Abstract
Synthesis of heat shock proteins (Hsps) in response to elevated temperatures and other denaturing agents is a common feature of prokaryotic and eukaryotic cells. The heat-induced expression of Hsp70 family members in the gills and mantle of Ostrea edulis, a highly valued fisheries resource inhabiting primarily estuarine environments, has been studied. O edulis is exposed to a variety of natural and anthropogenic stresses in the environment. Two isoforms of about 72 kDa and 77 kDa were constitutively present in unstressed organisms, reflecting the housekeeping function performed by these proteins under normal circumstances. Their expression in animals undergoing thermal stress was highly variable, and on the average, little change occurred under different experimental conditions. A third isoform of about 69 kDa was induced in both tissues after exposure to ≥32°C; its synthesis was detected within 4 hours of poststress recovery at 15°C, reaching the maximum expression after 24 hours in the gills and after 48 hours in the mantle and declining thereafter. Hsp69 expression was low at 38°C, a temperature lethal for about 50% of the individuals tested. Densitometric analysis of Western blots revealed that Hsp69 was mostly responsible for the significant heat-induced overexpression of Hsp70s in O edulis. Comparison with heat shock responses in tissues of Crassostrea gigas indicated a similar pattern of Hsp70 expression. In this organism, however, Hsp69 was induced after exposure to ≥38°C. We conclude that tissue expression of Hsp69 in O edulis, and possibly other bivalves, is an early sign of thermal stress; determining whether these changes also correlate with other major environmental stresses is the goal of ongoing studies.
INTRODUCTION
Since the first studies on the heat shock response (Ritossa 1962), much work has been done to understand the role of heat shock proteins (Hsps), whose synthesis is dramatically enhanced by high temperatures. Crucial information is now available about the proteins themselves, the coding genes, and the control of the response to heat (Scharf et al 1998; Feder and Hofmann 1999). A number of Hsp inducers other than heat have also been recognized, including hypoxia, heavy metals, oxygen radicals, radiation, osmotic changes, etc, and different Hsps have been identified and grouped according to their molecular weight (De Maio 1999). Hsps appear to be ubiquitous in animals and plants (Nover et al 1996) and are critical for heat, oxidative, and high-light stress resistance (Heckathorn et al 1999). It is now clear that not all Hsps are stress-inducible, and constitutive isoforms, referred to as molecular chaperones, appear to be essential for protein folding or trafficking and regulated proteolysis in unstressed cells (Hendrick and Hartl 1995; Fink 1999). The expression of the Hsp-coding genes is under the control of specific transcription factors (Scharf et al 1998), and there is evidence that such regulation differs among organisms, depending greatly upon their life history and adaptation capacity (Hofmann and Somero 1995).
As to the role of stress-induced Hsps, more work is needed to understand the exact mechanism by which these proteins provide protection to the cell, in particular at the tissue level (Hightower et al 2000). In general, however, they are believed to interact with hydrophobic domains of polypeptides unfolded by stress stimuli and to promote protein renaturation or definitive elimination (De Maio 1999). Exposure of cells to a mild thermal stress protects them from further exposure to stronger, otherwise lethal, insults other than heat. These findings have prompted new attempts at manipulation of the Hsp response in medicine (eg, the development of drugs for the control and prevention of degenerative diseases; Morimoto and Santoro 1998) as well as in biology (eg, related to aquaculture practices; Clegg et al 1998; DuBeau et al 1998). Hsps have also been considered potential biomarkers of organism stress, and some applications of Hsp expression for environmental biomonitoring have indeed been reported (Kohler et al 1992, 1996; Nadeau et al 2001). In contrast, the inadequacy of these proteins as a tool for biomonitoring because of the variable amount of constitutive isoforms in different organisms or life stages, the variability of the response among individuals, and the influence of previous stress has also been reported (Feder and Hofmann 1999).
Although heat-induced overexpression of Hsps has been demonstrated in almost all species studied to date, the characterization of the heat shock response in different organisms may be important for the understanding of its evolution, adaptation to different environments, and its role in animal survival (Sanders et al 1991; Dietz and Somero 1993; Hightower et al 1999). Moreover, it may contribute to the exploration of several other physiological and ecological issues, including its validation as a biomarker of stress.
In this context, we characterized the expression of Hsp70 family members in Ostrea edulis, an autochthon species of great economic value along the coast of the North Adriatic Sea and widely distributed as natural or reared populations in other geographical areas. Particular interest was focused on a specific Hsp70 member, ie, an inducible 69-kDa protein isoform.
MATERIALS AND METHODS
Oysters were collected along the North Adriatic Sea coast (Italy) and immediately transferred to the laboratory in seawater tanks (about 1 L/oyster) at environmental temperature with continuous aeration. Animals (25 oysters/aquarium) were acclimated for at least 18 days in 60-L, 34-ppt seawater aquaria at 15°C, fed once a day with appropriate amounts of algal slurry, and made to fast for 24 hours before treatment. For heat shock experiments, oysters were transferred for 15–60 minutes to a water bath set at the appropriate temperature (20–38°C) and then returned to 15°C. To minimize the influence of size and shell thickness on the results, the O edulis selected for the experiments were of similar size (8–10 cm shell diameter). Crassostrea gigas individuals of weights and volumes as close as possible to those of the O edulis were used. Three animals were used for each treatment in an experiment. For evaluation of Hsp70 expression, the gills and mantle were separately processed on ice using a handheld 5-mL homogenizer and 10 mM sodium phosphate buffer (pH 7.4) containing 1% Nonidet-P40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) plus protease inhibitor cocktail (at final concentrations of 1 μg/mL pepstatin A, E-64, bestatin, leupeptin, and aprotinin plus 0.25 μg/mL phenylmethane sulfonyl fluoride). Homogenates were filtered through a single layer of gauze, and 50 μL was removed for protein determination (Lowry et al 1951). Aliquots of each homogenate were mixed with equal volumes of Laemmli buffer (Laemmli 1970), boiled for 5 minutes, and cooled and stored at −80°C until use. For Hsp70 determination, 15 μg of thawed homogenate proteins and molecular weight markers was loaded onto 10% polyacrylamide gels using a discontinuous buffer system (Laemmli 1970); electrophoresis was run in a Mini Protean III apparatus (BioRad, Hercules, CA, USA) at a constant voltage (200 V) for 90 minutes at 4°C. The resolved proteins were transferred onto nitrocellulose membranes at 300 mA for 60 minutes at 4°C with phosphate-buffered saline containing 3% bovine serum albumin. Blots were probed with anti-Hsp70 rat monoclonal antibody (1:10 000; 1 hour at 22°C) and anti-rat IgG conjugate with horseradish peroxidase (1:6000; 30 minutes at 22°C) as primary antibody and secondary antibody, respectively. After repeated washings with 20 mM Tris phosphate buffer (pH 7.4) plus 1% polyoxyethylenesorbitan monolaurate (Tween 20), immunoblots were finally developed by enhanced chemiluminescence (ECL) reagent.
Densitometric scanning and statistical analysis
Densitometric analysis of the films was performed by ImageMaster (Amersham-Pharmacia, Milan, Italy) equipped with TotalLab software (Amersham-Pharmacia). Densitometry was routinely evaluated for each band; when comments on the densitometry of Hsp70 as a whole are reported, they refer to the sum of the 3 bands of about 70 kDa analyzed within the same lane. Given the inherent variation between film developments, data were normalized to standard samples included in each experimental protocol. Data were subjected to 1-way analysis of variance (SigmaStat, Jandel Scientific, San Rafael, CA, USA) to test for significance, and statistical difference was accepted if P < 0.05.
Materials
Anti-Hsp70 rat monoclonal antibody was from Affinity Bioreagents (Vinci Biochem, Vinci, Italy). Anti-rat IgG, conjugate with horseradish peroxidase, amoeba anti-actin antibodies, acrylamide, N′,N′-bisphenyl acrylamide, N,N,N',N'-tetramethylethylenediamine (TEMED), ammonium persulate (APS), protease inhibitor cocktail, Nonidet-P40, Tween 20, SDS, 2-mercaptoethanol, glycine, developer, fixative, Kodak emulsion film, and all other reagents were from Sigma-Aldrich (Milan, Italy). Hybond nitrocellulose and ECL were purchased from Amersham-Pharmacia.
RESULTS
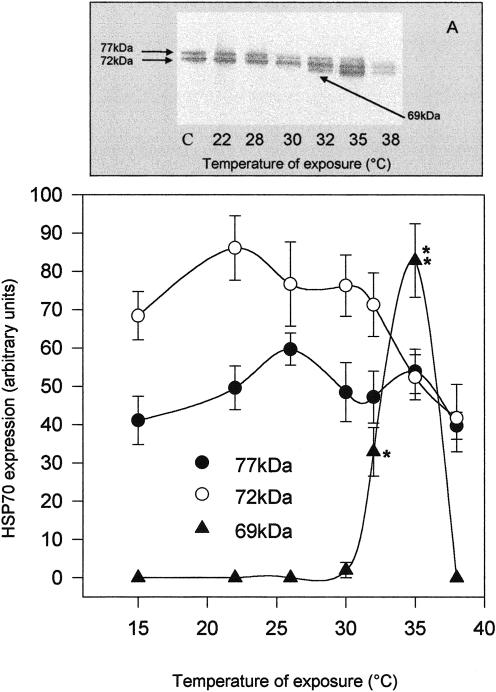
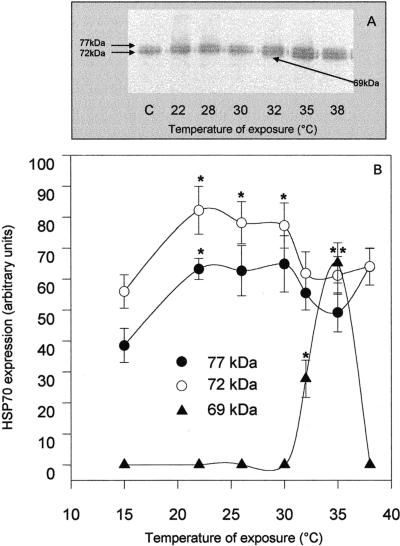
Expression of Hsp70 members after thermal shock was evaluated in tissues of O edulis exposed to different temperatures for 1 hour and returned to 15°C for 24 hours. Two apparent constitutive Hsp70 isoforms of about 72 kDa and 77 kDa (Hsp72 and Hsp77) were detected by Western blot analysis in tissues of control individuals, and no substantial difference between the gills and mantle was observed (Figs 1A and 2A). Levels of Hsps appeared progressively higher both in the gills (Fig 1A) and in the mantle (Fig 2A), and in particular a third inducible band corresponding to a protein of about 69 kDa (Hsp69) was detected in both tissues after exposure to ≥32°C. No appreciable change in the level of actin immunoreactivity was detected under the different conditions analyzed (data not shown). Single-band densitometries plotted on Figures 1B and 2B indicated that changes in the levels of Hsp72 and Hsp77 were not closely related to temperature increases but clearly showed a sharp increase of Hsp69 expression, with a maximum at 35°C. In this condition, Hsp69 contributed about one-third of the whole Hsp70 content detected by the antibody in both tissues. After exposure to 38°C, few animals expressed Hsp69. Interestingly, exposure to 38°C for 1 hour corresponded to the median lethal conditions for O edulis, whereas 40°C was the lowest lethal temperature for the animals. In each experiment, 2 individuals per treatment were maintained at 15°C for at least 2 weeks after heat shock. No mortality was registered among oysters shocked at 32°C or 35°C, whereas about 50% of the animals died within 4 days after exposure to 38°C and within 6 hours after exposure to 40°C.
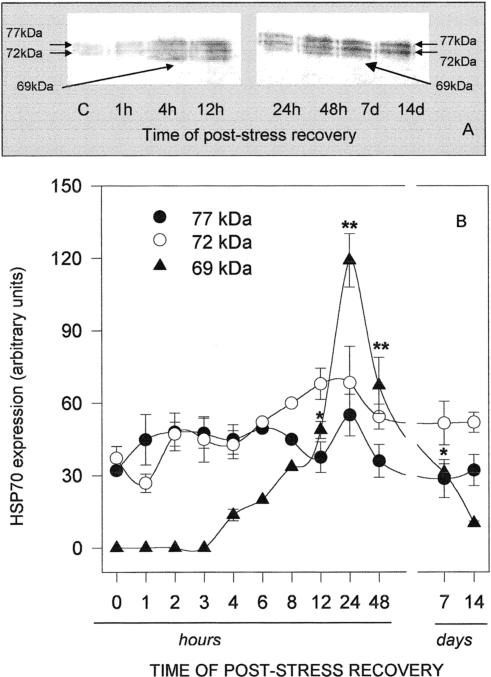
Fig 1.
Western blotting (A) and densitometry (B) of Hsp immunodetection in the gills of Ostrea edulis exposed to different temperatures for 1 hour. Plotted data are the mean ± SEM of 4 separate experiments, for a total of 12 individuals analyzed for each point. *P < 0.05; **P < 0.01 vs control levels
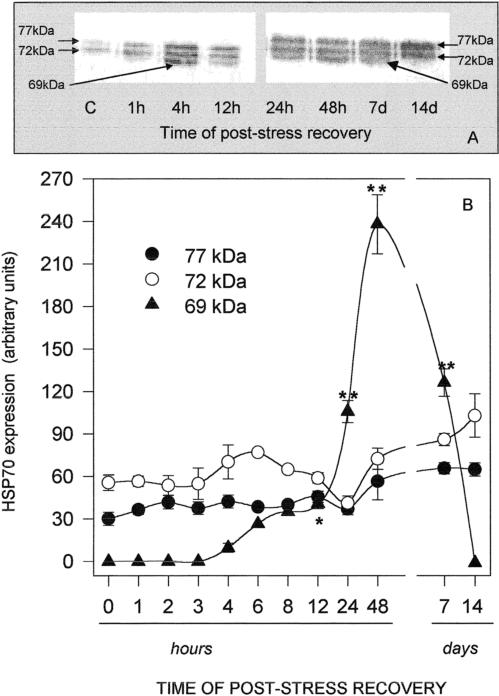
Fig 2.
Western blotting (A) and densitometry (B) of Hsp immunodetection in the mantle of Ostrea edulis exposed to different temperatures for 1 hour. Plotted data are the mean ± SEM of 4 separate experiments, for a total of 12 individuals analyzed for each point. *P < 0.05; **P < 0.01 vs control levels
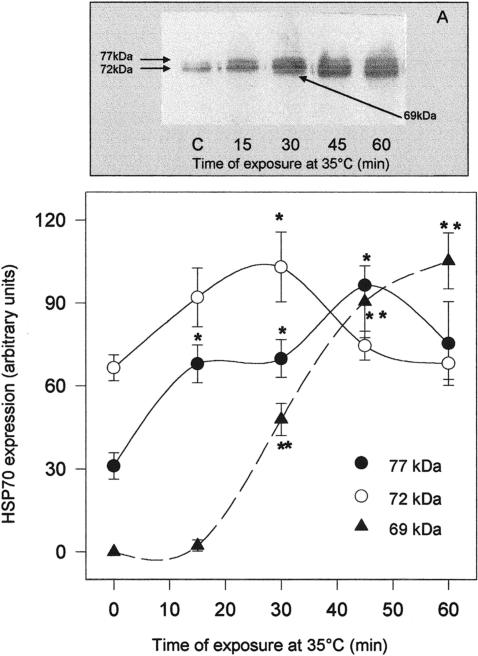
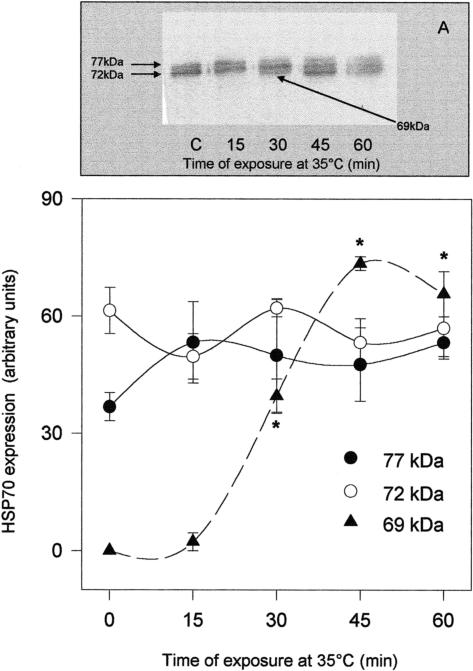
To reveal the duration of thermal stress needed to trigger a significantly increased expression of Hsp70 members, oysters were exposed to 35°C for a minimum of 15 minutes and a maximum of 1 hour and returned to the aquaria at 15°C for 24 hours. In Figures 3 and 4, Western blots and densitometric analysis plots relative to the gills (Fig 3) and mantle (Fig 4) are reported. The total amount of gill Hsp70 detected by the specific antibody, calculated as the sum of band densities corresponding to Hsp69, Hsp72, and Hsp77, increased after 15 minutes of exposure to 35°C and remained significantly higher than in controls subjected to 1 hour of stress. Such an increase was initially because of the overexpression of gill Hsp77, already significant at 15 minutes, and later because of the massive increment of Hsp69 (Fig 3B). As for the mantle (Fig 4B), Hsp70 expression as a whole was significantly increased after 30 minutes of treatment; in this case, however, Hsp72 and Hsp77 levels were unaffected by heat, whereas a strong increase of Hsp69 expression was observed after 30 minutes of thermal stress at 35°C.
Fig 3.
Western blotting (A) and densitometry (B) of Hsp immunodetection in the gills of Ostrea edulis exposed to 35°C for different durations. Plotted data are the mean ± SEM of 4 separate experiments, for a total of 12 individuals analyzed for each point. *P < 0.05; **P < 0.01 vs control levels
Fig 4.
Western blotting (A) and densitometry (B) of Hsp immunodetection in the mantle of Ostrea edulis exposed to 35°C for different durations. Plotted data are the mean ± SEM of 4 separate experiments, for a total of 12 individuals analyzed for each point. *P < 0.01 vs control levels
In order to evaluate the time course of Hsp accumulation and degradation after heat shock, oysters were exposed to 35°C for 1 hour and allowed to recover at 15°C for a minimum of 30 minutes and a maximum of 14 days. As shown in Figure 5A, the gill Hsp70 band density increased with time of poststress recovery, reaching a maximum after 24 hours and declining thereafter. Data from densitometric analysis plotted in Figure 5B clearly indicate that Hsp69 biosynthesis was easily detectable after 4 hours and was maximal after 24 hours, whereas Hsp72 and Hsp77 did not seem to be consistently affected by heat. A significant amount of Hsp69 was still present after 7 days of recovery but was barely detectable after 14 days. The time course of the heat shock response was similar in the mantle (Fig 6A), and again, Hsp69 was mostly responsible for the change of Hsp70 total levels observed in the tissue (Fig 6B); in contrast to the gills, the maximum expression of mantle Hsp69 was registered after 48 hours, when it was about 4-fold that of the constitutive isoforms. Hsp69 was still present after 7 days but not expressed in the mantle after 14 days of poststress recovery.
Fig 5.
Western blotting (A) and densitometry (B) of Hsp immunodetection in the gills of Ostrea edulis exposed to 35°C for 1 hour and then allowed to recover at 15°C for different durations. Plotted data are the mean ± SEM of 5 separate experiments, for a total of 10 individuals analyzed for each point, with the exception of data at 6 hours and 8 hours of recovery, which are the averages of only 2 separate experiments. *P < 0.05; **P < 0.01 vs control levels
Fig 6.
Western blotting (A) and densitometry (B) of Hsp immunodetection in the mantle of Ostrea edulis exposed to 35°C for 1 hour and then allowed to recover at 15°C for different durations. Plotted data are the mean ± SEM of 5 separate experiments, for a total of 10 individuals analyzed for each point, with the exception of data at 6 hours and 8 hours of recovery, which are the averages of only 2 separate experiments. *P < 0.05; **P < 0.01 vs control levels
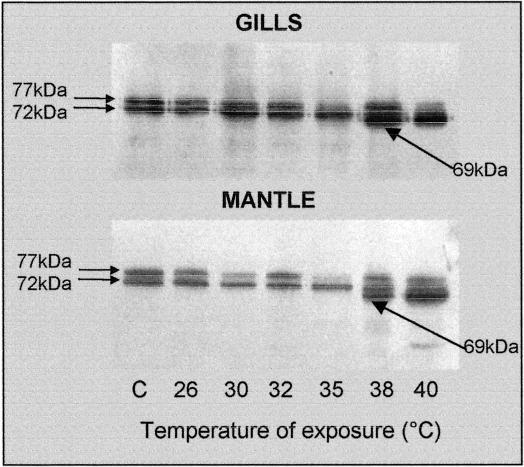
A representative Western immunoblot for detecting Hsp70 expression in the gills and mantle of C gigas is reported in Figure 7. Nonstressed animals displayed 2 constitutive proteins of about 72 kDa and 77 kDa, which run in parallel with the O edulis constitutive isoforms transferred onto the same blots (not shown). In contrast to O edulis, however, 38°C was the lowest temperature needed to induce the appearance of the 69-kDa protein in tissues of C gigas, and its density was increased further at 40°C. The lowest lethal temperature for these animals was about 44°C.
Fig 7.
Western immunoblotting to detect proteins of the Hsp70 family in tissues of Crassostrea gigas exposed to different temperatures for 1 hour. A representative blot out of 3 separate experiments is reported
DISCUSSION
Synthesis of Hsps occurs in almost all organisms studied to date after thermal stress, probably to protect the cells from potential damage produced by polypeptides in their nonnative conformation (Feder and Hofmann 1999). Hsps are classified into families, whose members are grouped according to their molecular weights and may have different locations within the cells as well as different levels of expression (De Maio 1999). Among the various families, the one referred to as Hsp70 appears to be the most conserved amongst species and is significantly involved in an organism's response to stress; in most mammalian cells, 2 prominent forms are distinguished, namely the constitutive member Hsp73 and the highly inducible member Hsp72 (Riabowol et al 1988).
Nonmammalian cells may also show prominent Hsp70 isoforms as observed in bivalves (Sanders et al 1994; Clegg et al 1998). These organisms may routinely undergo thermal stress and are also widely exposed to other sources of natural and anthropogenic environmental challenges imposed by aquatic environments (Hofmann 1999). Among them, Ostreidae are largely being used to monitor pollution (Tanguy et al 2001), especially in those areas where mussels are rare. Within their range of distribution, water temperature may occasionally exceed 40°C, evoking strong synthesis of inducible Hsps, as demonstrated in hemocytes of Crassostrea virginica (Tirard et al 1995a). Other authors reported the phenomenon of acquired thermotolerance in C gigas and explored the possibility that enhancement of Hsps by mild heat shock might be useful in reducing oyster mass mortality in the field (Clegg et al 1998).
In the present investigation we characterized the response to thermal stress in O edulis by evaluating the expression of Hsp70 family members under different experimental conditions. The monoclonal antibody used for this approach, raised in rabbit Hsp70 against rat Hsp70, recognized 2 isoforms of about 72 kDa and 77 kDa in both the gills and mantle of O edulis maintained in control conditions, thus suggesting a probable housekeeping role of these proteins and also a high sequence homology between molluscan and mammalian Hsps. Because heat-induced Hsp expression was detectable up to day 14 of poststress recovery, oysters were kept in aquaria at 15°C for a minimum of 18 days after being collected from the field and were considered nonstressed individuals for heat shock treatment. Animals randomly selected and analyzed for Hsp70s at days 0, 2, and 4 after sampling did not show a different pattern, indicating that handling and transportation did not cause enhanced synthesis of the proteins. Expression of O edulis Hsp70s increased after 1 hour of exposure to heat shock at different temperatures. Densitometric analysis of the blots indicated that the increment of Hsp70s was mainly caused by the expression of a 69-kDa protein induced at temperatures ≥32°C. The appearance of the lower molecular weight isoform was shared by all tested individuals exposed to ≥32°C, and in each case the maximum expression was induced at 35°C. Individuals exposed to 38°C showed low, if any, expression of Hsp69, and this feature could be related to the high mortality observed at this temperature. Whether the lower synthesis of Hsp69 compromises the animals' survival or is itself a consequence of the overall biochemical damage leading to death needs further elucidation.
Additional Hsp70 isoforms induced by stress have been reported in aquatic organisms including fish (Dyier et al 1991; Koban et al 1991) and bivalves. A lower molecular weight Hsp70 isoform was induced by Cu++ in the gills but not in the mantle of Mytilus edulis (Sanders et al 1994), although it was not expressed in other mussel species after heat shock (Hofmann 1999). A heat-inducible 69-kDa Hsp was clearly observed in the gills of C gigas (Clegg et al 1998). However, expression patterns were not reported by the authors cited here.
Hsp72 and Hsp77 expression in the gills of O edulis significantly increased after short exposure of oysters to 35°C, remaining at a steady level or decreasing after 45–60 minutes. A different trend was shown by mantle proteins, where exposure to 35°C for 15–60 minutes did not affect the expression of Hsp72 and Hsp77, while inducing a sharp increase in Hsp69 content.
A lag time after heat shock was needed for the synthesis of Hsp69. Densitometric analysis of Hsp expression in oysters exposed to 35°C and allowed to recover at 15°C for variable periods of time indicated that 72-kDa and 77-kDa protein levels did not undergo significant modifications, whereas increments of Hsp69 were detected after recovery for 4 hours. Maximum expression of Hsp69 was measured after 24 hours and 48 hours of poststress recovery in the gills and mantle, respectively, and the protein was still clearly detectable in both tissues 7 days after the heat challenge, persisting in the gills for up to 14 days. Such a phenomenon could be related to the low metabolism typical of molluscs. Because we did not measure Hsp synthesis, it is difficult to distinguish whether the apparently low turnover of Hsp in O edulis is caused by the low holding temperature of the animals or by a prolonged burst of Hsp synthesis to replace Hsps that turn over at a rapid rate. It has previously been reported that during this period Hsps maintain their protective role and, moreover, contribute to the acquired thermotolerance. High levels of Hsp70 were expressed in tissues of C gigas for up to 14 days after heat shock, during which period the animals were tolerant to an otherwise lethal heat shock (Clegg et al 1998). Expression of Hsp72 and Hsp77 in the gills and mantle of O edulis remained easily detectable at the end of the 14-day period. This confirms the constitutive role of these proteins. From the aforementioned data, levels of Hsp72 and Hsp77 appear to increase transiently after mild or short thermal stress. One could speculate that they represent the first cell response to temperature modifications. After continued stress stimulus, cells could set up a stronger reaction, here represented by Hsp69 synthesis.
Because oysters were collected from different locations, over a range of about 300 km, we believe that the thermal profile characterized in the present work is representative of the O edulis population inhabiting the North Adriatic Sea. The possibility that oysters living in other areas of the Mediterranean Sea or Atlantic Ocean show differences cannot be excluded.
Some features of the O edulis heat shock response have been compared to those of C gigas, an allochthon species reared along the northwestern Adriatic Sea coast. The 2 species display strong similarity as to the molecular weight and number of constitutive and inducible isoforms detected by the same antibody. This study, however, used only a single anti-Hsp70 antibody and 1-dimensional electrophoresis; therefore, the presence of other members of the Hsp70 family and other Hsp families cannot be excluded in the tissues of O edulis and C gigas. The expression of Hsp69 in the gills and mantle of C gigas was induced at temperatures ≥38°C—which interestingly corresponded to the apparent half-lethal temperature stated for O edulis—and densitometric analysis indicated that the maximum was reached at 40°C in both tissues. In agreement with previous reports (Clegg et al 1998), 44°C was found to be the minimum lethal temperature for C gigas. According to the models for the transcriptional activation of Hsp genes, denatured proteins would represent the trigger for the enhancement of Hsp synthesis, and induction of the Hsp response has been suggested to mirror the thermal stability of cell proteins (Dietz and Somero 1993). In this context, the biochemical machinery of O edulis could have a higher susceptibility to heat than that of C gigas. Alternatively, it has been proposed that heat directly activates a single transcriptional factor, HSF1, which trimerizes and binds to specific regions in the promoter of hsp genes (Zhong et al 1998). In this case, different thermal sensitivity of the promoter or the heat shock factor between the 2 oysters could be hypothesized. As a matter of fact C gigas is more resistant to stress stimuli than are other oyster species (Tirard et al 1995b), and one could speculate that a higher threshold of stress sensitivity contributes to the ability of C gigas to colonize new habitats in competition with autochthon species. Unfortunately, no information is available at the moment to compare homologous protein or complementary deoxyribonucleic acid (cDNA) sequences between the 2 species. A C gigas constitutive hsp70 cDNA sequence has been reported by Gourdon et al (2000), and a C virginica–inducible hsp70 cDNA sequence has been reported by Rathinam et al (2000). An O edulis–inducible hsp70 cDNA has recently been sequenced in our laboratory (F. Tinti and E. Fabbri, in preparation).
Temperature fluctuations are a true challenge for sedentary species like Ostreidae and Mytilidae living in natural or reared conditions. Because many other stimuli are known to provoke strong Hsp expression in animal tissues (Goodman and Blank 1998; Mager et al 2000; Matranga et al 2000), future research on Hsp69 expression in oysters exposed to stress different from heat will be undertaken. Notwithstanding the good correlation between oyster Hsp69 expression and heat-exposure, more has to be done to fully understand the sensitivity of hsp70 genes to environmental stimuli. Knowledge about the oyster heat shock response and gene regulation is increasing (Tirard et al 1995a, 1997; Clegg et al 1998; Gourdon et al 2000; Rathinam et al 2000; F. Tinti and E. Fabbri, in preparation), and taken together these data could be useful for evolutionary studies and health assessment of aquatic resources. As a consequence of the dramatic and progressive decline of C virginica along the North American east coast (Rathinam et al 2000) and of C gigas mass mortality in bays in California (Clegg et al 1998) in the last few decades, application of strategies for their protection through Hsp-based acquired thermotolerance has been planned (Clegg et al 1998; Rathinam et al 2000). The severe reduction of nominal catches of O edulis within the Mediterranean Sea (FAO Yearbook 1996) observed in the last few years further emphasizes the need for the development of more sensitive species-specific Hsp probes that could be used to assess the health of oyster populations.
Acknowledgments
We are greatly indebted to Dr Fausto Tinti for his valuable help and discussion and are grateful to Mrs Leda De Ware for the English revision of the manuscript. We also wish to thank the fishermen of “La Bussola” (Rimini, Italy) for collecting the oysters. The research was funded by MURST (ex40% 2000) (E.F.).
REFERENCES
- Clegg JS, Uhlinger KR, Jackson SA, Cherr GN, Rifkin E, Friedman CS. Induced thermotolerance and the heat shock protein-70 family in the Pacific oyster Crassostrea gigas. Mol Mar Biol Biotechnol. 1998;7:21–30. [Google Scholar]
- De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Dietz TJ, Somero GN. Species- and tissue specific synthesis patterns for heat-shock proteins HSP70 and HSP90 in several marine teleost fishes. Physiol Zool. 1993;66:863–880. [Google Scholar]
- DuBeau SF, Pen F, Tremblay GC, Bradley TM. Thermal shock of salmon in vivo induces the heat shock proteins hsp70 and confers protection against osmotic shock. Aquaculture. 1998;168:311–323. [Google Scholar]
- Dyier SD, Dickson KL, Zimmermann EG, Sanders BM. Tissue specific patterns of heat shock protein synthesis and thermal tolerance of the fathead minnow (Pimephales promelas) Can J Zool. 1991;69:2021–2027. [Google Scholar]
- FAO Yearbook. 1996 Fishery statistics, vol. 82. FAO, Rome, Italy. [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Goodman R, Blank M. Magnetic field stress induces expression of hsp70. Cell Stress Chaperones. 1998;3:79–88. doi: 10.1379/1466-1268(1998)003<0079:mfsieo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdon I, Gricourt L, Kellner K, Roch P, Escoubas J-M. Characterization of a cDNA encoding a 72 kDa heat shock cognate protein (Hsc72) from the Pacific Oyster, Crassostrea gigas. DNA Sequence. 2000;11:265–270. doi: 10.3109/10425170009033241. [DOI] [PubMed] [Google Scholar]
- Heckathorn SA, Downs CA, Coleman JS. Small heat shock proteins protect electron transport in chloroplast and mitochondrion during stress. Am Zool. 1999;39:865–876. [Google Scholar]
- Hendrick JP, Hartl F-U. The role of molecular chaperones in protein folding. FASEB J. 1995;9:1559–1569. doi: 10.1096/fasebj.9.15.8529835. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Brown MA, Renfro JL, Perdrizet GA, Rewinski M, Guidon PT Jr,, Mistry T, House SD. Tissue-level cytoprotection. Cell Stress Chaperones. 2000;5:412–414. doi: 10.1379/1466-1268(2000)005<0412:tlc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower LE, Norris CE, DiIorio PJ, Fielding E. Heat shock responses of closely related species of tropical and desert fish. Am Zool. 1999;39:877–888. [Google Scholar]
- Hofmann G, Somero G. Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in intertidal mussel Mytilus trossulus. J Exp Biol. 1995;198:1509–1518. doi: 10.1242/jeb.198.7.1509. [DOI] [PubMed] [Google Scholar]
- Hofmann GE. Ecologically relevant variation in induction and function of heat shock proteins in marine organisms. Am Zool. 1999;39:889–900. [Google Scholar]
- Koban M, Yup AA, Agellon LB, Powers DA. Molecular adaptation to environmental temperature: heat-shock response of the eurythermal teleost Fundulus heteroclitus. Mol Mar Biol Biotechnol. 1991;1:1–17. [Google Scholar]
- Kohler HR, Rahamn B, Graff S, Berkus M, Triebskorn R. Expression of the stress-70 protein family (HSP70) due to heavy metal contamination in the slug Deoroceras reticulatum: an approach to monitor sublethal stress conditions. Chemosphere. 1996;33:1327–1340. [Google Scholar]
- Kohler HR, Triebskorn R, Stocker W, Koetzel PM, Alberti G. The 70 kDa heat shock protein (hsp70) in soil invertebrates: a possible tool for monitoring environmental toxicants. Arch Environ Contam Toxicol. 1992;22:334–338. doi: 10.1007/BF00212095. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the heat of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mager WH, de Boer AH, Siderius MH, Voss HP. Cellular responses to oxidative and osmotic stress. Cell Stress Chaperones. 2000;5:73–75. doi: 10.1379/1466-1268(2000)005<0073:crtoao>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga V, Troia G, Bonaventura R, Muller WEG. Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress Chaperones. 2000;5:113–120. doi: 10.1379/1466-1268(2000)005<0113:cabrte>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Nadeau D, Corneau S, Plante I, Morrow G, Tanguay RM. Evaluation for Hsp70 as a biomarker of effect of pollutants on the earthworm Lumbricus terrestris. Cell Stress Chaperones. 2001;6:153–163. doi: 10.1379/1466-1268(2001)006<0153:efhaab>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Scharf KD, Gagliardi D, Vergne P, Czarnecka-Verner E, Gurley WB. The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperones. 1996;1:215–223. doi: 10.1379/1466-1268(1996)001<0215:thwcap>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam AV, Chen TT, Grossfeld RM. Cloning and sequence analysis of a cDNA for an inducible 70 kDa heat shock protein (Hsp70) of the American Oyster (Crassostrea virginica) DNA Sequence. 2000;11:261–264. doi: 10.3109/10425170009033240. [DOI] [PubMed] [Google Scholar]
- Riabowol KT, Mizzen LA, Welch WJ. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988;242:433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- Sanders BM, Hope C, Pascoe VM, Martin LS. Characterization of the stress proteins response in two species of Collisella limpets with different temperature tolerance. Physiol Zool. 1991;64:1471–1489. [Google Scholar]
- Sanders BM, Martin LS, Howe SR, Nelson WG, Hegre ES, Phelps DK. Tissue-specific differences in accumulation of stress proteins in Mytilus edulis exposed to a range of copper concentrations. Toxicol Appl Pharmacol. 1994;125:206–213. doi: 10.1006/taap.1994.1066. [DOI] [PubMed] [Google Scholar]
- Scharf K-D, Hohfeld I, Nover L. Heat stress response and heat stress transcription factors. J Biosci. 1998;23:313–329. [Google Scholar]
- Tanguy A, Mura C, Moraga D. Cloning of a metallothionein gene and characterization of two other cDNA sequences in the Pacific oyster Crassostrea gigas (CgMT1) Aquat Toxicol. 2001;55:35–47. doi: 10.1016/s0166-445x(01)00160-6. [DOI] [PubMed] [Google Scholar]
- Tirard CT, Grossfeld RM, Levine JF, Kennedy-Stoskopf S. Effects of hypothermia in vitro on stress protein synthesis and accumulation in oyster hemocytes. Fish Shellfish Immunol. 1995a;5:9–25. [Google Scholar]
- Tirard CT, Grossfeld RM, Levine JF, Kennedy-Stoskopf S. Effects of osmotic shock on protein synthesis of oyster hemocytes in vitro. Comp Biochem Physiol. 1997;116A:43–49. [Google Scholar]
- Tirard CT, Grossfeld RM, Volety AK, Chu F-LE. Heat shock proteins of the oyster parasite Perkinsus marinus. Dis Aquat Org. 1995b;22:147–151. [Google Scholar]
- Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol Cell. 1998;2:101–108. doi: 10.1016/s1097-2765(00)80118-5. [DOI] [PubMed] [Google Scholar]