Abstract
Extending earlier studies, this report demonstrates that Leishmania infantum heat shock proteins (Hsps), Hsp70 and Hsp83, expressed as recombinant proteins fused to the Escherichia coli maltose-binding protein (MBP), are potent mitogens for murine splenocytes. The response was not due to lipopolysaccharide (LPS) because the stimulatory activity of Hsp preparations was sensitive to boiling and trypsin treatments, whereas the corresponding activity of LPS was resistant to both treatments. It was found that in vitro incubation of spleen cells with the Leishmania Hsps leads to the expansion of CD220-bearing populations, suggesting a direct effect of these proteins on B lymphocytes. In fact, splenocytes from B cell–deficient mice did not proliferate in response to the Leishmania Hsps. In contrast, spleen cells from athymic nude mice were significantly stimulated by these recombinant proteins as an indication that the MBP-Hsp70 and MBP-Hsp83 recombinant proteins behave as T cell–independent mitogens of B cells. Furthermore, both proteins were able to induce proliferation on B cell populations purified from BALB/c spleen.
INTRODUCTION
Heat shock proteins (Hsps) are highly conserved molecules that play important roles in protein folding, assembly of protein complexes, and translocation of proteins across cellular compartments. Because of these helper functions, Hsps also have been termed molecular chaperones. Increasing evidence also favors an important role of chaperones in several immunological processes. Aside from the assembly of components of the immune system, such as immunoglobulins, T cell receptors, and major histocompatibility complex molecules, chaperones are involved in antigen processing and presentation pathways (reviewed by Zügel and Kaufmann 1999). In addition, it has been suggested that the Hsps may function as a sort of link between innate and adaptive immune responses (Srivastava et al 1998; Anderson and Srivastava 2000). This suggestion is based on 2 features displayed by these proteins: (1) Hsps are specialized carriers for re-presentation of antigenic peptides (Wells and Malkovsky 2000), and (2) Hsps induce release of cytokines by different immune cells (Retzlaff et al 1994; Galdiero et al 1997; Breloer et al 1999; Multhoff et al 1999; Asea et al 2000).
Another interesting aspect of Hsps arises from the fact that they represent prominent antigens during the course of infectious diseases caused by bacteria, protozoa, fungi, and nematodes (reviewed by Zügel and Kaufmann 1999). Because Hsp sequences are highly conserved throughout evolution, it has been proposed that the recognition of epitopes shared by Hsps from different pathogens may provide the immune system with a universal signal for infection (Kaufmann 1990). This hypothesis may be in line with the adjuvant properties showed by several microbial Hsps. In particular, it has been demonstrated that immunization with peptides or oligosaccharides conjugated to the Mycobacterium tuberculosis Hsp70 promotes a strong humoral response in the absence of adjuvants (Barrios et al 1992). Suzue and Young (1996) have further shown the immunogenic potential of M tuberculosis Hsp70 by immunizing mice with a recombinant human immunodeficiency virus type 1 p24-Hsp70 fusion protein. They demonstrated that covalent linking of Hsp70 to p24 was essential to elicit humoral and cellular immune responses to p24. Recently, we have also demonstrated that the Leishmania infantum Hsp70 possesses the conspicuous property of promoting strong immune responses against the maltose-binding protein (MBP) when administered genetically fused to the Hsp70 protein (Rico et al 1998). The immunostimulatory properties of the L infantum Hsp70 were observed even in athymic nu/nu mice in which immunization with the MBP-Hsp70 fusion protein triggered a detectable anti-MBP humoral response. In a more recent work, we further demonstrated that other L infantum Hsp, ie, the Hsp83, is also endowed with a similar adjuvant property and that the 2 Leishmania Hsps, Hsp70 and Hsp83, have the ability to induce in vitro proliferation of naive BALB/c mice splenocytes (Rico et al 1999). In the present work, we show data indicating that the proliferative capacity of the recombinant L infantum Hsp70 and Hsp83 proteins is related to the fact that they behave as potent B cell mitogens. Remarkably, activation of B cell proliferation by these proteins is promoted in the presence as well as in the absence of T cells.
MATERIALS AND METHODS
Mice
BALB/c (H-2d), athymic BALB/c nu/nu, and C57BL/6 (H-2b) mice were purchased from Harlan Interfauna Iberica S.A. (Barcelona, Spain) and used at the age of 8 to 10 weeks. B cell–deficient mice (μMT; Kitamura et al 1991) were kindly provided by Dr M.A. Rodríguez-Marcos (Centro de Biología Molecular).
Recombinant proteins
The L infantum Hsp70, Hsp83, and the N-terminal domain of Hsp70 were obtained as recombinant proteins fused to the Escherichia coli MBP: MBP-Hsp70, MBP-Hsp83, and MBP-Nt70, respectively. The construction of plasmids and purification of proteins have been described elsewhere (Rico et al 1999). To eliminate endotoxins, the recombinant proteins MBP-Hsp70 and MBP-Nt70 were passed through a polymyxin B-agarose column (Sigma, St Louis, MO, USA). This step was omitted in the purification of MBP-Hsp83 because of the unexpected binding of the fused protein to the polymyxin B column.
Splenocytes proliferation assays
Spleen cells were isolated and cultured in complete Roswell Park Memorial Institute (RPMI) medium (RPMI 1640 supplemented with 10% fetal calf serum [FCS], 2 mM l-glutamine, and 10 μM 2-mercaptoethanol). Lysis of erythrocytes was achieved by incubating the splenocytes in lysis buffer (150 mM ammonium chloride, 10 mM potassium hydrogen carbonate, 1 mM ethylenediaminetetraacetic acid, pH 7.4) for 2 minutes at 37°C. The cells were then washed 2 times with complete medium and counted. The splenocytes (105 cells/well) were seeded in 96-well flat-bottom microtiter plates in a final volume of 200 μL. The concentrations of the different stimuli were: recombinant proteins (MBP-Hsp70, MBP-Nt70, and MBP-Hsp83) at 12 μg/mL, concanavalin A (ConA; Sigma) at 2 μg/mL, or lipopolysaccharide (LPS; Sigma) at 2 μg/mL. Polymyxin B sulphate (Sigma) at 2 μg/mL was added to the wells containing the MBP-Hsp83 stimulus. Splenocytes were incubated for 72 hours at 37°C in 5% CO2. Sixteen hours before the end of incubation period, 1 μCi of [3H]thymidine (5 Ci/mmol; Amersham Pharmacia Biotech, Piscataway, NJ, USA) was added to each well. Cells were harvested onto glass fiber filters. The number of counts per minute (cpm) was determined in a liquid scintillation counter.
Pretreatments of stimuli
In control experiments of splenocyte proliferation, stimuli (recombinant proteins and mitogens) were pretreated with either heat or trypsin. Thermal denaturation of the stimuli was achieved heating at 100°C for 4 minutes followed by chilling in ice. Alternatively, the different stimuli were proteolytically digested by incubation for 2 hours at 37°C with trypsin attached to beaded agarose (Sigma). After incubation, the trypsin-agarose beads were eliminated by centrifugation.
Flow cytometric determinations
Splenocytes, at 106 cells/mL, were seeded in 24-well plates in the presence of the different stimuli (used at the concentrations indicated above) and were incubated at 37°C in 5% CO2. On days 1, 2, 3, 4, and 5, the cell number and viability were determined in culture aliquots by trypan blue dye exclusion. After 2 washes with phosphate-buffered saline (PBS) + 10% FCS, 2 × 105 viable cells were incubated (for 30 minutes at 4°C) in 50 μL of PBS + 10% FCS with either fluorescein isothiocyanate–labeled antimouse CD3 (1:100 dilution; PharMingen, San Diego, CA, USA) or phycoerythrin-labeled antimouse CD45R/B220 (1:250 dilution; PharMingen). After washing 2 times with PBS + 10% FCS, the cells were analyzed by flow cytometry in a fluorescence-activated cell sorter scan (Becton Dickinson, Lincoln Park, NJ, USA) using the Cell Quest software.
Stimulation of purified B cells
Spleen from BALB/c mice was elicited, and spleen cells were obtained using a cell strainer (Becton Dickinson). After lysing the red blood cells with water for 5 seconds, the cells were washed twice in RPMI and suspended in RPMI + 10% FCS. Adherent cells were depleted by incubation in polystyrene 100-mm dishes for 16 hours. T cells were depleted by incubation of nonadherent cells with 1 μg/mL of rat anti-Thy1.2 antibody (Pharmingen) per 106 cells in PBS at 4°C for 30 minutes. Cells were washed twice in PBS and incubated with sheep anti-rat IgG-coated Dynabeads M-450 (Dynal) at a bead to cell ratio 4:1 for 20 minutes at 37°C. Thy 1.2–positive cells were collected by magnetic separation. The B cell population was transferred to RPMI + 10% FCS, plated in 96-well plates at a concentration of 2 × 105 cells/well, and cultured in medium alone or in the presence of 12 μg/mL of each recombinant protein, LPS (2 μg/mL) or ConA (2 μg/mL). Also, 2 additional sets of B cell cultures with the different stimuli were prepared, in which 20 ng/mL of either interleukin (IL)-4 or IL-10 was added. Proliferation was measured by incorporation of 1 μCi [3H]thymidine per well during the last 24 hours of 3-day culture. Cells were then harvested and processed for radioactivity measuring as indicated above.
Assays were done in triplicate, and the stimulatory index was calculated dividing the arithmetic mean of cpm obtained from stimulated cultures by the arithmetic mean of cpm obtained from the control cultures without stimulation.
RESULTS
Splenocytes from naive BALB/c mice proliferate in response to L infantum Hsps
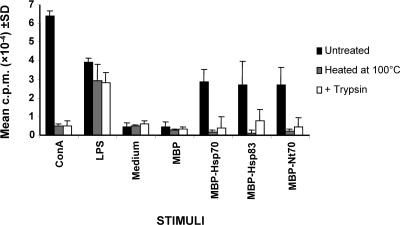
Figure 1 illustrates the capacity of the L infantum Hsp70 and Hsp83 fused to the MBP to stimulate the proliferation of splenocytes from naive BALB/c mice. But the MBP moiety did not show any proliferative capacity, indicating that the proliferative effect elicited is the result of the Leishmania Hsps. The proliferation induced by the amino-terminal domain of Hsp70 (MBP-Nt70) was also assayed because we have previously reported that this is the region of the protein responsible for the immunostimulatory properties (Rico et al 1999). Furthermore, in a recent work, within the amino-terminal domain of the M tuberculosis Hsp70, a 200–amino acid fragment has been mapped as responsible for the in vivo cytotoxic T lymphocyte response that Hsp70 fusion elicits against the fusion partner (Huang et al 2000). Heat treatment at 100°C of MBP-Hsp70, MBP-Hsp83, and MBP-Nt70 preparations abolished their splenocyte-proliferating activities as occurred with ConA, whereas the proliferative activity of LPS was not affected (Fig 1). That the proliferative effect is because of the recombinant proteins was further suggested by the fact that after proteolytic digestion with trypsin of the MBP-Hsp70, MBP-Hsp83, MBP-Nt70 preparations and of ConA no proliferation was observed. In contrast, as expected, the proliferation capacity of LPS was not abolished by the trypsin treatment. Altogether, the present data indicated that the proliferation capacity of the MBP-Hsp70, MBP-Hsp83, and MBP-Nt70 preparations must be attributed to the proteins and not to LPS contamination (Fig 1). It must be noticed, in addition, that the preparations of the recombinant proteins MBP-Hsp70 and MBP-Nt70 were passed through a polymyxin B-agarose column as the final step in their purification process. This step was omitted in the purification of MBP-Hsp83 because of the unexpected binding of this protein to the polymyxin B-agarose column. Because of this, splenocyte-proliferating assays in the presence of the MBP-Hsp83 were performed in the presence of 2 μg/mL of soluble polymyxin B.
Fig. 1.
Proliferative responses of splenocytes to various stimuli. The concentrations of the different stimuli were: ConA and LPS at 2 μg/mL; MBP, MBP-Hsp70, MBP-Nt70, and MBP-Hsp83 at 12 μg/mL. Polymyxin B at 2 μg/mL was added to the wells containing the MBP-Hsp83 stimulus. The different stimuli were untreated, denatured at 100°C, or treated with trypsin before being added to the splenocyte cultures. Splenocytes were incubated for 72 hours at 37°C in 5% CO2. Values represent the mean counts per minute and standard deviations of triplicate samples and are representative of 3 experiments. ConA, concanavalin A; LPS, lipopolysaccharide; MBP, maltose-binding protein; Hsp, heat shock protein; Nt, N-terminal domain
Leishmania Hsps behave as B cell mitogens
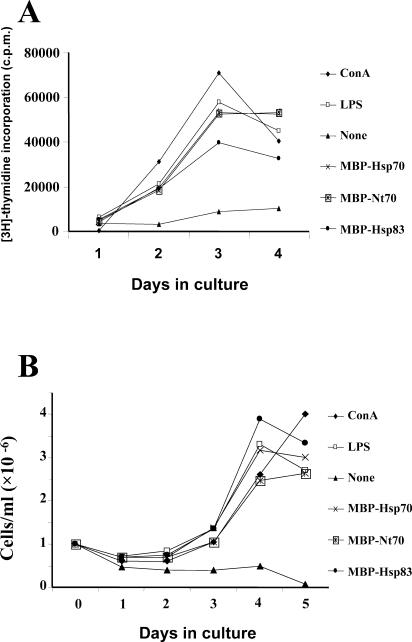
Although [3H]-thymidine incorporation is a measurement of DNA synthesis, and it is often used to indicate lymphocyte proliferation, we wanted to ensure that the proliferation shown in Figure 1 was associated with an increase in the number of cells. Thus, the number of splenocytes was also determined after stimulation with the L infantum Hsps. As shown in Figure 2A, after incubation of the splenocytes (106 cells/mL) with the MBP-Hsp70, MBP-Nt70, or MBP-Hsp83 proteins, a maximum of [3H]-thymidine incorporation was observed on days 3–4. For comparison, the kinetics of [3H]-thymidine incorporation in splenocytes stimulated with the mitogens ConA and LPS were also determined. It was observed that the time course of [3H]-thymidine incorporation in the cultures stimulated with the ConA and LPS mitogens was similar to that of the cells stimulated with the recombinant proteins.
Fig. 2.
Kinetics of splenocyte proliferations. Splenocytes were incubated with the different stimuli (ConA, LPS, MBP-Hsp70, MBP-Nt70, and MBP-Hsp83 used at the concentrations described in Fig 1). [3H]-Thymidine incorporation (A) and viable cell numbers (B) were determined in the cultures on the indicated days. ConA, concanavalin A; LPS, lipopolysaccharide; MBP, maltose-binding protein; Hsp, heat shock protein; Nt, N-terminal domain
An increase in the number of splenocytes followed the peak of [3H]-thymidine incorporation after stimulation with either Hsps or mitogens (Fig 2B). But differences in kinetic profile were observed: although the number of splenocytes stimulated with MBP-Hsp70, MBP-Nt70, MBP-Hsp83, and LPS reached a plateau on days 4–5, the number of ConA-stimulated splenocytes continued growing on day 5. This fact was taken as an indication that the cell population stimulated by the ConA treatment could be of a different origin than that stimulated by the Hsps and LPS preparations. This hypothesis was supported by previous results that showed that the splenocyte proliferation induced by Leishmania Hsps is less sensitive to cyclosporin A, a specific inhibitor of T cell activation, than the proliferation induced by ConA (Rico et al 1999).
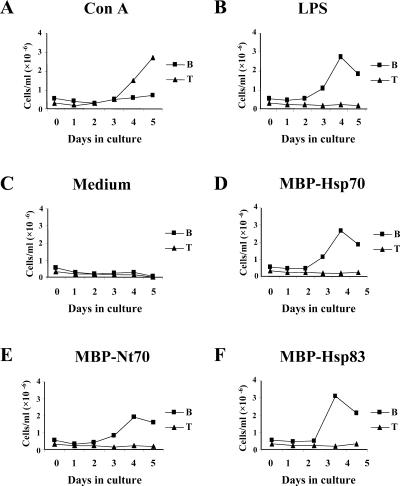
B and T lymphocytes are the most abundant cells present in the murine spleen accounting for about 90% of the total number of cells (Baumgarth 2000). To identify the subset that was proliferating in response to Leishmania Hsps, splenocytes from BALB/c mice were labeled with fluorophore-conjugated antibodies against T cell– or B cell–specific markers. Afterward, the percentages of lymphocyte subpopulations were determined by flow cytometry on different days of stimulation (Fig 3). In BALB/c untreated animals, we determined that 31% of the cells were CD3+ positive and that 56% were B220+ positive. An increase in CD3+ cells (accounting for 68% after 120 hours of culture) was observed in ConA-stimulated splenocytes, as expected for a T cell mitogen. In contrast, after LPS stimulation, the percentage of B220+ cells increased to 83% after 96 hours of culture, as expected from a B cell–specific mitogen. Interestingly, stimulation of splenocytes with MBP-Hsp70, MBP-Nt70, or MBP-Hsp83 promoted an average increase in B220+ cells similar to that induced by LPS. The stimulation with the Leishmania Hsps did not alter significantly the total number of CD3+ cells (Fig 3). Thus, the results suggested that the main spleen cell population proliferating in response to Leishmania Hsps is constituted by B lymphocytes.
Fig. 3.
Determination of B cell and T cell populations in splenocyte cultures after stimulation with the different stimuli. After incubation during the indicated days with ConA (A), LPS (B), none (C), MBP-Hsp70 (D), MBP-Nt70 (E), and MBP-Hsp83 (F), cells were labeled with either anti-CD3 (T cells) or anti-B220 (B cells) and were analyzed by flow cytometry to determine the percentage of T and B cells. The numbers of B and T cells in the splenocyte cultures were obtained by multiplying the percentages of CD3+ and B220+, respectively, and the total number of viable cells as determined by trypan blue staining. ConA, concanavalin A; LPS, lipopolysaccharide; MBP, maltose-binding protein; Hsp, heat shock protein; Nt, N-terminal domain
Leishmania Hsp70 and Hsp83 are T cell–independent mitogens
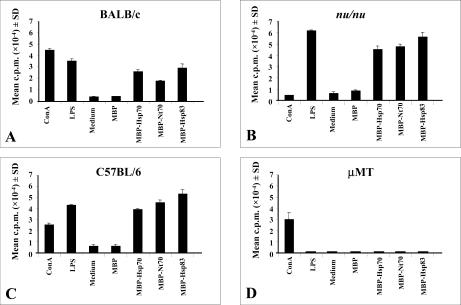
To confirm that the B lymphocytes were the cell type responsible for the proliferation observed after incubation with L infantum Hsps, spleen cells from either B cell–deficient or T cell–immunodeficient mice were cultured in the presence of several stimuli (Fig 4). Because the μMT mice, lacking B lymphocytes (Kitamura et al 1991), were generated on the C57BL/6 genetic background, we considered it interesting to analyze the proliferation capacity of the L infantum Hsps on splenocytes from C57BL/6 mice. The results presented in Figure 4 show that spleen cells from mice of either BALB/c or C57BL/6 backgrounds responded to MBP-Hsp70, MBP-Nt70, and MBP-Hsp83 with similar proliferation indexes, demonstrating that the response is not influenced by genetic variability between different inbred strains.
Fig. 4.
Proliferation of spleen cells from different mouse strains induced by the stimuli. Spleen cells from BALB/c (A), BALB/c nu/nu (B), C57BL/6 (C), and B cell–deficient μMT (D) were stimulated with ConA (2 μg/mL), LPS (2 μg/mL), MBP (12 μg/mL), MBP-Hsp70 (12 μg/mL), MBP-Nt70 (12 μg/mL), and MBP-Hsp83 (12 μg/mL) for 72 hours. [3H]-Thymidine was added for the last 16 hours of culture. The data represent the mean plus standard deviations of triplicate cultures and are representative for 2 to 5 experiments. ConA, concanavalin A; LPS, lipopolysaccharide; MBP, maltose-binding protein; Hsp, heat shock protein; Nt, N-terminal domain
Splenocytes from BALB nu/nu mice, lacking mature T lymphocytes, showed significant proliferation rates after stimulation with LPS, MBP-Hsp70, MBP-Nt70, and MBP-Hsp83 but did not proliferate in the presence of ConA (Fig 4B). These data suggest that stimulatory properties of Hsp70 and Hsp83 are T cell–independent. In contrast, assays carried out with spleen cells from μMT mice showed that none of the recombinant proteins was able to induce proliferation. As expected, splenocytes from μMT mice were actively stimulated in the presence of ConA (Fig 4D). In conclusion, the present data, altogether, indicate that the L infantum Hsps are T cell–independent activators of murine B lymphocytes.
Leishmania Hsps induce proliferation of purified B cells
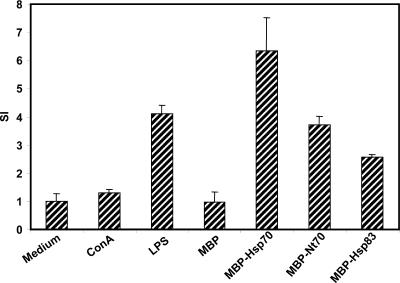
We next tried to determine whether the mitogenic effect of MBP-Hsp70, MBP-Nt70, and MBP-Hsp83 on B cells is dependent on accessory cells. For this purpose, splenocytes were depleted of adherent and T cells, and the resulting B cell population was incubated with the different stimuli (Fig 5). Remarkably, we found that Leishmania Hsps, and mainly Hsp70, stimulated proliferation of these B cell–enriched cultures, suggesting that these proteins exert a direct proliferative effect on B cells. Finally, we assayed whether IL-4 and IL-10, as activators of B cell growth, were able to further stimulate the Hsps-induced B cell proliferation. But we found that B cell proliferation induced by Leishmania Hsps was not increased after addition of IL-4 or IL-10 to the cultures (data not shown), suggesting that these cytokines are not required for the B cell stimulation with the Leishmania proteins.
Fig. 5.
Proliferation of purified B cells in the presence of Leishmania Hsps. B cells, purified by negative selection from BALB/c mice spleen, were cultured at 2 × 105 cells per well and stimulated with ConA (2 μg/mL), LPS (2 μg/mL), MBP (12 μg/mL), MBP-Hsp70 (12 μg/mL), MBP-Nt70 (12 μg/mL), and MBP-Hsp83 (12 μg/mL) for 72 hours. [3H]-Thymidine was added for the last 24 hours of culture. Results are presented as the stimulatory index and standard deviations of triplicate cultures of B cells stimulated with the different stimuli
DISCUSSION
In a previous work, we have reported that the L infantum Hsps, Hsp70, and Hsp83, expressed as recombinant proteins fused to the Escherichia coli MBP, behave as potent activators of proliferation for BALB/c splenocytes (Rico et al 1999). This study was undertaken to determine the spleen cell subpopulation responding to Leishmania Hsp70 and Hsp83. Among the proliferating lymphocytes stimulated by these molecules, a significant increase in the percentage of B220+ cells was determined by flow cytometry analysis. Accordingly, these molecules did not stimulate the proliferation of splenocytes from B cell–deficient mice. Thus, the data demonstrate that these Leishmania Hsps represent a new class of mitogens for murine B lymphocytes. Moreover, the fact that similar, or even higher, levels of proliferation were induced by the Leishmania Hsps in splenocytes of athymic BALB/c mice, compared with those induced in splenocytes of BALB/c mice (Fig 4), is indicative of a T cell–independent mitogenic activity. Even more, as shown in Figure 5, the proliferation of B cells occurs in the absence of T lymphocytes and adherent cells, suggesting a direct effect of Leishmania Hsps on B lymphocytes.
Because LPS is a well-known B cell mitogen and the recombinant proteins used in this study were obtained from gram-negative bacterial cultures, we considered the possibility that the splenocyte proliferation observed could be due to LPS contamination. Three experimental approaches suggest that LPS is not responsible for the splenocyte proliferation observed in response to these recombinant protein preparations (Fig 1). First, the mitogenic activity was not abolished by either polymyxin-agarose purification (for preparations of MBP-Hsp70 and MBP-Nt70) or coincubation with polymyxin (for MBP-Hsp83). Second, thermal denaturation of the protein preparations resulted in the loss of their mitogenic activity, whereas this treatment did not alter the mitogenic capacity of LPS. Third, after proteolytic treatment, the protein preparations were not able to stimulate the proliferation of splenocytes, whereas the trypsin-treated LPS maintained the potential to induce proliferation in these cells. In addition, we have previously reported that the capacity of these Leishmania Hsps to induce splenocyte proliferation is inhibited by specific antibodies (Rico et al 1999). Altogether, these studies converge in the idea that the mitogenic activity is a property of these Leishmania Hsps.
The question that arises from our results is what might be the biological role that the L infantum Hsp70 and Hsp83 proteins may play as immunostimulatory agents during host-parasite interactions. It is known that LPS present on bacterial walls (Vos et al 2000) as well as unmethylated CpG-containing DNA (Liang and Lipsky 2000) are well-characterized B cell activators. Also, in the last few years, several proteins from pathogenic organisms have been described as potent mitogens of B lymphocytes. Examples of such mitogens are the 13-kDa protein fraction (called ISTF) of Actinobacillus actinomycetemcomitans (Jeong et al 2000), the exoenzyme S of Pseudomonas aeruginosa (Barclay et al 1999), and the rTc24 antigen and the proline racemase of Trypanosoma cruzi (Cordeiro da Silva et al 1998; Reina-San-Martín et al 2000b). The hypothesis that these molecules are used by the pathogens to weaken the host immune response and that these are of central importance in the pathogenesis caused by the infection has been proposed (Reina-San-Martín et al 2000a). In this regard, it is worthy to note that infection with L major increases the splenic B220+ B cell subset in BALB/c mice (Palanivel et al 1996). In line with this result, it can be speculated that Hsp70 and Hsp83 can serve Leishmania to deviate the immune response into a nonspecific activation of immune cells.
On the other hand, this is not the first time that the proliferation-inducing properties of Hsps have been described. In an interesting work, Marañón et al (2000) have shown that the T cruzi Hsp70 recombinant protein has the capacity to stimulate splenocytes and lymph node cells from naive mice in a non–haplotype-restricted way. The phenotype of the expanded cells was characterized as CD3+ TCRαβ+ CD4+. In addition, it has been shown that the recombinant Hsp70 from M tuberculosis has the property to induce proliferation on spleen cells from unprimed mice (Bonorino et al 1998). Although not shown, the authors indicate that the populations that increase in frequency on incubation with the mycobacterial Hsp70 are γδT cells and B cells. Remarkably, the proliferation of natural killer cells from healthy humans has been shown to be stimulated by the human recombinant Hsp70 (Multhoff et al 1999). In fact, we and others have found that the human recombinant Hsp70 is also a potent activator of proliferation of splenocytes from BALB/c mice (Marañón et al 2000; S. Iborra, C. Alonso and J.M. Requena, laboratory data).
In recent years, an increasing number of immunostimulatory activities other than proliferative-inducing responses are being ascribed to the mammalian Hsp70 and Hsp83/90 protein families. Among these activities the stimulation of macrophages to secrete cytokines and the activation of antigen-presenting and costimulatory molecules on dendritic cells (Basu et al 2000; Singh-Jasuja et al 2000; Kuppner et al 2001), the in vivo maturation and migration of CD11c+ (Binder et al 2000a), and the activation of T cells (Breloer et al 1999) are worth mentioning. All these effects are produced through specific interactions with surface receptors on target cells (Arnold-Schild et al 1999; Binder et al 2000b; Castellino et al 2000; Sondermann et al 2000). In addition, it has been shown that bacterial Hsps directly induce cytokine secretion in macrophages (Retzlaff et al 1994), monocytes, and endothelial cells (Galdiero et al 1997). These findings have led to postulate that Hsps, either derived from infectious organisms or released during tissue damage, are acting as danger signals, whose abnormal presence would turn on the immune response (Todryk et al 2000).
In view of all these data, we think that the Leishmania Hsps could play 2 alternative roles during parasite invasion. On the one hand, the Leishmania Hsp70 and Hsp83, as a “parasite's strategy,” have evolved acquiring the ability to deviate immune responses into a nonspecific activation of immune cells leading to immunosuppression. On the other hand, given their evolutionary conservation they could behave as general “danger molecules” that the mammalian immune system recognizes to trigger the initiation of an immune response against the invading pathogen. It is likely that factors like antigen concentration, the type of Hsp-recognizing cells, and the cytokine balance in the infection microenvironment may be determinant in the development of either one or the other antagonistic function.
Acknowledgments
We thank Dr M.A. Rodríguez-Marcos (Centro de Biología Molecular, Madrid, Spain) for providing B cell–deficient mice. A.I.R. was the recipient of a doctoral fellowship from the Ministerio de Educación y Ciencia. This research was supported by grants 1FD97-0630-C02-0 and BIO99-1133 from Plan Nacional de I+D, 08.2-0013-1/99 from Comunidad Autónoma de Madrid, and 00/0831 from Fondo de Investigaciones Sanitarias. An institutional grant and direct support to the project from Fundación Ramón Areces are acknowledged.
REFERENCES
- Anderson KM, Srivastava PK. Heat, heat shock, heat shock proteins and death: a central link in innate and adaptive immune responses. Immunol Lett. 2000;74:35–39. doi: 10.1016/s0165-2478(00)00246-7. [DOI] [PubMed] [Google Scholar]
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee H-G, de la Salle H, Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- Asea A, Kraefi S-K, Kurt-Jones EA, Stevenson MA, Chien LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Barclay NG, Spurrell JCL, Bruno TF, Storey DG, Woods DE, Mody CH. Pseudomonas aeruginosa exoenzyme S stimulates murine lymphocyte proliferation in vitro. Infect Immun. 1999;67:4613–4619. doi: 10.1128/iai.67.9.4613-4619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios C, Lussow AR, and Van Embden J. et al. 1992 Mycobacterial heat-shock proteins as carrier molecules. II: the use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guerin priming. Eur J Immunol. 22:1365–1372. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000;176:171–180. doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Anderson KM, Basu S, Srivastava PK. Heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000a;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000b;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- Bonorino C, Nardi NB, Zhang X, Wysocki LJ. Characteristics of the strong antibody response to mycobacterial hsp70: a primary, T cell-dependent IgG response with no evidence of natural priming of gammadelta T cell involvement. J Immunol. 1998;161:5210–5216. [PubMed] [Google Scholar]
- Breloer M, Fleischer B, von Bonin A. In vivo and in vitro activation of T cells after administration of Ag-negative heat shock proteins. J Immunol. 1999;162:3141–3147. [PubMed] [Google Scholar]
- Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro da Silva A, Espinoza AG, Taibi A, Ouaissi A, Minoprio P. A 24000 MW Trypanosoma cruzi antigen is a B-cell activator. Immunology. 1998;94:189–196. doi: 10.1046/j.1365-2567.1998.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero M, Cipollaro de L'Ero G, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect Immun. 1997;65:699–707. doi: 10.1128/iai.65.2.699-707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Richmond JFL, Suzue K, Eisen HN, Young RA. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4+ T cell independent. J Exp Med. 2000;191:404–408. doi: 10.1084/jem.191.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SJ, Yee ST, and Jo WS. et al. 2000 A novel factor isolated from Actinobacillus actinomycetemcomitans stimulates mouse B cells and human peripheral blood mononuclear cells. Infect Immun. 68:5132–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SHE. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Köhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin my chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kuppner MC, Gastpar R, Gelwer S, Nossner E, Ochmann O, Scharner A, Issels RD. The role of heat shock protein (hsp70) in dendritic cell maturation: Hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602–1609. doi: 10.1002/1521-4141(200105)31:5<1602::AID-IMMU1602>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Liang H, Lipsky PE. Responses of human B cells to DNA and phosphorothioate oligodeoxynucleotides. Curr Top Microbiol Immunol. 2000;247:227–240. doi: 10.1007/978-3-642-59672-8_16. [DOI] [PubMed] [Google Scholar]
- Marañón C, Planelles L, Alonso C, López MC. HSP70 from Trypanosoma cruzi is endowed with specific cell proliferation potential leading to apoptosis. Int Immunol. 2000;12:1685–1693. doi: 10.1093/intimm/12.12.1685. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Mizzen L, and Winchester CC. et al. 1999 Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 27:1627–1636. [DOI] [PubMed] [Google Scholar]
- Palanivel V, Posey C, Horauf AM, Solbach W, Piessens WF, Harn DA. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp Parasitol. 1996;84:168–177. doi: 10.1006/expr.1996.0102. [DOI] [PubMed] [Google Scholar]
- Reina-San-Martín B, Cosson A, Minoprio P. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol Today. 2000a;16:62–67. doi: 10.1016/s0169-4758(99)01591-4. [DOI] [PubMed] [Google Scholar]
- Reina-San-Martín B, Degrave W, and Rougeot C. et al. 2000b A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat Med. 6:890–897. [DOI] [PubMed] [Google Scholar]
- Retzlaff C, Yamamoto Y, Hoffman PS, Friedman H, Klein TW. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infect Immun. 1994;62:5689–5693. doi: 10.1128/iai.62.12.5689-5693.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico AI, Angel SO, Alonso C, Requena JM. Immunostimulatory properties of the Leishmania infantum heat shock proteins HSP70 and HSP83. Mol Immunol. 1999;36:1131–1139. doi: 10.1016/s0161-5890(99)00136-4. [DOI] [PubMed] [Google Scholar]
- Rico AI, del Real G, Soto M, Quijada L, Martinez-A C, Alonso C, Requena JM. Characterization of the Immunostimulatory properties of Leishmania infantum HSP70 by fusion to the Escherichia coli maltose-binding protein in normal and nu/nu BALB/c mice. Infect Immun. 1998;66:347–352. doi: 10.1128/iai.66.1.347-352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Jasuja H, Hilf N, Scherer HU, Arnold-Schild D, Rammensee HG, Toes REM, Schild H. The heat shock protein gp96: a receptor-targeted cross-priming carrier and activator of dendritic cells. Cell Stress Chaperons. 2000;5:462–470. doi: 10.1379/1466-1268(2000)005<0462:thspga>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Becker T, Mayhew M, Wieland F, Hartl FU. Characterization of a receptor for heat shock protein 70 on macrophages and monocytes. Biol Chem. 2000;381:1165–1174. doi: 10.1515/BC.2000.144. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- Suzue K, Young RA. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873–879. [PubMed] [Google Scholar]
- Todryk SM, Melcher AA, Dalgleish AG, Vile RG. Heat shock proteins refine the danger theory. Immunology. 2000;99:334–337. doi: 10.1046/j.1365-2567.2000.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos Q, Lees A, Wu Z-Q, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: an integrated view. Immunol Today. 2000;21:129–132. doi: 10.1016/s0167-5699(99)01558-3. [DOI] [PubMed] [Google Scholar]
- Zügel U, Kaufmann SHE. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]