Abstract
We have previously observed that subunits of the chaperonin required for actin production (type-II chaperonin containing T-complex polypeptide 1 [CCT]) localize at sites of microfilament assembly. In this article we extend this observation by showing that substantially substoichiometric CCT reduces the initial rate of pyrene-labeled actin polymerization in vitro where eubacterial chaperonin GroEL had no such effect. CCT subunits bound selectively to F-actin in cosedimentation assays, and CCT reduced elongation rates from both purified actin filament “seeds” and the short and stabilized, minus-end blocked filaments in erythrocyte membrane cytoskeletons. These observations suggest CCT might remain involved in biogenesis of the actin cytoskeleton, by acting at filament (+) ends, beyond its already well-established role in producing new actin monomers.
INTRODUCTION
The chaperonins are double-ringed ∼20S particles containing 7–9 ∼60 kDa adenosine triphosphatase subunits per ring, enclosing a substrate-binding cavity (Ellis and Hartl 1999; Leroux and Hartl 2000). They have been subdivided into types I and II on the basis of distribution, sequence similarity, subunit complexity, and ring symmetry (Leroux and Hartl 2000). The type-II chaperonin containing T-Complex Polypeptide 1 (CCT), found in the eukaryotic cytosol, has 8-membered rings and is unique in having 8 distinct, though related, subunits (Kubota et al 1995; Gutsche et al 1999; Leroux and Hartl 2000). Unlike the archetypal GroEL chaperonin (type I), which has a rather general range of substrates (Houry et al 1999), CCT is proposed to have more limited specificity: its chief substrates in vivo are the major cytoskeletal proteins actin and tubulin (Sternlicht et al 1993). Other reports propose a wider extent of folding substrates (eg, Melki et al 1997; Thulasiraman et al 1999; Leroux and Hartl 2000; McCallum et al 2000) and other evidence still suggests additional cellular interactions involving CCT. Oligomeric (∼20S) CCT particles associate with assembly intermediates of the hepatitis B viral capsid but not with unassembled native capsid proteins or fully assembled capsids (Lingappa et al 1994). Other findings also suggest a transient role for CCT in assembling multimeric structures, such as modulating interaction between Von-Hippel Lindau (VHL) protein and elongins B and C (Feldman et al 1999), myosin light and heavy chain assembly (Srikakulam and Winkelmann 1999) and, possibly, Ca2+-dependent chromaffin granule secretion (Creutz et al 1994). The CCTα subunit is detected at the centrosome, and CCT-specific antibodies prevent centrosome-nucleated microtubule regrowth, a process not dependent in vitro on new protein synthesis (Brown et al 1996). It is not clear whether all these activities relate to CCT in the form of an oligomer of fixed composition (Liou and Willison 1997) or involve free subunits or microcomplex subassemblies (Liou and Willison 1997) that can arise by K+ ion and adenosine triphosphate (ATP) hydrolysis–dependent disassembly (Roobol et al 1999a). CCT subunits behave selectively as microtubule-associated proteins (Roobol et al 1999b), and this again suggests that CCT may have a role in tubulin assembly beyond production of correctly folded monomers. The possibility that CCT may also be involved in actin polymerization was first hinted at by immunofluorescence studies, which detected CCT subunits at ruffling edges of fibroblastlike cells (Roobol and Carden 1999) and at the leading edge of developing neurites (Roobol et al 1995). Both are locations where constant modification of the actin cytoskeleton (microfilament assembly and disassembly) occurs. To examine the possibility that CCT has functions relating to actin assembly (polymerization into filaments), beyond the folding of newly synthesized proteins, we performed a set of in vitro experiments. The results, described in this study, provide the first evidence that CCT can modulate elongation rate at the (+) end of actin filaments.
MATERIALS AND METHODS
Preparation of CCT
Approximately 20S CCT (16-mer) was enriched from rat testis by sucrose gradient fractionation (Roobol and Carden 1993) and further purified by anion exchange chromatography (Pharmacia Resource Q) using a linear gradient of 100–400 mM NaCl (Roobol et al 1995; Roobol and Carden 1999). CCT for all experiments was a selective population (pooled lowest salt eluting fractions) lacking bound actin or tubulin detectable by immunoblot, unless noted otherwise. Escherichia coli GroEL was from Epicentre Technologies (Cambio, UK).
Preparation of pyrene-labeled actin and measurement of actin polymerization
Actin was purified from rabbit muscle (Pardee and Spudich 1982) and labeled in filamentous form at cysteine374 with N-(pyrenyl)maleimide using a 1:1 (with respect to G-actin) molar ratio (Kouyama and Mihashi 1981). Spectrophotometry revealed pyrene incorporation into ∼30% of the actin monomers. Equal amounts of labeled and nonlabeled G-actin (final concentration of 4.5 μM) were combined (in 2 mM Tris pH 8.0, 0.2 mM ATP, 5 mM β-mercaptoethanol, 0.2 mM CaCl2, and 0.02% NaN3) to reduce the fluorescent signal into the detection range of the equipment and made up to 100 mM KCl, 2 mM MgCl2, and 2 mM ATP (assembly buffer). For all assembly experiments, except 1 noted in the results section, this actin was subjected to a size exclusion chromatography step (Pardee and Spudich 1982) before use (to generate substantially monomeric G-actin). After excitation at 344 nm, fluorescence emission was measured at ambient temperature using a Perkin-Elmer LS50B instrument set at 388 nm, 5-nm slit width, sample interval 5 seconds, and response time 0.5 seconds. To monitor the effect of CCT on actin polymerization, purified CCT was dialyzed overnight into assembly buffer without ATP and added to a final concentration of 0.05 μM.
Preparation of rat erythrocyte ghosts
The procedure of Pinder and Gratzer (1983) was used: red blood cells were collected by centrifugation at 500 × g from 20 mL of rat blood diluted 1:1 with 1.5% (w/v) sodium citrate and 0.88% (w/v) NaCl. They were washed 3 times by centrifugation with 10 mM sodium phosphate of pH 7.4, 150 mM NaCl, and lysed by resuspension in 7.5 mM sodium phosphate of pH 7.4, 1 mM ethylenediamine-tetraacetic acid (EDTA), 1 μg/mL pepstatin A, and 1 μg/mL phenylmethane sulfonyl fluoride. Soluble cell contents were removed by repeated washing with 7.5 mM sodium phosphate of pH 7.4 and 1 mM EDTA.
Cosedimentation of F-actin and CCT
CCT and G-actin were clarified before use in polymerization assays, at 96 600 × g for 30 minutes at 4°C, conditions under which F-actin forms a pellet, whereas CCT and G-actin remain soluble. After 2 hours of polymerization, samples were recentrifuged under these same conditions.
Other methods
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) used 9% polyacrylamide gels and was sometimes followed by Western blotting (Roobol et al 1999b). Protein concentrations were determined by Bradford assay (Bradford 1976) using bovine serum albumin (BSA) standards. Immunoblot detection of CCT subunits was carried out with subunit-specific antibodies (Roobol et al 1999b) to sequences from the COOH-termini of mouse CCT subunits (Kubota et al 1994). Essentially identical procedures (Roobol et al 1999b) were used to generate and characterize new CCTη subunit-specific, affinity-purified rabbit antisera with the peptide NH2-CSAGRGRGQARFH-COOH (GenBank NM_007638), where the underlined cysteine residue was introduced for conjugation. Two different batches of antisera (eta1 and eta2) were produced in separate rabbits. Densitometric analysis of Coomassie Blue G250–stained SDS-PAGE gels was performed using Phoretix PC data analysis software.
RESULTS
Substoichiometric amounts of CCT inhibit actin polymerization in vitro
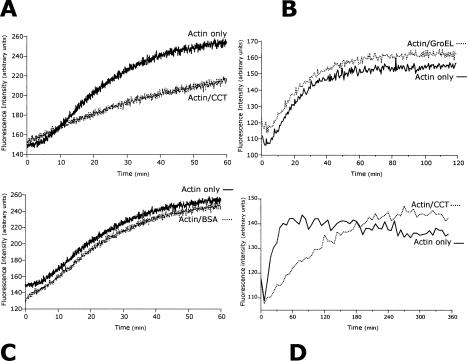
To investigate the effect of CCT on actin filament assembly, the in vitro polymerization of 4.5 μM pyrene-labeled G-actin was monitored by fluorescence enhancement (Fig 1). A significant reduction in the initial rate of actin polymerization was observed in the presence of 0.05 μM CCT 16-mer. No such reduction was apparent in the presence of 0.05 μM GroEL or BSA, indicating that the effect of CCT is specific.
Fig 1.
Type-II chaperonin containing T-complex polypeptide 1 (CCT) reduces the initial rate but not the final level of actin polymerization. Polymerization of 4.5 μM G-actin [15% labeled, at Cys374, with N-(1-pyrenyl)maleimide] was initiated by adding 2 mM MgCl2, 100 mM KCl, and 2 mM adenosine triphosphate. Fluorescence enhancement (filament formation) was measured over 3600 seconds, and example traces, smoothed by 1/10 to reduce noise, show actin polymerization in the presence and absence of 0.05 μM CCT (A), 0.05 μM GroEL (B), or 0.05 μM bovine serum albumin (C). Fluorescence enhancement of actin with (dotted line) or without (solid) 0.05 μM CCT was also measured over 6 hours (D) with an interval time of 90 seconds to reduce photobleaching and smoothed by 1/5
Initiation of actin polymerization involves the formation of a nucleation complex that comprises 3 actin monomers to which further salt-activated monomers are added during the elongation of polymers (Bray 2000). Because the number of such trimers is small and free actin monomers are in large excess, polymerization of actin is a pseudo first-order reaction. Polymerization rate constants were fitted to a single exponential and rate constants calculated using data obtained from 180 seconds to 3600 seconds. Values were 6.67 × 10−4 for 4.5 μM G-actin alone, 3.76 × 10−4 for 4.5 μM G-actin-50 nM CCT, 5.9 × 10−4 for 4.5 μM G-actin-50 nM BSA, and 6.6 × 10−4 for 4.5 μM G-actin-50 nM GroEL. Data from the first 180 seconds were omitted to allow for thermal equilibration. Residual values from fitted lines of best fit were random with correlation coefficients close to unity (data not shown), supporting the use of a single exponential model. During extended assays, where polymerization reached a plateau, CCT affected the initial rate of polymerization and not the final level of filament formation (Fig 1D).
CCT-mediated reduction of the initial rate of actin polymerization was also determined by a different, sedimentation-based assay over a range of concentrations (0–65 nM) limited by the difficulty of isolating highly pure (tubulin-free) ∼20S CCT. F-actin (4.5 μM) pellet formation after 1 hour was reduced by 14% in the presence of 14 nM CCT (1:321 CCT 16-mer:G-actin), 26% at 32 nM CCT, and 32% at 65 nM CCT. These values are clearly proportional to CCT concentration and confirm results from fluorimetric assays.
Selected CCT subunits remain associated with microfilaments assembled in vitro
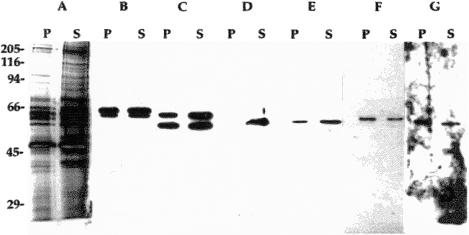
We had previously observed that selected CCT subunits remain associated with polymerizing microtubules (Roobol et al 1999b). Therefore, the possible binding of CCT (either as an intact 16-mer or as smaller subassemblies) to F-actin was investigated by sedimentation assays followed by immunoblotting. CCT subunits were cosedimented with actin filaments formed from nonlabeled G-actin after 2 hours of incubation under the same polymerizing conditions as those used for fluorimetric analyses (Fig 2). CCT incubated alone and then centrifuged under the same conditions did not form a pellet (not shown—but see Grantham 1998). But CCT subunits did associate with F-actin pellets and to quite different extents: CCTζ and CCTη were comparatively enriched in the pellet, whereas CCTβ, CCTε, and CCTθ were slightly more abundant in the supernatant. Distribution of CCTα and CCTγ appeared comparable between pellet and supernatant. Most significantly, CCTδ did not bind detectably to F-actin under these conditions. Samples of actin polymerized in the presence of CCT were examined after negative staining using an electron microscope, but no CCT complexes were obviously associated with the filaments. Single CCT subunits and subassemblies would not, however, have been detectable because of resolution limitations (data not shown, but see Grantham 1998).
Fig 2.
Type-II chaperonin containing T-complex polypeptide 1 (CCT) subunits selectively bind F-actin. After incubation under polymerizing condition for 2 hours at room temperature, a sample containing 4.5 μM actin and 0.05 μM CCT was centrifuged at 96 600 × g for 30 minutes and corresponding volumes of pellet (P) and supernatant (S) prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Duplicate samples resolved on a 9% polyacrylamide gel were either silver stained (A) or transferred to nitrocellulose (B–G) and probed with CCT subunit-specific antibodies: (B) anti-CCTα (lower band) plus anti-CCTγ (upper); (C) anti-CCTɛ (upper) plus anti-CCTβ (lower); (D) anti-CCTδ; (E) anti-CCTθ; (F) anti-CCTζ; and (G) anti-CCTη
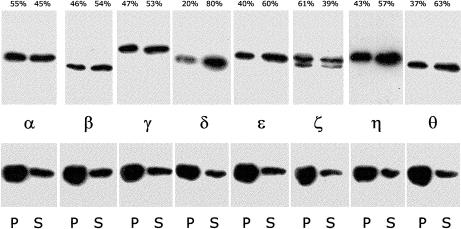
To confirm these results actin was polymerized in the presence of CCT and the cosedimentation of CCT subunits analyzed at a point when polymerization had reached a plateau (Fig 3). The actin used for this experiment (kind gift of Miss Nancy Adamek, UKC) varied from that used in all other experiments. It was prepared (from rabbit muscle) essentially as described (Pardee and Spudich 1982) but notably without ATP added to the buffers for purification steps beyond the initial extraction from an acetone powder and without chromatography by size exclusion as a final purification step. This actin polymerized faster than that used in other experiments. The CCT preparation used for this analysis also differed from that used in all other experiments insofar as it derived from a single fraction in the actin-free (by blot) leading peak from a monoQ column. Even so, the degree of F-actin association again varied between CCT subunits, with CCTδ again showing the least binding and CCTζ the greatest, as quantitated in Fig 3. Testing of other CCT fractions in the same preparation yielded similar results (not shown), namely, selective CCT subunit binding to F-actin, though with some variability. This suggestion that subfractionated CCT (which reproducibly elutes in an unusually very wide profile from monoQ columns) does not have uniform properties is reminiscent of a similar variability (A. Roobol, personal observation) in K+-, Mg++-, and ATP hydrolysis–dependent disassembly (Roobol et al 1999).
Fig 3.
Immunoblot quantitation of selective type-II chaperonin containing T-complex polypeptide 1 (CCT) subunit binding to F-actin in a polymerization experiment. After incubation under polymerizing conditions for 2 hours at room temperature, a sample containing 4.5 μM actin (method 2—see text) and 0.05 μM CCT (a single column fraction—see text) was centrifuged at 96 600 × g for 30 minutes and corresponding volumes of pellet (P) and supernatant (S) prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blot. Upper panels are strips that were probed separately with CCT subunit-specific antibodies, as indicated. Lower panels are the same blot strips reprobed with anti-actin (monoclonal antibody C4 from ICN Pharmaceuticals Inc. Costa Mesa, CA, USA) yielding 73% of the immunoreactivity in the pellet vs 27% in the supernatant. These latter values were consistent across lanes and intensities indistinguishable, suggesting uniformity of transfer to nitrocellulose during electroblotting
CCT reduces actin filament elongation
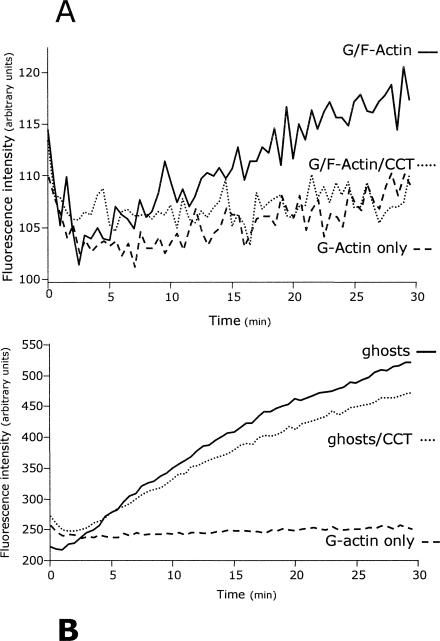
Reduction of actin polymerization by CCT cannot be explained by CCT 16-mer or even by individual CCT subunits acting as monomer sequestering proteins because effective levels of CCT are simply too low. Therefore, CCT may modulate actin polymerization by affecting nucleation (trimer formation), filament elongation, or possibly both. The filament elongation process can be assessed directly by monitoring the addition of pyrene-labeled G-actin onto preexisting, nonlabeled F-actin “seeds” in the presence of CCT. If used below its critical concentration for polymerization (ie, in the absence of significant nucleation), any fluorescence enhancement must arise from the addition of labeled monomer onto existing filaments. Indeed, no fluorescence enhancement was detected when 1 μM pyrene-labeled G-actin was incubated alone in assembly buffer (Fig 4A), although elongation did occur in the presence of an additional 200 nM F-actin seeds. At 0.05 μM, CCT virtually eliminated this elongation (Fig 4A), strongly indicating that CCT interacts with the filament because actin trimers involved in nucleation are excluded from this assay. Under these conditions there was no evidence for CCT acting as an actin-severing protein because this would result in fluorescence enhancement by the creation of new filament ends available for elongation (Pinder and Gratzer 1983).
Fig 4.
Type-II chaperonin containing T-complex polypeptide 1 (CCT) reduces elongation of actin filaments. (A) Addition of pyrene-labeled 1 μM G-actin onto 200 nM nonlabeled F-actin (filament elongation) was monitored by fluorescence enhancement (interval 30 seconds) over 30 minutes in the presence and absence of 0.05 μM CCT. (B) Addition of pyrene-labeled 1 μM G-actin onto the short, minus-end–capped actin filaments of erythrocyte ghost membrane–associated cytoskeletons (filament elongation) monitored by fluorescence enhancement over 30 minutes. Example traces smoothed by 1/5 are shown
To confirm this interpretation another elongation assay was conducted using the short actin filaments present in the erythrocyte membrane–associated cytoskeleton (Pinder and Gratzer 1983; Kuhlman 2000). This system is based on the principle that actin filament minus-ends are blocked by interactions with spectrin and protein 4.1 and in the absence of Mg2+ ions (conditions used here) the barbed filament (+) ends are available for elongation. When a preparation of erythrocyte “ghosts” containing such filaments (50 μg erythrocyte ghost protein/mL) was used as seeds for elongation assays with 1 μM pyrene-labeled G-actin (subcritical concentration), the presence of 0.05 μM CCT again significantly reduced the fluorescence enhancement (Fig 4B).
DISCUSSION
It is well accepted that CCT is a chaperone essential for functional actin production in cells. As with other chaperones, there is no detectable interaction between CCT and its final native G-actin product (eg, Melki and Cowan 1994 and our own observations not shown here, but see Grantham 1998). It is therefore widely held that the interaction of actin with CCT is completed upon production of the folded actin polypeptide. But the assembly of G-actin to F-actin involves significant structural changes to monomers (Lorenz et al 1995), and such changes might well alter or reexpose surfaces that render CCT once more capable of binding this known substrate polypeptide. In each of the various experiments described in this study the results suggest such interactions between CCT and filamentous actin. This is particularly important because it suggests a wider extent to the functional interactions between CCT as a chaperone and actin as a substrate than that recognized previously.
Interactions between CCT and polymerizing actin are demonstrated by the reduction of the initial rate of actin filament elongation by CCT (Fig 1). GroEL shows no such effect and provides a fairly stringent control because it is a chaperonin that can bind, but is not able to fold, denatured actin monomer (Melki and Cowan 1994). Thus, any effect of CCT on actin polymerization does not seem attributable to its recognizing nonnative actin possibly generated by the pyrene labeling used here. Indeed, in the sedimentation assays (see text of results section) CCT again diminished the rate of actin filament polymerization, despite completely unmodified purified native actin being used. Sedimentation analysis in the presence of GroEL (not shown, but see Grantham 1998) likewise confirmed its lack of effect on actin polymerization (Fig 1B). The similar sizes and shapes of GroEL and CCT oligomers also control for any unspecific chaperonin entanglement in F-actin pellets. That CCT slows down actin polymerization without affecting final yield (Fig 1D) is consistent with the action of other molecular chaperones. These may influence (usually by increasing) the fidelity of processes involved in protein folding, structure adoption, interactions (or all), sometimes at the expense of reducing the overall rate at which they are completed (eg, Thirumalai and Lorimer 2001).
Selective binding of CCT subunits to F-actin (Fig 2) is of great interest, in particular, the absence of the CCTδ subunit from F-actin pellets. CCTδ is directly implicated in binding newly synthesized or denatured actin (Vinh and Drubin 1994; Llorca et al 1999). Absence of CCTδ interaction, and other aspects of the selectivity with which CCT subunits were found in this study to interact with F-actin, strongly suggests a significant difference between this mechanism and that by which CCT interacts with nonnative monomeric actin during its initial folding and production. The high levels of KCl and ATP required here for actin polymerization resemble conditions necessary in cell lysates for CCT to undergo ATP hydrolysis–dependent disassembly (Roobol et al 1999a). But purified CCT (as used in this study) appears quite resistant to ATP-hydrolysis–induced disassembly (Roobol et al 1999a) in the absence of unknown factors in total cell extracts. It seems likely therefore that the differential CCT subunit associations with F-actin revealed in this study arise by interaction of intact CCT with actin filaments followed by partial disassembly. We cannot, however, exclude the possibility that there is prior disassembly and then selective binding, especially if actin is capable of acting as a cofactor in regulating CCT disassembly.
Results in Figure 4 suggest that CCT interacts with F-actin at filament ends. This would certainly explain the effectiveness of substantially substoichiometric CCT. Reduction in elongation from erythrocyte ghost filaments (Fig 3B) was less extensive than that from pure F-actin seeds (Fig 3A), but this is likely to reflect the relative abundance of filament ends in the 2 preparations. In the seeds assay numbers could be sufficiently low for CCT binding at most, if not all, ends. In the ghosts assay the reduction of actin filament elongation by CCT is consistent with its action at the barbed, fast-growing (+) ends of actin filaments.
Questions arise regarding the possible purpose and significance in vivo of CCT modulating actin filament formation. CCT could, perhaps, act as a “quality control” mechanism for G-actin molecules during their addition onto filaments. This would affect rate and perhaps fidelity, but not the final level, of actin polymerization. After the production of native G-actin monomer it may be necessary for CCT to act to reduce the rate of actin polymerization in the cell to allow or assist in the sequestration of actin molecules, or their interactions, with 1 or more of their many regulatory proteins. A more likely function for CCT is that it is required for successful filament formation in vivo; that is, in the crowded context of the cytosol (Ellis 2001). Addition of actin monomers onto elongating actin filaments must involve both protein conformational change and transient exposure of the hydrophobic surfaces that will lie between adjacent actin molecules in the filament. Exposure of such vulnerable, aggregation-prone surfaces in the crowded environment of the cell is obviously highly undesirable. Interaction with CCT at these vulnerable stages of filament formation may therefore be essential to the efficiency of in vivo actin assembly. Because actin can be long-lived (Rubinstein et al 1976; Cook et al 1991), if easily denatured, the phenotypes of yeast CCT gene knockouts and mutants (eg, Vinh and Drubin 1994) may owe something to loss of this function, in modulating cytoskeletal disposition and dynamics, not simply to a lack of functional building block production. Certainly, findings in this study suggest that CCT may contribute to the recent observation in quite different experiments (Jahraus et al 2001) that “…actin, a protein that has been extensively characterized as a pure component, behaves differently when in a cytosolic extract, …”. That CCT has not been identified previously as a factor in actin assembly is not surprising: pure actin assembles well in isolation and, specifically, in the absence of actin modulating factors, such as capping and stabilizing proteins, now known to have significant effects in vivo (eg, Bray 2000). Further, we have found that CCT and actin are cotransported along axons (Carden et al 2000), in a quite distinctly posttranslational process. Although this does not prove direct interaction, it could minimally reflect a requirement that CCT remains as a chaperone for this vulnerable but crucial cellular protein, already a known substrate, throughout its lifetime or at least before its final incorporation into the microfilament network.
In conclusion, we have presented here in vitro findings that extend previous subcellular colocalization of actin and CCT subunits at sites of microfilament assembly (Roobol et al 1995; Roobol and Carden 1999). This makes it not unreasonable to propose the possibility of a functional link between CCT and actin filament formation. It is therefore both necessary and of great interest to investigate in vivo the role of CCT in the process of microfilament dynamics, although such investigations to prove the hypothesis, or otherwise, will by no means be simple.
Acknowledgments
This research was supported by the Wellcome Trust, and J.G. held a BBSRC PhD studentship. We are grateful to Drs Anthony J. Baines and Jenni Fordham (Pinder) for helpful advice during the practical work, analysis, and/or its preparation for publication. We also thank Dr Rob Goold at King's College London for making the Phoretix PC analysis facilities available for densitometry and Miss Nancy Adamek for the actin used for the experiment shown in Fig 3.
REFERENCES
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bray D 2000 Cell Movements: From Molecules to Motility, 2nd ed. Garland Scientific Publishing (Taylor and Francis Group), New York. [Google Scholar]
- Brown CR, Doxsey SJ, Hong-Brown LQ, Martin RL, Welch WJ. Molecular chaperones and the centrosome: a role for TCP-1 in microtubule nucleation. J Biol Chem. 1996;271:824–832. doi: 10.1074/jbc.271.2.824. [DOI] [PubMed] [Google Scholar]
- Carden MJ, Roobol A, El Alami W, Wilson SJ, Bray JJ. Axonal transport of chaperonin CCT subunits. Mol Biol Cell. 2000;11S:2905. [Google Scholar]
- Cook RK, Sheff DR, Rubenstein PA. Unusual metabolism of the yeast actin amino terminus. J Biol Chem. 1991;266:16825–16833. [PubMed] [Google Scholar]
- Creutz CE, Liou A, Snyder SL, Brownawell A, Willison K. Identification of the major chromaffin granule binding protein, chromobindin A, as the cytosolic chaperonin CCT (Chaperonin Containing TCP-1) J Biol Chem. 1994;269:32035–32038. [PubMed] [Google Scholar]
- Ellis RJ. Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol. 2001;11:114–119. doi: 10.1016/s0959-440x(00)00172-x. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Hartl F. Principles of protein folding in the cellular environment. Curr Opin Struct Biol. 1999;9:102–110. doi: 10.1016/s0959-440x(99)80013-x. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Thulasiraman V, Ferreyra RG, Frydman J. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol Cell. 1999;4:1051–1061. doi: 10.1016/s1097-2765(00)80233-6. [DOI] [PubMed] [Google Scholar]
- Grantham J 1998 Studies on the chaperonin containing TCP-1. PhD dissertation, University of Kent, Canterbury, UK. [Google Scholar]
- Gutsche I, Essen L-O, Baumeister W. Group II chaperonins: new TRiC(k)s and turns of a protein folding machine. J Mol Biol. 1999;293:295–312. doi: 10.1006/jmbi.1999.3008. [DOI] [PubMed] [Google Scholar]
- Houry WA, Frishman D, Eckerskorn C, Lottspeich F, Hartl F. Identification of in vivo substrates of the chaperonin GroEL. Nature. 1999;402:147–154. doi: 10.1038/45977. [DOI] [PubMed] [Google Scholar]
- Jahraus A, Egeberg M, and Hinner B. et al. 2001 ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol Biol Cell. 12:155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama T, Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114:33–38. [PubMed] [Google Scholar]
- Kubota H, Hynes G, Carne A, Ashworth A, Willison K. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol. 1994;4:89–99. doi: 10.1016/s0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Willison KR. The chaperonin containing t-complex polypeptide 1 (TCP-1). Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem. 1995;230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- Kuhlman PA. Characterisation of the actin filament capping state in human erythrocyte ghost and cytoskeletal preparations. Biochem J. 2000;349:105–111. doi: 10.1042/0264-6021:3490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux MR, Hartl F 2000 Versatility of the cytosolic chaperonin TRiC/CCT. Curr Biol. 10 R. 260–R264. [DOI] [PubMed] [Google Scholar]
- Lingappa JR, Martin RL, Wong ML, Ganem D, Welch WJ, Lingappa VR. A eukaryotic cytosolic chaperonin is associated with a high molecular weight intermediate in the assembly of hepatitis B virus capsid, a multimeric particle. J Cell Biol. 1994;125:99–111. doi: 10.1083/jcb.125.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AKF, Willison KR. Elucidation of the subunit orientation in CCT (chaperonin containing TCP1) from the subunit composition of CCT micro-complexes. EMBO J. 1997;16:4311–4316. doi: 10.1093/emboj/16.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O, McCormack EA, and Hynes G. et al. 1999 Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature. 402:693–696. [DOI] [PubMed] [Google Scholar]
- Lorenz M, Poole KJ, Popp D, Rosenbaum G, Holmes KC. An atomic model of the unregulated thin filament obtained by X-ray fiber diffraction on oriented actin-tropomyosin gels. J Mol Biol. 1995;246:108–119. doi: 10.1006/jmbi.1994.0070. [DOI] [PubMed] [Google Scholar]
- McCallum CD, Do H, Johnson AE, Frydman J. The interaction of the chaperonin tailless complex polypeptide 1 (TCP1) ring complex (TRiC) with ribosome-bound nascent chains examined using photo-cross-linking. J Cell Biol. 2000;149:591–602. doi: 10.1083/jcb.149.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R, Batelier G, Soulié S, Williams RC Jr.. Cytoplasmic chaperonin containing TCP1: structural and functional characterisation. Biochemistry. 1997;36:5817–5826. doi: 10.1021/bi962830o. [DOI] [PubMed] [Google Scholar]
- Melki R, Cowan NJ. Facilitated folding of actins and tubulins occurs via a nucleotide-dependant interaction between cytoplasmic chaperonin and distinctive folding intermediates. Mol Cell Biol. 1994;14:2895–2904. doi: 10.1128/mcb.14.5.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85:164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pinder JC, Gratzer WB. Structural and dynamic states of actin in the erythrocyte. J Cell Biol. 1983;96:768–775. doi: 10.1083/jcb.96.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol A, Carden MJ. Identification of chaperonin particles in mammalian brain cytosol and of T-complex polypeptide 1 as one of their components. J Neurochem. 1993;60:2327–2330. doi: 10.1111/j.1471-4159.1993.tb03524.x. [DOI] [PubMed] [Google Scholar]
- Roobol A, Carden MJ. Subunits of the eukaryotic cytosolic chaperonin CCT do not always behave as components of a uniform hetero-oligomeric particle. Eur J Cell Biol. 1999;78:21–32. doi: 10.1016/S0171-9335(99)80004-1. [DOI] [PubMed] [Google Scholar]
- Roobol A, Grantham J, Whitaker HC, Carden MJ. Disassembly of the cytosolic chaperonin in mammalian cell extracts at intracellular levels of K+ and ATP. J Biol Chem. 1999a;274:19220–19227. doi: 10.1074/jbc.274.27.19220. [DOI] [PubMed] [Google Scholar]
- Roobol A, Holmes FE, Hayes NV, Baines AJ, Carden MJ. Cytoplasmic chaperonin complexes enter neurites developing in vitro and differ in subunit composition within single cells. J Cell Sci. 1995;108:1477–1488. doi: 10.1242/jcs.108.4.1477. [DOI] [PubMed] [Google Scholar]
- Roobol A, Sahyoun ZP, Carden MJ. Selected subunits of the cytosolic chaperonin associate with microtubules assembled in vitro. J Biol Chem. 1999b;274:2408–2415. doi: 10.1074/jbc.274.4.2408. [DOI] [PubMed] [Google Scholar]
- Rubinstein N, Chi J, Holzer H. Coordinated synthesis and degradation of actin and myosin in a variety of myogenic and non-myogenic cells. Exp Cell Res. 1976;97:387–393. doi: 10.1016/0014-4827(76)90630-3. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Myosin II folding is mediated by a molecular chaperonin. J Biol Chem. 1999;274:27265–27273. doi: 10.1074/jbc.274.38.27265. [DOI] [PubMed] [Google Scholar]
- Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffe MB. The t-complex polypeptide 1 is a chaperone for tubulin and actin in vivo. Proc Natl Acad Sci U S A. 1993;90:9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai D, Lorimer GH. Chaperonin-mediated protein folding. Annu Rev Biophys Biomol Struct. 2001;30:245–269. doi: 10.1146/annurev.biophys.30.1.245. [DOI] [PubMed] [Google Scholar]
- Thulasiraman V, Yang C-F, Frydman J. In vivo translated polypeptides are sequestered in a protected folding environment. EMBO J. 1999;18:85–95. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DB, Drubin DG. A yeast TCP-1-like protein is required for actin function in vivo. Proc Natl Acad Sci U S A. 1994;91:9116–9120. doi: 10.1073/pnas.91.19.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]