Abstract
A chimeric protein consisting of enhanced green fluorescent protein (EGFP) fused to the N-terminus of human Hsp27 conferred stress protection in human A549 lung carcinoma and murine L929 cells that were stably transfected to express the chimera constitutively. The resultant protection was comparable with that in the same cell lines when they were transfected to express corresponding levels of Hsp27. Unlike L929 cells, A549 cells exhibit endogenous Hsp27 expression, whose expression was inhibited in proportion to the amount of fluorescent chimera expressed, suggesting that the A549 cells recognized the latter as Hsp27. Upregulation of Hsp27 or chimeric Hsp27 in all transfected cell lines (stable or transient transfection) caused no measurable change in cellular glutathione levels, indicating that glutathione played no role in the stress protection associated with either protein. Chimeric Hsp27 had a monomeric molecular weight of 55 kDa (that of Hsp27 plus EGFP) in both cell types and formed a 16-mer complex twice as massive as that formed by Hsp27. Heat shock or sodium arsenite induced phosphorylation of both chimeric Hsp27 and Hsp27, which resulted in the disaggregation of Hsp27 multimers in both cell types and disaggregation of 20% of the chimeric multimers in L929 cells. But chimeric Hsp27 multimers did not disaggregate after stress in A549 cells. Epifluorescence and confocal microscopy demonstrated that chimeric Hsp27 was restricted to the cytoplasm under normal growth conditions and after heat shock in all cells. This study supports the conclusions that Hsp27 stress protection requires neither its translocation into the nucleus nor the dissociation of its multimeric complex. Furthermore, it demonstrates that fluorescent chimeras of heat shock proteins can be functional and used to observe the protein's distribution within living cells.
INTRODUCTION
Exposing cells or tissues to elevated temperatures induces an adaptive response known as heat shock response, which renders surviving cells resistant to the cytotoxicity of subsequent exposure to either heat shock (Lindquist 1986; Henle 1987; Morimoto et al 1990) or to many other stresses (Donaldson et al 1978; Dienel et al 1986; Lee and Hahn 1988; Chopp et al 1989; Donati et al 1990; Borrelli et al 1998). The term thermotolerance has been used to denote this induced protective state because it was first observed as heat shock–induced protection to hyperthermia (Gerner and Schneider 1975; Henle and Leeper 1976). But the term Tolerance will be used within this article to indicate the broader cross-protection that is evinced in this phenomenon.
Many studies have implicated heat shock proteins (Hsps) as the purveyors of Tolerance (Landry et al 1982; Li and Werb 1982; Subjeck et al 1982), with overexpression of Hsp27 (Landry et al 1989; Huot et al 1991), Hsp70 (Angelidis et al 1991; Li et al 1992), Hsp90 (Yahara et al 1986), or other Hsps (Laszlo and Li 1985) correlating directly with increased resistance to hyperthermia and other stresses. Many cellular stresses cause cellular proteins to denature and aggregate (Roti Roti et al 1979; Lee and Hahn 1988; Roti Roti and Laszlo 1988; Floyd 1990; Kampinga 1993; Lepock et al 1993; Caraceni et al 1997; Senisterra et al 1997; Borrelli et al 1998), and it has been proposed that the Hsps confer stress protection by functioning as molecular chaperones to disaggregate or refold these proteins (or both). Recent studies have indicated that the major Hsps fall into 2 chaperone categories: Hsps that bind to denatured or aggregated proteins and stabilize them in a refolding competent state and Hsps that unfold and then refold proteins in the aggregates (Jakob et al 1993; Carver et al 1995; Freeman and Morimoto 1996; Ehrnsperger et al 1997; Lee et al 1997).
Hsp27 forms a multimeric complex (Arrigo and Welch 1987) within cells, and its primary chaperone function, like other small Hsps, appears to be the stabilization of denatured or aggregated proteins in a refolding competent state (Ehrnsperger et al 1997; Lee et al 1997). Although this function of Hsp27 has been demonstrated consistently in cell-free systems, it has not yet been shown to occur within cells or tissues. A significant portion of the cellular Hsp27 becomes phosphorylated after exposure to heat shock and other stresses (Chretien and Landry 1988; Crete and Landry 1990), which can result in substantial dissociation of the multimeric complexes (Lavoie et al 1995; Chaufour et al 1996; Lambert et al 1999). But there is still considerable controversy concerning whether or not Hsp27 phosphorylation or dissociation (or both) of the multimeric complexes influence its chaperone function or its ability to confer stress protection (Knauf et al 1994; Lavoie et al 1995; Mehlen et al 1997).
Under normal conditions Hsp27 is cytoplasmic, but there are reports of it translocating into the nucleus after heat shock and other stresses (Arrigo et al 1988). Kampinga et al (1994) demonstrated that cells overexpressing Hsp27 exhibited faster disaggregation of nuclear proteins rendered insoluble (Borrelli et al 1996a) by heat shock. These observations suggest that stress-induced Hsp27 translocation into the nucleus is required for the more rapid disaggregation of nuclear proteins that were denatured and aggregated by hyperthermia. Because the temporal integral of the heat-insolubilized nuclear protein is proportional to heat killing (Kampinga et al 1989), it is plausible that facilitated recovery of heat denatured or aggregated nuclear proteins is 1 mechanism by which Hsp27 confers stress protection. An ensuing corollary is that stress-induced phosphorylation of Hsp27 is required to produce smaller complexes of Hsp27 that can translocate to the nucleus to interact with the denatured or aggregated nuclear proteins.
A chimeric protein consisting of enhanced green fluorescent protein (EGFP) fused to the N-terminus of human Hsp27 was constructed to facilitate investigations into the functions of Hsp27 in mammalian cells. This chimera proved equivalent to Hsp27 in conferring protection against hyperthermic killing and permitted the visualization of Hsp27 distribution within living cells. The latter property should prove useful in determining the compartmentalization of Hsp27 and its interactions with other cellular molecules, especially if fluorescence resonance energy transfer (FRET) is used to study and quantify interactions with other fluorescent molecules.
Forming the chimera affected Hsp27 phosphorylation, inhibited stress-induced dissociation of multimeric complexes, and prevented Hsp27 from translocating into the nucleus after stress. These properties of the chimera permitted experiments that provided definitive evidence that dissociation of the Hsp27 multimer and its subsequent translocation into the nucleus are not a requisite for the stress protection conferred by upregulated Hsp27 expression.
MATERIALS AND METHODS
Cell culture
A549 human lung carcinoma cells and L929 murine fibroblasts were adapted to growth in 10% iron-supplemented calf serum (Hyclone, Logan, UT, USA) in Dulbecco modified Eagle medium-F12. The medium for the A549 cells was also supplemented with minimum essential medium (MEM) nonessential amino acids (Invitrogen, Carlsbad, CA, USA), MEM vitamin solution (0.5× recommended concentration; Life Technologies Inc), and 1 mM l-glutamine. The adaptation process required 2 weeks for the A549 cells, whereas it was immediate for the L929 cells.
Both cell lines were grown as monolayers at 37.0°C in humidified incubators with 5% CO2 to maintain medium pH at 7.4. For experimentation, cells were seeded into 25-cm2, 75-cm2, or 150-cm2 culture flasks to obtain cultures with 80–85% confluency 48 hours later.
Chimera vector construction
The promoter was removed from the hsp27 gene, and the remainder of the genomic DNA was excised from the carrier plasmid (Bluescript KS+, Stratagene Inc, La Jolla, CA, USA) using AatII and HindIII. Both ends of the DNA fragment were then blunted using T4 DNA polymerase because the DNA was to be cloned into several different vectors, and the double-blunted fragment represented the most efficient means of accomplishing the desired clonings. This double-blunted DNA fragment was gel-isolated and then cloned downstream of the egfp gene (egfp: stop codon removed) into the SmaI site of each of the following EGFP fusion vectors: pEGFP-C1, pEGFP-C2, and pEGFP-C3 (Clontech Laboratories Inc, Palo Alto, CA, USA). The resultant recombinants were first screened for the proper orientation of the Hsp27 coding region and then their ability to produce the desired chimera within A549 cells (transient transfection with 30 μg of the plasmid, using the calcium phosphate technique). The hsp27 cloned into the pEGFP-C3 produced the in-frame chimera, which was confirmed using Western blots to ensure that the chimera had the expected molecular weight (55 kDa) and cross-reacted with antibodies against both Hsp27 and EGFP.
Establishing permanently transfected cell lines
Thirty micrograms of the pEGFP-C3 plasmid containing the Hsp27 chimera were transfected into A549 and L929 cells using the calcium phosphate technique. The cells were then subcultured at a 1:4 dilution 24 hours after the transfection. Addition of the selection drug G-418 (400 μg/mL for A549 cells and 200 μg/mL for L929 cells) was delayed for 1 hour until the cells had first attached to the substrate. Forty-eight hours later the cells were seeded out at lower densities to permit clonal selection. All selected clones required 1–2 rounds of subcloning to attain a cell line that maintained a constant level of chimera expression.
Electrophoresis analyses
Western blot analyses were accomplished by running lysates of the permanently transfected cells on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Borrelli et al 1996b), electroblotting the proteins onto nitrocellulose paper, and then probing the blots with a primary antibody to either EGFP (Clontech) or human Hsp27 (Stressgen, Victoria, BC, Canada). The labeled proteins were recorded on film using chemiluminescent detection (ECL system; Biosciences, Uppsala, Sweden).
Two-dimensional (2D) gel analyses were performed by first running cell lysates onto isoelectric-focusing tube gels containing 1.6%, pH 5–7, ampholine and 0.4%, pH 3.5–10, ampholine. The extracted tube gel was then used as the sample for running the second dimension on a 12% SDS-PAGE square plate gel. Two first- and second-dimension gels were made for each sample. One of the resultant second-dimension gels was subjected to silver staining, whereas the other was electroblotted onto nitrocellulose paper. The resultant blots were treated for immunolabeling with primary antibody to either EGFP or human Hsp27, and the labeled proteins were recorded on film using chemiluminescent detection.
Glutathione measurements
Cell monolayers were washed once with ice cold phosphate buffered saline (PBS), scraped into cold PBS, and centrifuged at 4°C for 5 minutes at 400 × g to obtain cell pellets which were then frozen at −80°C. Pellets were then thawed and homogenized in 50 mM potassium phosphate buffer (pH 7.8) containing 1.34 mM diethylenetriaminepenta-acetic acid. Protein assay and thiol analyses were performed immediately.
Total glutathione content was determined by the method of Anderson (1985). Reduced and oxidized glutathione were distinguished by the addition of 2 mL 2-vinyl pyridine mixed 1:1 (v/v) with ethanol per 50 mL of sample followed by incubation for 1 hour and assay as previously described by Griffith (1980). All biochemical determinations were normalized to the protein content of whole homogenates using the method of Lowry et al (1951).
Oligomer size determination by glycerol gradient centrifugation
Hsp27 and chimeric Hsp27 oligomer size were determined using glycerol gradients according to the procedure described previously by Lambert et al (1999). Briefly, 0.5 mL of cell lysate was loaded atop a 12.6-mL linear gradient of glycerol (10–40%) made up in 25 mM N-2-hydroxythylpiperazine-N′-2-ethane-sulfonic acid buffer (pH 7.4) containing 1 mM ethylenediamine-tetraacetic acid and 1 mM dithiothreitol. The gradients were centrifuged for 18 hours at 30 000 rpm in an SW40 rotor (Beckman, Fullerton, CA, USA) at 4.0°C. Each gradient was fractionated into 44 fractions. Each fraction was then diluted with Tris-glycine-SDS buffer (25 mM Tris, 192 mM glycine, 0.01% SDS), and then a sample was dot-blotted onto nitrocellulose membrane. The blotted nitrocellulose was treated with a primary antibody raised against Hsp27, and the antibody-Hsp27 complex was detected using a secondary antibody (goat anti-rabbit IgG) labeled with 125I. Detection and quantitation of the dots on the membrane were performed using a Storm imaging system (Molecular Dynamics Inc, Sunnyvale, CA, USA). Varying amounts of the supernatant and other standards were used to confirm that the measured dots fell within the linear dynamic range of the detection system.
RESULTS
Characterization of the chimeric Hsp27 protein
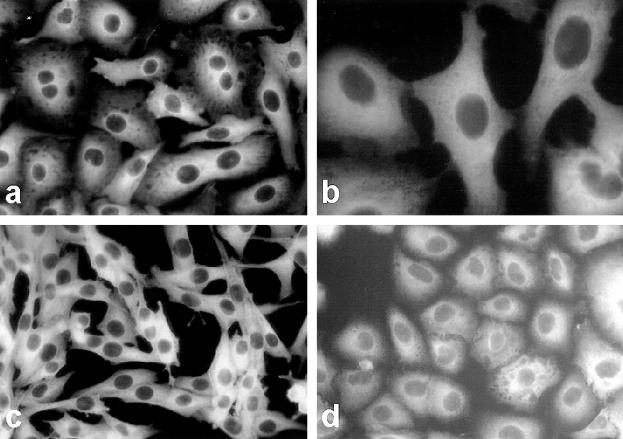
The distribution of chimeric Hsp27 in stably transfected A549 and L929 cells is presented in Figure 1 a–c. These micrographs are of live cells that were grown and maintained at 37.0°C. In both cell lines chimeric Hsp27 was restricted to the cytoplasm, and the higher magnification micrograph of A549 cells (Fig 1b) is provided to better illustrate this point. The distribution of chimeric Hsp27 in A549 and L929 cells was very similar to that of endogenous Hsp27 in A549 cells (Fig 1d). The slight, background fluorescence seen in the nuclei of Figure 1d was attributed to the nonspecific staining by the fluorescent secondary antibody (data not shown). A similar comparison could not be made for L929 cells because they lack endogenous Hsp27 and α-b crystalline (Lee et al 1992; Blackburn et al 1996).
Fig 1.
Cellular distribution of chimeric Hsp27 and Hsp27. (a) Distribution of chimeric Hsp27 in live A549 cells. (b) Higher magnification of live A549 cells to better illustrate the cytoplasmic distribution of chimeric Hsp27 and emphasize its absence within the nuclei. (c) Distribution of chimeric Hsp27 in live L929 cells. (d) Distribution of endogenous Hsp27 in fixed A549 cells, as visualized by indirect immunofluorescence. Chimeric Hsp27 was observed in live cells by means of its native fluorescence, which was present in the cytoplasm but not in the nucleus of both A549 and L929 cells. Indirect immunofluorescent labeling showed that the endogenous Hsp27 in A549 cells was also restricted to the cytoplasm. The slight fluorescence seen in the nuclei of (d) was caused by nonspecific staining by the fluorescent secondary antibody (data not shown).
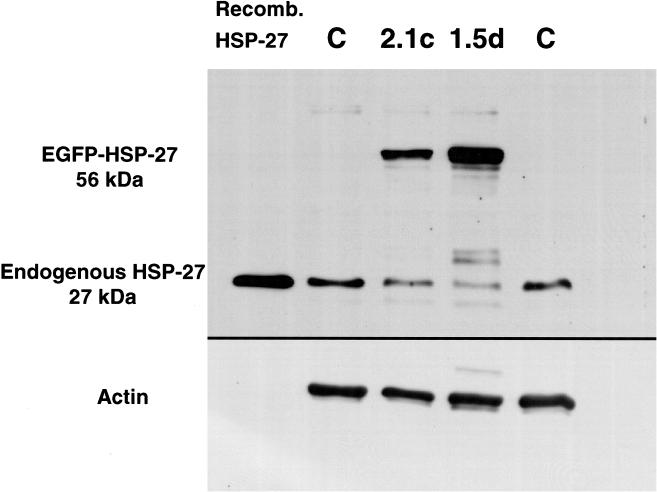
Western blot analyses demonstrated that the chimera had a monomeric molecular weight of approximately 55 kDa in A549 cells, which reflects the combined molecular weights of EGFP (28 kDa) and Hsp27 (27 kDa) (Fig 2). The molecular weight of chimeric Hsp27 was also 55 kDa in all transfected L929 clones (data not shown).
Fig 2.
Western blot of chimeric and endogenous Hsp27 in A549 Cells. Western blot analyses of chimeric Hsp27 expression in 2 different, stably transfected A549 clones (2.1c and 1.5d). Recombinant Hsp27 and endogenous Hsp27 in control, untransfected A549 cells (C) were included for comparison. As the level of chimeric Hsp27 expression increased, that of endogenous Hsp27 decreased proportionately. The blot was counterprobed for actin to demonstrate equal protein loading per lane
Constitutive expression of the chimeric Hsp27 increased the total Hsp27 content of A549 (chimeric Hsp27 plus endogenous Hsp27) by more than 2-fold in clone 2.1c and by more than 4-fold in clone 1.5d. But expressing chimeric Hsp27 proportionately reduced endogenous Hsp27 expression in A549 cells (Fig 2). This suggests that A549 cells recognized the chimera as Hsp27 and downregulated the expression of endogenous Hsp27 by the Hsp27 promoter accordingly, ostensibly caused by feedback regulation of Hsp27. A similar reduction in endogenous Hsp27 expression was observed in HeLa cells that were stably transfected to express chimeric Hsp27 (data not shown) demonstrating that this phenomenon was not unique to A549 cells and suggesting that it might be expected in all cell lines that express Hsp27.
Overexpression of either Hsp27 or chimeric Hsp27 had no significant effect upon the steady-state levels of Hsp110, Hsp90, Hsp70, Hsc70, or Hsp40 in A549 or L929 cells, or in α-b crystalline levels in A549 cells (data not shown), as determined from Western blots that were quantified using a scanning laser densitometer (Borrelli et al 1996b).
Stress protection by chimeric Hsp27
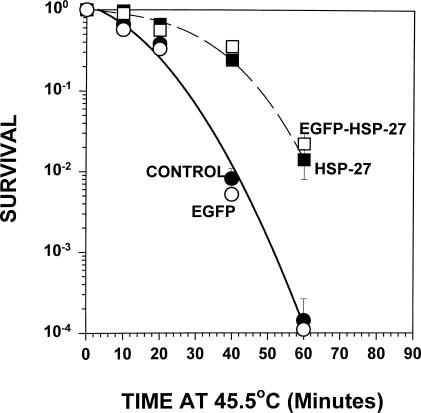
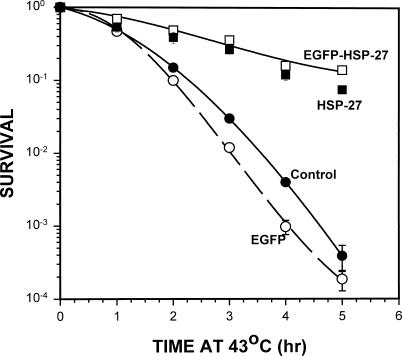
All stably transfected A549 and L929 that expressed chimeric Hsp27 exhibited marked protection against hyperthermic cell killing, as illustrated by the data presented in Figures 3 and 4, respectively. In both cell lines chimeric Hsp27 provided protection equivalent to that observed in cells expressing comparable levels of Hsp27, whereas expressing similar levels of EGFP had no effect on cellular heat sensitivity (Figs 3 and 4). Adenovirus expression vectors were used to introduce EGFP and Hsp27 expression cassettes into A549 cells. But EGFP and Hsp27 expression in L929 cells was attained by stable transfection of the cells with the corresponding recombinant DNA because, in our hands, L929 cells could not be infected with the adenovirus.
Fig 3.
Effects of overexpressing chimeric or endogenous Hsp27 on the heat shock survival of A549 cells. A549 cells constitutively expressing chimeric Hsp27 (clone 1.5d: enhanced green fluorescent protein-Hsp27) were markedly more resistant to heat killing at 45.5°C than were control cells. The level of heat protection was essentially identical to that of cells infected with adenovirus vectors to express an equivalent level of Hsp27. Adenovirus infection to express an equivalent level of EGFP had no effect upon the cells' heat sensitivity
Fig 4.
Effects of overexpressing chimeric or endogenous Hsp27 on the heat shock survival of L929 cells. L929 cells constitutively expressing chimeric Hsp27 (enhanced green fluorescent protein-Hsp27) were markedly more resistant to heat killing at 43.0°C than were control cells. The level of heat protection was essentially identical to that of cells expressing an equivalent level of Hsp27, whereas expressing an equivalent level of EGFP had no effect upon the cells' heat sensitivity
Cellular distribution of Hsp27 and chimeric Hsp27 after heat shock
Epifluorescent and confocal microscopy were used to observe the distribution of Hsp27 and chimeric Hsp27 in control and heat-shocked cells. Confocal microscopy was used to unequivocally determine whether either protein actually entered the nucleus after stress or merely collapsed tightly onto the nucleus. The latter could be mistaken for nuclear translocation when viewed with conventional, epifluorescence microscopy. All confocal images show slices taken approximately midway through at least 1 of the pictured cells. Traditional, indirect immunohistochemistry was used to visualize Hsp27 in A549 and L929 cells through the fluorescent chromophore attached to the secondary antibody, whereas chimeric Hsp27 was visualized through its native EGFP fluorescence.
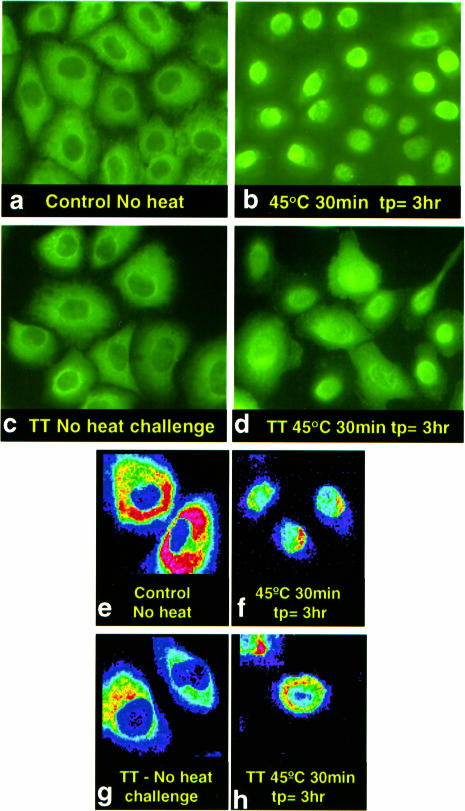
Figure 5 a,e shows the distribution of endogenous Hsp27 in control A549 as observed with, respectively, epifluorescence and confocal microscopy. As shown in Figure 1c the Hsp27 was restricted to the cytoplasm. The low fluorescence seen within the nuclei of Figure 5e was caused by nonspecific staining with the fluorescent secondary antibody, ie, background (data not shown). More than 80% of the Hsp27 was translocated into the nucleus immediately after a 30-minute heat shock at 45.0°C, with maximal nuclear translocation observed 3 hours later (Fig 5 b,f). Longer heat shocks produced no additional nuclear translocation of Hsp27; however, the return of Hsp27 to the cytoplasm was proportionately delayed.
Fig 5.
Epifluorescent and confocal images of endogenous Hsp27 in heat-shocked A549 cells. A549 cells were subjected to indirect immunofluorescent labeling for Hsp27 after different heat treatments at 45.0°C, as indicated. The cells were then observed by epifluorescence (a–d) or confocal (e–h) microscopy. Some nuclear translocation of Hsp27 was observed immediately after heat shock but was maximal 3 hours post heating (tp = 3 hours) when all Hsp27 was either nuclear or perinuclear (b and f). Nuclear Hsp27 translocation was not observed 3 hours after a Tolerance-inducing heat shock (TT: 43.0°C for 30 minutes, tp = 3 hours) (g). Tolerant cells exhibited less Hsp27 nuclear translocation after a challenge heat shock (TT: h), with most cells showing more perinuclear or even cytoplasmic Hsp27
No Hsp27 nuclear translocation was observed immediately or 3 hours after a mild, tolerance-inducing heat shock of 30 minutes at 43.0°C (Fig 5 c,g). A subsequent, challenge heat shock of 45.0°C still induced Hsp27 nuclear translocation, but approximately 50% of the Hsp27 remained cytoplasmic, albeit concentrated perinuclearly (Fig 5 d,h).
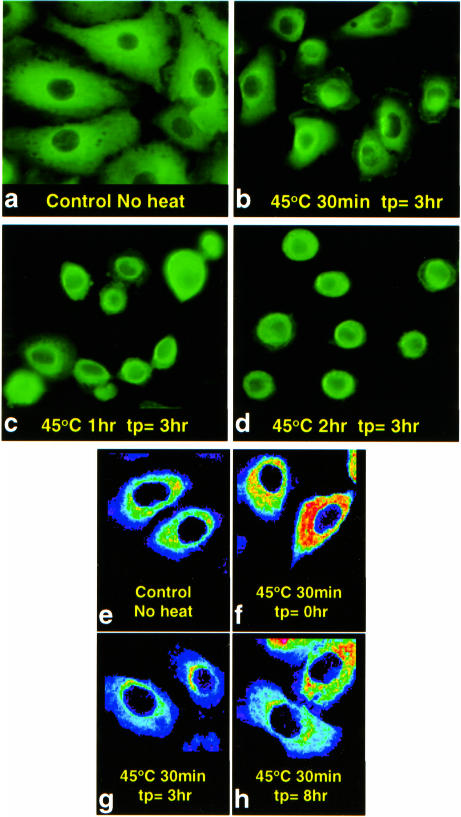
Chimeric Hsp27 was confined to the cytoplasm in control A549 cells (Fig 6 a,e) and remained cytoplasmic after all hyperthermic treatments up to and including 3 hours at 45.0°C (Fig 6 b–d, f–h). When the cells became rounded in response to more severe heat shocks (1–3 hours at 45.0°C) chimeric Hsp27 remained cytoplasmic even though it was concentrated tightly about the cell nuclei (Fig 6 c,d). Heat-shocked cells were observed every 0.5 hour for 8 hours after heat shock (Fig 6 f–h) to determine if any chimeric Hsp27 nuclear translocation was merely delayed relative to that of Hsp27. But chimeric Hsp27 nuclear translocation was never observed within 8 hours after heat shocks of 0.5–3 hours at 45.0°C.
Fig 6.
Epifluorescent and confocal images of chimeric Hsp27 in heat-shocked A549 cells. A549 cells were subjected to different heat treatments at 45.0°C, as indicated. The cells were then observed by epifluorescence (a–d) or confocal (e–h) microscopy as a function of time after heat shock (tp). No nuclear translocation of chimeric Hsp27 was observed within an 8-hour time span after heat shocks up to 3 hours at 45.0°C (tp: time post heat)
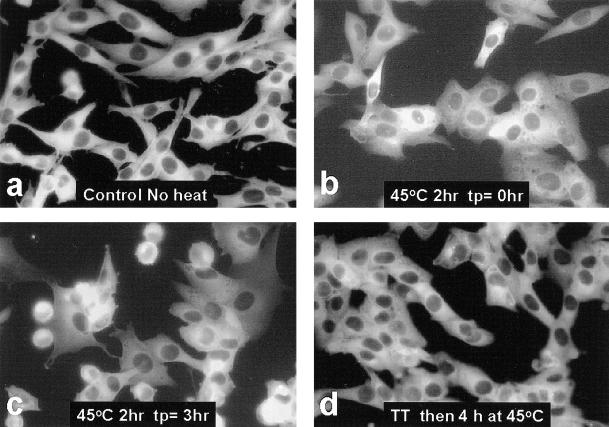
Fluorescent micrographs of L929 cells expressing chimeric Hsp27 are presented in Figure 7. As with A549 cells chimeric Hsp27 was restricted to the cytoplasm in both control (Fig 7a) and heat-shocked (Fig 7 b–d) L929 cells. Immediately after heat shock of 2 hours at 45.0°C some of the chimeric Hsp27 migrated to a more perinuclear location, whereas the remainder took on a mottled appearance within the cytoplasm (Fig 7b). The mottled distribution disappeared within 3 hours after the cells were returned to 37.0°C; although some cells rounded up during this 3 hour period, no nuclear-translocated chimeric Hsp27 was observed (Fig 7c). When cells were made Tolerant (10 minutes at 43.0°C followed by 3 hours at 37.0°C) before a challenge heat shock of 4 hours at 45.0°C the distribution of chimeric Hsp27 remained indistinguishable from that in nonheated cells, despite the harsher challenge heat shock (Fig 7d).
Fig 7.
Epifluorescent images of chimeric Hsp27 in heat-shocked L929 cells. L929 cells were subjected to different heat treatments at 45.0°C, as indicated. The cells were then observed by epifluorescence microscopy as a function of time after heat shock. No nuclear translocation of chimeric Hsp27 was observed within an 8-hour time span after heat shocks up to 3 hours at 45.0°C (tp: time post heat), and this remained true for Tolerant cells (TT: 43.0°C for 30 minutes, tp = 3 hours followed by challenge heat shock) (d)
Phosphorylation of chimeric and wild-type Hsp27 in mammalian cells
It is plausible that the inability of chimeric Hsp27 to translocate into the nucleus after heat shock occurred because forming the chimera altered the phosphorylation or oligomerization properties (or both) of Hsp27. Consequently, the phosphorylation and oligomerization of chimeric Hsp27 in A549 and L929 cells were investigated and compared with those of Hsp27 expressed in these same cells.
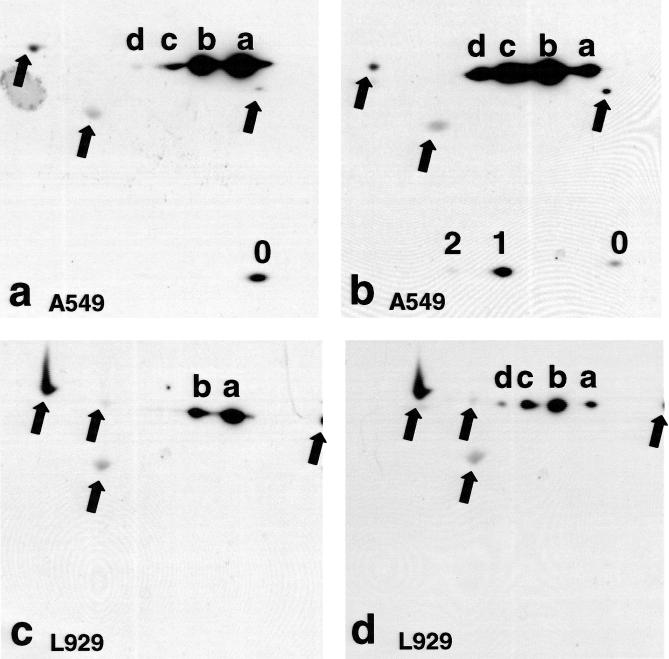
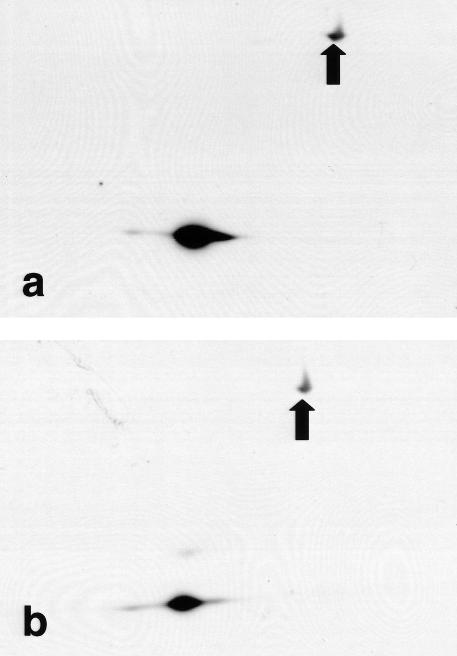
Phosphorylation of Hsp27 and chimeric Hsp27 was examined using 2D Western blots (isoelectric focusing then molecular weight separation: Lee et al 1992; Knauf et al 1994) using a monoclonal antibody to Hsp27 to visualize the proteins. Figure 8 a,b shows proteins from A549 cells expressing chimeric Hsp27. But the clone used for these data had sufficient, residual, endogenous Hsp27 for it to also be visible on the 2D chemilumigraphs, and it served as a reference for analyzing the chimeric Hsp27. To visualize all the endogenous Hsp27 isoforms the scanner settings had to be set near their upper limits. This produced a visible interference pattern that did not obscure any relevant data.
Fig 8.
Two-dimensional Western blot analysis of chimeric Hsp27 in A549 and L929 cells. Chimeric Hsp27 was visualized using a monoclonal antibody to Hsp27. The transfected A549 cells (clone 1.5d) had sufficient, residual, endogenous Hsp27 to be observed too. Only chimeric Hsp27 was present in transfected L929 cells. The arrows indicate proteins that were also visualized after the immonostaining, even in nontransfected cells. (a) Four different isoforms of chimeric Hsp27 were present (a–d) in control A549 cells, but only the nonphosphorylated isoform of Hsp27 (0) was observed. (b) Immediately after heat shock (43.0°C, 2 hours) to A549 cells the mono- and bisphosphorylated isoforms of Hsp27 were observed, and the b, c, and d isoforms of chimeric Hsp27 became more prominent, whereas the a isoform was diminished. (c) Only the a and b isoforms of chimeric Hsp27 were observed in L929 cells. (d) Immediately after heat shock (43.0°C, 2 hours) 4 isoforms (a–d) of chimeric Hsp27 were observed in L929, with the b isoform being dominant
Only the nonphosphorylated isoform of the endogenous Hsp27 (0) was present in control A549 cells (Fig 8a), whereas its mono- (1) and bisphosphorylated (2) isoforms were observed in heat-shocked cells (Fig 8b), as reported for other cell lines (Lee et al 1992; Knauf et al 1994). Immediately after heat shock the monophosphorylated isoform was dominant followed by the nonphosphorylated and then the bisphosphorylated isoforms. The latter was barely detectable, even after protracted exposures for the chemilumigrahs.
Four isoforms of chimeric Hsp27, labeled a–d (Fig 8a), were observed in nonheated (control) A549 cells. The a isoform was dominant in the control, nonheated cells, and the remaining isoforms decreased in prominence with increasingly positive isoelectric points. Immediately after a heat shock the amount of the a chimeric Hsp27 isoform was markedly reduced, whereas that of the b, c, and d isoforms was increased (Fig 8b). The b isoform was now dominant followed closely by the c isoform and then nearly equivalent amounts of the a and d isoforms (Fig 8b). With reference to the endogenous Hsp27 the a and c isoforms of chimeric Hsp27 had isoelectric points similar to, respectively, the nonphosphorylated and monophosphorylated forms of Hsp27, those of the b and d isoforms fell midway between, respectively, the nonphosphorylated and monophosphorylated, and the monophosphorylated and bisphosphorylated isoforms of Hsp27.
Only the a and b isoforms of chimeric Hsp27 were visible in control L929 cells (Fig 8c), but 4 isoforms were present immediately after heat shock (Fig 8d). The relative amounts of these 4 isoforms were similar to those observed in heated A549 cells. Only the nonphosphorylated isoform was present in control L929 cells transfected to express Hsp27, with the mono- and bisphosphorylated isoforms appearing after heat shock, as reported previously (Lee et al 1992) (data not shown).
The arrows in Figure 8 indicate other proteins that produced visible spots after staining with the primary and secondary antibodies. These spots were present in cells that did not express chimeric Hsp27 or recombinant Hsp27 (data not shown) and served as additional reference points on the 2D Westerns.
The greater number of chimeric Hsp27 isoforms, their shifted positions in 2D gels, and the fact that chimeric Hsp27 was phosphorylated in nonstressed cells were clearly caused by the presence of EGFP in the chimera. But these changes were not necessarily the result of the EGFP moiety itself being phosphorylated, a point supported by the fact that EGFP expressed in stably transfected L929 cells was not phosphorylated after heat shock (Fig 9 a,b).
Fig 9.
Two-dimensional Western blot analysis of EGFP expressed in L929 cells. EGFP constitutively expressed in stably transfected L929 cells exhibited a single isoform in nonheated cells (a) and immediately after heat shock (b: 1 hour, 43.0°C). A polyclonal antibody to EGFP was used to detect the protein, and the arrows indicate another protein that was also detected by this antibody
The exact nature of chimeric Hsp27 phosphorylation was not determined; also, it was not necessary for the purposes of this study. These data were obtained solely to determine whether chimeric Hsp27 phosphorylated in response to stress and whether such phosphorylation corresponded to any stress-induced changes in oligomeric size.
Oligomeric status of chimeric and wild-type Hsp27 in mammalian cells
The size of the Hsp27 and chimeric Hsp27 oligomers was determined by running cell lysates on linear glycerol gradients (Lambert et al 1999). Gradient fractions were subjected to slot blot analyses to quantitate Hsp27 content (Lambert et al 1999), whereas chimeric Hsp27 content was analyzed using a spectrofluorimeter.
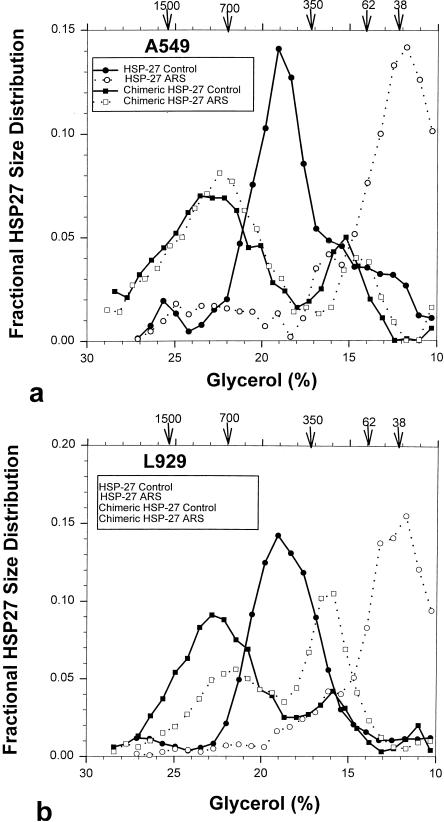
In control A549 and L929 cells endogenous and recombinant human Hsp27, respectively, was distributed amongst the gradient fractions containing between 13% and 25% glycerol (Fig 10 a,b). The distribution curves in both cell lines were well defined, and the peak protein amount occurred in the fraction containing 19% glycerol, corresponding to a molecular weight of approximately 450 kDa (16-mer complex). After treatment with 200 μM sodium arsenite (2 hours at 37.0°C) essentially all the Hsp27 complexes in both cell lines disaggregated into either dimers or tetramers, as reported by Lambert et al (1999).
Fig 10.
Glycerol gradient analyses of oligomer size for chimeric Hsp27 and Hsp27 in A549 (a) and L929 (b) cells. The data points show the fraction of the total cellular Hsp27 or chimeric Hsp27 that was found in each gradient fraction as a function of the latter's glycerol concentration. The arrows along the abscissa indicate the localization of standard proteins within the gradient (molecular weight indicated in kilodaltons). In both cell lines aresenite treatment resulted in nearly complete dissociation of the 16-mer Hsp27 oligomers into dimers and tetramers. In contrast, arsenite caused no detectable dissociation of chimeric Hsp27 in A549 (Fig 10a) cells and dissociation of approximately 50% of the large oligomers in L929 cells (Fig 10b).
The size distribution of chimeric Hsp27 in control A549 and L929 cells was broader than that of Hsp27, and its peak occurred at 23.5% glycerol, which corresponded to a molecular weight of approximately 900 kDa (Fig 10 a,b). Thus, the chimeric Hsp27 maintained the same multimeric size as Hsp27 in these cells. A smaller peak centered about 350 kDa (6-mer) was observed consistently in A549 cells. Arsenite treatment had no significant effect on the size distribution of chimeric Hsp27 in A549 cells (Fig 10a). The same arsenite treatment caused approximately 50% of the chimeric Hsp27 in L929 cells to disaggregate into smaller complexes with an average size of 350 kDa (6-mer), whereas the remaining chimeric Hsp27 maintained itself as 16-mer complexes (Fig 10b).
The multimer of human Hsp27 in both A549 and L929 cells was smaller than the 600- to 700-kDa reported by Lambert et al (1999) for the hamster Hsp27 in CCL39 and 3T3 cells. When hamster Hsp27 was expressed in A549 and L929 cells its size distribution was essentially identical to that observed for human Hsp27, with its peak at approximately 500 kDa (data not shown). Thus, the difference between the Hsp27 multimeric size reported in this study and by Lambert et al (1999) appears to be attributable to cell line variations.
Total cellular glutathione measurements
Other studies have suggested that overexpressing Hsp27 increases total intracellular glutathione, which in turn contributes to cellular stress protection (Mehlen et al 1995, 1997). This represents a potential stress protection for cells overexpressing Hsp27 or chimeric Hsp27 that is independent of Hsp27 chaperone activity. Consequently, glutathione levels were measured in the stably transfected L929 cells that expressed Hsp27 or chimeric Hsp27, which were used throughout this study. Glutathione measurements were also made in L929 cells stably transfected to express Hsp27 under control of the inducible LacSwitch promoter (Stratagene Inc) to determine whether any glutathione increase was short lived after a transient Hsp27 upregulation. No glutathione measurements were made in wild-type or transfected A549 cells because these cells have a naturally high glutathione content (>500 nM/mg protein) that could potentially mask smaller, Hsp27-induced increases in cellular glutathione.
Results of the glutathione measurements are presented in Table 1. Expressing Hsp27 or chimeric Hsp27 (constitutively or transiently) consistently produced a slight reduction in the glutathione content in L929 cells. A nearly 2-fold increase in glutathione was observed in L929 cells stably transfected to express the Lac repressor protein. But this was not associated with any increase in stress protection or Hsp27 (data not shown), and no further increase in glutathione was observed after transfection with recombinant Hsp27, even when the LacSwitch promoter was activated with isopropyl-β-d-thiogalactoside to express Hsp27.
Table 1.
Cellular glutathione measurements
DISCUSSION
No difficulties or problems were encountered while establishing stable transfectants of A549 or L929 cells that constitutively expressed a fluorescent chimera containing EGFP fused directly to the N-terminus of Hsp27. Conversely, establishing a stable transfectant of L929 cells that constitutively expressed EGFP was extremely difficult and time consuming, whereas all attempts to do so in A549 cells failed. The difficulty in establishing transfectants that constitutively expressed EGFP may have been related to the toxicity of protracted EGFP expression that has been reported by others (Liu et al 1999). One might speculate that the ease of producing and maintaining the transfectants that expressed EGFP-Hsp27 was related to the fact that EGFP was being expressed as a chimera with a chaperone protein and that the properties of the latter somehow muted or obviated any EGFP toxicity. Regardless, the chimeric Hsp27 provided a means for observing the distribution of Hsp27 within living cells using fluorescence microscopy and permitted several specific conclusions concerning Hsp27 stress protection. Furthermore, the fluorescent chimera provides a means for investigating chaperone function in living cells using quantitative fluorescent measurements, as discussed subsequently.
Forming the chimera did not affect the multimeric state of Hsp27 or its ability to provide stress protection. Hence, it can be presumed that neither of these 2 properties of the Hsp27 multimer requires specific interactions between free, Hsp27 N-termini. It is also clear that the multimeric complex did not have to be homogeneously Hsp27 to provide stress protection. Half of the chimeric Hsp27 multimer consisted of the equimassive EGFP (a protein that by itself provided no stress protection); yet, chimeric Hsp27 proved equally effective as did Hsp27 for stress protection.
The organization and orientation of chimeric Hsp27 within the multimer was not determined; however, it is extremely unlikely that the oligomer formed with every Hsp27 moiety oriented into the complex's central core, with the EGFP moieties projecting outward. In that case the EGFP moieties would have served as a restrictive barrier preventing access of the Hsp27 moieties to any stress-damaged proteins or intracellular structures. This would have ostensibly obviated any Hsp27 stress protection, which did not occur. The converse, ie, a multimer with an EGFP core and all Hsp27 moieties oriented outward, is possible and could conceptually function identically to an Hsp27 homomultimer because all the Hsp27 moieties would be available for stress protection. Many intermediate possibilities exist, and it is necessary that the exact organization of the chimeric Hsp27 multimer be determined.
Chimeric Hsp27 did not translocate into the nucleus after stress, demonstrating that stress-induced nuclear translocation is not a requisite for achieving the maximum stress protection attainable with Hsp27 (Figs 3 and 4). This does not exclude the possibility that Hsp27 translocation into the nucleus provides some stress protection, eg, by stabilizing or facilitating recovery of denatured or aggregated proteins in the nucleus (Kampinga et al 1994), or that stress protection within the nucleus is not required to achieve the maximum protection attainable by overexpressing Hsp27. The results of this study merely demonstrate that it is not necessary for Hsp27 to enter the nucleus and directly perform a function therein to achieve its maximal level of stress protection. Ostensibly, overexpressed chimeric Hsp27 could stabilize denatured or aggregated proteins and subcellular structures within the cytoplasm of stressed cells and by so doing free up other Hsps. The latter could then translocate into the nucleus to protect the damage that would have been protected by nuclear-translocated Hsp27 were that expressed instead of chimeric Hsp27. The possibility for such indirect protection of the nucleus by chimeric Hsp27 is currently being tested and represents a second phase of investigations using chimeric Hsp27.
A simple explanation for the inability of chimeric Hsp27 to translocate into the nucleus of either A549 or L929 cells is the failure of stress to reduce the size of the chimeric Hsp27 multimers. In A549 cells chimeric Hsp27 multimers remained at 900 kDa, which, as in control cells, was too large to enter the nucleus. Although stress reduced nearly 50% of chimeric Hsp27 multimers to 6-mers (350 kDa) in L929 cells, the latter were probably still too large to enter the nucleus. It is also plausible that monomers of the EGFP-Hsp27 chimera were incapable of nuclear translocation. This was not tested because stress exposure (arsenite treatment) did not produce chimeric Hsp27 oligomers smaller than 6-mers in L929 cells, and production of mutant, chimeric Hsp27 that formed smaller multimers was not attempted. But the fact that Hsp27 dimers and tetramers (produced after arsenite treatment; Lambert et al 1999) or EGFP dimers could enter the nucleus suggests that chimeric Hsp27 monomers would not be excluded from the nucleus.
Forming the chimera altered the phosphorylation properties of Hsp27 markedly. Hsp27 was not phosphorylated in nonstressed A549 or L929 cells, whereas chimeric Hsp27 exhibited 3 and 1 phosphorylated isoforms, respectively. The shift in chimeric Hsp27 to more positive isoforms in stressed cells was similar to that observed for Hsp27, with the exception that the former exhibited 4 rather than 3 isoforms. The positions of the 3 phosphorylation sites on chimeric Hsp27 were not determined, but it is reasonable to presume that 2 of these sites involved phosphorylation of Ser78 and Ser82. The d isoform of the chimera may have required phosphorylation of a third residue, and the identity of all phosphorylatable residues will be determined subsequently.
Hsp27 and chimeric Hsp27 both formed 16-mers in nonstressed cells despite the fact that chimeric Hsp27 exhibited 1 phosphorylated isomer in L929 cells and 3 in A549 cells. Increased phosphorylation after stress had no effect on multimers in A549 cells and reduced 50% of the 16-mers to 6-mers in L929 cells. These observations suggest the postulate that phosphorylation of Hsp27 is not sufficient for reducing its multimeric size after stress. But there are other plausible scenarios. It is possible that interactions between adjacent EGFP molecules or between adjacent EGFP and Hsp27 molecules (or both) provided sufficient binding force to hold the multimer intact despite any phosphorylation-induced reduction in multimeric binding between Hsp27 molecules.
The data in Table 1 provide strong evidence that transient or protracted overexpression of Hsp27 does not induce upregulation of intracellular glutathione in L929 cells. In actuality, glutathione levels were markedly reduced in L929 cells that constitutively expressed either chimeric Hsp27 or Hsp27. Expressing the Lac-repressor protein did increase cellular glutathione levels by nearly 2-fold, but this did not affect the heat sensitivity of these cells. Consequently, the data in Table 1 support the conclusions that that the protection against heat killing conferred by chimeric Hsp27 and Hsp27 did not involve upregulation of glutathione and that a 69% increase in cellular glutathione levels (in Lac-repressor transfected cells) did not alter cellular heat sensitivity. It is possible that the changes in glutathione levels observed in these experiments could alter cellular sensitivity to other stresses, eg, reactive oxygen species (Mehlen et al 1997), but this possibility was not tested in this study.
The success with the fluorescent Hsp27 chimera bodes well for the potential to produce fluorescent chimeras of other chaperones that retain, if not all, most of the functions of their chaperone moieties. Besides the obvious ability to observe the distribution and redistribution of fluorescent chimeras within, respectively, normal and stressed cells, fluorescent chimeras would present the opportunity to perform very sophisticated experiments to elucidate the interaction of chaperones with each other and with other proteins and structures within living cells. Fluorescence recovery after photobleaching could be used to measure how heat shock and other stresses affects chaperone diffusion rates within cells and whether or not specific chaperones become immobilized or migrate in concert. FRET measurements could be used to determine which chaperones interact with each other during normal or stress conditions and how the chaperones interact with other fluorescently labeled structures within the cell, eg, the cytoskeleton, endoplasmic reticulum, or fluorescently labeled, denatured, and aggregated proteins. Some of these studies are currently underway using the fluorescent EGFP-Hsp27 chimera described in this study.
Acknowledgments
This study was supported by NIH/NCI grant CA71650.
REFERENCES
- Anderson ME 1985 Tissue glutathione. In: CRC Handbook of Method for Oxygen Radical Research, ed Greenwald RA. CRC Press, Boca Raton, FL, 317–323. [Google Scholar]
- Angelidis CE, Lazaridis I, Pagoulatos GN. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991;199:35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Suhan JP, Welch W. Dynamic changes in the structure and intracellular locale of the mammalian low–molecular weight heat shock protein. Mol Cell Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, Welch WJ. Characterization and purification of the small 28,000-Dalton mammalian heat shock protein. J Biol Chem. 1987;262:15359–15369. [PubMed] [Google Scholar]
- Blackburn R, Galoforo S, Berns CM, Ireland M, Cho JM, Corry PM, Lee YJ. Thermal response in murine L929 cells lacking αB-crystallin expression and αB-crystallin expressing L929 transfectants. Mol Cell Biochem. 1996;155:51–60. doi: 10.1007/BF00714333. [DOI] [PubMed] [Google Scholar]
- Borrelli MJ, Lepock JR, Frey HE, Lee YJ, Corry PM. Excess protein in nuclei isolated from heat-shocked cells results from reduced extractability of nuclear proteins. J Cell Physiol. 1996a;167:369–379. doi: 10.1002/(SICI)1097-4652(199606)167:3<369::AID-JCP1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Borrelli MJ, Stafford DM, Karczewski LA, Rausch CM, Lee YJ, Corry PM. Thermotolerance expression in mitotic cells without increased translation of heat shock proteins. J Cell Physiol. 1996b;169:420–428. doi: 10.1002/(SICI)1097-4652(199612)169:3<420::AID-JCP2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Borrelli MJ, Stafford DM, Rausch CM, Bernock LJ, Freeman ML, Lepock JR, Corry PM. Diamide-induced cytoxicity and thermotolerance in CHO cells. J Cell Physiol. 1998;177:483–492. doi: 10.1002/(SICI)1097-4652(199812)177:3<483::AID-JCP11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Caraceni P, De Maria N, Ryu HS. et al. Proteins but not nucleic acids are molecular targets for the free radical attack during reoxygenation of rat hepatocytes. Free Radic Biol Med. 1997;23:339–344. doi: 10.1016/s0891-5849(96)00571-0. [DOI] [PubMed] [Google Scholar]
- Carver JA, Guerreiro N, Nicholls KA, Truscott RJW. On the interaction of α-crystallin with unfolded proteins. Biochim Biophys Acta. 1995;1252:251–260. doi: 10.1016/0167-4838(95)00146-l. [DOI] [PubMed] [Google Scholar]
- Chaufour S, Mehlen P, Arrigo AP. Transient accumulation, phosphorylation and changes in the oligomerization of Hsp27 during retinoic acid–induced differentiation of HL-60 cells: possible role in the control of cellular growth and differentiation. Cell Stress Chaperones. 1996;1:225–235. doi: 10.1379/1466-1268(1996)001<0225:tapaci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Chen H, Ho K, Dereski M, Pulsinelli WA. Transient hyperthermia protects against subsequent forebrain ischemic cell damage in the rat. Neurology. 1989;39:1396–1398. doi: 10.1212/wnl.39.10.1396. [DOI] [PubMed] [Google Scholar]
- Chretien P, Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol. 1988;137:157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Crete P, Landry J. Induction of HSP27 phosphorylation and thermoresistance in Chinese hamster cells by arsenite, cycloheximide, A23187, and EGTA. Radiat Res. 1990;121:320–327. [PubMed] [Google Scholar]
- Dienel GA, Kiessling M, Jacewicz M, Pulsinelli WA. Synthesis of heat shock proteins in rat brain cortex after transient ischemia. J Cereb Blood Flow Metab. 1986;6:505–510. doi: 10.1038/jcbfm.1986.86. [DOI] [PubMed] [Google Scholar]
- Donaldson SS, Gordon LF, Hahn GM. Protective effect of hyperthermia against the cytotoxicity of actinomycin D on Chinese hamster cells. Can Treat Rep. 1978;62:1489–1495. [PubMed] [Google Scholar]
- Donati YR, Slosman DO, Polla BS. Oxidative injury and the heat shock response. Biochem Pharmacol. 1990;40:2571–2577. doi: 10.1016/0006-2952(90)90573-4. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to HSP-25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB. 1990;4:2587–2597. [PubMed] [Google Scholar]
- Freeman BC, Morimoto RI. The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein folding. EMBO J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- Gerner EW, Schneider MJ. Induced thermal resistance in HeLa cells. Nature. 1975;256:500–502. doi: 10.1038/256500a0. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Henle KJ 1987 Thermotolerance in cultured mammalian cells. In: Thermotolerance: Thermotolerance and Thermophily, vol 1, ed Henle KJ. CRC Press, Boca Raton, FL, 13–71. [Google Scholar]
- Henle KJ, Leeper DB. Interaction of hyperthermia and radiation CHO cells: recovery kinetics. Radiat Res. 1976;66:505–518. [PubMed] [Google Scholar]
- Huot J, Roy G, Lambert H, Chretien P, Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991;51:5245–5252. [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Kampinga HH. Thermotolerance in mammalian cells: protein denaturation and aggregation, and stress proteins. J Cell Sci. 1993;104:11–17. doi: 10.1242/jcs.104.1.11. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Brunsting JF, Stege GJ, Konings AW, Landry J. Cells overexpressing Hsp-27 show accelerated recovery from heat-induced nuclear protein aggregation. Biochem Biophys Res Commun. 1994;204:1170–1177. doi: 10.1006/bbrc.1994.2586. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Turkel-Uyger N, Roti Roti JL, Konings AWT. The relationship of increased protein content induced by hyperthermia to killing of HeLa S3 cells. Radiat Res. 1989;117:511–522. [PubMed] [Google Scholar]
- Knauf U, Jakob U, Engel K, Buchner J, Gaestel M. Stress- and mitogen-induced phosphorylation of the small heat shock protein Hsp25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance. EMBO J. 1994;13:54–60. doi: 10.1002/j.1460-2075.1994.tb06234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982;42:2457–2461. [PubMed] [Google Scholar]
- Landry J, Chretien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo A, Li GC. Heat resistant variants of Chinese hamster fibroblasts altered in expression of heat shock protein. Proc Natl Acad Sci U S A. 1985;82:8029–8033. doi: 10.1073/pnas.82.23.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Hahn GM. Abnormal proteins as the trigger for the induction of stress responses: heat, diamide, and sodium arsenite. J Cell Physiol. 1988;136:411–420. doi: 10.1002/jcp.1041360304. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Hou ZZ, Curetty L, Borrelli MJ, Corry PM. Development of acute thermoresistance in L929 cells: lack of HSP28 synthesis and phosphorylation. J Cell Physiol. 1992;152:118–125. doi: 10.1002/jcp.1041520116. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock JR, Frey HE, Ritchie KP. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat shock. J Cell Biol. 1993;122:1267–1276. doi: 10.1083/jcb.122.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Li L, Liu RY, Mak JY, Chen L, Lee WMF. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1992;88:1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3219–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ. Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun. 1999;260:712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement using the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mehlen P, Hickey E, Weber L, Arrigo AP. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFα in NIH-3T3-ras cells. Biochem Biophys Res Commun. 1997;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz J, Arrigo AP. Constitutive expression of human hsp27, Drosophila, hsp27, or human αB-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- Morimoto RI, Tissieres A, and Georgopoulos C 1990 The stress response, function of the proteins, and perspectives. In: Stress Proteins in Biology and Medicine, ed Morimoto RI, Tissieres A, Georgopolous C. Cold Spring Harbor Press, NY, 1–36. [Google Scholar]
- Roti Roti JL, Henle KJ, Winward RT. The kinetics of increases in chromatin protein content in heated cells: a possible role in cell killing. Radiat. Res. 1979;78:522–531. [PubMed] [Google Scholar]
- Roti Roti JL, Laszlo A 1988 The effects of hyperthermia on cellular macromolecules. In: Hyperthermia and Oncology, vol 1, ed Urano M, Douple EB. VNU Scientific, The Netherlands, 13–56. [Google Scholar]
- Senisterra GA, Huntley SA, Escaravage M, Sekhar KR, Freeman ML, Borrelli MJ, Lepock JR. Destabilization of the Ca+2-ATPase of sarcoplasmic reticulum by thiol-specific, heat shock inducers results in thermal denaturation at 37.0°C. Biochemistry. 1997;36:11002–11011. doi: 10.1021/bi9711590. [DOI] [PubMed] [Google Scholar]
- Subjeck JR, Sciandra J, Johnson RJ. Heat shock proteins and thermotolerance: comparison of induction kinetics. Br J Radiol. 1982;55:579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- Yahara I, Iida H, Koyasu S. A heat shock-resistant variant of Chinese hamster cell line constitutively expressing heat shock protein of Mr 90,000 at high level. Cell Struct Funct. 1986;11:65–73. doi: 10.1247/csf.11.65. [DOI] [PubMed] [Google Scholar]