Abstract
Inflammation of the human bronchial epithelium, as observed in asthmatics, is characterized by the selective death of the columnar epithelial cells, which desquamate from the basal cells. Tissue repair initiates from basal cells that resist inflammation. Here, we have evaluated the extent of apoptosis as well as the Hsp27 level of expression in epithelial cells from bronchial biopsy samples taken from normal and asthmatic subjects. Hsp27 is a chaperone whose expression protects against oxidative stress. We report that in asthmatic subjects the basal epithelium cells express a high level of Hsp27 but no apoptotic morphology. In contrast, apoptotic columnar cells are devoid of Hsp27 expression. Moreover, we observed a decreased resistance to hydrogen peroxide–induced apoptosis in human bronchial epithelial 16–HBE cells when they were genetically modified to express reduced levels of Hsp27.
INTRODUCTION
Airway inflammation associated with asthma is characterized by the damage of the bronchial epithelium. This process is associated with the selective loss of columnar epithelial cells while basal cells usually remain attached to the basement membrane, playing an important role in the initiation of tissue repair (Jeffery et al 1989; Bousquet et al 2000). It has therefore been suggested that in the attempt to restore the integrity of the epithelium layer after injury, epithelial cells must protect themselves against noxious and proapoptotic agents.
Several proteins have been described as modulators of programmed cell death or apoptosis. Among them are the members of the Bcl-2 (Kluck et al 1997) and inhibitor of apoptosis proteins (IAP) (Deveraux et al 1998) families. Recently, members of the family of stress (or heat shock) proteins have emerged as potent modulators of the cell death machinery. For example, the major stress protein Hsp70 negatively regulates apoptosis by interfering with the release of cytochrome c from the mitochondria (Mosser et al 2000). Hsp70 also acts downstream of the cytochrome c release and upstream of the caspase-3 activation (Li et al 2000) probably by altering the formation of the apoptosome complex through its interaction with Apaf-1 (Beere et al 2000; Saleh et al 2000). Hsp70 also interacts with BAG-1 (Takayama et al 1997) and stimulates the Bcl-2 action or exerts its antiapoptotic activities downstream of the caspase-3 activation. Of interest, another stress protein Hsp90 also acts as a negative modulator of apoptosis through its binding to Apaf-1 (Pandey et al 2000). In contrast to Hsp70 and Hsp90, the mitochondrial Hsp60 and Hsp10 are proapoptotic chaperones that favor the conversion of procaspase-3 to active caspase-3 (Samali et al 1999; Xanthoudakis et al 1999).
Hsp27 is an oligomeric phosphoprotein belonging to the stress proteins family (Arrigo 1998), which negatively regulates cell death induced by different conditions or agents, such as heat shock (Arrigo and Landry 1994), oxidative stress (Huot et al 1991, 1996; Mehlen et al 1993; Hastie et al 1997), tumor necrosis factor α (Mehlen et al 1995a, 1995b, 1995c, 1996a, 1997a; Wang et al 1996; Park et al 1998), chemotherapeutic drugs (Arrigo and Landry 1994; Arrigo 1998), and apoptotic agents (Mehlen et al 1996b; Samali and Cotter 1996; Arrigo 1998; Garrido et al 1999; Wagstaff et al 1999; Bruey et al 2000a, 2000b; Charette et al 2000; Pandey et al 2000; Paul et al 2002). The mechanism involved in these protective functions appears to be related to the fact that, in vitro, Hsp27 acts as an adenosine triphosphate (ATP)-independent chaperone that counteracts protein denaturation and help in the refolding of malfolded polypeptides (Jakob et al 1993; Jakob and Buchner 1994; Ehrnsperger et al 2000). Hsp27 large oligomers are supposed to bind denatured polypeptides (Ehrnsperger et al 1997; Lee et al 1997) and to present them to the ATP-dependent protein chaperones (Hsp70, Hsp40, Hsp90, and cochaperones) for refolding.
In the case of oxidative stress, the protection probably results from the ability of Hsp27 to decrease the intracellular level of reactive oxygen species (ROS) and to its chaperone activity, which favors the degradation of oxidized proteins (Preville et al 1999; Rogalla et al 1999; Arrigo 2002; Arrigo et al 2002a). Hsp27 has also been described to protect cells by acting as a stabilizer of cytoskeletal architecture (Lavoie et al 1993, 1995; Huot et al 1996; Mairesse et al 1996; Guay et al 1997; Mounier and Arrigo 2002). Recently, Hsp27 expression has been described to generate cellular protection against apoptosis when it is induced independently of ROS production (Arrigo 2000; Arrigo et al 2002b), for example, by apoptotic agents such as staurosporine, Fas/APO-1 (Mehlen et al 1996b; Arrigo 1998; Charette et al 2000; Paul et al 2002), actinomycin D, camptothecin, etoposide (Samali and Cotter 1996; Garrido et al 1999; Bruey et al 2000a, 2000b), cisplatin (Garrido et al 1997), and doxorubicin (Hansen et al 1999). Moreover, during early differentiation, the transient expression of Hsp27 protects against the apoptotic process inherent to cell differentiation (Mehlen et al 1997b, 1999). Hsp27 reduces apoptosis by interacting with cytochrome c once this apoptogenic protein is released from the mitochondria, a phenomenon which counteracts procaspase-9 activation (Garrido et al 1999; Bruey et al 2000a, 2000b). Depending on its level of expression, Hsp27 expression also interferes with the release of cytochrome c from the mitochondria (Paul et al 2002). This phenomenon is related to the Hsp27-mediated alteration of the signaling pathways upstream of the mitochondria, such as the F-actin integrity (Paul et al 2002).
In recent studies we and others have shown that the epithelium of the asthmatic subject is characterized by an increased expression of the marker proteins, such as Bcl-2, proliferating cell nuclear antigen, CD40-L, nuclear factor–κB (Vignola et al 2001), granulocyte macrophage-colony stimulating factor (GM-CSF) (Vachier et al 1998), and Hsp70 (Vignola et al 1995; Bertorelli et al 1998; Aron et al 1999). The positive staining of Hsp70 observed in bronchial biopsy samples of asthmatic subjects was localized on the epithelium and basement membrane. This staining was correlated with the severity of asthma (Vignola et al 1995). Hence, in bronchial epithelial cells of asthmatic subjects, markers of cell survival and proliferation are coexpressed with markers of cell activation, suggesting that epithelium repair is associated with a persistent activation state of epithelial cells. To further characterize bronchial cells from asthmatics, we have analyzed the expression of Hsp27 together with the extent of cell death in the epithelium of bronchial biopsy samples taken from normal and asthmatic subjects. We report that an increased Hsp27 expression in the epithelium of asthmatic subjects is associated with the absence of apoptosis of these cells. To further analyze the role of Hsp27, we have analyzed the resistance to hydrogen peroxide–induced apoptosis of the 16–human bronchial epithelial (16-HBE) cells genetically modified to express different levels of Hsp27. We provide evidence that a down-regulation of the level of endogenously expressed Hsp27 stimulates caspase-3 activation and the death of 16-HBE cells induced by H2O2. Hence, in bronchial epithelial cells of asthmatics patients, Hsp27 is probably 1 of the proteins whose expression generates a protection against the oxidative stress induced by the chronic inflammatory state of this tissue.
MATERIALS AND METHODS
Subjects
Eleven asthmatic subjects (age range, 22–55 years; median, 29 years; 25–75% percentiles, 25–36.2 years) were selected according to the criteria of the American Thoracic Society, as described previously (Bousquet et al 1990). The clinical severity of asthma was assessed according to the Global Initiative for Asthma (GINA) guidelines (Global Strategy for Asthma Management and Prevention 1995). The selected patients presented mild to moderate persistent asthma. They were defined as untreated asthmatics in spite of the fact that they received, as needed, intermittent inhaled short-acting β2 agonists. These patients were not previously treated with inhaled corticosteroids, or oral corticosteroids, nedocromil sodium, or cromoglycate. None of the subjects participating in this study was a current smoker. Subjects who had any bronchial or respiratory tract infection during the month preceding the test were excluded from the study. Moreover, patients were excluded from the study if they had a severe exacerbation of asthma, resulting in hospitalization during the month preceding the study. Eleven healthy subjects (age range, 22–45 years; median, 34 years; 25–75% percentiles, 28.5–37.2 years) were used as a control group. Their pulmonary function was within the normal range. Subjects who had any bronchial or respiratory tract infection during the month preceding the test were excluded from the study. This control group was formed by normal subjects who volunteered to undergo bronchoscopy. These subjects have been informed about the procedure, and all have signed an informed consent. The ethic committee of our institution approved this study.
Pathological examination of bronchial mucosa and immunoreactivity of Hsp27
Biopsy samples were recovered using fiberoptic bronchoscopy performed, as described previously, in subsegmental bronchi (Bousquet et al 1990). Specimens were fixed in 3.7% wt/v formaldehyde (pH 7.0) and embedded in paraffin blocks. Four-micrometer-thick sections were cut, deparaffined, and rehydrated before performing hematoxylin and eosin staining or immunohistochemistry. Immunoreactivity of Hsp27 was studied on sections incubated for 45 minutes at room temperature with a polyclonal anti-Hsp27 antibody used at the dilution of 1:50 (Arrigo and Welch 1987). Immunoreactivity of this antibody was revealed using the labeled streptavidin-biotin method (Dako LSAB®, Glostrup, Denmark), following the manufacturer's instructions. Control slides were prepared using an irrelevant mouse antibody of the same isotype and at the same concentration as the specific secondary antibody (Dako, Glostrup, Denmark).
Detection of apoptosis in bronchial epithelial cells
Apoptosis was detected as previously described (Vignola et al 1999) using the terminal deoxynucleotidyl transferase–mediated deoxynucleoside triphosphate nick end–labeling (TUNEL) assay (Oncor Inc, Gaithersburg, MD, USA), which detects deoxyribonucleic acid (DNA) strand breaks in cells undergoing apoptosis (Gavrieli et al 1992). Apoptosis was also evaluated on the basis of the morphological appearance of the cells using light microscopy, in particular, the marked loss of cytoplasm and nuclear condensation. Apoptotic cells were therefore identified as those cells stained using the TUNEL technique and showing the morphological appearance of apoptosis.
Detection of apoptosis in Hsp27-immunostained cells
To evaluate whether epithelial cells expressing Hsp27 were in an apoptotic state, the slides were first stained for Hsp27 immunoreactivity using the LSAB method (Dako LSAB®) described earlier and subsequently the TUNEL technique to detect the apoptotic cells. Control slides were prepared using an irrelevant mouse antibody of the same isotype and at the same concentration as the specific primary monoclonal antibody (Dako LSAB®). To analyze the colocalization of the dark-brown nuclear staining (caused by the TUNEL technique) and of the red cytoplasmic staining (caused by the LSAB immunoreactivity with anti-Hsp27 antibody), the slides were coded and the immunoreactivity of each marker was evaluated blindly by 2 independent investigators (A.M.V. and G.C.). The interinvestigator coefficient of agreement was excellent (Kappa = 0.95).
Quantification of immunoreactive cells in the bronchial epithelium
Airway epithelial expression of immunoreactive Hsp27 in biopsy sections was quantified by computer-assisted image analysis (Quantimet software, Cambridge, UK). For each biopsy specimen, the entire epithelium in 2 nonserial sections was systematically assessed on the basis of the red, blue, and green color balance. At the beginning of each session, the image analysis system was standardized using the same section of the bronchial mucosa stained for Hsp27 to ensure reproducibility of the analysis. Using the Quantimet software, the surface of the total epithelium was measured and expressed as square micrometers of the epithelium. Subsequently, the number of Hsp27-positive cells was evaluated and expressed per square micrometers of the epithelium. All the slides were coded, and the immunoreactivity of each of the markers studied was evaluated blindly by 2 independent investigators (A.M.V. and G.C.). The interinvestigator coefficient of agreement was considered excellent (Kappa = 0.94).
Culture and treatment of parental 16-HBE cells
In order to evaluate if Hsp27 overexpression by bronchial epithelial cells of asthmatics was involved in the protection against oxidative stress–induced apoptosis, we used the bronchial epithelial cell line (16-HBE) (Haws et al 1992), which constitutively expresses high levels of Hsp27 (as shown subsequently). 16-HBE epithelial cells were cultured as adherent monolayers in modified Eagle medium (MEM) + 10% heat-inactivated (56°C, 30 minutes) fetal bovine serum + gentamicin solution (25 μg per 100 ml) + 1 mM l-glutamine, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES, 25 mM) (all from Invitrogen-GIBCO, Grand Island, NY, USA) in 25-cm2 tissue culture flasks. To assess the survival of 16-HBE cells in response to oxidative stress, these cells were plated in flasks of 25 cm2 at a density of 3 × 105 cells/ml in 3 ml of MEM without fetal calf serum (FCS). The cells were then exposed for 1 hour at 37°C to 100 μM H2O2 (which was found to be optimal in preliminary dose-response experiments). Then, the medium was removed and replaced with MEM + 10% FCS, and the cells were incubated for 18 hours at 37°C before being evaluated for apoptosis using Annexin V–fluorescein isothiocyanate (FITC) (Roche Diagnostic, Meylan, France) staining (Clerget and Polla 1990; Polla et al 1996).
Vectors, transfection, and selection of 16-HBE cells expressing a decreased level of Hsp27
Exponentially growing 16-HBE cells, plated at a density of 2.1 × 106 cells per 78 cm2 1 day before transfection, were cotransfected using 20.4 μl of Fugene 6 (Roche Diagnostic) containing 0.68 μg of pGhygro plasmid bearing the hygromycin B resistance gene and 6.8 μg of either the pSVK3 void vector (Pharmacia, Uppsala, Sweden) or the hsp27 antisense vector. This latter vector contains the entire coding sequence of the hsp27 gene placed in reverse orientation under the control of the SV40 promoter. This vector was constructed using an EcoRI-EcoRI DNA fragment of the plasmid pSVhsp27 (Mehlen et al 1995c), which was inserted in the corresponding site of the pSVK3 polylinker. After 24 hours, the medium was removed and a fresh culture medium containing hygromycin B (30 μg/ml) was added to the cells. Hygromycin B–resistant clones, isolated by the end-point dilution technique, were grown in the presence of the antibiotic, and screened for the Hsp27 level of expression. Normal and Hsp27-underexpressing 16-HBE cells were cultured as adherent monolayers in MEM containing 10% heat-inactivated (56°C, 30 minutes) fetal bovine serum, gentamicin solution (25 μg per 100 ml), 1 mM l-glutamine, HEPES (25 mM), 1× nonessential amino acids, and hygromycin B (30 μg/ml) (all from Invitrogen-GIBCO) in 25-cm2 tissue culture flasks.
Evaluation of Hsp27 expression in 16-HBE cells
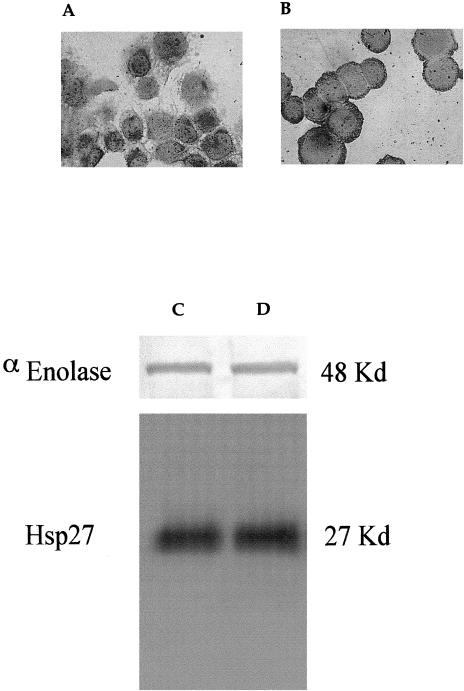
Parental 16-HBE cells, as well as 16-HBE cells transfected with an empty vector (control cells) or an hsp27 antisense vector, were lysed in ice-cold buffer containing 10 mM Tris pH 7.4, 50 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 1% Nonidet P-40, and the protease inhibitor cocktail (Roche, Milan, Italy), under repeated agitation and centrifuged at 10 000 × g. The supernatant extracts were analyzed for their protein content using the bicinchonic acid (BCA) assay (Pierce, Rockford, IL, USA). Protein samples were denatured under reducing conditions by boiling for 5 minutes in 50 mM Tris-HCl pH 6.8, 1% sodium dodecyl sulfate (SDS), 2% β-mercaptoethanol, 0.01% bromophenol blue, and separated by SDS–polyacrylamide gel electrophoresis. The expression of Hsp27 was determined before and after exposure to H2O2. Western immunoblotting was performed by electroblotting total cellular proteins to Immobilon-P membranes (Millipore, St Quentin en Yvelines, France), which were then blocked overnight at room temperature in phosphate-buffered saline (PBS) supplemented with 3% bovine serum albumin, 0.5% Tween, and 0.02% NaN3 and washed 3 times with PBS containing 0.1% (v/v) of Tween 20. The filters were incubated for 1 hour at room temperature with the mouse monoclonal antibody anti-Hsp27 (dilution 1:1000) (Stressgen, clone G3.1, Victoria, BC, Canada). After washing (3 times in PBS), the filters were then incubated for 45 minutes at room temperature with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Dako) at the dilution of 1:5000. As control protein, we analyzed the quantity of the housekeeping protein α-enolase. To identify α-enolase, we used a rabbit antiserum (anti-p48) at the dilution of 1:1000, which is directed against the α-isoenzyme of enolase, as described previously (Giallongo et al 1986). The protein-antibody complex was detected by anti-rabbit IgG alkaline phosphatase (AP) conjugated at the dilution of 1:7500 (Promega Corporation, Madison, WI, USA). The final reaction was developed by an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK). The signal intensity was measured using a BioRad densitometer (model GS-670). Immunocytochemistry was performed using the LSAB technique (Dako) with a polyclonal anti-Hsp27 antibody (Arrigo and Welch 1987) used at the dilution of 1:50 for 45 minutes at room temperature. Controls were performed using cells incubated with the irrelevant mouse IgG1. Results were expressed as a percentage of the positive cells.
Measurement of DEVD caspase-3–like activities
For the determination of procaspase-3–like activation, cells (106) treated or not with hydrogen peroxide were harvested and subsequently washed twice in ice-cold PBS, pH 7.4. They were then pelleted at 200 × g for 5 minutes, and the cell pellets were boiled for 5 minutes in SDS-gel sample buffer. Total cellular proteins were then analyzed in immunoblots probed with anti–caspase-3 antibody (anti-CPP32, Pharmingen, San Diego, CA), which recognizes the 38-kDa procaspase-3 polypeptide and the active caspase-3 polypeptide of 17 kDa.
Statistical analysis
Statistical analysis was performed by means of nonparametric tests. The Wilcoxon U test was used for paired comparisons.
RESULTS
Comparative analysis of apoptosis and Hsp27 expression in bronchial mucosa cells of normal and asthmatic patients
The characteristics of the normal and asthmatic subjects analyzed in this study are presented in Table 1. The pulmonary function of normal subjects was within normal range. Patients with persistent asthma had a mild to moderate form of the disease and had a significant decrease in forced expiratory volume in 1 second (FEV1) values (52% to 106% of the predicted values) in comparison with the normal subjects (100% to 108% of the predicted values, P = 0.001, Mann-Whitney U-test).
Table 1.
Demographic characteristics of the subjects
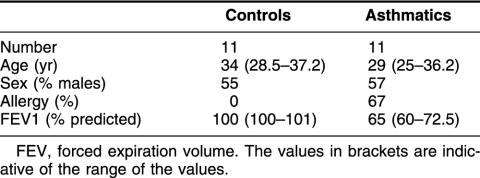
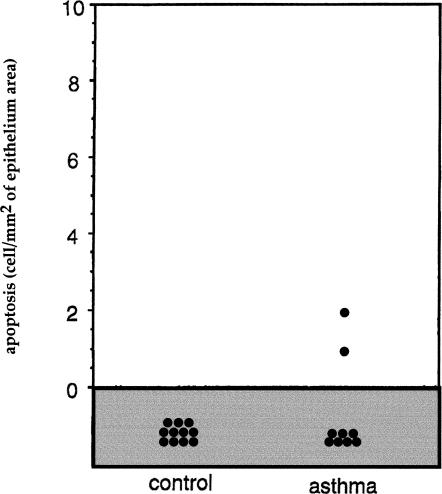
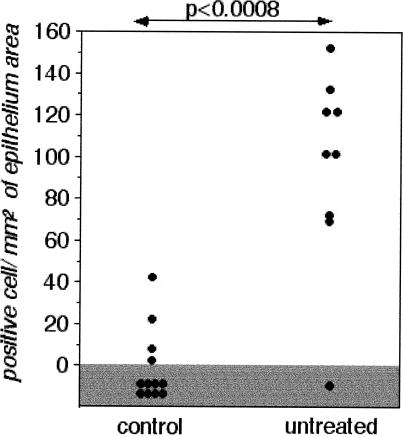
We have examined the bronchial epithelial cells of these different subjects and have determined the level of apoptotic cell death and immunoreactivity for the antiapoptotic small stress protein Hsp27. Biopsy samples from subsegmental bronchi were therefore recovered and processed for apoptosis detection using the TUNEL assay and on the basis of the morphological appearance of the cells. We observed that in bronchial biopsy samples taken from control subjects, TUNEL-positive epithelial cells were not detected in any of the samples studied. In bronchial biopsy samples from asthmatic subjects, rare TUNEL-positive cells were observed. It was concluded that the number of TUNEL-positive cells was not significantly different between the 2 groups (Fig 1). Analysis of Hsp27 immunoreactivity revealed that it was significantly higher in the epithelium of the asthmatic subjects when compared with what was observed in the control subjects (P < 0.008, Mann-Whitney U-test) (Fig 2). Analysis of the biopsy samples obtained from the normal and asthmatic subjects for the presence of Hsp27 and apoptosis using LSAB and TUNEL technique in combination is shown in Figure 3. It is seen in Figure 3A that the epithelium of a normal subject appears devoid of Hsp27 staining and is not TUNEL positive, indicating that no apoptotic event is taking place in this normal tissue. As previously described (Vignola et al 1998), analysis of the bronchial epithelium of asthmatic subjects (Fig 3 B,C) revealed that a significant proportion of this epithelium was damaged. This phenomenon is characterized by either a lack of the columnar epithelial cells (Fig 3B) or a fragile morphology (Fig 3C) (or both). Of interest, the analysis of the damaged epithelium of asthmatic patients showed that ciliated bronchial epithelial cells that were completely desquamated (Fig 3B) or still present in a fragile epithelium (Fig 3C) were TUNEL positive but were not immunoreactive for Hsp27. In contrast, the analysis of the large fraction of the asthmatic epithelium that was intact and has not undergone desquamation revealed a strong signal for Hsp27 but no nuclear staining using the TUNEL technique (Fig 3D). It is of note that in a previous article we showed that the bronchial epithelium of asthmatic subjects was also characterized by an increased expression of Hsp70. Interestingly, the pattern and topography of Hsp70 expression were very similar to that of Hsp27 (not shown, Vignola et al 1995). This suggests that Hsp27 and Hsp70 expression by the bronchial epithelium of asthmatic subjects contribute to the lack of an apoptotic morphology.
Fig 1.
Number of TUNEL-positive cells in bronchial biopsy specimens from control and asthmatic subjects. Sections of biopsy samples were prepared as described in Materials and Methods, and the number of TUNEL-positive epithelial cells was determined. Results are expressed as the number of TUNEL-positive cells per square millimeter of the epithelial area. Note the slight increase in the number of apoptotic cells in the biopsy samples from asthmatic patients. TUNEL, terminal deoxynucleotidyl transferase–mediated deoxynucleoside triphosphate nick end labeling
Fig 2.
Number of Hsp27-positive cells. Expression of Hsp27 in epithelial cells of control and asthmatic subjects. Results are expressed as the number of Hsp27-positive cells per square millimeter of the epithelium. Statistical analysis by Mann-Whitney U-test
Fig 3.
Analysis of Hsp27 expression and apoptosis in bronchial biopsy samples from normal and asthmatic patients. Hsp27 expression and TUNEL immunoreactivity in bronchial epithelial cells of a control (A) and an asthmatic subject (B, C, D) were performed as described in Materials and Methods. Cells expressing Hsp27 show a red immunostaining as the result of the labeled streptavidin-biotin immunoreactivity. TUNEL-positive cells are characterized by a brown staining of the nuclei (arrows). (A) Area of intact epithelium of a bronchial biopsy sample taken from normal subjects showing no immunoreactivity for Hsp27 and for TUNEL. (B) Area of damaged epithelium of a bronchial biopsy sample taken from asthmatic subjects with desquamated epithelial cells, which are not immunoreactive for Hsp27 but show a positivity for the TUNEL technique. (C) Wide area of fragile epithelium not immunoreactive for Hsp27 showing a nuclear TUNEL staining of many bronchial epithelial cells. (D) Area of intact epithelium showing a strong immunostaining for Hsp27, and a complete lack of nuclear staining by the TUNEL technique. TUNEL, terminal deoxynucleotidyl transferase–mediated deoxynucleoside triphosphate nick end labeling
Characterization of 16-HBE cells modified to underexpress Hsp27 and analysis of their resistance to oxygen peroxide–induced cell death
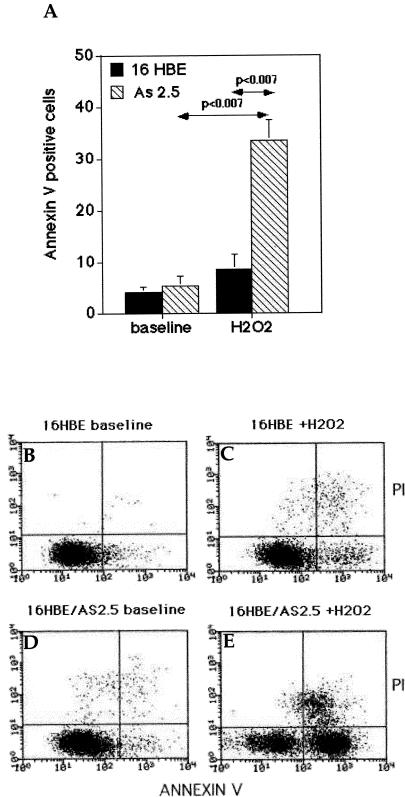
To better understand the role played by Hsp27 in vivo in the bronchial epithelium of asthmatic patients, we have undergone in vitro investigations using the already described 16-HBE cells (Vignola et al 1998). Analysis of Hsp27 immunoreactivity revealed a high level of constitutive Hsp27 expression in most of these cells (97 ± 2% positive cells). No change in this pattern of expression was detected after exposing these cells to 100 μM H2O2 for 1 hour (98 ± 3% positive cells) (Fig 4 A,B). Similar results were obtained by immunoblot analysis of the total cell protein extracts (Fig 4 C,D). This mild oxidative stress caused a slight increase in the percentage of 16-HBE cells presenting an apoptotic morphology (see Annexin V–propidium iodide (PI) analysis presented in Fig 6).
Fig 4.
Analysis of Hsp27 level in parental 16-HBE cells. Panels A and B: Immunocytochemical expression of Hsp27 before and after H2O2 (100 μM) exposure. Immunoreactivity, revealed by the labeled streptavidin biotin technique, is shown by the red staining of positive cells. Final magnification: 400×. Panels C and D: Western blot analysis of 16-HBE parental cells, 3 μg of each cellular extract was run on 10% SDS-PAGE and immunoblotted, as described in Materials and Methods. The relative intensity of the Hsp27 signal, compared with that of the housekeeping protein α-enolase, was of a factor of 100 before H2O2 exposure (panel C) and 110 after H2O2 (100 μM) exposure (panel D) in 16-HBE parental cells. 16-HBE, 16–human bronchial epithelial; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Fig 6.
Analysis of the oxidative stress–mediated cell death of the control and AS2.5 16-HBE cells. (A) Estimation of the cell death of the control and Hsp27-underexpressing AS2.5 cells. Nontreated or H202-treated (100 μM for 1 hour, followed by 18 hours recovery) cells were resuspended in PBS-containing FITC-conjugated Annexin V and PI. Data are expressed as the percentage of Annexin V–positive–PI-negative cells. Black plot: control cells; hatched plot: AS2.5 cells. (B and C) Representative cytofluorimetric bivariate analysis of Annexin V binding and PI uptake in control cells at baseline (B) and after 100 μM H2O2 (1 hour, followed by 18 hours recovery) exposure (C). (D and E) As in (B) and (C), but in this case the analysis was performed using AS2.5 cells at baseline (D) and after 100 μM (1 hour) H2O2 treatment (E). 16-HBE, 16–human bronchial epithelial; PBS, phosphate-buffered saline; FITC, fluorescein isothiocyanate; PI, propidium iodide
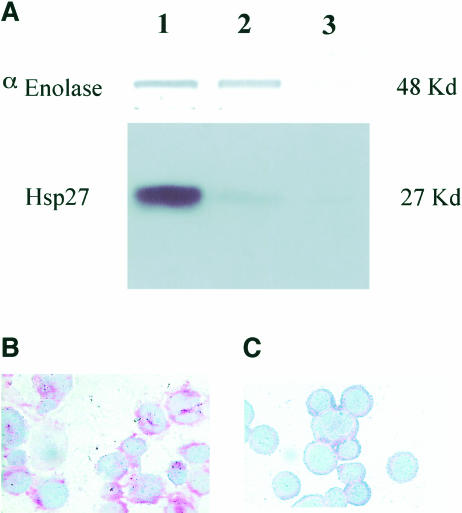
Numerous publications have described that Hsp27 expression confers cellular resistance to oxidative stress (reviewed in Arrigo 2002; Arrigo et al 2002a). But it has also been reported that in human KMST-6 cells (Arata et al 1995), squamous carcinoma cells (Trautinger et al 1997), or subspecies of L929 cells (Mairesse et al 1998) Hsp27 expression could generate a sensitization to oxidative stress. We, therefore, investigated whether the high level of constitutively expressed Hsp27 protected or sensitized 16-HBE cells to oxidative challenge. Studies were then performed to reduce the level of Hsp27 in these cells. Using an antisense strategy, we generated stable 16-HBE cell lines that showed a decreased level of Hsp27 (see Materials and Methods). For example, AS2.5 cells displayed an 80% reduced level of Hsp27 compared with the control cells (Fig 5A) or the parental cells (not shown). In AS2.5 cells (Fig 5), but not in control cells (not shown), exposed to 100 μM H2O2 for 1 hour followed by an 18-hour incubation without hydrogen peroxide, Hsp27 as well as the control polypeptide enolase were barely detectable. This phenomenon probably results from the intense degradation of cellular polypeptides as observed after Coomassie Blue staining of the gels (not shown). This observation prompted us to analyze the apoptotic morphology of these cells using Annexin V–PI, as described in Materials and Methods. It is seen in Fig 6 that whereas the percentage of apoptotic AS2.5 cells was slightly increased compared with that of the control 16-HBE cells, exposure to H2O2 induced a drastic increase in the percentage of apoptotic AS2.5 cells on comparison with the baseline condition (AS2.5 cells before H2O2 exposure) (P < 0.02, Wilcoxon U-test). This increase (6.6-fold) was far more important than that observed in parental 16-HBE cells (2-fold) exposed to the same hydrogen peroxide treatment (P < 0.02, Wilcoxon U-test) (Fig 6). Similar results were obtained when another stable 16-HBE cell line (AS2.21) underexpressing Hsp27 was analyzed (not shown).
Fig 5.
Analysis of the level of Hsp27 expressed in the control and AS2.5 16-HBE cells. 16-HBE cells transfected with an empty vector are indicated as control cells. (A) Immunoblot analysis of alpha-enolase and Hsp27 cellular contents. Lane 1: 16-HBE control cells; lane 2: AS2.5 cells; lane 3: AS2.5 cells treated with H2O2 (100 μM for 1 hour and subsequently incubated for 18 hours without hydrogen peroxide). Note the sharp decline (about 80%) in the level of Hsp27 in AS2.5 cells and the disappearance of Hsp27 and enolase signals in H2O2-treated AS2.5 cells because of apoptosis-induced protein degradation. Immunocytochemical analysis of Hsp27 in AS2.5 cells (C) exposed or (B) not exposed to H2O2, as described earlier. Note the disappearance of the red staining after the hydrogen peroxide treatment. 16-HBE, 16–human bronchial epithelial
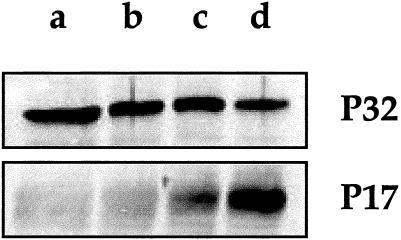
In order to further test the increased ability of AS2.5 cells to undergo cell death when they are exposed to hydrogen peroxide, we probed for the presence of the active form of procaspase-3 by immunoblot analysis. Figure 7 shows that in AS2.5 cells exposed to hydrogen peroxide, the procaspase-3 antibody detected the 17-kDa polypeptide of active caspase-3. Moreover, a significant decrease in the procaspase 38-kDa band was observed. In contrast, no significant cleavage of procaspase-3 was detected in control 16-HBE cells exposed to oxidative stress. This result favors the hypothesis that the down-regulation of Hsp27 sensitizes 16-HBE cells to hydrogen peroxide–mediated apoptosis, a phenomenon that results in a drastic proteolysis of the cellular proteins (Fig 5).
Fig 7.
Immunoblot analysis of caspase-3 activation. (a, b) Control or (c, d) AS2.5 cells were (b, d) exposed or (a, c) not exposed to 100 μM hydrogen peroxide during 1 hour, followed by 18 hours recovery. Cells were then harvested and total cellular proteins were applied to SDS-PAGE. Immunoblots were performed with the anti–caspase-3 antibody. The blots show that procaspase-3 is converted to its active form (17 kDa) in AS2.5 cells exposed to oxidative stress. A weak conversion is already detectable in nontreated AS2.5 cells. These phenomena were not detectable in control 16-HBE cells. 16-HBE, 16–human bronchial epithelial; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis
DISCUSSION
The bronchial epithelium is an important target of airway inflammation in asthma and is frequently damaged and structurally altered (Jeffery 1992). Epithelial damage appears to increase as the clinical severity of asthma worsens (Vignola et al 1998). Injury often causes the loss of the pseudostratified structure of the bronchial epithelium, leaving a residual layer of epithelial cells that have not undergone desquamation and remain attached to the basement membrane. It is likely that these cells constitute a sort of stem cells with the potential to activate the injury-repair cycle in response to inflammatory agents. In bronchial biopsy samples taken from the asthmatic subjects these cells have been shown to persistently express abnormally high levels of the epidermal growth factor receptor, suggesting that this may cause the epithelium to be “locked” into a repair phenotype (Puddicombe et al 2000). Although unexpected, the fact that the number of apoptotic cells is low and not statistically different in the epithelium of normal or asthmatic subjects suggests that in asthma residual epithelial cells, which have not undergone desquamation, do not show a greater propensy to undergo apoptosis, a feature which may teologically ensure their protection and their ability to develop an injury-repair cycle.
Unlike the bronchial epithelium in normal individuals, in the asthmatic epithelium, we show in this study a striking increase in the level of Hsp27, a polypeptide capable of enhancing the survival of mammalian cells exposed to a variety of injuries, particularly the oxidative ones. Contrasting with normal epithelium, Hsp27 immunostaining in asthmatic subjects was found to occur throughout the epithelial layer, which was indicative of widespread functional changes. In addition, we found that Hsp27 overexpression occurred in the fraction of the epithelium that was not damaged and characterized by a low or absent positivity for the TUNEL technique. This suggests a low trend toward apoptosis of these intact Hsp27-expressing cells. Interestingly, the small fraction of the bronchial epithelium from asthmatic patients that was damaged, as revealed by the presence of an altered morphology of the epithelium and desquamated columnar cells, did not show Hsp27 immunoreactivity but was positive for the TUNEL staining. This supports the hypothesis that Hsp27, probably in concert with Hsp70 (Vignola et al 1995), plays an important role in vivo in protecting bronchial epithelial cells of asthmatic patients against apoptosis. Our data are in perfect agreement with those of Hastie et al (1997) who reported that a mild inflammation to the allergen elevates Hsp27 levels, thereby potentially protecting the epithelial function from additional adverse conditions (eg, the H2S04 treatment).
To better understand the role played by Hsp27 overexpression, we used the human bronchial epithelial cell line 16-HBE as an in vitro model. These cells, similar to those of the epithelium of the asthmatic subjects, constitutively express very high levels of Hsp27 and are associated with a low rate of apoptosis, both spontaneously and after exposure to H2O2.
In spite of the fact that in some particular cells Hsp27 expression can sensitize to oxidative stress (Arata et al 1995; Trautinger et al 1997; Mairesse et al 1998), the vast majority of the studies have reported that the expression of this protein confers resistance to oxidative stress. It is probable that such a protection results from the chaperone activity of Hsp27 (Preville et al 1999; Rogalla et al 1999) and the ability of Hsp27 to decrease the intracellular level of ROS and uphold glutathione in its reduced form (Mehlen et al 1996a; Preville et al 1999; Paul and Arrigo 2000; Arrigo 2002). It is also interesting to note that the expression of Hsp27 in murine fibroblasts increases glucose 6-phosphate dehydrogenase activity and by doing so elevates the reducing power of the cell by generating a high level of the reduced form of nicotinamide adenine dinucleotide(H) molecules (Preville et al 1999). Hsp27 expression also protects numerous enzymes, such as those involved in ROS detoxification, against oxidative stress–mediated denaturation (Preville et al 1999). Whether the large oligomers of Hsp27 (Mehlen et al 1995b, 1997a; Preville et al 1998) bearing the chaperone activity of this protein (Rogalla et al 1999) are responsible in vivo of the degradation of the oxidized protein will need to be proven experimentally.
By decreasing the endogenous level of Hsp27 present in 16-HBE cells, we provide evidence demonstrating that the constitutive expression of this protein protects 16-HBE cells against caspase-3 activation and cell death induced by H2O2. This was assessed by analyzing the 16-HBE cell lines that were stably transfected with a vector containing the Hsp27-coding sequence cloned in the reverse orientation (AS2.5 cells). These 16-HBE transfected cells, which are characterized by an 80% reduction in Hsp27 level compared with the control or parental cells, show a weak ability to display spontaneous figures of apoptosis but were far more sensitive to hydrogen peroxide than were the control cells. This further supports the concept that the high level of Hsp27 expression by basal cells from asthmatic patients participate in an important protective and antiapoptotic effect and suggests an important role for Hsp27 in the injury-repair cycle of human bronchial epithelial cells in asthma. Future work will investigate whether Hsp27 acts alone or in concert with Hsp70 and the other survival proteins that are also up-regulated in bronchial epithelial cells from asthmatic patients.
Acknowledgments
This work was supported by the Association pour la Recherche sur le Cancer, (grant 5204) the Région Rhône-Alpes and the CNR.
REFERENCES
- Arata S, Hamaguchi S, Nose K. Effects of the overexpression of the small heat shock protein, Hsp27, on the sensitivity of human fibroblast cells exposed to oxidative stress. J Cell Physiol. 1995;163:458–465. doi: 10.1002/jcp.1041630305. [DOI] [PubMed] [Google Scholar]
- Aron Y, Busson M, Polla BS, Dusser D, Lockhart A, Swierczewski E, Favatier F. Analysis of hsp70 gene polymorphism in allergic asthma. Allergy. 1999;54:165–170. doi: 10.1034/j.1398-9995.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- Arrigo A-P. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Arrigo A-P. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol Biol. 2000;48:280–288. [PubMed] [Google Scholar]
- Arrigo A-P. Small stress proteins: novel regulators of intracellular redox state. IUBMB Life. 2002;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- Arrigo A-P, Landry J 1994 Expression and function of the low-molecular-weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 335–373. [Google Scholar]
- Arrigo A-P, Paul C, Ducasse C, Sauvageot O, and Kretz-Remy C 2002a Small stress proteins: modulation of intracellular redox state and protection against oxidative stress induced cytotoxicity. In: Small Stress Proteins. Progress in Molecular and Subcellular Biology, vol 28, ed Arrigo A-P, Muller WEG. Springer Verlag, Berlin, Germany, 171–184. [DOI] [PubMed] [Google Scholar]
- Arrigo A-P, Paul C, and Ducasse C. et al. 2002b Small stress proteins: novel regulators of apoptosis induced independently of reactive oxygen species. In: Small Stress Proteins. Progress in Molecular and Subcellular Biology, vol 28, ed Arrigo A-P, Muller WEG. Springer Verlag, Berlin, Germany, 185–204. [DOI] [PubMed] [Google Scholar]
- Arrigo A-P, Welch WJ. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987;262:15359–15369. [PubMed] [Google Scholar]
- Beere HM, Wolf BB, and Cain K. et al. 2000 Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. [DOI] [PubMed] [Google Scholar]
- Bertorelli G, Bocchino V, Zhuo X, Chetta A, Del Donno M, Foresi A, Testi R, Oliveri D. Heat shock protein 70 upregulation is related to HLA-DR expression in bronchial asthma. Effect of inhaled glucocorticoids. Clin Exp Allergy. 1998;28:551–560. doi: 10.1046/j.1365-2222.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Chanez P, and Lacoste JY. et al. 1990 Eosinophilic inflammation in asthma. N Engl J Med. 323:1033–1039. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. J Allergy Clin Immunol. 2000;105:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, and Bonniaud P. et al. 2000a Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2:645–652. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Paul C, Fromentin A, Hilpert S, Arrigo A-P, Solary E, Garrido C. Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene. 2000b;19:4855–4863. doi: 10.1038/sj.onc.1203850. [DOI] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerget M, Polla BS. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proc Natl Acad Sci U S A. 1990;87:1081–1085. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, and Stennicke HR. et al. 1998 IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Gaestel M, Buchner J. Analysis of chaperone properties of small Hsp's. Methods Mol Biol. 2000;99:421–429. doi: 10.1385/1-59259-054-3:421. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo A-P, Solary E. HSP27 inhibits cytochrome c–dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Garrido C, Ottavi P, Fromentin A, Hammann A, Arrigo A-P, Chauffert B, Mehlen P. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Res. 1997;57:2661–2667. [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallongo A, Feo S, Moore R, Croce CM, Showe LC. Molecular cloning and nucleotide sequence of a full-length cDNA for human alpha enolase. Proc Natl Acad Sci U S A. 1986;83:6741–6745. doi: 10.1073/pnas.83.18.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Strategy for Asthma Management and Prevention. 1995 WHO/NHLBI Workshop Report. National Institutes of Health, National Heart, Lung and Blood Institute, Publication Number 95-3659. [Google Scholar]
- Guay J, Lambert H, Gingras BG, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase–mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Hansen RK, Parra I, Lemieux P, Oesterreich S, Hilsenbeck SG, Fuqua SA. Hsp27 overexpression inhibits doxorubicin-induced apoptosis in human breast cancer cells. Breast Cancer Res Treat. 1999;56:187–196. doi: 10.1023/a:1006207009260. [DOI] [PubMed] [Google Scholar]
- Hastie AT, Everts KB, Zangrilli J, Shaver JR, Pollice MB, Fish JE, and Peters SP 1997 Hsp27 elevated in mild allergic inflammation protects airway epithelium from H2SO4 effects. Am J Physiol. 273 L. 401–L409. [DOI] [PubMed] [Google Scholar]
- Haws C, Krouse ME, Xia Y, Gruenert DC, and Wine JJ 1992 CFTR channels in immortalized human airway cells. Am J Physiol. 263 L. 692–L707. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation–mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Huot J, Roy G, Lambert H, Chretien P, Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991;51:5245–5252. [PubMed] [Google Scholar]
- Jakob U, Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jeffery PK. Histological features of the airways in asthma and COPD. Respiration. 1992;1:13–16. doi: 10.1159/000196096. [DOI] [PubMed] [Google Scholar]
- Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy WE, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome crelease and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- Mairesse N, Bernaert D, Del Bino G, Horman S, Mosselmans R, Robaye B, Galand P. Expression of HSP27 results in increased sensitivity to tumor necrosis factor, etoposide, and H2O2 in an oxidative stress–resistant cell line. J Cell Physiol. 1998;177:606–617. doi: 10.1002/(SICI)1097-4652(199812)177:4<606::AID-JCP11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Mairesse N, Horman S, Mosselmans R, Galand P. Antisense inhibition of the 27 kDa heat shock protein production affects growth rate and cytoskeletal organization in MCF-7 cells. Cell Biol Int. 1996;20:205–212. doi: 10.1006/cbir.1996.0025. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Briolay J, Smith L, Diaz lC, Fabre N, Pauli D, Arrigo A-P. Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type or deletion mutants of the Drosophila 27-kDa heat-shock protein. Eur J Biochem. 1993;215:277–284. doi: 10.1111/j.1432-1033.1993.tb18032.x. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Coronas V, Ljubic Thibal V, Ducasse C, Granger L, Jourdan F, Arrigo A-P. Small stress protein Hsp27 accumulation during dopamine-mediated differentiation of rat olfactory neurons counteracts apoptosis. Cell Death Differ. 1999;6:227–233. doi: 10.1038/sj.cdd.4400483. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Hickey E, Weber LA, Arrigo A-P. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFalpha in NIH-3T3-ras cells. Biochem Biophys Res Commun. 1997a;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Briolay J, Fostan P, Mirault ME, Arrigo A-P. Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (hsp27) structural organization and phosphorylation in basal and tumour necrosis factor alpha–treated T47D human carcinoma cells. Biochem J. 1995a;312:367–375. doi: 10.1042/bj3120367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Preville X, Arrigo A-P. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNF alpha–induced cell death. EMBO J. 1996a;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Godet J, Arrigo A-P. Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997b;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Guillet D, Preville X, Arrigo A-P. Tumor necrosis factor-alpha induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J Cell Biochem. 1995b;58:248–259. doi: 10.1002/jcb.240580213. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo A-P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress–induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995c;154:363–374. [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo A-P. Small stress proteins as novel regulators of apoptosis. Heat shock. J Biol Chem. 1996b;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier N, Arrigo A-P. sHSP and actin: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey PR, Farber A, and Nakazawa S. et al. 2000 Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 19:1975–1981. [DOI] [PubMed] [Google Scholar]
- Pandey P, Saleh A, and Nakazawa A. et al. 2000 Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 19:4310–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YM, Han MY, Blackburn RV, Lee YJ. Overexpression of HSP25 reduces the level of TNF alpha–induced oxidative DNA damage biomarker, 8-hydroxy-2′-deoxyguanosine, in L929 cells. J Cell Physiol. 1998;174:27–34. doi: 10.1002/(SICI)1097-4652(199801)174:1<27::AID-JCP4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Paul C, Arrigo A-P. Comparison of the protective activities generated by two survival proteins Bcl-2 and Hsp27 in L929 murine fibroblasts exposed to menadione or staurosporine. Exp Gerontol. 2000;35:757–766. doi: 10.1016/s0531-5565(00)00150-9. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo A-P. Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla BS, Kantengwa S, Francois D, Salvioli S, Franceschi C, Marsac C, Cossarizza A. Mitochondria are selective targets for the protective effects of heat shock against oxidative injury. Proc Natl Acad Sci U S A. 1996;93:6458–6463. doi: 10.1073/pnas.93.13.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preville X, Salvemini F, Giraud S, Chaufour S, Paul C, Stepien G, Ursini MV, Arrigo A-P. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp Cell Res. 1999;247:61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- Preville X, Schultz H, Knauf U, Gaestel M, Arrigo A-P. Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFα- and hydrogen peroxide–induced cell death. J Cell Biochem. 1998;69:436–452. [PubMed] [Google Scholar]
- Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, and Preville X. et al. 1999 Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 274:18947–18956. [DOI] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime SC, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautinger F, Kokesch C, Herbacek I, Knobler RM, Kindas-Mugge I. Overexpression of the small heat shock protein, hsp27, confers resistance to hyperthermia, but not to oxidative stress and UV-induced cell death, in a stably transfected squamous cell carcinoma cell line. J Photochem Photobiol. 1997;39:90–95. doi: 10.1016/s1011-1344(96)00010-3. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998;157:403–409. doi: 10.1164/ajrccm.157.2.96-08040. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, and Chiappara G. et al. 1999 Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 103:563–573. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, Polla BS, Vic P, Godard P, Bousquet J. Increased expression of heat shock protein 70 on airway cells in asthma and chronic bronchitis. Am J Respir Cell Mol Biol. 1995;13:683–691. doi: 10.1165/ajrcmb.13.6.7576706. [DOI] [PubMed] [Google Scholar]
- Wagstaff MJ, Collaco MY, Smith J, de BJ, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus–based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- Wang G, Klostergaard J, Khodadadian M, Wu J, Wu TW, Fung KP, Carper SW, Tomasovic SP. Murine cells transfected with human Hsp27 cDNA resist TNF-induced cytotoxicity. J Immunother Emphas Tumor Immunol. 1996;19:9–20. doi: 10.1097/00002371-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S, Roy S, and Rasper D. et al. 1999 Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 18:2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]