Abstract
The surprisingly efficient uptake of peptide-loaded heat shock proteins (Hsps) by antigen-presenting cells (APCs) has been recently associated with a specific receptor-ligand–based mechanism, and the identity of at least 1 receptor has been determined. In this study, we tested how the domain composition of the stress protein affected its surface association and internalization by APCs, and this was facilitated by the availability of the 70-kDa human heat shock protein (Hsp70) and its various deletion mutants. We show that both these processes strictly depend on the presence of all 3 domains of Hsp70. We propose that the previously described interdomain interactions as a determinant of a favorable conformational status might also govern a sterical adaptation of Hsps to components of the internalization machinery.
INTRODUCTION
A rapidly growing area of research has implicated a number of heat shock or stress proteins in specifically activating the innate and adaptive immune system through their receptor-mediated internalization by antigen-presenting cells (APCs) (Suto and Srivastava 1995; Castellino et al 2000), their capability of stimulating the cytokine production by APCs (Basu et al 2000; Harpreet et al 2000), and their function in inducing the maturation of dendritic cells (Binder et al 2000a). The fundamental basis of these phenomena is the capability of heat shock proteins (Hsps) to associate with and deliver tumor-related peptides to the efferent arm of the immune system that leads to the major histocompatibility complex class I–associated presentation, immune recognition, and a protective and tumor peptide–specific CD8+ cytotoxic T-cell response in injected animals (Blachere et al 1997; Tamura et al 1997; Srivastava 2000). Recent studies have shed some light on the mechanism of binding and internalization of Hsps (and associated peptides) by APCs. The unusually efficient uptake of Hsp by APCs led to the assumption of a specific receptor (Srivastava et al 1994), the identity of which has been revealed recently (Binder et al 2000b). It has also been demonstrated that this receptor, CD91, accepts a panel of Hsps, such as Hsp70, Hsp90, Gp96, and calreticulin, but with varying degrees of affinity and specificity (Sondermann et al 2000; Basu et al 2001). Because we have recently cloned and purified the human Hsp70 and its various deletion mutants to near homogeneity (Zimmer et al 2001), with the aid of these proteins on hand, we addressed the question of how surface binding and uptake by APCs depended on the domain composition of Hsp70. We demonstrated that all domains of Hsp70 were required for surface association and internalization by APCs. These data indicate that similarly to what had been described regarding its various physiological functions, possible reciprocal interactions of the bulky N-terminal adenosine triphosphatase (ATPase) domain of Hsp70 with the C-terminal segments might also participate in conformational changes that can adapt the molecule favorably to the internalization machinery.
MATERIALS AND METHODS
Cell culture, cytospin preparation, and fluorescence microscopy
HeLa cells (American Type Culture Collection [ATCC], CCL-2) and the mouse monocytic cell line, P388 (ATCC, CL-46), were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 5% fetal calf serum (FCS), 1 mM l-glutamine, 40 μg/mL gentamycin (GIBCO Austria Invitrogen GmBH, Lofer, Austria), 1 mM sodium pyruvate at 37°C, and 5% CO2. Bone marrow–derived primary mouse macrophages (PMMs) were generously provided by Veronika Jesenberger. PMM cells were cultured in DMEM supplemented with 10% FCS and 20% LADMAC cell line-conditioned medium (ATCC, CRL-2420) as a source of colony stimulating factor-1 (CSF-1). Routinely, after incubation of cells with fluorescein isothiocyanate (FITC)–labeled proteins (see section on surface binding and uptake assays), approximately 5 × 104 cells were spun onto glass slides using the cytospin centrifuge (Shandon Inc, Pittsburgh, PA, USA) for 5 minutes at 500 × g. Cells were then fixed with 1% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 20 minutes and washed twice for 5 minutes with Tris-buffered saline (TBS) to neutralize PFA. Samples were subsequently incubated in TBS containing 0.25% Triton X-100 for 20 minutes and washed twice with TBS. Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (100 ng/mL in PBS for 5 minutes), and the samples were washed twice with PBS. Fluorescence microscopy was performed using a Zeiss Axiophot fluorescence microscope (Zeiss, Oberkochen, Germany) equipped with the Spot Advanced imaging system and software. Confocal imaging was done using a Zeiss LSM 501 confocal microscope fitted with a Zeiss Axiovert 100 M laser.
Flow cytometry
PMMs and P388 cells were stained for CD11b and F4/80. Cells were harvested, and approximately 106 cells (usually in 200 μL) were transferred into FACS tubes (Falcon) and placed immediately on ice, where all subsequent manipulations were performed. Four milliliters of fluorescence activated cell sorting (FACS) buffer (0.5% bovine serum albumin [BSA], 0.2% sodium azide in PBS) was added to the tubes, and the cells were spun at 1000 × g for 5 minutes. Pellets of the mouse cells were resuspended in 50 μL (1 μg) anti-mouse CD16/CD32 Fc-block (Pharmingen Europe, Becton, Dickinson GmBH, Heidelberg, Germany), and the cells were incubated further for 10 minutes. After washing in 4 mL FACS buffer, cells were resuspended in 50 μL FACS buffer containing 0.2 μg APC-conjugated, rat anti-mouse CD11b antibody (Pharmingen) and 10 μL/106 cells of FITC-conjugated, rat anti-mouse F4/80 antibody (Serotec Ltd, Oxford, UK) and were incubated for 30 minutes. Cells were then washed twice with 4 mL FACS buffer and resuspended in 500 μL, 1% PFA in PBS. A total of 104 cells were analyzed in a FACS Calibur cell sorter (Becton, Dickinson Biosciences, BD Austria GmBH Schwechat, Austria) using the CellQuest software.
Surface binding and uptake assays
Approximately 5 × 105 cells were washed 3 times in ice-cold PBS–milk buffer (1% nonfat dry milk in PBS) and placed on ice. FITC-labeled proteins were added to the cells at a concentration of 100 μg/mL (usually 10–20 μg protein) and incubated for 20 minutes on ice. For internalization, 5 × 105 cells were washed as for surface binding and incubated in the presence of 100 μg/mL FITC-labeled proteins for 60 minutes at 37°C. Samples were then washed extensively with ice-cold PBS–milk, fixed with 1% PFA in PBS, and stored at 4°C or examined by flow cytometry and fluorescence microscopy. In competition assays, FITC-labeled full-length (FL) Hsp70 was added to the cells simultaneously with unlabeled competitors.
RESULTS
The P388 mouse macrophage cell line is a suitable model for investigating Hsp70 binding and internalization
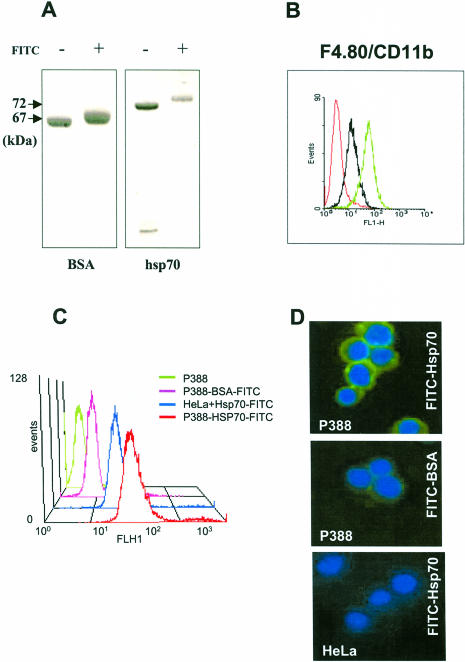
In order to generate a reproducible model system to investigate surface binding and uptake of human Hsp70 and its various deletion mutants, we tested these parameters on the available and conveniently propagated P388 macrophage-like mouse cell line. First, we labeled recombinant Hsp70 with FITC and used FITC-labeled BSA as a control; the labeling efficiency was ensured by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). In accordance with similar data obtained earlier (Binder et al 2000c), Figure 1A demonstrates that the estimated average of 5 FITC molecules in conjugation with each protein molecule resulted in an approximately 2-kDa increase in protein size that was detected on Coomassie-stained SDS-PAGs and was comparable between Hsp70 and BSA. Next, P388 cells were tested for CD11b surface integrin positivity (Fig 1B) because it had been demonstrated that CD11b− cells did not bind and internalize Hsps (Binder et al 2000c). Labeled proteins were then incubated with P388 cells at low temperature to prevent endophagocytotic mechanisms from occurring. After extensive washing to remove unbound proteins, cells were analyzed by FACS. As shown in Figure 1C, FITC-labeled Hsp70 specifically bound to P388 cells because only negligible intensity was measured with FITC-labeled BSA. Binding also depended on the cell type because CD11b− HeLa cells in a similar assay demonstrated considerably lower binding of FITC-Hsp70 (Fig 1C). These findings were also confirmed by examining the same cell populations under the fluorescence microscope. As illustrated in Figure 1D, FITC-labeled Hsp70 stained the surface of P388 cells universally, although some cells occasionally showed weaker surface staining (about 10% of total; not shown). In agreement with the FACS results, this analysis also confirmed binding specificity because negligible surface binding of P388 cells with FITC-labeled BSA or of CD11b− HeLa cells with FITC-labeled Hsp70 could be observed (Fig 1D).
Fig 1.
P388 macrophage-like cells specifically bind heat shock protein 70 (Hsp70). Human recombinant Hsp70 was expressed and purified as described elsewhere (Zimmer et al 2001). Five hundred to seven hundred micrograms of protein was incubated with fluorescein isothiocyanate (FITC) (Molecular Probes Inc. Eugene, OR, USA) in phosphate-buffered saline at a molar ratio of 1:10 for 2 hours at room temperature. Nonincorporated FITC was removed with a Micro Bio-spin column (BioRad Laboratories GmBH, Vienna, Austria; cutoff value of 40 kDa). The number of conjugated FITC molecules was estimated by measuring the optical density of the samples at 280 nm and 480 nm, and the labeling efficiency was ensured by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (A). Labeled proteins were incubated at 4°C for 1 hour with mouse P388 cells that were previously analyzed by FACS (B) for CD11b surface positivity (green line) and F4/80 surface staining as a positive control (black line). Red line indicates unstained control cells. Unbound proteins were removed by extensive washing, and surface binding was ensured by FACS analysis (C) and fluorescence microscopy (Zeiss Axiophot) (magnification, 400×) (D). Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride
Next, we were interested in addressing whether cell surface binding by P388 cells was also accompanied by internalization of the labeled Hsp70 protein. To test this, we incubated FITC-labeled Hsp70 with P388 cells for 1 hour at 37°C before fixation and fluorescence microscopy. This experiment demonstrated that the characteristic circumferential staining pattern was no longer evident, but a heterogeneous cytoplasmic signal appeared with a somewhat granular appearance, suggesting that P388 cells internalized the labeled Hsp70 protein (data not shown). This process was, again, specific because no binding or uptake was visible with FITC-labeled BSA.
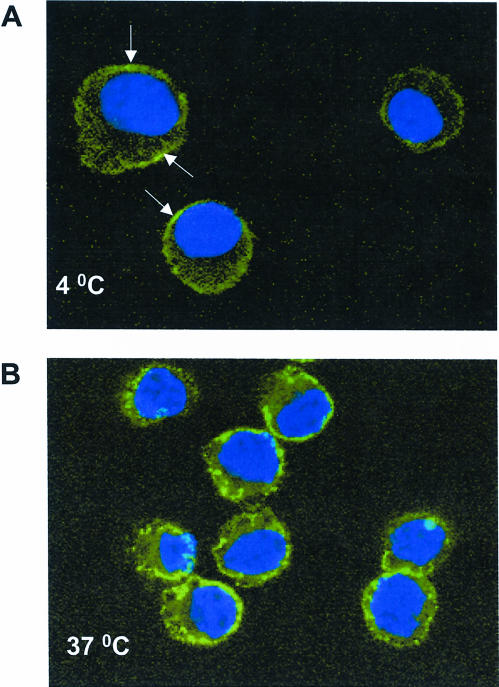
The described changes in fluorescence pattern, however, did not per se verify intracellular localization of the labeled proteins. To unequivocally demonstrate the uptake of Hsp70 by P388 cells, we analyzed the same cells with laser confocal microscopy. As seen in Figure 2, planar resolution of cells that were incubated in the presence of the labeled protein at 4°C vs 37°C clearly demonstrated cell surface association and cytoplasmic internalization of Hsp70, respectively. When 2 additional CD11b+ cell types were analyzed, it was shown that, as with P388 cells, both PMMs and human dendritic cells internalized Hsp70 (data not shown). These results, therefore, were in good agreement with earlier data (Binder et al 2000c) and, importantly, they established mouse P388 cells as a suitable model for our further experiments.
Fig 2.
Laser confocal imaging of heat shock protein 70 (Hsp70) uptake by P388 cells. Mouse P388 cells were incubated with fluorescein isothiocyanate–labeled Hsp70 or bovine serum albumin (BSA) at 4°C or 37°C for 1 hour before fixation (1% paraformaldehyde in phosphate-buffered saline followed by 0.25% Triton X-100 in TBS) and were observed by laser confocal microscopy (Zeiss LSM 501 confocal microscope fitted with a Zeiss Axiovert 100M laser). Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride. Note characteristic surface staining of cells incubated at 4°C (A, white arrows) or granular cytoplasmic localization of green fluorescence in cells that internalized Hsp70 but not BSA (not shown) at 37°C (B). Magnification, 800×
FL Hsp70 but not its various deletion mutants are taken up by APCs
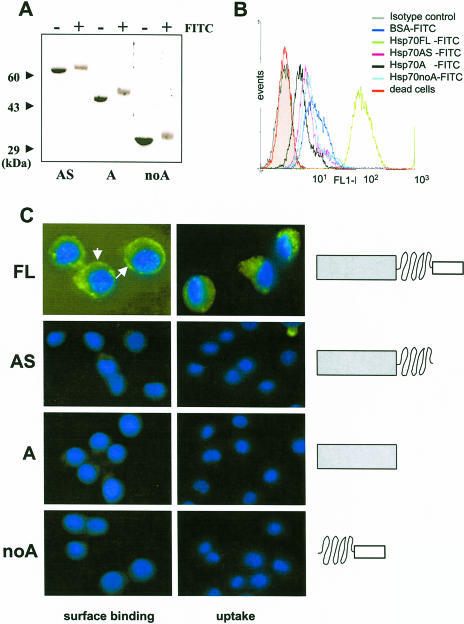
In the next set of studies, we aimed at testing how cell surface association and internalization of Hsp70 depended on the domain composition of the molecule. To this end, in addition to the wild-type protein, we used 3 deletion mutants of Hsp70, which were generated recently in our laboratory (Zimmer et al 2001). First, all these mutants were FITC labeled, and the labeling efficiency was comparable for all proteins as judged by measuring the optical density at 280 nm and 480 nm (data not shown) and by SDS-PAGE (Fig 3A). P388 cells were then incubated with each of these proteins as described in materials and methods section, and surface binding as well as internalization was monitored by FACS analysis or fluorescence microscopy. Interestingly, these results independently demonstrated that it was only the FL Hsp70 that bound to the cell surface and was internalized (Fig 3 B,C). When the C-terminal–most helical lid domain was removed alone (mutant AS) or together with the intermediary substrate-binding domain (mutant A), surface binding and, consequently, uptake was completely abolished. Similarly, deletion of the bulky N-terminal ATPase domain of the molecule (mutant noA) also led to the abrogation of binding and internalization. We note here that no difference in the described binding patterns was seen whether cells were incubated with equiamounts (micrograms of total weight) or equimolar (compensated for molecule size) amounts of the various Hsp70 proteins.
Fig 3.
Surface binding and internalization of full-length (FL) heat shock protein 70 (Hsp70) and its deletion mutants by P388 cells. Deletion mutants of Hsp70 were generated as described elsewhere (Zimmer et al 2001) and were labeled with fluorescein isothiocyanate (FITC) as described for the FL protein (see legend to Fig 1). Labeling efficiency was ensured by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (A). Cells were incubated in the presence FITC-labeled proteins as described in materials and methods section, washed extensively, and analyzed by FACS (B) and fluorescence microscopy (C) as described in Figure 1. Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride. White arrows indicate the spherical surface localization of labeled Hsp70 by P388 cells. Magnification was 630-fold for the FL protein and 400-fold for the other mutants. Schematic illustration of the domain composition of wild-type and mutant Hsp70 proteins is presented in the right of panel C
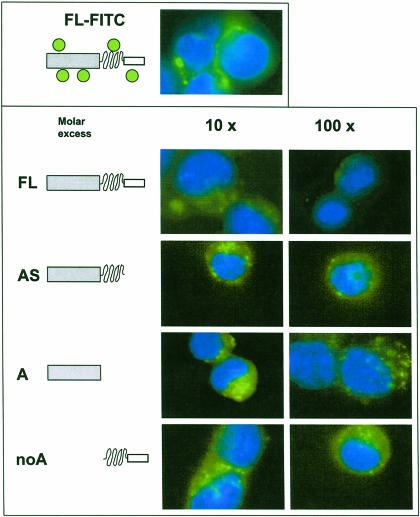
Next, we reasoned that if it is indeed only the FL Hsp70 that binds to and is internalized efficiently by these cells, then nonlabeled mutants should not displace the FITC-labeled FL Hsp70 in a competition assay. To test this assumption, in a set of experiments, P388 cells were incubated with FITC-labeled FL Hsp70 in the presence of nonlabeled FL Hsp70 as well as all the mutants shown not to bind to these cells. As expected, none of the deletion mutants were effective in competing with the FITC-labeled FL protein in binding and internalization by P388 cells (Fig 4). In the presence of a 100-fold molar excess of any of these mutants, the labeled FL protein was still clearly visible delineating the cell surface or as punctate fluorescence within the cytoplasm. Moreover, supporting our results shown in Figure 3, FL Hsp70 at 100-fold molar excess clearly diminished the characteristic signal given by the FITC-labeled protein (Fig 4). These results also eliminated the possibility that FITC conjugation of the mutants would have affected their binding to P388 cells.
Fig 4.
Competition for uptake of fluorescein isothiocyanate (FITC)–labeled heat shock protein 70 (Hsp70) by unlabeled wild-type and mutant Hsp70 proteins. P388 cells were incubated for 60 minutes at 37°C with FITC-labeled FL Hsp70 in the presence of unlabeled wild type and various mutants at various molar excesses as shown on the top of the lower panel. Cells were extensively washed and analyzed by fluorescence microscopy as described in materials and methods section. The domain composition of the recombinant Hsps is shown schematically on the left side of the figure. Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride. Magnification, 630×
DISCUSSION
During the course of their remarkable functions, molecular chaperones undergo a cyclic transition of conformational changes, a phenomenon that extends to all domains of the molecule. These conformational changes come across as inductive events transmitted by the association of the chaperone not only with ATP and cochaperones but also with those usually hydrophobic polypeptide residues (also referred to as substrates) that are exposed on misfolded proteins and sensed as stress signals (Buchberger et al 1995; Fung et al 1996; Lopez-Buesa et al 1998; Davis et al 1999). These conformational transitions—as monitored, eg, by changes in proteolytic cleavage patterns or tryptophan fluorescence—are coordinated in a sequential manner and are catalytic in successful folding assistance or aggregation prevention elicited by the chaperone (Buchberger et al 1995). Because of these intriguing molecular properties (best studied are members of the 70-kDa chaperone family) and the availability of various domain-deletion mutants of human Hsp70 in our laboratory, we asked ourselves how the domain composition of this important molecule affected surface binding and internalization by the mouse monocytic P388 cells with antigen-presenting properties. This question seemed timely because recent studies have not only proposed a receptor-based mechanism for the explanation of the unusual efficiency of Hsp uptake by APCs (Srivastava et al 1994) but have also identified a specific receptor, CD91, as an effective acceptor for a panel of Hsps (Binder et al 2000b; Basu et al 2001). Because receptor-ligand interactions call for strict conformational match of the macromolecule pair to allow highly specific, saturable, and competitive interactions (Binder et al 2000c), we reasoned that the mechanisms underlying these processes might, at least in part, rely on the same or similar conformational phenomena seen during the chaperone cycle.
Thus, we first established the mouse monocytic cell line, P388, as a suitable model system to investigate surface binding and internalization of Hsp70 and then demonstrated that all 3 domains were required for the molecule to effectively bind and enter APCs. Although these findings seem to be consistent with the possibility that the mutants resulting from the domain deletions are not compatible with a putative conformation that is required for proper recognition and binding by the receptor, it cannot be ruled out that the deleted domains harbor primary sequences that are essential for binding, which would explain the loss of mutant interaction with APCs. This possibility can be addressed in further systematic studies.
A number of independent studies, however, provided some additional clues about the nature of cell-type–specific uptake of Hsp70 and its targeting into various cellular compartments (see eg, Guzhova et al 1998). Fujihara and Nadler (1999) have demonstrated that a fusion protein consisting of the p50 subunit of nuclear factor kappa B (NFκB) and the C-terminal 92 amino acids of Hsp70 can be internalized by certain cell types and subsequently targeted into the nucleus in a manner that is similar to that of the wild-type Hsp70, and this is of importance to our study. This indicates that the C-terminal–most domain harbors sequences that are responsible for conferring binding and internalization to the chimeric protein. Although, in our study, the Hsp70 noA mutant mechanistically corresponds to a similar context where a protein segment (the intermediate substrate- or peptide-binding domain) is linked directly to the C-terminus of the Hsp70, this mutant also failed to bind to and be internalized by CD11b+ APCs. If we assume that the chimerical components interact to confer binding to and internalization by the cell, as we suggested previously, our results then raise 2 possibilities. One is that the substrate-binding domain of Hsp70 is too small when compared with that of p50 of NFκB to be subjected to such a conformational interaction. Alternatively, both p50 and the ATPase domain of Hsp70 have some structural features that are required for functional interaction with the C-terminus of the molecule; hence, FL Hsp70 binds and is taken up efficiently. The intermediary substrate-binding domain might also have a role in this process. This possibility, however, could not be addressed directly because we did not analyze a mutant here where the N-terminal ATPase domain is fused to the C-terminal–most domain of Hsp70.
The experimental approach used here did not permit quantitative measurements to directly and precisely compare the binding affinities of FL Hsp70 with those of its mutants. Because of this constraint, we were not able to resolve whether any of the mutants had a minimal binding capacity, which would not be obvious in the fluorescent microscopic experiments (as seems to be indicated by our results) but could still be detectable in an assay that is more sensitive in this regard. But our competition results argue that a binding capacity (if any) that may be associated with any of the mutants is not comparable to that of the wild-type protein. In this study we have identified, for the first time to our knowledge, the domain context that is required for the surface binding and internalization of an Hsp (Hsp70) by APCs. Our results demonstrate that none of the deletion mutants we generated were capable of significantly binding and entering APCs, suggesting that the deleted domains normally conduct structural or functional information required for the association of the parent protein to components of the receptor system. These data are consistent with the recently identified receptor-mediated uptake mechanism as well as with the long-suspected highly organized and dynamic conformational changes by members of the Hsp70 family that require fine interactions of all domains of the molecule.
Acknowledgments
We are grateful to Sylvia Vlcek for the excellent assistance in the confocal imaging. The technical assistance of Dieter Gelbmann is greatly appreciated. We also thank the members of the Molecular Biology group at INTERCELL Inc for stimulating discussions. The Wiener Wirtschaftsförderungsfond and the Forschungförderungsfond supported this work.
REFERENCES
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hap90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000a;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000b;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Harris ML, Ménoret A, Srivastava PK. Saturation, competition, and specificity in interaction of heat shock proteins (hsp) gp96, hsp90, and hsp70 with CD11b+ cells. J Immunol. 2000c;165:2582–2587. doi: 10.4049/jimmunol.165.5.2582. [DOI] [PubMed] [Google Scholar]
- Blachere NE, Li Z, Chandarwarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberger A, Theyssen H, Schröder H, McCarty JS, Virgallita J, Milkereit P, Reinstein J, Bukau B. Nucleotide-induced conformational changes in the ATPase and substrate binding domains of the DnaK chaperone provide evidence for interdomain communication. J Biol Chem. 1995;270:16903–16910. doi: 10.1074/jbc.270.28.16903. [DOI] [PubMed] [Google Scholar]
- Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Voisine C, Craig EA. Intragenic suppressors of Hsp70 mutants: interplay between the ATPase- and peptide-binding domains. Proc Natl Acad Sci U S A. 1999;96:9269–9276. doi: 10.1073/pnas.96.16.9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara SM, Nadler SG. Intranuclear targeted delivery of functional NFκB by 70 kDa heat shock protein. EMBO J. 1999;18:411–419. doi: 10.1093/emboj/18.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung KL, Hilgenberg L, Wang NM, Chirico WJ. Conformations of the nucleotide and polypeptide binding domains of a cytosolic Hsp70 molecular chaperone are coupled. J Biol Chem. 1996;271:21559–21565. doi: 10.1074/jbc.271.35.21559. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Arnholdt ACV, and Darieva ZA. et al. 1998 Effects of exogenous stress protein 70 on the functional properties of human promonocytes through binding to cell surface and internalization. Cell Stress Chaperones. 3:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpreet S-J, Scherer HU, Hilf N, Schild D, Rammensee H-G, Toes REM, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Buesa P, Pfund C, Craig EA. The biochemical properties of the ATPase activity of a 70-kDa heat shock protein (Hsp70) are governed by the C-terminal domains. Proc Natl Acad Sci U S A. 1998;95:15253–15258. doi: 10.1073/pnas.95.26.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Becker T, Mayhew M, Wieland F, Hartl F-U. Characterization of a receptor for heat shock protein 70 on macrophages and monocytes. Biol Chem. 2000;381:1165–1174. doi: 10.1515/BC.2000.144. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Immunotherapy of human cancer: lessons from mice. Nat Immunol. 2000;1:363–366. doi: 10.1038/808795. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Kang L, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Zimmer C, von Gabain A, Henics T. Analysis of sequence-specific binding of RNA to Hsp70 and its various homologues indicates the involvement of N- and C-terminal interactions. RNA. 2001;7:1628–1637. [PMC free article] [PubMed] [Google Scholar]