Abstract
Heat shock proteins play a major role in the process of protein folding, and they have been termed molecular chaperones. Two members of the Hsp70 family, Hsc70 and Hsp70, have a high degree of sequence homology. But they differ in their expression pattern. Hsc70 is constitutively expressed, whereas Hsp70 is stress inducible. These 2 proteins are localized in the cytosol and the nucleus. In addition, they have also been observed in close proximity to cellular membranes. We have recently reported that Hsc70 is capable of interacting with a lipid bilayer forming ion-conductance channels. In the present study, we found that both Hsc70 and Hsp70 interact with lipids and can be differentiated by their characteristic induction of liposome aggregation. These proteins promote the aggregation of phosphatidylserine liposomes in a time- and protein concentration–dependent manner. Although both proteins are active in this process, the level and kinetics of aggregation are different between them. Calcium ions enhance Hsc70 and Hsp70 liposome aggregation, but the effect is more dramatic for Hsc70 than for Hsp70. Addition of adenosine triphosphate blocks liposome aggregation induced by both proteins. Adenosine diphosphate (ADP) also blocks Hsp70-mediated liposome aggregation. Micromolar concentrations of ADP enhance Hsc70-induced liposome aggregation, whereas at millimolar concentrations the nucleotide has an inhibitory effect. These results confirm those of previous studies indicating that the Hsp70 family can interact with lipids directly. It is possible that the interaction of Hsp70s with lipids may play a role in the folding of membrane proteins and the translocation of polypeptides across membranes.
INTRODUCTION
A very active rate of protein synthesis is observed in cells under normal physiological conditions. These newly synthesized polypeptides are required to activate different metabolic pathways in response to changes in the cellular environment. In addition, these new polypeptides are necessary to replace degraded proteins as a part of the normal cell turnover. The final conformation of a protein is determined by its primary amino acid sequence. But this process may be affected by nonconstructive interactions between unfolded regions on the same polypeptide or on another protein. Consequently, accessory proteins, named chaperones, are required to assist the folding of newly synthesized polypeptides (Agashe and Hartl 2000). Chaperones are also necessary for the assembly of oligomeric proteins and the translocation of polypeptides across membranes, such as the endoplasmic reticulum (ER).
Heat shock proteins (Hsps) are the important components of the cellular chaperone pool. Hsps are present in cells under normal physiological conditions. In addition, their expression is induced after different physiological stresses. The Hsp70 family of Hsps is composed of 4 members, Hsc70, Hsp70, BIP, and Mtp70, which are localized in different subcellular compartments. Hsc70, BIP, and Mtp70 are present under normal conditions, whereas Hsp70 is primarily expressed after environmental stresses such as hyperthermia (De Maio 1999). Although Hsc70 and Hsp70 are predominately cytosolic proteins, the presence of these in association with or in close proximity to cellular membranes has been reported (Welch and Suhan 1985; Domanico et al 1993; Kurucz et al 1999). Moreover, Hsp70 has been detected on the cell surface of tumor cells (Di Cesare et al 1992; Camins et al 1995; Multhoff et al 1995; Kaur et al 1998). In EL-4 lymphoma cells, the presence of Hsps on the cell surface was found to increase through different apoptotic stages, with the highest levels observed during the loss of cell membrane phospholipid asymmetry (Sapozhnikov et al 1999). Exogenous addition of Hsp70 to a variety of cells (skate retinal cells, motor neuron in the spinal cord) resulted in particular responses (Johnson and Tytell 1993; Houenou et al 1996; Tidwell et al 2000), which may occur after binding to the plasma membrane. Incubation of human monocytes with Hsp70 elicits a rapid intracellular calcium flux, activates nuclear factor-kB, and up-regulates the expression of proinflammatory cytokines. In these experiments, fluorescence microscopic analysis and laser confocal microscopy revealed that Hsp70 binds specifically to the cell surface (Asea et al 2000). Activation of potassium channels in patch-clamped membranes of human promonocyte U937 cells has also been observed after exogenous addition of Hsp70 (Negulyaev et al 1996).
We have recently reported that Hsc70 incorporates into artificial phospholipid membranes, forming a nucleotide-regulated ion-conducting channel (Arispe and De Maio 2000a). Moreover, Hsc70 and Hsp70 copurified with nonesterified, saturated fatty acids of long chain length during the isolation from cells (Guidon and Hightower 1986a, 1986b). These findings suggest that these chaperone proteins also possess specific affinity for lipids. In the present study, the direct interaction of Hsc70 and Hsp70 with lipids was analyzed using a liposome aggregation assay. This technique allows us to study the direct protein-lipid interactions using purified proteins in a controlled lipid environment, as well as the self-association of proteins that are already intimately associated with the lipids of membranes. Additionally, the effect of several factors (nucleotides, ions, etc) can be tested. Liposome aggregation assay has been extensively used to study protein-lipid interactions with a variety of proteins that are known to self-associate to form oligomers (Hong et al 1981; Glenney et al 1987; Blackwood and Ernst 1990; De la Fuente and Parra 1995; Caohuy et al 1996; Lee and Pollard 1997). In the present study, we found that both Hsc70 and Hsp70 interact with liposomes to induce aggregation. This process is time and protein concentration dependent. The induced liposome aggregation is characteristic for each of these 2 Hsps. Thus, Hsp70 and Hsc70 can be discriminated by their interaction with lipids.
MATERIALS AND METHODS
Preparation of liposomes
Large unilamellar liposomes were prepared using the extrusion method. Palmitoyl-oleoyl-phosphatidylserine (Avanti Polar Lipids Inc, Alabaster, AL, USA) was dissolved in chloroform and dried under nitrogen gas. The dried lipid was hydrated in 25 mM Tris-HCl, pH 7.5, 250 mM NaCl (1 mL/10 mg of lipid) for a minimum of 1 hour at 25°C (or 24 hours at 4°C). The suspension was passed 12 to 15 times through a 0.05-mm polycarbonate filter in an extruder apparatus (Avanti Polar Lipids) to uniformly size the liposomes. The final lipid concentration was 10 mg/mL.
Liposome aggregation assay
Aggregation of liposomes was determined by the change in absorbance that follows the increase in turbidity of the liposome suspension after addition of Hsps. Absorbance was measured at 350 nm in a Hewlett-Packard spectrophotometer, and data were collected every 30 seconds. The aggregation reactions were performed in 1-mm path length optical glass cuvettes at 25°C. Unless otherwise stated, the standard assay was conducted in 40 mM histidine-HCl, pH 6, 300 mM sucrose, 0.5 mM MgCl2, 1 mM CaCl2. Hsc70 and Hsp70 were first added directly to the aggregation medium followed by freshly prepared liposomes (phosphatidylserine [PS] final concentration of 1.25 mM). Recombinant bovine Hsc70 (SPP-751) and human Hsp70 (SPP-755) free of any contamination by nucleotides were purchased from Stressgen Biotechnologies Co, Victoria, BC, Canada. These proteins were certified by Stressgen to present normal adenosine 5′-triphosphatase (ATPase) activity.
RESULTS
Liposome aggregation induced by Hsc70 and Hsp70 is different
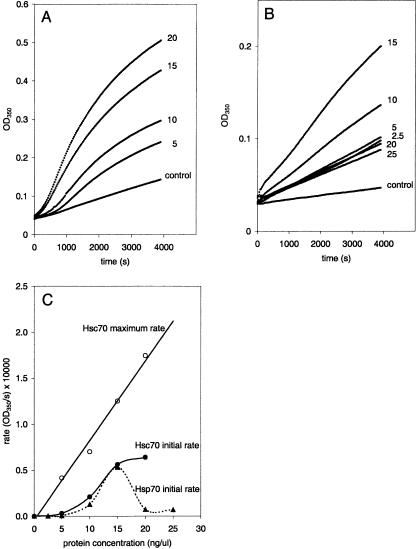
The interaction of Hsc70 and Hsp70 with PS was studied using a liposome aggregation assay in the presence of CaCl2 (1 mM) and MgCl2 (0.5 mM) to screen charges on the liposome surface and to minimize basal liposome-liposome aggregation. Aggregation was measured by the change in optical density (OD) of liposome suspensions in the presence of Hsc70 or Hsp70. These data indicate that both proteins induce liposome aggregation. Figure 1A and Figure 1B illustrate the OD change of liposome suspension induced by the addition of Hsc70 and Hsp70, respectively. These results reveal that in terms of magnitude and time course, the processes of liposome aggregations are different. The initial and the maximum rates of liposome aggregation are displayed in Figure 1C. The initial rates were measured as the OD change during the first 250 seconds. To obtain the maximum rates, the derivative of the OD (dOD/dt, where dt = 30 seconds) was plotted as a function of time for as long as 1 hour. The peak value from the resulting curve was taken as the maximum rate and plotted for each protein concentration in Figure 1C. The data show a sigmoidal increase in the initial rate as a function of Hsc70 (filled circles) or Hsp70 (filled triangles) concentrations. This behavior suggests that the interaction between the Hsps and liposomes is a cooperative process. The initial rate of liposome aggregation induced by Hsc70 reaches saturation (at a protein concentration of 15 ng/mL) and decays thereafter. This observation suggests that the interaction between Hsp70 and liposomes is initially cooperative but becomes additionally more complex for larger protein concentrations. Because the change in the OD of liposome suspension containing Hsp70 follows a first-order trajectory (see Fig 1B), the initial and the maximum rates of the Hsp70-induced aggregation up to a protein concentration of 15 ng/μL have similar values. The OD change of liposome suspension containing Hsc70 follows a sigmoidal trajectory (see Fig 1A), therefore, the initial (filled circles) and maximum (empty circles) rates of Hsc70-induced aggregation are always different for any concentration (Fig 1C). At concentrations below 15 ng/μL, the maximum aggregation rate of the process induced by Hsc70 (1.2 × 104 OD/s) was about 2 times higher (0.5 × 104 OD/s) than the rate of aggregation induced by an equivalent concentration of Hsp70.
Fig. 1.
Liposome aggregation induced by heat shock proteins (Hsps). (A) Time course of change in optical density (OD) due to aggregation of phosphatidylserine (PS) liposomes in the presence of different concentrations of Hsc70. (B) Time course of change in OD due to aggregation of PS liposomes in the presence of different concentrations of Hsp70. Values next to each curve indicate the protein concentration (ng/μL). The aggregation solution contained 1 mM CaCl2 and 0.5 mM MgCl2. To initiate the aggregation process, PS liposomes were added to the aggregation solution to a final concentration of 1 μg/μL. The OD change was measured every 30 seconds at 350 nm. (C) Rates of the OD change (OD/s) as a function of Hsc70 and Hsp70 concentrations. The initial rate (filled squares and circles) was calculated as the slope for the first 250 seconds of the aggregation reaction. The maximum rate values (empty circles) correspond to the maximum OD change per sampling period (30 seconds) value calculated over 1 hour of aggregation reaction
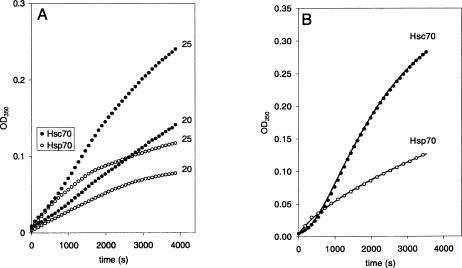
The preceding observations suggest that the capacity of Hsc70 and Hsp70 to aggregate PS liposomes is different. To further substantiate this finding, liposome aggregation by Hsc70 and Hsp70 was performed simultaneously using the same ionic conditions and the same liposome preparation. The OD change observed in the liposome suspension containing Hsc70 was always larger than that observed in presence of Hsp70 under identical experimental conditions (Fig 2). Thus, aggregation induced by Hsc70 was slower but more pronounced, whereas aggregation induced by Hsp70 was more rapid toward a lower saturation level. The time course of OD changes (ΔOD(t)) were best fitted by the equations ODmax (1 − exp(−αt)2 + ODt = 0) and ODmax (1 − exp(−α1t)) + ODmax (1 − exp(−α2t)) + ODt = 0 for Hsc70 and Hsp70, respectively, supporting the observation that these 2 proteins have different behavior in their interaction with lipids.
Fig. 2.
Differential liposome aggregation induced by Hsc70 and Hsp70. (A) Time course of optical density (OD) change due to aggregation of phosphatidylserine (PS) liposomes induced by Hsc70 (filled circles) and Hsp70 (empty circles) at 2 different concentrations (20 and 25 ng/μL). The reactions were simultaneously run under equal conditions to illustrate the higher capacity of Hsc70 to induce liposome aggregation. (B) Simultaneous measurements of the OD change generated by the aggregation of liposome induced by equal concentrations (10 ng/mL) of Hsc70 (filled circles) and Hsp70 (empty circles). The curves illustrate differences in the kinetics of the liposome aggregation. The solid lines represent the best fit to the points calculated using equations described in the text. For solid line on Hsc70 points: ΔODmax = 0.35, ODt = 0 = 0.0048, α = 1/1580 s−1. For solid line on Hsp70 points: ΔODmax,1 = 0.242, ΔODmax,2 = 0.012, ODt = 0 = 0.0046, α1 = 1/5800 s−1, α2 = 1/350 s−1
Hsc70- and Hsp70-induced liposome aggregation is calcium dependent
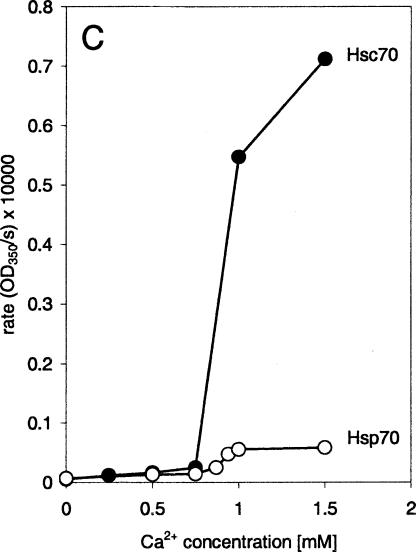
The effect of different calcium concentrations on liposome aggregation by Hsc70 and Hsp70 was also studied. For both proteins, the induction of liposome aggregation was found to be calcium dependent on a concentration above 0.75 mM. The effect of calcium ions was more pronounced on Hsc70 than on Hsp70. The velocity of aggregation by Hsc70 is still increasing at a calcium concentration of 1.5 mM, whereas Hsp70-induced aggregation is already maximal (Fig 3).
Fig. 3.
Effect of calcium on Hsc70- and Hsp70-induced liposome aggregation. Maximum rates of liposome aggregations induced by equal concentrations (10 ng/mL) of Hsc70 (filled circles) and Hsp70 (empty circles) as a function of the calcium concentration. The maximum rate values (empty circles) correspond to the maximum OD change per sampling period (30 seconds) value calculated over 1 hour of aggregation reaction
Addition of ATP and ADP modifies liposome aggregation induced by Hsc70
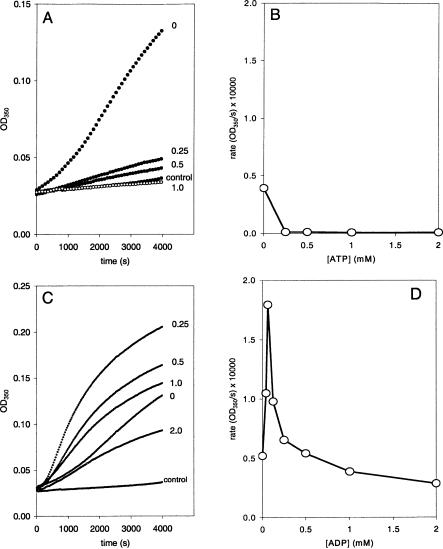
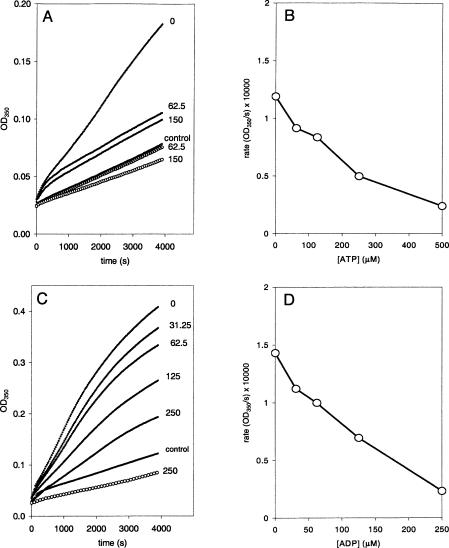
The effect of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) on Hsc70-induced liposome aggregation was also studied. Addition of ATP resulted in inhibition of Hsc70-induced liposome aggregation in a range of concentrations between 0.25 mM and 1 mM (Fig 4A). As little as 250 μM ATP was sufficient to reduce the initial rate of Hsc70-induced liposome aggregation to values close to 0 (Fig 4B). This inhibitory effect of ATP is unlikely the result of calcium ion chelation because addition of 0.5 mM ATP was calculated to reduce the concentration of calcium in less than 10% (from 1 mM to 0.92 mM). Addition of ADP resulted in an enhancing effect on Hsc70-induced liposome aggregation in a range of nucleotide concentration between 0.25 mM and 1.0 mM. On the contrary, higher concentrations of ADP have an inhibitory effect (Fig 4C). Although the ADP used in these studies is of the highest purity, the inhibitory effect could be the result of small contamination of ADP by ATP. The initial rate of Hsc70-induced liposome aggregation was increased up to an ADP concentration of 62.5 μM, which displayed a maximum value. Addition of ADP at concentration higher than 62.5 μM reduced this enhancing effect to levels even below the observed in absence of ADP (Fig 4D).
Fig. 4.
Effect of nucleotides on liposome aggregation induced by Hsc70. (A) Representative time course of the optical density (OD) change generated by liposome aggregation induced by Hsc70 (10 ng/μL) in the absence and the presence of 0.25 or 0.5 mM of adenosine triphosphate (ATP) (filled circles). The curve described by the empty circles corresponds to the time course of the liposome aggregation, in the absence of Hsc70 protein, generated by the sole addition of 1 mM ATP. (B) Maximum rates of the liposome aggregations induced by Hsc70 (10 ng/mL) as a function of the ATP concentration (average, n = 3). (C) Representative time course of the OD change generated by liposome aggregation induced by Hsc70 (10 ng/μL) in the absence and the presence of adenosine diphosphate (ADP). Numbers next to the curves indicate the ADP concentrations (mM). (D) Maximum rates of the liposome aggregations induced by Hsc70 (10 ng/mL) as a function of the ADP concentration (average, n = 5)
Addition of ATP and ADP blocks Hsp70-induced liposome aggregation
The effect of nucleotides on Hsp70-induced liposome aggregation was also studied. A significant inhibition of liposome aggregation was observed in the presence of ATP proportionally to the concentration of this nucleotide (Fig 5A). Thus, aggregation was reduced in 50% by an ATP concentration of 225 μM (Fig 5B). Similarly, addition of ADP resulted in a reduction of Hsp70-induced liposome aggregation (Fig 5C). The concentration of ADP necessary to reduce 50% the initial rate of aggregation was 125 μM (Fig 5D).
Fig. 5.
Effect of nucleotides on liposome aggregation induced by Hsp70. (A) Time course of the optical density (OD) change generated by liposome aggregation induced by Hsp70 (10 ng/μL) in the absence and the presence of 62.5 and 150 μM of adenosine triphosphate (ATP) (filled circles). The curves described by the empty circles correspond to the time course of the liposome aggregation, in the absence of Hsc70 protein, generated by the sole addition of 62.5 and 150 μM ATP. (B) Maximum rates of the liposome aggregations induced by of Hsp70 (10 ng/mL) as a function of the ATP concentration. (C) Time course of the OD change generated by liposome aggregation induced by Hsp70 (10 ng/μL) in the absence and the presence of adenosine diphosphate (ADP). The numbers next to the curves indicate the ADP concentrations (μM). The curve described by the empty circles corresponds to the time course of the liposome aggregation, in the absence of Hsp70 protein, generated by the sole addition of 250 μM of ADP. (D) Maximum rates of the liposome aggregations induced by Hsp70 (10 ng/mL) as a function of the ADP concentration
ATP and ADP compete to modulated Hsc70-induced liposome aggregation
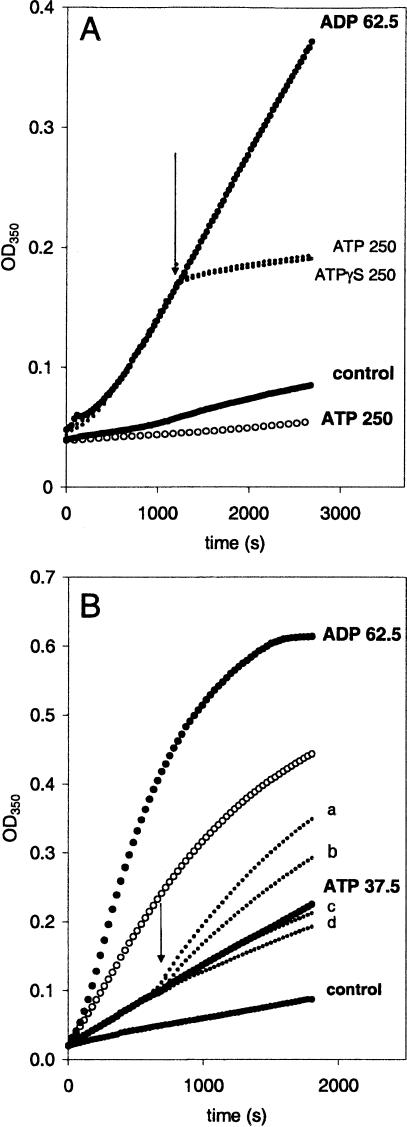
The preceding experiments demonstrate that ATP and ADP have different effects on liposome aggregation induced by Hsc70. Experiments were performed to study the competition between these 2 nucleotides on Hsc70-induced liposome aggregation. Hsc70 (10 ng/μL) and liposomes were simultaneously incubated in presence of ADP (62.5 μM). This concentration of ADP was shown to enhance Hsc70-induced liposome aggregation (see Fig 3D). After 20 minutes of the liposome aggregation process, ATP (250 μM) or the nonhydrolyzable analogue ATPγS (250 μM) was added, and aggregation was allowed to proceed for 20 minutes. The addition of ATP or its analogue similarly detained the progress of Hsc70 liposome aggregation as compared with the process in the presence of ADP but not ATP (Fig 6A). A second experiment was performed in which Hsc70 (10 ng/μL) and liposomes were mixed in presence of ATP at a concentration that partially blocks the aggregation process. During the course of liposome aggregation, ADP was added at concentrations between 62.5 μM and 200 μM (Fig 6B, arrows). Addition of ADP neutralizes the inhibitory effect of ATP and results in a pattern similar to the one presented above (see Fig 3).
Fig. 6.
Competition between nucleotides on liposome aggregation induced by Hsc70. (A) Simultaneous recording of the time course of the optical density (OD) change in 3 reaction cells in which liposome aggregation was induced by Hsc70 (10 ng/μL) in the presence of 62.5 μM of adenosine diphosphate (ADP). After 20 minutes of liposome aggregation process, adenosine triphosphate (ATP) (250 μM) or a nonhydrolyzable analogue ATPγS (250 μM) was added (see arrow) to 2 of the cuvettes. Liposome aggregation in the presence of the same concentrations of ATP, but in the absence of Hsp70, was monitored simultaneously (empty circles). The control curve corresponds to the liposome aggregation observed in the absence of protein and nucleotides. (B) Time course of the OD change generated by liposome aggregation induced by Hsp70 (10 ng/μL) in the absence (empty circles) and the presence (filled circles) of nucleotides. ADP at concentrations of 62.5, 75, 100, and 200 μM (curves a, b, c, and d, respectively) was added 10 minutes after the initiation of the reaction (arrow) to 5 different cuvettes containing ATP (37.5 μM). The aggregation in 1 of the cuvettes (top curve) was performed in the presence of 62.5 μM ADP. The control (bottom curve) corresponds to the resulting liposome aggregation observed in the absence of protein and nucleotides
DISCUSSION
The Hsp70 family of proteins participates in the folding of newly synthesized polypeptides and their translocation across membranes (Hartl 1996). They may also cooperate in the folding of membrane proteins, particularly those that span the lipid bilayer several times (Martin and Hartl 1997; Fink 1999). Thus, Hsp70 has been found associated with integral membrane proteins within the ER, such as the cystic fibrosis transmembrane regulator (Yang et al 1993). The possible mechanism for the participation of Hsp70s in the folding of transmembrane proteins has not been elucidated yet. The interaction of chaperones, particularly the Hsp70 family, with membranes may be a necessary step in the folding of membrane proteins. Morphologic studies have shown the presence of Hsp70s in close proximity to cellular membranes (Welch and Suhan 1985; Domanico et al 1993; Kurucz et al 1999). We have recently reported that Hsc70 can interact with lipids forming an ion-conductance pathway (Arispe and De Maio 2000). Other Hsps have also been found to interact with membranes (Vigh et al 1998). In the present study, we have compared the interaction of Hsc70 and Hsp70 with lipids using a liposome aggregation assay. Liposome aggregation requires protein-lipid as well as protein-protein interactions.
Both Hsc70 and Hsp70 were found to induce PS liposome aggregation in a protein concentration– and time-dependent manner. Therefore, the present results regarding liposome aggregation confirm our previous observations indicating the spontaneous interaction of Hsp70s with membrane lipids. Our results also reveal different characteristics of the aggregation process between these 2 proteins. The magnitude of liposome aggregation was greater for Hsc70 than for Hsp70. The initial rate of liposome aggregation was similar for both proteins at lower protein concentrations but showed a very distinct pattern at higher protein concentrations. Liposome aggregation induced by Hsc70 could be described by a second-order exponential equation, suggesting the prevalence of at least 2 important steps. On the other hand, liposome aggregation induced by Hsp70 was described by single exponential equations, suggesting a single rate-limiting step. The first step in the aggregation process is likely the interaction between protein and liposomes. This first step in the aggregation process proceeds much faster for Hsp70 than for Hsc70. This result suggests a differential affinity of these proteins for the membrane lipid, which may be a consequence of the small differences in the amino acid sequence between these 2 proteins.
Previous studies have shown that Hsp70s can bind noncovalently to fatty acids, predominantly palmitic and stearic acids and to a lesser extent myristic acid. This binding seems to be of high affinity and selectivity. Noncovalent binding to fatty acids by Hsp70s may also produce a change in protein conformation similarly to the one reported for albumin (Guidon and Hightower 1986a, 1986b). Our findings are consistent with those of previous studies indicating the interaction of these proteins with palmitic acid (Guidon and Hightower 1986b) because the synthetic PS used in our analysis is composed of ester-bound palmitic and oleic acids (1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine). Furthermore, a high affinity of both Hsc70 and Hsp70 for PS has been observed (Arispe et al 2001). Under normal conditions, plasma membrane phospholipids are asymmetrically distributed across the bilayer, with PS found almost entirely in the inner leaflet. In preapoptosis stages, PS appears in the outer leaflet of the plasma membrane. Higher levels of Hsp70s have been detected on the outer surface of the plasma membrane in apoptotic EL-4 cells than in nonapoptotic cells (Sapozhnikov et al 1999). Presence of Hsp70s on the cell surface has been reported to increase through several stages of early and late apoptosis, and the highest level was observed during the loss of the cell membrane phospholipid asymmetry (Sapozhnikov et al 1999). These observations are consistent with a specific interaction of Hsp70s with PS lipids and are supported by our results on PS liposome aggregation.
The presence of calcium ions increases the extent of liposome aggregation induced by Hsc70 and Hsp70. We observed that this effect is more pronounced for Hsc70 than for Hsp70. Calcium ions in the millimolar range have been found to induce liposome aggregation and fusion in the absence of proteins (Lee and Pollard 1997). Some proteins can also promote aggregation of secretory granules and liposomes in a calcium-dependent manner (Drust and Creutz 1988; Blackwood and Ernst 1990; De la Fuente and Parra 1995; Lee and Pollard 1997). The effect of calcium on protein-induced liposome aggregation could be an effect of this ion on protein conformation or self-association properties. Although 2 calcium-binding sites have been identified within the ATPase domain of human Hsp70s (Sriram et al 1997), the effect of calcium in the self-association properties of these proteins has not been studied yet. Hsp70 is phosphorylated in vitro in the presence of divalent ions, calcium being the most effective. Phosphorylation has been argued to affect the self-association properties of Hsp70s (Sriram et al 1997), which may explain the promotion of liposome aggregation observed in the presence of calcium. Further studies are required to test this possibility.
The effect of nucleotides on the aggregation process was also different between Hsc70 and Hsp70. ATP in a wide range of concentrations blocked liposome aggregation mediated by both proteins. But the concentration of ATP necessary to produce a 50% inhibition was approximately 10-fold lower for Hsc70 than for Hsp70. ADP at low concentrations (micromolar) resulted in an increase of Hsc70-mediated liposome aggregation, whereas higher concentration of this nucleotide (>0.5 mM) resulted in an inhibitory effect. Contamination of ADP by traces of ATP could provide an explanation for the inhibitory effect of ADP. Hsp70-mediated liposome aggregation was inhibited by the addition of ADP; no activation was ever observed. Additional studies illustrated the dual effect of ADP on Hsc70-induced aggregation of liposomes. Hsc70-mediated aggregation at lower concentrations of ADP, which results in a stimulatory effect, was reversed by increases in the concentration of the same nucleotide. Addition of ADP could override the inhibitory effect of ATP on Hsc70-induced liposome aggregation. Similar studies indicated that the addition of ATP could also override the stimulatory effect of lower ADP concentrations. Addition of ATPγS had an identical effect as ATP, suggesting that binding of the nucleotide, rather than hydrolysis, is a necessary step for Hsc70 self-aggregation and interaction with lipids. We have found that the liposome aggregation assay provides an excellent method to study the behavior of nucleotides on Hsc70 and Hsp70. The interaction of Hsc70 and Hsp70 with lipids is likely to occur by the oligomeric rather than by the monomeric form of these proteins. Hsp70s in the absence of target peptides or denatured proteins have been reported to self-associate forming oligomers of low order (Freiden et al 1992). Oligomerization is disrupted by incubation with ATP (Benaroudj et al 1996). Additionally, ATP binding produced a conformational change of Hsp70s that modulates their interaction with target polypeptides (Fung et al 1996). Thus, it is possible that the effect of nucleotides on liposome aggregation is the result of either a change in oligomerization or a conformational change (or both).
Hsc70 is constitutive, whereas Hsp70 is induced after stress. These 2 proteins have a high degree of sequence homology and are localized within the subcellular compartments, cytosol, and nucleus. Consequently, it would be expected that they play similar roles. This redundancy has never been understood. The results presented in this study unveil a new means to differentiate between these 2 proteins. The liposome aggregation induced by Hsc70 and Hsp70 was different. Hsc70 has been found to be more potent in promoting liposome aggregation than is Hsp70, suggesting that Hsc70 is more effective in establishing an interaction with lipids. Although recombinant proteins were used in this study, we cannot discard the possibility that the differences in liposome aggregation between Hsc70 and Hsp70 are the result of diversity in the origin of these 2 proteins. Hsp70 was cloned from human embryonic cells, whereas Hsc70 is of bovine origin. Other Hsp may also interact with lipid membranes. Thus, Hsp40 is posttranslationally modified by the addition of an isoprenyl group at the C terminal, which may be involved in the interaction of this protein with lipids. Moreover, homologues of Hsp40 have been observed interacting with biological membranes (De Maio 2002). Whether additional Hsps can interact with lipids remains to be established.
The question that remains to be elucidated is the possible function of the interaction between Hsc70 and Hsp70 with lipids. The role of Hsp70s in the folding of cytosolic (water soluble) proteins has been well established. It is possible that Hsp70s are similarly involved in assisting membrane proteins to reach their appropriate conformation within the lipid bilayer. Although this hypothesis remains to be tested, the findings presented in this study in combination with previous reports strongly support the assumption that Hsp70s can firmly interact with lipids.
Acknowledgments
This work was supported by NIGMS, National Institutes of Health, Grant GM-50878 and the Garrett Research Foundation.
REFERENCES
- Agashe VR, Hartl FU. Roles of molecular chaperones in cytoplasmic protein folding. Semin Cell Dev Biol. 2000;1(1):15–25. doi: 10.1006/scdb.1999.0347. [DOI] [PubMed] [Google Scholar]
- Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acid phospholipid membranes. J Biol Chem. 2000a;275:30839–30843. doi: 10.1074/jbc.M005226200. [DOI] [PubMed] [Google Scholar]
- Arispe N, De Maio A 2000b Differential liposome aggregation by two members of the heat shock 70 family of proteins. Biophys J. 78(1):36. A. [Google Scholar]
- Arispe N, Doh M, and De Maio A 2001 Extracellular interaction of the heat shock proteins Hsc70 and Hsp70 with PC12 cells leads to a reduction in viability [abstract]. Mol Biol Cell. 12:201. A. 11160833 [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Caldewood SK. Hsp70 stimulates cytokine production through a CD 14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Benaroudj N, Triniolles F, Ladjimi MM. Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone HSC70. J Biol Chem. 1996;271(31):18471–18476. doi: 10.1074/jbc.271.31.18471. [DOI] [PubMed] [Google Scholar]
- Blackwood RA, Ernst JD. Characterization of Ca2+-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990;266:195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camins A, Diez-Fernandez C, Camarasa J, Escubedo E. Cell surface expression of heat shock proteins in dog neutrophils induced by mitochondrial benzodiazepine receptor ligands. Immunopharmacology. 1995;29:159–166. doi: 10.1016/0162-3109(94)00055-k. [DOI] [PubMed] [Google Scholar]
- Caohuy H, Srivastava M, Pollard HB. Membrane fusion protein synexin (annexin VII) as a Ca2+/GTP sensor in exocytotic secretion. Proc Natl Acad Sci U S A. 1996;93:10797–10802. doi: 10.1073/pnas.93.20.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente M, Parra A. Vesicle aggregation by annexin I. Role of a secondary membrane binding site. Biochemistry. 1995;34:10393–10399. doi: 10.1021/bi00033a010. [DOI] [PubMed] [Google Scholar]
- De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- De Maio A 2002 HSP40 (DNAJ). In: Wiley Encyclopedia of Molecular Medicine, vol 5, John Wiley & Son, Inc., New York, 1672–1673. [Google Scholar]
- Di Cesare S, Poccia F, Mastino A, Colizzi V. Surface expressed heat-shock proteins by stressed or human inmunodeficiency virus (HIV)-infected lymphoid cells represent the target for antibody-dependent cellular cytotoxicity. Immunology. 1992;76:341–343. [PMC free article] [PubMed] [Google Scholar]
- Domanico SZ, Denagel DC, Dahlseid JN, Green JM, Pierce SK. Cloning of the gene encoding peptide-binding protein-74 shows that it is a new member of the heat shock protein-70 family. Mol Cell Biol. 1993;13:3598–3610. doi: 10.1128/mcb.13.6.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust DS, Creutz CE. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988;331:88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79(2):425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Freiden PJ, Gaut JR, Hendershot LM. Interconversion of three differentially modified and assembled forms of BiP. EMBO J. 1992;11:63–70. doi: 10.1002/j.1460-2075.1992.tb05028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung KL, Hilgenberg L, Wang NM, Chirico WJ. Conformations of the nucleotide and polypeptide binding domains of a cytosolic Hsp70 molecular chaperone are coupled. J Biol Chem. 1996;271:21559–21565. doi: 10.1074/jbc.271.35.21559. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Tack B, Powell MA. Calpactins: two distinct Ca++-regulated phospholipid-and actin-binding proteins isolated from lung and placenta. J Cell Biol. 1987;104:503–511. doi: 10.1083/jcb.104.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidon PT Jr,, Hightower LE. Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry. 1986a;25(11):3231–3239. doi: 10.1021/bi00359a023. [DOI] [PubMed] [Google Scholar]
- Guidon PT Jr,, Hightower LE. The 73 kilodalton heat shock cognate protein purified from rat brain contains nonesterified palmitic and stearic acids. J Cell Physiol. 1986b;128(2):239–245. doi: 10.1002/jcp.1041280215. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hong K, Duzgunes N, Papahadjopoulos D. Role of synexin in membrane fusion. Enhancement of calcium-dependent fusion of phospholipid vesicles. J Biol Chem. 1981;256:3641–3644. [PubMed] [Google Scholar]
- Houenou LJ, Li L, Lei M, Kent CR, Tytell M. Exogenous heat shock cognate protein Hsc70 prevents axotomy-induced death of spinal sensory neurons. Cell Stress Chaperones. 1996;1(3):161–166. doi: 10.1379/1466-1268(1996)001<0161:ehscph>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Tytell M. Exogenous HSP70 becomes cell associated, but not internalized, by stressed arterial smooth muscle cells. In Vitro Cell Dev Biol Anim. 1993;29A(10):807–812. doi: 10.1007/BF02634348. [DOI] [PubMed] [Google Scholar]
- Kaur J, Das SN, Srivastava A, Ralhan R. Cell surface expression of 70 kDa heat shock protein in human oral dysplasia and squamous cell carcinoma; correlation with clinicopathological features. Oral Oncol. 1998;34(2):93–98. doi: 10.1016/s1368-8375(97)00055-9. [DOI] [PubMed] [Google Scholar]
- Kurucz I, Tombor B, and Prechl J. et al. 1999 Ultrastructural localization of Hsp-72 examined with a new polyclonal antibody raised against the truncated variable domain of the heat shock protein. Cell Stress Chaperones. 4(2):139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Pollard HB. Highly sensitive and stable phosphatidylserine liposome aggregation assay for annexins. Anal Biochem. 1997;252:160–164. doi: 10.1006/abio.1997.2311. [DOI] [PubMed] [Google Scholar]
- Martin J, Hartl FU. The effect of macromolecular crowding on chaperonin-mediated protein folding. Proc Natl Acad Sci U S A. 1997;94(4):1107–1112. doi: 10.1073/pnas.94.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA, Kinev AV, Voronin AP. Exogenous heat shock protein Hsp70 activates potassium channels in U937 cells. Biochim Biophys Acta. 1996;1282:156–162. doi: 10.1016/0005-2736(96)00055-7. [DOI] [PubMed] [Google Scholar]
- Sapozhnikov AM, Ponomarev ED, Tarasenko TN, Telford WG. Spontaneous apoptosis and expression of cell surface heat-shock proteins in cultured EL-4 lymphoma cells. Cell Prolif. 1999;32(6):363–378. doi: 10.1111/j.1365-2184.1999.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram M, Osipiuk J, Freeman B, Morimoto R, Joachimiak A. Human Hsp70 molecular chaperone binds two calcium ions within the ATPase domain. Structure. 1997;5(3):403–414. doi: 10.1016/s0969-2126(97)00197-4. [DOI] [PubMed] [Google Scholar]
- Tidwell JL, Yu Q, Clifton C, Houenou LJ, Milligan CE, and Tytell M 2000 Abstract. In: Molecular chaperones & the heat shock response. May 3–7. Cold Spring Harbor, NY, 245. [Google Scholar]
- Vigh L, Maresca B, Harwood JL. Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Suhan JP. Morphological study of the mammalian stress response: characterization of the changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblast after heat-shock treatment. J Cell Biol. 1985;101:1198–1211. doi: 10.1083/jcb.101.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Janich S, Cohn JA, Wilson JM. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci U S A. 1993;90(20):9480–9484. doi: 10.1073/pnas.90.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]