Abstract
The NMDA receptor (NMDAR) hypofunction hypothesis of schizophrenia is supported by multiple lines of evidence. Notably, administration of the NMDAR antagonist, ketamine, to healthy human subjects has psychotogenic action, producing both positive and negative symptoms associated with schizophrenia. NMDARs have multiple subtypes, but the subtypes through which ketamine produces its psychotogenic effects are not known. Here we address this question using quantitative data that characterize ketamine's ability to block different NMDAR subtypes. Our calculations indicate that, at a concentration that has psychotogenic action in humans, ketamine blocks a substantial fraction of GluN2C subunit-containing receptors but has less effect on GluN2A-, GluN2B-, and GluN2D-containing receptors. Thus, GluN2C-containing receptors may have preferential involvement in psychotic states produced by ketamine. A separate line of experiments also points to a special role for GluN2C. That work demonstrates the ability of NMDAR antagonists to mimic the elevation in the awake-state δ frequency EEG power that occurs in schizophrenia. Physiological experiments in rodents show that NMDAR antagonists generate δ oscillations by their action on the GluN2C-containing NMDARs that are prevalent in the thalamus. Optogenetic experiments suggest that such oscillations could contribute to symptoms of schizophrenia.
Keywords: channel block, delta oscillations, NMDA hypofunction hypothesis, thalamus
Introduction
Schizophrenia symptoms can be categorized as positive symptoms (i.e., hallucination and delusions), negative symptoms (i.e., avolition), and cognitive symptoms (i.e., working memory deficits). The NMDA glutamate receptor hypofunction hypothesis of schizophrenia arose from the observation that the administration of NMDA receptor (NMDAR) channel-blocking antagonist (phencyclidine or ketamine) to healthy subjects produces both positive and negative symptoms of schizophrenia (Luby et al., 1959; Krystal et al., 1994). Indeed, the symptoms associated with acute ketamine administration or the persisting psychoses associated with ketamine abuse have a very similar factor structure to schizophrenia (Xu et al., 2015). NMDAR antagonists, like ketamine, impair aspects of attention, working memory, declarative memory, and other domains of cognition that are also impaired in schizophrenia (Krystal et al., 1994, 1998, 1999). Furthermore, ketamine impairs aspects of sensory-evoked potentials (Oranje et al., 2000; Watson et al., 2009) and cognitive activations of the cortex evaluated with fMRI (Honey et al., 2004, 2005; Driesen et al., 2013) that also resemble deficits observed in schizophrenia. Recent work has shown that psychosis can also be caused by antibodies to NMDARs in autoimmune encephalitis (Wandinger et al., 2011). The NMDAR hypofunction hypothesis is further supported by various other lines of evidence, including the identification of risk genes directly tied to NMDAR function (Javitt et al., 2012; Timms et al., 2013; Balu and Coyle, 2015).
NMDARs are usually tetramers that are composed of four subunits: two that bind glycine (GluN1 subunits) and two that bind glutamate (GluN2 subunits) (Traynelis et al., 2010). In some NMDARs, GluN3 subunits, which bind glycine, can substitute for one or more GluN2 subunits (Low and Wee, 2010). There are four types of GluN2 subunits (GluN2A-GluN2D). These determine many properties of the receptor, including open probability, single-channel conductance, and deactivation rate (Gielen et al., 2009; Yuan et al., 2009; Siegler Retchless et al., 2012; Glasgow et al., 2015). Importantly (see below), channel block by extracellular Mg2+ is much weaker for receptors containing GluN2C and GluN2D subunits (Kutsuwada et al., 1992; Monyer et al., 1994; Kuner and Schoepfer, 1996). It is also clear that different NMDAR subtypes have different pharmacological sensitivities (Gielen et al., 2009; Yuan et al., 2009). However, it is unclear whether psychosis depends on particular subtypes of NMDARs.
The GluN2C subunit and psychosis
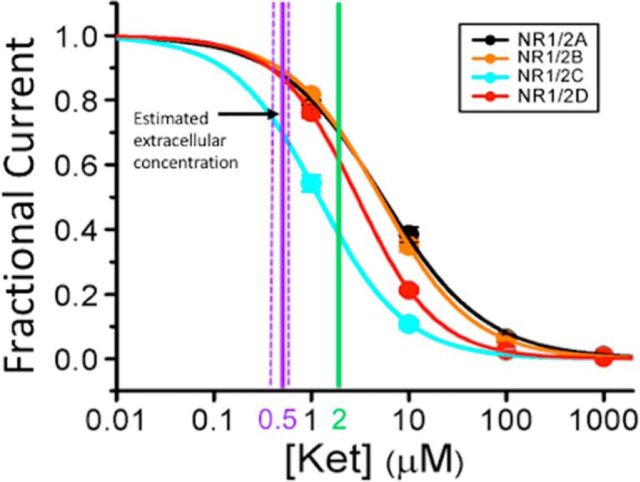
Here we make the case that two lines of published experiments, when considered together, are relevant to the question of which of the NMDAR subunits produce the psychotogenic action of NMDAR antagonists. These experiments addressed the effect of ketamine, the NMDAR antagonist used most extensively to investigate psychosis in the laboratory setting (Moghaddam and Krystal, 2012). Both ketamine and Mg2+ exert their blocking action by binding within the NMDAR ion channel to overlapping sites (Yamakura et al., 1993), and they compete in their blocking action (MacDonald et al., 1991). In experiments critical for our argument (Kotermanski and Johnson, 2009), the effect of NMDAR subunit composition on ketamine's blocking action was investigated. The response to agonist application was measured in a heterologous expression system at a typical neuronal resting potential (−66 mV) and at physiological Mg2+ concentration (1 mm). Various ketamine concentrations were used, and the data were fit with a binding curve to measure the IC50, the concentration that gives half-maximal inhibition. As shown in Fig. 1, NMDARs containing GluN2C subunits have a significantly lower IC50 (1.18 ± 0.04 μm) than GluN2A-containing (5.35 ± 0.34 μm) or GluN2B-containing (5.08 ± 0.02 μm) receptors. GluN2D-containing receptors were intermediate (2.95 ± 0.02 μm). These differences are considerably smaller when measured in zero Mg2+ and thus are mostly attributable to the weaker Mg2+ binding to GluN2C/GluN2D than to GluN2A/GluN2B-containing receptors. However, even in zero Mg2+, small differences in IC50 remain, indicating that other receptor properties also contribute to selectivity.
Figure 1.
The effect of subunit composition of NMDARs on the ketamine concentration inhibition curve in 1 mm Mg2+ (data from Kotermanski and Johnson, 2009). HEK293T cells were transfected with Rattus norvegicus cDNA for the GluN1 (NR1) subunit and one of the GluN2 (NR2) subunits (GluN2A-GluN2D). The receptors were activated by 1 mm glutamate and 100 μm glycine at a holding voltage (−66 mV) that is similar to resting potential in neurons. The fractional current, the current in the presence of ketamine normalized to current in zero ketamine, is plotted as a function of ketamine concentration. The heavy vertical purple line at 0.5 μm marks the estimated extracellular ketamine concentration that induces psychotogenic symptoms in normal human subjects, with dashed vertical lines at 0.4–0.6 μm indicating the median absolute percent error, as calculated for the Clements 250 model for ketamine administration (Absalom et al., 2007); this error is shown because it can be estimated, but other sources of error cannot be excluded. If the extracellular ketamine concentration in brain were equal to some estimates of total brain concentration (fourfold higher than plasma levels), a possibility we think unlikely, the extracellular concentration would be as marked by the green line. Selectivity for GluN2C-containing receptors remains substantial even under these assumptions.
The preferential effect of ketamine on GluN2C-containing receptors raises the possibility that these receptors may contribute preferentially to psychotogenic effects. To evaluate this possibility, it would be important to know whether the concentration at which ketamine produces psychosis in humans is close to the concentrations at which ketamine preferentially inhibits GluN2C-containing receptors. Achieving a defined brain concentration in human experiments is not straightforward given the dynamics of ketamine spread and removal. Nearly steady-state plasma levels can be produced by an initial bolus injection followed by a slow infusion of a lower ketamine concentration. Analysis of plasma ketamine levels was used to constrain a pharmacodynamic model (Clements, 250) that accounts for the measured steady-state ketamine concentration (Absalom et al., 2007). In subsequent experiments, this model was used to establish dosing designed to produce a blood plasma concentration of ∼150 ng/ml for up to 1.5 h, during which the psychotogenic symptoms could be observed (Pollak et al., 2015). The blood–brain barrier is highly permeable to ketamine; it was shown that, in rabbit, the average CSF concentration was on average 74% of the plasma concentration in samples taken between 30 and 120 min following intravenous administration (Adachi et al., 2005). Therefore, the concentration of ketamine in the brain extracellular space is likely to be nearly equal to that in plasma. Considering the molar mass of ketamine (237.7 g/mol) and assuming CSF concentration to be ∼74% of the plasma concentration, it can be concluded that psychotogenic action occurs at a ketamine concentration of ∼0.5 μm. According to the curve of Fig. 1, GluN2C-containing NMDARs will be ∼30% inhibited at this concentration. In contrast, NMDARs containing GluN2A, GluN2B, and GluN2D subunits will be only ∼10% inhibited. These results suggest that the ketamine dose that produces psychotogenic effects substantially blocks GluN2C-containing receptors and that the effects on GluN2A- or GluN2B-containing receptors are considerably smaller.
Several caveats should, however, be noted. First, it is possible that there are inaccuracies in the estimate of the ketamine concentration in the extracellular space of the brain. Some measurements in rodents (Cohen et al., 1973; Cohen and Trevor, 1974) indicate that the total concentration of ketamine in the brain may be up to fourfold higher than that in plasma (measured 10 min after administration). This is confirmed in a recent paper (Zanos et al., 2016) (total brain ketamine concentration was found to be approximately twofold higher than plasma concentration 10 min following intraperitoneal injection). However, the total brain concentration may not be the best estimate of the ketamine available to block NMDARs for several reasons. The lipophilic properties of ketamine allow it to partition into membranes and thus contribute to the total brain concentration. However, because the principal route of ketamine access to its blocking site is through open NMDAR channels (MacDonald et al., 1991), it is important to consider only the free extracellular ketamine concentration. It has also been suggested that ketamine may undergo acid trapping (Lester et al., 2015), a process in which ketamine becomes protonated and thus membrane impermeant. It may therefore accumulate in acidic intracellular compartments, such as lysosomes and thus not be free to bind NMDARs. Although both of these pools could contribute to total brain ketamine concentration, they would not contribute to the pool of ketamine available to act as an open-channel blocker of NMDARs. Thus, CSF ketamine concentration may provide a better estimate of the extracellular brain concentration than total brain concentration. However, this argument notwithstanding, it is of interest to analyze what would happen if the effective concentration is indeed fourfold higher than in CSF. As shown in Figure 1 (green vertical line), this would produce an ∼30% block of GluN2A/GluN2B-containing receptors but a much larger block (∼60%) of GluN2C-containing receptors (the block of GluN2D is probably intermediate).
A second caveat is that the effect of Mg2+ on the IC50 values of ketamine was measured using recombinant rat receptors, the properties of which might differ from those of human receptors. However, ketamine IC50 values are similar for human and rat NMDARs (Hedegaard et al., 2012), and the NMDAR subtype dependence of inhibition by Mg2+ is similar for human and rat NMDARs (Daggett et al., 1998); furthermore, Mg2+ reduces the potency of the channel blocker memantine both for human (Otton et al., 2011) and rodent (Kotermanski and Johnson, 2009) GluN1/2A receptors. Thus, the NMDAR subtype dependence of the ketamine IC50 is unlikely to be species-specific.
Third, preferential inhibition by ketamine of GluN2C-containing NMDARs has thus far been observed only using recombinant diheteromeric receptors (Fig. 1). Many brain regions express triheteromeric NMDARs (Luo et al., 1997; Huang and Gibb, 2014). It is thus possible that many GluN2C-containing NMDARs also contain a GluN2A or GluN2B subunit. The IC50 of ketamine for such triheteromeric receptors is not known. However, Mg2+ inhibition of putative GluN1/2B/2D triheteromeric receptors is relatively weak (Huang and Gibb, 2014), suggesting that ketamine may inhibit GluN2C- or GluN2D-containing triheteromeric NMDARs more effectively than receptors that contain only GluN1, GluN2A, and GluN2B subunits.
A related caveat stems from the possibility that NMDARs in vivo might differ in their properties from those measured in the in vitro study of Figure 1. Notably post-translational changes, such as phosphorylation by tyrosine or PKA, might alter the properties of NMDARs (Wang et al., 1996; Murphy et al., 2014).
A final caveat is that some of the behavioral effects of ketamine might not be due to ketamine itself, but due to a metabolite. Ketamine has strong antidepressant effects (Berman et al., 2000; Krystal et al., 2013), and these can result from the same infusion paradigm as used to study the cognitive and psychotogenic effects of ketamine (Krystal et al., 1994). The psychotogenic effects are rapid, whereas the antidepressant effects are substantially delayed (Berman et al., 2000; Krystal et al., 2013). Some antidepressant effects have been produced by relatively selective antagonists of GluN2B-containing NMDARs in animals (Li et al., 2010) and humans (Preskorn et al., 2008), suggesting that such NMDARs can affect depression. However, a recent paper suggests that some antidepressant effects of ketamine are not due to effects on NMDARs and are indeed not due to ketamine itself, but rather to a metabolite (HNK) of ketamine (Zanos et al., 2016). HNK does not block NMDARs and does not produce psychotogenic-like effects (Zanos et al., 2016). HNK is thus not relevant to the psychotogenic effects that we seek to account for. There are other metabolites of ketamine, such as R/S-norketamine (Ebert et al., 1997; Dravid et al., 2007; Moaddel et al., 2013). However, if such metabolites inhibit NMDARs by acting as channel blockers, as appears likely (Ebert et al., 1997; Dravid et al., 2007), then competition with Mg2+ should still result in preferential inhibition of GluN2C-containing (and GluN2D-containing; see below) receptors.
The question arises whether antagonists selective for GluN2B-containing receptors can produce psychotogenic effects. The GluN2B-selective antagonist CP-101,606 (Mott et al., 1998), in contrast to ketamine, was observed at low doses to have robust antidepressant activity without inducing psychotogenic effects (Preskorn et al., 2008). Although higher doses of CP-101,606 produced psychotogenic effects in some subjects, administration of placebo also produced psychotogenic effects in some subjects, and adverse events did not differ significantly between the CP-101,606 and placebo groups (Preskorn et al., 2008). Thus, whether antagonism of GluN2B-containing NMDARs alone is able to produce psychotogenic effects remains unclear.
In summary, available quantitative data on NMDAR subtype dependence of ketamine inhibition and on approximate CSF ketamine concentrations that result from a psychotogenic dose suggest two conclusions: (1) that this dose inhibits GluN2C-containing receptors more effectively that GluN2A/GluN2B (and probably GluN2D)-containing receptors; and (2) that the inhibition of GluN2C-containing receptors is substantial, whereas the inhibition of GluN2A/GluN2B-containing receptors is relatively minor.
Other reported effects of interfering with NMDAR subtypes
If GluN2C subunits have a special role in producing the effects of ketamine, then knock-out of these NMDAR subunits should mimic NMDAR hypofunction and produce schizophrenia-related symptoms in animal models. Consistent with this prediction, hypofunction of GluN2C (by GluN2C subunit knock-out) is sufficient to produce deficits in working memory (as occurs in schizophrenia) and to produce other schizophrenia-related behaviors (Hillman et al., 2011; Hillman, 2012).
There are, however, also reasons to suspect a role for the GluN2D subunit in some schizophrenia-related behaviors. Recent work has provided evidence for an important physiological role of GluN2D-containing receptors in cortical interneuron function and in the basal ganglia (Swanger et al., 2015; von Engelhardt et al., 2015). Some of these targets may affect behavioral and neuronal processes. Indeed, work on a GluN2D knock-out shows attenuation of several of ketamine's effects, particularly on locomotion and γ frequency oscillations, changes that are sometimes considered as schizophrenia-related behavior (H. Yamamoto et al., 2015; Sapkota et al., 2016; T. Yamamoto et al., 2016).
Our conclusion that the psychotogenic dose of ketamine has a relatively weak effect on GluN2A/GluN2B-containing receptors is consistent with other observations. These receptors mediate LTP in the hippocampus and procedures that preferentially inhibit these receptors profoundly interfere with LTP (Berberich et al., 2007) and memory (Tsien et al., 1996). In contrast, psychotogenic doses of ketamine have only moderate effects on memory (Anand et al., 2000; Krystal et al., 2005; Murray et al., 2014). The fact that learning and memory are only partially inhibited by psychotogenic doses of ketamine suggests that the block of GluN2A/B-containing receptors is weak, as our calculations suggest.
The δ frequency EEG abnormality in schizophrenia: role of GluN2C
The possibility that the psychotogenic action of ketamine depends strongly on GluN2C-containing NMDARs is of particular interest because of another line of research pointing to a special role of the GluN2C subunit in schizophrenia. That research has sought to understand an EEG abnormality seen in schizophrenia, specifically an elevation of power in the δ frequency (1–4 Hz) band in the awake state (Lehmann et al., 2014). Meta-analyses of EEG and MEG studies show this to be a highly reproducible finding (Boutros et al., 2008; Seikmeier and Stufflebeam, 2013). Moreover, δ power is elevated in anti-NMDAR autoimmune encephalitis, a disease that produces psychosis (Dalmau et al., 2008).
An important discovery in rodent models is that elevation of δ power in cortex and hippocampus can be produced by injection of NMDAR antagonist into the thalamus (Buzsáki, 1991; Zhang et al., 2012b). Furthermore, the δ elevation produced by systemic ketamine administration can be reduced by injection of the inhibitory agent, muscimol, into the thalamus (Zhang et al., 2012b), which strongly suggests that the site of ketamine action is localized to the thalamus. In adult rat brain, the strongest expression of GluN2C-containing NMDARs is found in the thalamus and cerebellum (Monyer et al., 1994; Karavanova et al., 2007). Slice experiments on the nucleus reticularis of the thalamus (Zhang et al., 2009), a region where GluN2C-containing NMDARs contribute strongly to synaptic responses (Zhang et al., 2012a), provided insight into the mechanism by which NMDAR antagonists produce δ oscillations. Because GluN2C-containing receptors exhibit weak Mg2+ block (Monyer et al., 1992, 1994), these channels generate a tonic inward current at resting potential (Zhang et al., 2009) in response to ambient glutamate (Sah et al., 1989). Block of GluN2C-containing receptors therefore produces hyperpolarization. This hyperpolarization deinactivates T-type calcium channels (Steriade and Llinás, 1988; McCormick and Bal, 1997), which then generate bursting at δ frequency (Zhang et al., 2009). Given the strong voltage dependence of T-type calcium channel inactivation, even the modest hyperpolarization produced by blocking a fraction of GluN2C-containing receptors (Fig. 1) might be sufficient to produce bursting.
Do such abnormal δ oscillations interfere with brain function? To address this question, optogenetic methods were used to impose δ oscillations on a thalamic nucleus important for working memory, the nucleus reuniens. Such optogenetically induced oscillations produced a robust and reversible deficit in working memory (Duan et al., 2015). Thus, abnormal δ oscillations in schizophrenia could cause the deficits in working memory seen in this disease (Silver et al., 2003).
Genome-wide association studies have provided support for a key role of T-type calcium channels in schizophrenia, consistent with the above model. Two recent genome-wide association studies identified a relatively small number of ion channels as risk genes for schizophrenia. However, both studies identified the same T-type calcium channel isoform (Cav3.3/CACNA1I) (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014; Narayanan et al., 2015). This is one of three isoforms present in the brain and is the specific isoform found in the nucleus reticularis of the thalamus.
In conclusion, here we have reviewed the evidence that GluN2C-containing NMDARs are more sensitive than other NMDAR subtypes to ketamine. Our analysis suggests that, at concentrations sufficient to produce psychosis in normal human subjects, ketamine substantially inhibits GluN2C-containing NMDARs but has a smaller effect on other NMDAR subtypes. This complements other evidence suggesting the importance of GluN2C in schizophrenia, specifically the evidence that GluN2C-containing NMDARs in the thalamus may underlie the abnormality of the low-frequency EEG oscillation in schizophrenia. Further testing of the key role of GluN2C-containing receptors in producing psychotogenic action will become possible with the development of improved NMDAR subtype-specific antagonists (Acker et al., 2013).
Footnotes
Dual Perspectives Companion Paper: Ketamine: NMDA Receptors and Beyond, by Charles F. Zorumski, Yukitoshi Izumi, and Steven J. Mennerick
This work was supported by National Institutes of Health R01MH045817 to J.W.J., National Center for Advancing Translational Science 4UH3TR000960-R02 to J.H.K., and National Institutes of Health R01MH086518 and R21MH104319 to J.L. We thank Edwin Johnson for comments on the paper.
The authors declare no competing financial interests.
References
- Absalom AR, Lee M, Menon DK, Sharar SR, De Smet T, Halliday J, Ogden M, Corlett P, Honey GD, Fletcher PC. Predictive performance of the Domino, Hijazi, and Clements models during low-dose target-controlled ketamine infusions in healthy volunteers. Br J Anaesth. 2007;98:615–623. doi: 10.1093/bja/aem063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker TM, Khatri A, Vance KM, Slabber C, Bacsa J, Snyder JP, Traynelis SF, Liotta DC. Structure-activity relationships and pharmacophore model of a noncompetitive pyrazoline containing class of GluN2C/GluN2D selective antagonists. J Med Chem. 2013;56:6434–6456. doi: 10.1021/jm400652r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi H, Inagaki Y, Okazaki N, Ishibe Y. Racemic ketamine and S (+)-ketamine concentrations in cerebrospinal fluid after epidural and intravenous administration in rabbits. Yonago Acta Med. 2005;33:33–40. [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-d-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Balu DT, Coyle JT. The NMDA receptor “glycine modulatory site” in schizophrenia: d-serine, glycine, and beyond. Curr Opin Pharmacol. 2015;20:109–115. doi: 10.1016/j.coph.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Jensen V, Hvalby Ø, Seeburg PH, Köhr G. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology. 2007;52:77–86. doi: 10.1016/j.neuropharm.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41:351–364. doi: 10.1016/0306-4522(91)90332-I. [DOI] [PubMed] [Google Scholar]
- Cohen ML, Trevor AJ. On the cerebral accumulation of ketamine and the relationship between metabolism of the drug and its pharmacological effects. J Pharmacol Exp Ther. 1974;189:351–358. [PubMed] [Google Scholar]
- Cohen ML, Chan SL, Way WL, Trevor AJ. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;39:370–376. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- Daggett LP, Johnson EC, Varney MA, Lin FF, Hess SD, Deal CR, Jachec C, Lu CC, Kerner JA, Landwehrmeyer GB, Standaert DG, Young AB, Harpold MM, Veliçelebi G. The human N-methyl-d-aspartate receptor 2C subunit: genomic analysis, distribution in human brain, and functional expression. J Neurochem. 1998;71:1953–1968. doi: 10.1046/j.1471-4159.1998.71051953.x. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF, Traynelis SF. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D'Souza DC, Gueorguieva R, He G, Leung HC, Ramani R, Anticevic A, Suckow RF, Morgan PT, Krystal JH. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38:2613–2622. doi: 10.1038/npp.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan AR, Varela C, Zhang Y, Shen Y, Xiong L, Wilson MA, Lisman J. Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: relevance to schizophrenia. Biol Psychiatry. 2015;77:1098–1107. doi: 10.1016/j.biopsych.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333:99–104. doi: 10.1016/S0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- Gielen M, Siegler Retchless B, Mony L, Johnson JW, Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459:703–707. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow NG, Siegler Retchless B, Johnson JW. Molecular bases of NMDA receptor subtype-dependent properties. J Physiol. 2015;593:83–95. doi: 10.1113/jphysiol.2014.273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard M, Hansen KB, Andersen KT, Bräuner-Osborne H, Traynelis SF. Molecular pharmacology of human NMDA receptors. Neurochem Int. 2012;61:601–609. doi: 10.1016/j.neuint.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman BG. Studies of GluN2C-containing NMDA receptors in schizophrenia-like behaviors and fear learning: relevance to the glutamate hypofunction hypothesis of schizophrenia. ProQuest Diss Publ. 2012:1–36. [Google Scholar]
- Hillman BG, Gupta SC, Stairs DJ, Buonanno A, Dravid SM. Behavioral analysis of NR2C knockout mouse reveals deficit in acquisition of conditioned fear and working memory. Neurobiol Learn Mem. 2011;95:404–414. doi: 10.1016/j.nlm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RA, Honey GD, O'Loughlin C, Sharar SR, Kumaran D, Bullmore ET, Menon DK, Donovan T, Lupson VC, Bisbrown-Chippendale R, Fletcher PC. Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: an FMRI study. Neuropsychopharmacology. 2004;29:1203–1214. doi: 10.1038/sj.npp.1300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Honey RA, O'Loughlin C, Sharar SR, Kumaran D, Suckling J, Menon DK, Sleator C, Bullmore ET, Fletcher PC. Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study. Cereb Cortex. 2005;15:749–759. doi: 10.1093/cercor/bhh176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Gibb AJ. Mg2+ block properties of triheteromeric GluN1-GluN2B-GluN2D NMDA receptors on neonatal ratsubstantia nigra pars compacta dopaminergic neurones. J Physiol. 2014;10:1–37. doi: 10.1113/jphysiol.2013.267864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull. 2012;38:958–966. doi: 10.1093/schbul/sbs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavanova I, Vasudevan K, Cheng J, Buonanno A. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Mol Cell Neurosci. 2007;34:468–480. doi: 10.1016/j.mcn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer's drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Bennett A, D'Souza DC, Abi-Dargham A, Morrissey K, Abi-Saab D, Bremner JD, Bowers MB, Suckow RF, Stetson P, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacology (Berl) 1998;135:213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Vegso S, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D'Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Faber PL, Pascual-Marqui RD, Milz P, Herrmann WM, Koukkou M, Saito N, Winterer G, Kochi K. Functionally aberrant electrophysiological cortical connectivities in first episode medication-naive schizophrenics from three psychiatry centers. Front Hum Neurosci. 2014;8:635. doi: 10.3389/fnhum.2014.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Lavis LD, Dougherty DA. Ketamine inside neurons? Am J Psychiatry. 2015;172:1064–1066. doi: 10.1176/appi.ajp.2015.14121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low CM, Wee KS. New insights into the not-so-new NR3 subunits of N-methyl-d-aspartate receptor: localization, structure, and function. Mol Pharmacol. 2010;78:1–11. doi: 10.1124/mol.110.064006. [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-d-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B) Mol Pharmacol. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS. fδ. J Physiol. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, Wainer IW. Sub-anesthetic concentrations of (R, S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Krystal JH. Capturing the angel in angel dust: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38:942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. Phenylethanolamines inhibit NMDA receptors by enchancing proton inhibition. Nat Neurosci. 1998;1:659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- Murphy JA, Stein IS, Lau CG, Peixoto RT, Aman TK, Kaneko N, Aromolaran K, Saulnier JL, Popescu GK, Sabatini BL, Hell JW, Zukin RS. Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J Neurosci. 2014;34:869–879. doi: 10.1523/JNEUROSCI.4538-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang XJ. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex. 2014;24:859–872. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan B, Soh P, Calhoun VD, Ruaño G, Kocherla M, Windemuth A, Clementz BA, Tamminga CA, Sweeney JA, Keshavan MS, Pearlson GD. Multivariate genetic determinants of EEG oscillations in schizophrenia and psychotic bipolar disorder from the BSNIP study. Transl Psychiatry. 2015;5:e588. doi: 10.1038/tp.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Otton HJ, Lawson McLean A, Pannozzo MA, Davies CH, Wyllie DJ. Quantification of the Mg2+-induced potency shift of amantadine and memantine voltage-dependent block in human recombinant GluN1/GluN2A NMDARs. Neuropharmacology. 2011;60:388–396. doi: 10.1016/j.neuropharm.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Pollak TA, Simoni S, De, Barimani B, Zelaya FO, Stone JM, Mehta MA. Phenomenologically distinct psychotomimetic effects of ketamine are associated with cerebral blood flow changes in functionally relevant cerebral foci: a continuous arterial spin labelling study. Psychopharmacology (Berl) 2015;232:4515–4524. doi: 10.1007/s00213-015-4078-8. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Sapkota K, Mao Z, Synowicki P, Lieber D, Liu M, Ikezu T, Gautam V, Monaghan DT. GluN2D N-methyl-d-aspartate receptor subunit contribution to the stimulation of brain activity and gamma oscillations by ketamine: implications for schizophrenia. J Pharmacol Exp Ther. 2016;356:702–711. doi: 10.1124/jpet.115.230391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seikmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in patients with schizophrenia. Clin Neuropharmacol. 2013;27:179–190. doi: 10.1097/WNP.0b013e3181e0b20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler Retchless B, Gao W, Johnson JW. A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nat Neurosci. 2012;15:406–413. S1–S2. doi: 10.1038/nn.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Swanger SA, Vance KM, Pare JF, Sotty F, Fog K, Smith Y, Traynelis SF. NMDA receptors containing the GluN2D subunit control neuronal function in the subthalamic nucleus. J Neurosci. 2015;35:15971–15983. doi: 10.1523/JNEUROSCI.1702-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms AE, Dorschner MO, Wechsler J, Choi KY, Kirkwood R, Girirajan S, Baker C, Eichler EE, Korvatska O, Roche KW, Horwitz MS, Tsuang DW. Support for the N-methyl-d-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiatry. 2013;70:582–590. doi: 10.1001/jamapsychiatry.2013.1195. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/S0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Bocklisch C, Tönges L, Herb A, Mishina M, Monyer H. GluN2D-containing NMDA receptors-mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice. Front Cell Neurosci. 2015;9:95. doi: 10.3389/fncel.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol. 2011;231:86–91. doi: 10.1016/j.jneuroim.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Wang YT, Yu XM, Salter MW. Ca(2+)-independent reduction of N-methyl-d-aspartate channel activity by protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1996;93:1721–1725. doi: 10.1073/pnas.93.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Krystal JH, Ning Y, Chen da C, He H, Wang D, Ke X, Zhang X, Ding Y, Liu Y, Gueorguieva R, Wang Z, Limoncelli D, Pietrzak RH, Petrakis IL, Zhang X, Fan N. Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J Psychiatr Res. 2015;61:64–72. doi: 10.1016/j.jpsychires.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Mori H, Masaki H, Shimoji K, Mishina M. Different sensitivities of NMDA receptor channel subtypes to non-competitive antagonists. Neuroreport. 1993;4:687–690. doi: 10.1097/00001756-199306000-00021. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Hagino Y, Kasai S, Ikeda K. Specific roles of NMDA receptor subunits in mental disorders. Curr Mol Med. 2015;15:193–205. doi: 10.2174/1566524015666150330142807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K, Yamamoto H. Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase. Neurosci Lett. 2016;610:48–53. doi: 10.1016/j.neulet.2015.10.049. [DOI] [PubMed] [Google Scholar]
- Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci. 2009;29:12045–12058. doi: 10.1523/JNEUROSCI.1365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Llinas RR, Lisman JE. Inhibition of NMDARs in the nucleus reticularis of the thalamus produces δ frequency bursting. Front Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Buonanno A, Vertes RP, Hoover WB, Lisman JE. NR2C in the thalamic reticular nucleus; effects of the NR2C knockout. PLoS One. 2012a;7:e41908. doi: 10.1371/journal.pone.0041908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes δ oscillations on the hippocampus. J Neurophysiol. 2012b;107:3181–3189. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]