates them. The signal for UPR activation is the accumulation of unfolded proteins that bind to the endoplasmic reticulum (ER) chaperone BiP. This signal is transduced by 2 transmembrane kinases (Ire1 and PERK) and an ER-tethered transcription factor (ATF6). PERK activation leads to eIF2-α phosphorylation, causing a general inhibition of protein translation, cell cycle arrest, and ATF4 synthesis. Other downstream effects of UPR activation include the transcriptional up-regulation of ER chaperones, CHOP induction, and XBP-1 messenger ribonucleic acid cleavage, resulting in an altered form of the XBP-1 transcription factor. UPR activation is accompanied by alterations in the transcription of a number of genes, most notably those regulated by ATF6, ATF4, and XBP-1. If the stress conditions persist, apoptotic signaling pathways are activated, in part, through the cleavage of procaspase 12
A third ER-localized stress-regulated kinase, PERK-PEK, was discovered that does not exixt in yeast but which can be found in all metazoans (Shi et al 1998; Harding et al 1999). The cytosolic domain of PERK shows homology to the double-stranded RNA–activated kinase PKR and is a member of the eIF2α family of kinases. Members of this family respond to distinct cellular stress conditions by phosphorylating eIF2α (Chen and London 1995; Harding et al 1999; Williams 1999; Fernandez et al 2002). This serves to inhibit protein synthesis, which in the case of ER stress limits the accumulation of unfolded proteins in the ER when conditions are not suitable for proper folding. The inhibition of protein synthesis also affects the synthesis of cytosolic proteins and results in the loss of cyclin D1 from cells (Brewer et al 1999). This causes them to arrest in the G1 phase of cell cycle and thus prevents the propagation of cells experiencing ER stress. In addition, the general block in translation suppresses the use of small open reading frames up-stream of the translation start site on the ATF4 mRNA and allows the ATF4 transcription factor to be synthesized and to directly transactivate the transcription of downstream genes like C/EBP-homologous protein (CHOP) (Harding et al 2000; Ma et al 2002). Induction of the mammalian UPR is regulated by levels of the ER chaperone BiP (Dorner et al 1992). In the absence of ER stress, BiP binds to the lumenal domain of both Ire1 kinases and PERK and keeps them in an inactive monomeric state. When stress occurs and unfolded proteins begin to accumulate, BiP is released from the kinases, allowing them to dimerize and signal the response (Bertolotti et al 2000; Okamura et al 2000).
A fourth component of the mammalian UPR is the ATF6 α and β transcription factors, which are synthesized as ER-localized transmembrane proteins (Haze et al 1999). Activation of the response leads to cleavage of ATF6 by the S1P and S2P proteases (Ye et al 2000), thus liberating the cytosolic transcription factor domain from the membranes and allowing it to regulate the transcription of UPR targets that possess an ERSE in their promoters (Yoshida et al 2000). Most recently, the ATF6 and Ire1 components have been shown to interact via regulation of the XBP-1 transcription factor. ATF6 up-regulates XBP-1 transcription via an ERSE in its promoter, and activated Ire1 cleaves the XBP-1 mRNA to produce a highly active transcription factor (Shen et al 2001; Yoshida et al 2001; Calfon et al 2002). The ER chaperone genes appear to be targets of XBP-1. Thus, unlike yeast where the UPR pathway is signaled by a single ER kinase, higher eucaryotes possess multiple distinct (Ire1α and β, PERK, and ATF6) signaling molecules that control downstream transcriptional responses.
Recently, a fifth element of the mammalian UPR was identified with the discovery of procaspase 12 (Nakagawa et al 2000). Procaspase 12 is localized to the cytosolic face of the ER membrane and is proteolytically cleaved to an active caspase form in response to ER stress. Cells from caspase 12 nullizygous mice are defective in activating apoptotic pathways in response to prolonged ER stress, suggesting that this protein is part of the delicate balancing act between cytoprotective and organismal protective responses, as discussed later.
ROLE OF THE ER STRESS RESPONSE IN NORMAL GROWTH AND IN DISEASE
It is clear that during cell growth and differentiation, conditions of stress can arise. These include normal physiological situations where increased demands are placed on the ER in cells synthesizing vast quantities of secretory pathway proteins (eg, plasma cells, hepatocytes, and pancreatic cells). Disruption of either Ire1α (Calfon et al 2002; Lee et al 2002) or XBP-1 (Reimold et al 2000) results in an embryonic lethal phenotype, and XBP-1 has been shown to affect liver development and terminal differentiation of B cells to antibody-secreting plasma cells (Reimold et al 2000, 2001). Because Ire1α−/− mouse embryonic fibroblasts (MEFs) have an intact UPR, these observations suggest that the Ire1-XBP-1 branch of the mammalian UPR may be essential for providing increased levels of molecular chaperones and folding enzymes in some secretory tissues and raise the interesting possibility that certain portions of the pathway can be activated without signaling the complete response. This would have clear implications for the potential of modulating either beneficial or destructive parts of the response during the treatment of certain diseases.
Mice that are nullizygous for the PERK kinase (Harding et al 2001) or have been engineered to express an eIF2-α mutant that cannot be phosphorylated (Scheuner et al 2001), and thus do not shut down protein synthesis in response to ER stress, have profound problems with glucose metabolism and increased death of pancreatic β islet cells. In humans a mutation in the PERK kinase results in hereditary type I infant diabetes (Delepine et al 2000). Thus, activation of the UPR is essential to protecting certain secretory organs during their normal growth. In addition, tissues often experience transient environmental stresses, like changes in blood glucose or alcohol, that would be expected to induce the UPR in order to protect the cell until the stress subsides. However, in cases of chronic alcohol abuse, cirrhosis and eventual death of hepatocytes occurs. It is possible that the activation of apoptotic pathways in response to ER stress participates in this cell death.
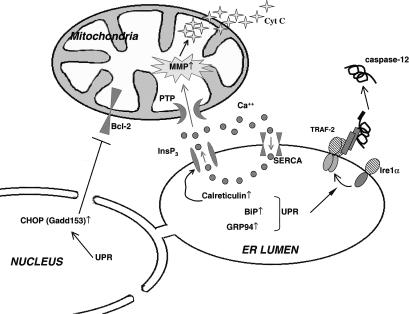
Much progress has been made during the past few years in understanding how perturbations in ER homeostasis induce apoptosis (Fig 2). Mobilization of internal calcium stores, leading to mitochondrial membrane permeabilization and cytochrome c release, is a common feature of apoptotic signaling pathways (Wang 2001). The ER is the storage site for intracellular calcium, which is loaded through the sarcoplasmic-endoplasmic Ca2+-adenosine triphosphatase (SERCA) and released through the inositol-1,4,5-trisphosphate (InsP3) receptor. Several of the ER chaperones also serve as major calcium-binding proteins (eg, calreticulin, BiP, and GRP94) (Lytton and Nigam 1992; Pozzan et al 1994). Like the ER chaperones, the SERCA pump is also up-regulated during ER stress (Caspersen et al 2000), which presumably allows for greater transport and storage of calcium in the ER to protect the cells against calcium depletion–induced apoptosis. Reports that induction of BiP protects against thapsigargin-induced apoptosis (Jamora et al 1996; Miyake et al 2000), whereas increased levels of calreticulin sensitize cells to apoptosis (Nakamura et al 2000), raise the interesting possibility that later in the stress response, these 3 proteins may play a role in balancing protective vs destructive responses. The activated IRE1α kinase, which is a proximal effector of the UPR signaling pathway, also serves to recruit the TRAF2 adaptor protein. This in turn activates c-jun N-terminal kinase pathways, leading to apoptotic death (Urano et al 2000), and induces clustering and activation of procaspase 12 (Yoneda et al 2001), the ER-localized member of the caspase family. Cells from mice lacking the caspase 12 gene are more resistant to cell death in response to glucose deprivation (Nakagawa et al 2000), but it has not been determined if these cells are also resistant to other chronic stresses like viral infection or are more sensitive to tumor formation, which would demonstrate a role for caspase 12 in these disease states. Finally, the CHOP transcription factor that is induced during UPR activation decreases the expression of the antiapoptotic protein, Bcl-2 (McCullough et al 2001), thus contributing to apoptosis during ER stress. Therefore, the cytoprotective and cytodestructive aspects of the ER stress response employ many of the same components, suggesting that they play a role in the balance between the 2 outcomes. How this is achieved and when good signals turn bad remain to be determined.
Fig. 2.
Illustration of unfolded protein response (UPR)-dependent apoptotic pathways. Upon endoplasmic reticulum (ER) stress, oligomerization of Ire1α induces the binding of TRAF-2 to its cytoplasmic domain, which in turn promotes procaspase-12 clustering, cleavage, and activation. UPR activation also up-regulates ER chaperones like BiP and GRP94, which can bind calcium and prevent further calcium mobilization during ER stress. Conversely, calreticulin, another UPR-induced ER chaperone, regulates the function of the ER inositol-1,4,5-trisphosphate (InsP3) calcium channel to increase calcium mobilization to the cytosol. The released calcium activates the mitochondria permeability transition pore (PTP) resulting in mitochondrial membrane permeabilization. This causes cytochrome c release from the mitochondria, leading to activation of apoptosomes. Finally, the UPR induces CHOP, which decreases the expression of the antiapoptotic protein Bcl-2, further contributing to cell death
Activation of the UPR appears to provide protection for cells experiencing hypoxic conditions (Koong et al 1994) and for animals that are experimental models for heart disease, ischemia, or seizures (Paschen and Frandsen 2001). There are data to suggest that the up-regulation of the ER chaperone GRP170-ORP150 is particularly important in providing this protection (Tamatani et al 2001), although the reason is not clear. It is possible that GRP170 chaperones the folding of a protein(s) that is particularly sensitive to low-oxygen conditions (Ozawa et al 2001a). Thus, activation of the UPR might provide a clinically important target for dealing with heart disease and stroke.
The UPR is also activated in virally infected cells (Watowich et al 1991; Lee 2001). Although each viral particle contains a number of proteins that are synthesized in the cytosol, in most cases many cell surface or viral coat proteins are made. These are often synthesized in the ER, which is probably the signal for UPR activation (Pahl and Baeuerle 1995). It is not currently clear if this represents a hijacking of host responses by the virus to aid in the production of viral coat proteins, particularly in the case of lytic viral infections that ultimately kill the cell anyway, or if the UPR induction represents an attempt by the host cell to initiate cell death pathways to limit the infection, in the case of chronic viral infections, like Hepatitis B or C viruses. A more complete characterization of the UPR components that are activated by lytic and chronic virus infection will be needed to understand the role of the UPR in viral infection and to determine if control of specific arms of the pathway could ultimately be manipulated as a means of supplementing antiviral defenses.
Finally, a number of studies have shown that the UPR is activated in cancer, particularly in solid tumors (Cai et al 1993; Chatterjee et al 1997; Fernandez et al 2000; Lee 2001). It is likely that this is because of changes in pH and decreased levels of oxygen, glucose, and nutrients that occur as a result of incomplete vascularization of the tumor. The cytoprotective aspects of the UPR may be critical to allowing the neoplastic cell to survive these stresses. Studies have shown that interfering with GRP170 up-regulation can profoundly affect tumor cell growth in animals (Ozawa et al 2001b). Because the tumor cell would be expected to experience chronic ER stress, some of the aspects of the pathway, like cell cycle arrest and apoptosis, must be overcome. Recent studies demonstrating that hsp70 and hsp27 are often induced in these same tumors (Volm et al 1995; Maehara et al 2000; Yamamoto et al 2001) may provide a mechanism for interfering with apoptotic signaling. Hsp70 binds to Apaf-1 (Beere et al 2000; Saleh et al 2000), and hsp27 can bind to cytochrome c (Bruey et al 2000) to prevent the formation of the apoptosome with procaspase-9, thereby blocking its activation (Xanthoudakis and Nicholson 2000). In addition, the well-documented up-regulation of antiapoptotic proteins like Bcl-2 and Bcl-XL in tumor cells (Jaattela 1999; Nakamura et al 2000) may play a role in suppressing UPR-induced cytodestructive responses. Clearly, further delineation of the signaling pathways that control the protective vs destructive outcomes of UPR activation could provide new targets for therapeutic interventions in conditions as diverse as heart disease, diabetes, viral infection, and cancer.
CONTINUED PROTECTION TO THE HOST AFTER UPR-INDUCED CELL DEATH
Induction of the ER stress response causes the up-regulation of a group of ER chaperones, including GRP94. Recent studies have shown that GRP94 binds a wide variety of peptides that it encounters in the ER (Li and Srivastava 1993; Wearsch and Nicchitta 1997). The peptides appear to be produced by the proteasome and are pumped into the ER by the transporter associated with antigen processing (TAP) to load nascent major histocompatibility complex class I molecules that are being assembled in the ER. These peptides serve as a source of antigen to activate killer T cells that can attack virally infected cells or tumors (Suto and Srivastava 1995; Li 1997). When cell death occurs as a result of prolonged ER stress, GRP94 bound to peptides is released from the cell. Experimental data show that GRP94 isolated from tumor cells can stimulate antitumor responses (Li 1997). Clinical trials are now being conducted to determine the efficacy of tumor-derived GRP94 in inducing antitumor immunity. It is possible that a similar approach could be used to enhance immunity to viruses that induce chronic infections.
SUMMARY
A unique and complex signaling cascade is initiated by physiological and chemical conditions that alter the folding of proteins in the ER. The response is initially characterized by cytoprotective measures that are designed to buffer the ER against the accumulation of unfolded proteins. However, these beneficial effects extend to other cellular organelles, suggesting that the cell may use the ER to monitor the environmental conditions that have broad effects on protein folding. If stress persists, decisions are made to destroy the cell in order to protect the organism. How these decisions are made is not currently understood but is at the heart of a number of disease processes. A clearer understanding of how this delicate balance between cytoprotective and cytodestructive processes is achieved could allow us to treat these diseases more effectively.
REFERENCES
- Beere HM, Wolf BB, and Cain K. et al. 2000 Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci U S A. 1999;96:8505–8510. doi: 10.1073/pnas.96.15.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Werner ED, Dubas ME, Goeckeler JL, Kruse KB, McCracken AA. The requirement for molecular chaperones during endoplasmic reticulum-associated protein degradation demonstrates that protein export and import are mechanistically distinct. J Biol Chem. 1999;274:3453–3460. doi: 10.1074/jbc.274.6.3453. [DOI] [PubMed] [Google Scholar]
- Brostrom CO, Prostko CR, Kaufman RJ, Brostrom MA. Inhibition of translational initiation by activators of the glucose-regulated stress protein and heat shock protein stress response systems. Role of the interferon-inducible double-stranded RNA-activated eukaryotic initiation factor 2alpha kinase. J Biol Chem. 1996;271:24995–25002. doi: 10.1074/jbc.271.40.24995. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, and Bonniaud P. et al. 2000 Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2:645–652. [DOI] [PubMed] [Google Scholar]
- Cai JW, Henderson BW, Shen JW, Subjeck JR. Induction of glucose regulated proteins during growth of a murine tumor. J Cell Physiol. 1993;154:229–237. doi: 10.1002/jcp.1041540204. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clask SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Carlberg M, Larsson O. Role of N-linked glycosylation in cell-cycle progression and initiation of DNA synthesis in tumor-transformed human fibroblasts. Anticancer Res. 1993;13:167–171. [PubMed] [Google Scholar]
- Casagrande R, Stern P, Diehn M, Shamu C, Osario M, Zuniga M, Brown PO, Ploegh H. Degradation of proteins from the ER of S. cerevisiae requires an intact unfolded protein response pathway. Mol Cell. 2000;5:729–735. doi: 10.1016/s1097-2765(00)80251-8. [DOI] [PubMed] [Google Scholar]
- Caspersen C, Pedersen PS, Treiman M. The sarco/endoplasmic reticulum calcium-ATPase 2b is an endoplasmic reticulum stress-inducible protein. J Biol Chem. 2000;275:22363–22372. doi: 10.1074/jbc.M001569200. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Hirota H, Belfi CA, Berger SJ, Berger NA. Hypersensitivity to DNA cross-linking agents associated with up-regulation of glucose-regulated stress protein GRP78. Cancer Res. 1997;57:5112–5116. [PubMed] [Google Scholar]
- Chen JJ, London IM. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem Sci. 1995;20:105–108. doi: 10.1016/s0968-0004(00)88975-6. [DOI] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- Dorner AJ, Wasley LC, Kaufman RJ. Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J. 1992;11:1563–1571. doi: 10.1002/j.1460-2075.1992.tb05201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- Fernandez PM, Tabbara SO, Jacobs LK, Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA, Patierno SR. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Yaman I, Merrick WC, Koromilas A, Wek RC, Sood R, Hensold J, Hatzoglou M. Regulation of internal ribosome entry site-mediated translation by eukaryotic initiation factor-2alpha phosphorylation and translation of a small upstream open reading frame. J Biol Chem. 2002;277:2050–2058. doi: 10.1074/jbc.M109199200. [DOI] [PubMed] [Google Scholar]
- Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol. 2000;2:379–384. doi: 10.1038/35017001. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/−mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–7704. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knittler MR, Haas IG. Interaction of BiP with newly synthesized immunoglobulin light chain molecules: cycles of sequential binding and release. EMBO J. 1992;11:1573–1581. doi: 10.1002/j.1460-2075.1992.tb05202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koong AC, Chen EY, Lee AS, Brown JM, Giaccia AJ. Increased cytotoxicity of chronic hypoxic cells by molecular inhibition of GRP78 induction. Int J Radiat Oncol Biol Phys. 1994;28:661–666. doi: 10.1016/0360-3016(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, and Shen X. et al. 2002 IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16:452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Priming of T cells by heat shock protein-peptide complexes as the basis of tumor vaccines. Semin Immunol. 1997;9:315–322. doi: 10.1006/smim.1997.0087. [DOI] [PubMed] [Google Scholar]
- Li Z, Srivastava PK. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993;12:3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J, Nigam SK. Intracellular calcium: molecules and pools. Curr Opin Cell Biol. 1992;4:220–226. doi: 10.1016/0955-0674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, and Diehl A 2002 Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol, . in press. [DOI] [PubMed] [Google Scholar]
- Maehara Y, Oki E, Abe T, Tokunaga E, Shibahara K, Kakeji Y, Sugimachi K. Overexpression of the heat shock protein HSP70 family and p53 protein and prognosis for patients with gastric cancer. Oncology. 2000;58:144–151. doi: 10.1159/000012091. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero JA. Isolation and cell cycle analysis of temperature-sensitive mutants from Chinese hamster cells. J Cell Physiol. 1979;98:17–30. doi: 10.1002/jcp.1040980104. [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000;77:396–408. doi: 10.1002/(sici)1097-4644(20000601)77:3<396::aid-jcb5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signalling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- Mori K, Sant A, Kohno K, Normington K, Gething MJ, Sambrook JF. A 22 bpcis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992;11:2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Bossy-Wetzel E, and Burns K. et al. 2000 Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 150:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Kimata Y, Higashio H, Tsuru A, Kohno K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem Biophys Res Commun. 2000;279:445–450. doi: 10.1006/bbrc.2000.3987. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Kondo T, Hori O, Kitao Y, Stern DM, Eisenmenger W, Ogawa S, Ohshima T. Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport. J Clin Investig. 2001a;108:41–50. doi: 10.1172/JCI11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Tsukamoto Y, Hori O, Kitao Y, Yanagi H, Stern DM, Ogawa S. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res. 2001b;61:4206–4213. [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Frandsen A. Endoplasmic reticulum dysfunction—a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem. 2001;79:719–725. doi: 10.1046/j.1471-4159.2001.00623.x. [DOI] [PubMed] [Google Scholar]
- Pathak VK, Schindler D, Hershey JW. Generation of a mutant form of protein synthesis initiation factor eIF-2 lacking the site of phosphorylation by eIF-2 kinases. Mol Cell Biol. 1988;8:993–995. doi: 10.1128/mcb.8.2.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Prostko CR, Brostrom MA, Malara EM, Brostrom CO. Phosphorylation of eukaryotic initiation factor (eIF) 2 alpha and inhibition of eIF-2B in GH3 pituitary cells by perturbants of early protein processing that induce GRP78. J Biol Chem. 1992;267:16751–16754. [PubMed] [Google Scholar]
- Reimold AM, Etkin A, and Clauss I. et al. 2000 An essential role in liver development for transcription factor XBP-1. Genes Dev. 14:152–157. [PMC free article] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, and Manis J. et al. 2001 Plasma cell differentiation requires the transcription factor XBP-1. Nature. 412:300–307. [DOI] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Sambrook JF. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990;61:197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Song B, and McEwen E. et al. 2001 Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 7:1165–1176. [DOI] [PubMed] [Google Scholar]
- Shen X, Ellis RE, and Kurnit DM. et al. 2001 Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 107:893–903. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Matsuyama T, and Yamaguchi A. et al. 2001 ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat Med. 7:317–323. [DOI] [PubMed] [Google Scholar]
- Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Valetti C, Sitia R. The differential effects of dithiothreitol and 2-mercaptoethanol on the secretion of partially and completely assembled immunoglobulins suggest that thiol-mediated retention does not take place in or beyond the Golgi. Mol Biol Cell. 1994;5:1311–1324. doi: 10.1091/mbc.5.12.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volm M, Mattern J, Stammler G. Up-regulation of heat shock protein 70 in adenocarcinomas of the lung in smokers. Anticancer Res. 1995;15:2607–2609. [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [Google Scholar]
- Wang X-Z, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich SS, Morimoto RI, Lamb RA. Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. J Virol. 1991;65:3590–3597. doi: 10.1128/jvi.65.7.3590-3597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearsch PA, Nicchitta CV. Interaction of endoplasmic reticulum chaperone GRP94 with peptide substrates is adenine nucleotide-independent. J. Biol. Chem. 1997;272:5152–5156. doi: 10.1074/jbc.272.8.5152. [DOI] [PubMed] [Google Scholar]
- Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S, Nicholson DW 2000 Heat-shock proteins as death determinants. Nat Cell Biol. 2 E. 163–E165. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y. Heat shock protein 27 was up-regulated in cisplatin resistant human ovarian tumor cell line and associated with the cisplatin resistance. Cancer Lett. 2001;168:173–181. doi: 10.1016/s0304-3835(01)00532-8. [DOI] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]