Abstract
Previously, we reported that the major stress-inducible heat shock protein 70 (Hsp70) acts as a recognition structure for natural killer (NK) cells, if localized on the cell surface of tumor cells. Incubation of purified NK cells with low-dose interleukin (IL)-2 (100 IU/mL) plus recombinant Hsp70-protein or the immunogenic 14-mer Hsp70-peptide TKDNNLLGRFELSG450–463, termed TKD (2 μg/mL), enhances the cytolytic activity against Hsp70 membrane-positive (CX+) but not against Hsp70-negative (CX−) tumor cells. Here, we show that the cytolytic activity against Hsp70-positive tumor cells is inducible by incubation of unseparated peripheral blood mononuclear cells (PBMNC) with low-dose IL-2 plus TKD. Cell sorting experiments revealed that within the PBMNC population CD94+/CD3− NK cells, and not CD94−/CD3+ T cells, mediate the cytotoxic activity against Hsp70-positive tumor cells. The antitumoral effect of PBMNC stimulated either with IL-2 plus TKD or with IL-2 alone was assessed in tumor-bearing severe combined immunodeficiency/beige mice. A single intravenous (iv) injection of 40 × 106 IL-2 plus TKD-stimulated PBMNC (containing 5.2 × 106 NK cells) on day 4 results in a 60% reduction in tumor size, from 3.89 g to 1.56 g. In contrast, the adoptive transfer of the identical amount PBMNC stimulated with low-dose IL-2 only (containing 4.4 × 106 NK cells) reduces the tumor size only less than 10% (3.64 g). A phenotypic characterization of the excised tumors revealed that predominantly Hsp70-positive tumor cells were eliminated by TKD-activated PBMNC. Kinetic studies demonstrate that the in vivo cytolytic capacity of TKD-stimulated PBMNC is dependent on the effector to target cell ratio. An iv injection of effector cells on day 1 or 2 after tumor cell inoculation results in significantly smaller tumors (0.77 g or 0.89 g) on day 21 as compared with mice that were immunoreconstituted on day 4 or 8 (1.39 g or 2.23 g). The tumor size of nonimmunoreconstituted control animals was 3.55 g.
INTRODUCTION
Heat shock proteins (Hsp) are highly conserved proteins that chaperone other proteins and nascent polypeptides during synthesis, folding, unfolding, assembly, and translocation (Frydman et al 1994; Hartl 1996). Besides their intracellular chaperoning function, Hsps with a molecular weight of 70 kDa in the extracellular milieu or present on the cell surface have been shown to elicit a potent antitumor immune response mediated by T cells, macrophages, and natural killer (NK) cells (Multhoff et al 1997; Asea et al 2000; Dressel et al 2000; Clark and Menoret 2001). Environmental stress, including heat shock and chemicals, renders tumor cells more sensitive to kill mediated by CD3 negative NK cells (Rabinovich et al 2000), by an increase in the amount of cell membrane–bound Hsp70 (Botzler et al 1996). Previously, we demonstrated that a single intravenous (iv) injection of 10 × 106 isolated NK cells in tumor-bearing mice with severe combined immunodeficiency (SCID/beige) completely suppresses growth of Hsp70-positive tumors (Multhoff et al 2000). SCID/beige mice are ideal hosts for the engraftment of human tumors and for an adoptive transfer using human effector cells (Mosier et al 1988) because they are lacking in functional, mature T and B cells, due to a defect in the deoxyribonucleic acid double-strand break repair system that affects the V(D)J recombination (Blunt et al 1995), and in NK cells (Talmadge et al 1980). In vitro, we assessed that incubation of purified NK cells with low-dose interleukin (IL)-2 plus recombinant Hsp70-protein (Multhoff et al 1999) or Hsp70-peptide TKDNNLLGRFELSG450–463 (TKD), derived thereof (Multhoff et al 2001), results in an enhanced cytolytic activity against Hsp70-positive tumors. It appeared that transient plastic adherent NK cells, but not T cells, mediate the cytotoxic response against Hsp70-positive tumor cells (Multhoff et al 1995a). This Hsp70-reactive NK cell subpopulation reveals an increased expression of the C-type lectin NK cell receptor CD94 and the NK cell markers CD57 and CD56 and exhibits a CD16 “dim” phenotype (Multhoff et al 1999). By a modified adherence selection method, previously described by Vujanovic et al (1993), this Hsp70-reactive NK cell subpopulation could be enriched (Multhoff et al 1997, 1999). But because of the purification procedures, including monocyte depletion and adherence selection, a number of cells get lost. With respect to the applicability of Hsp70-reactive NK cells in tumor therapy, we addressed the question whether it is possible to stimulate these NK cells by incubating unseparated peripheral blood mononuclear cells (PBMNC) with low-dose IL-2 plus the immunostimulatory Hsp70-peptide TKD. Furthermore, we were interested in knowing whether these effector cells mediate a tumor immune response in tumor-bearing SCID/beige mice.
MATERIALS AND METHODS
Tumor cell lines
The human colon carcinoma sublines CX+ and CX− and the original cell line CX2 (TZB 610005, Tumorzentrum Heidelberg, Heidelberg, Germany) were cultured at 37°C in 5% CO2 in Roswell Park Memorial Institute (RPMI)-1640 medium (GIBCO, Eggenstein, Germany) supplemented with heat-inactivated 10% fetal calf serum (FCS) (GIBCO), 2 mM l-glutamine, and antibiotics (penicillin-streptomycin). To keep the cell lines under exponential growth conditions, the cells were harvested regularly every 3 days with trypsin-ethylenediaminetetraacetic acid and seeded in fresh medium at a density of 0.5 × 106 cells/5 mL. All experiments were performed with cells between passages 10 and 40.
Cell lines were free from mycoplasma contamination as determined by repeated testing using the 6-methylpurin desoxyribosid assay (Boehringer-Mannheim, Mannheim, Germany).
Ex vivo activation of PBMNC with Hsp70-peptide TKD
TKD is a 14-mer sequence of the C-terminal substrate-binding domain of human Hsp70. It contains the 8-mer antibody-binding epitope NLLGRFEL454–461 of the Hsp70-specific monoclonal antibody (mAb) (clone C92F3B1) and exhibits immunostimulatory activity on NK cells (Multhoff et al 2001). TKD was kindly synthesized by Dr Manfred Eulitz (GSF—Institute of Molecular Immunology, Munich, Germany). The purity of TKD was determined by the Limulus Amebocyte Lysate Kit (QCL-1000, BioWhittaker, Walkersville, MD USA).
PBMNC were isolated from leukapheresis products derived from healthy human volunteers by Ficoll (PAA, Linz, Austria) density-gradient centrifugation. After 2 washing steps in RPMI-1640 medium, cells were counted and resuspended. With respect to the results obtained with purified NK cells (Multhoff et al 1999), 10 × 106 PBMNC/mL were incubated in RPMI-1640 medium supplemented with low-dose IL-2 (100 IU/mL) plus 2 μg/mL Hsp70-peptide TKD or with low-dose IL-2 alone for 4 days at 37°C. To avoid cell aggregates during cultivation, the cells were transferred into sterile 250-mL Teflon bags (VueLife, CellGenix, Freiburg, Germany) that were gently moved upside-down on a cellroll machine (Integra, Fernwald, Germany) during the ex vivo incubation period. After 2 washing steps in RPMI-1640 medium, the cells were counted and resuspended in fresh medium.
Separation of CD94 positive effector cells
IL-2 plus TKD-stimulated PBMNC were separated on day 4 by positive selection using an antibody directed against CD94 (HP3-D9, Immunotech, Marseille, France) and by the standard Miltenyi phycoerythrin (PE) separation kit (488-0, Miltenyi, Bergisch Gladbach, Germany). Briefly, 0.5 × 106 cells/mL were incubated with 10 μL of anti-CD94 mAb for 30 minutes at 4°C. After incubation with PE-conjugated beads for 15 minutes at 4°C and washing in bovine serum albumin–containing magnetic cell sorting (MACS) buffer, CD94 positive and CD94 negative cells were separated on a LSVS column (424-01, Miltenyi). After washing the cells and a recovery period of 12 hours in the incubator, cells were used for further experiments. Following the same protocol, a negative NK cell separation was performed using the mAbs CD14, CD3, and CD19 to deplete monocytes, T cells, and B cells.
Flow cytometry
The amount of T and NK cells in the PBMNC was determined by flow cytometric analysis. Briefly, fluorescein-conjugated mAb (CD3FITC/CD16/CD56PE, Becton Dickinson, Heidelberg, Germany) and the anti-killer cell receptor (KIR) CD94 mAb (HP3-D9, Immunotech) were added to cell suspensions (0.1 × 106 cells), incubated for 20 minutes on ice, washed, and propidium iodide–negative, viable cells were analyzed on a FACSCalibur instrument (Becton, Dickinson, BD Biosciences, San Jose, CA, USA).
Indirect immunofluorescence studies were performed with tumor cells using Hsp70-specific mAb (clone C92F3B1), isotype-matched negative control antibodies (Immunotech), anti-CD3 (OKT3, Ortho Biotech, NJ, USA), anti-CD16 (3G8, Immunotech), anti-CD56 (NCAM16.2, Pharmingen BD, Heidelberg, Germany), and a human leukocyte antigen class I–specific antibody (IgG2a, W6/32, Becton, Dickinson) as primary reagents and a secondary PE-conjugated rabbit anti-mouse antibody (Dako, Hamburg, Germany). The cells were stained with the primary and secondary reagents for 30 minutes at 4°C, as described above. The percentage of positively stained cells was defined as the number of specifically stained, propidium iodide–negative, viable cells minus the number of cells stained with an isotype-matched control antibody on a FACSCalibur instrument (Becton, Dickinson).
CX2-derived subclones (65% Hsp70 cell-surface expression; patent 196 47 426.4-41) were as follows: CX+, >90% Hsp70 cell-surface expression; CX−, <20% Hsp70 cell-surface expression (Multhoff et al 1997).
51Cr cytotoxicity assay
Cytolytic activity of either unseparated or separated IL-2 plus Hsp70-peptide TKD and IL-2–stimulated PBMNC was monitored in a standard 51Cr assay (MacDonald et al 1974). Dilutions of the effector cells were incubated with 51Cr-labeled (100 μCi of NaCr51O4, NEN-Dupont, Boston, MA, USA) tumor target cells (3 × 103 cells/well) in duplicates at a final volume of 200 μL of RPMI-1640 medium supplemented with 10% FCS at 37°C for 4 hours in 96-well U-bottom plates (Greiner, Nuertingen, Germany). After the incubation period, supernatants were collected, and the radioactivity was counted in a γ-counter (Packard Instruments, Perkin Elmer Life Sciences, Boston, MA, USA).
The percentage of specific lysis was determined according to the equation:
| (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. The percentage of spontaneous release of all tumor target cells used in the assays was always less than 20%. |
SCID/beige mice
Pathogen-free, 8- to 10-week-old female and male CB-17 IcrHsd scid/scid (SCID/beige, BALB/c) mice were obtained from an animal breeding colony (Harlan Winkelmann, Borchen, Germany) and bred in IVC microisolator cages (Tecniplast, Waldkraiburg, Germany) at the animal facility of the University Hospital Regensburg, kindly provided by the Bartling Stiftung. Mice were maintained under sterile conditions and given autoclaved food and water. All manipulations were carried out in an air-filtered laminar flow hood (Gelaire, Meckenheim, Germany).
Tumor cell inoculation and engraftment with PBMNC
Mice were injected into the intraperitoneal (ip) cavity with single-cell suspensions of exponentially growing human CX2 tumor cells (2.5 × 106 in 100 μL of sterile RPMI-1640 medium). The Hsp70 phenotype of the injected tumor cells was determined by flow cytometry.
On days 1, 2, 4, and 8 after tumor cell inoculation, ex vivo–activated, viable human PBMNC (40 × 106 cells) were injected intravenously in a total volume of 500 μL of RPMI-1640 medium containing 100 IU/mL IL-2 into the tail vein. The in vitro cytolytic activity of the effector cells was determined in a standard 51Cr release assay against Hsp70-positive (CX+) and -negative (CX−) tumor target cells. The amount of T and NK cells was determined by flow cytometry as described above.
Autopsy
On day 21 after ip injection of either tumor cells alone (2.5 × 106 cells in 100 μL of RPMI-1640) or tumor cells plus effector cells (iv), mice were sacrificed by cervical dislocation. As a parameter of health, the complete body weight of each mouse was determined. After careful macroscopic inspection, the complete tumor was excised from the ip cavity with a scalpel under sterile conditions, and the weight of each individual tumor was determined, separately. After cutting into pieces, single-cell suspensions were prepared by gentle teasing of the tissues in sterile RPMI-1640 medium and passing it through a sterile wire mesh (0.45-μm pores). The Hsp70 membrane expression was determined on freshly isolated, viable (propidium iodide negative) single-cell suspensions of each tumor by flow cytometry.
Statistical analysis
The statistical significance of the differences was determined by the Student's t-test. Differences were considered as significant for P < 0.05.
RESULTS
In vitro activity of Hsp70-peptide–stimulated PBMNC
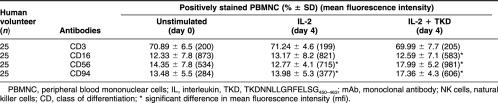
Membrane-bound Hsp70 acts as a target recognition structure for NK cells but not for T cells (Multhoff et al 1995). The sublines CX+ and CX− that were originally derived from the human colon carcinoma cell line CX2 differ profoundly with respect to their capacity to express Hsp70 on the plasma membrane (Multhoff et al 1997) and, thus, provide ideal target cells for the definition of the Hsp70 specificity of effector cells. In vitro, the cytolytic activity against Hsp70 could be significantly enhanced by preincubation of purified NK cells with a 14-mer Hsp70-peptide TKD (2 μg/mL) plus low-dose IL-2 (100 IU/mL) (Multhoff et al 2001). Concomitant with the enhanced cytolytic activity, the CD94, CD57, and CD56 expressions were up-regulated, and the CD16 expression was down-regulated on NK cells. In the present study, we asked the question whether the Hsp70 reactivity could be stimulated in unseparated PBMNC. In accordance with the protocol for purified NK cells, PBMNC of healthy human volunteers were incubated either with IL-2 alone or with IL-2 plus Hsp70-peptide TKD for 4 days at a cell density of 10 × 106. Cell-surface markers including the T cell marker CD3 and the NK cell markers CD16, CD56, and CD94 were determined on day 0 before stimulation and on day 4 after stimulation with IL-2 plus Hsp70-peptide TKD. As summarized in Table 1, after stimulation with either low-dose IL-2 or IL-2 plus Hsp70-peptide TKD, PBMNC derived from 25 different human volunteers revealed no significant differences in the amount of CD3 positive T cells. An incubation with IL-2 plus TKD results in a slight increase in the amount of CD56 and CD94 positive NK cells from 14.35 to 17.99 and from 13.48 to 17.36, respectively. But with respect to the mean fluorescence intensity (mfi) significant changes were observed: after incubation with IL-2 plus TKD, the mfi of CD16 dropped from 873 to 583. In contrast, mfi of the NK markers CD56 and CD94 increased from 534 to 981 and from 284 to 606, respectively. These results are in line with previous data obtained with purified NK cells after treatment with Hsp70-protein (Multhoff et al 1999).
Table 1.
Phenotypic characterization of unstimulated (day 0) peripheral blood lymphocytes (PBMNC) stimulated either with IL-2 (100 IU/mL) only, or with IL-2 plus Hsp70-peptide TKD (2 μg/mL), for 4 days. The cells were stained with the following mAb directed against T and NK cell-specific markers: CD3, CD16, CD56, and CD94. Flow cytometric analysis was performed using propidium iodide negative-viable cells of 25 different human volunteers
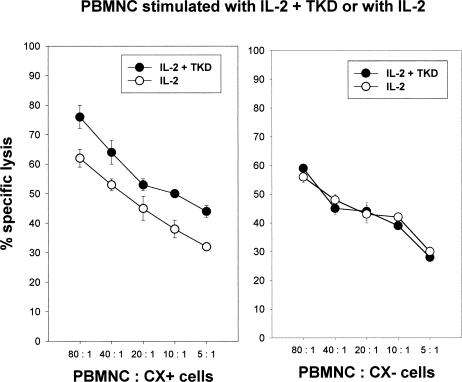
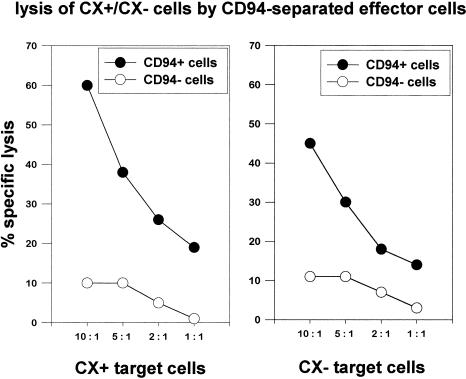
Concomitant with the determination of the phenotype by flow cytometry, the cytolytic activity of PBMNC stimulated with either IL-2 plus TKD or IL-2 alone was determined in a standard 51Cr cytotoxicity assay, using human colon carcinoma sublines CX+ and CX− cells as tumor target cells (Multhoff et al 1997). In 20 of the 25 cases, Hsp70-peptide TKD-activated PBMNC revealed a 5% to 10% increase in lysis of Hsp70 membrane-positive CX+ tumor cells. The lytic activity against CX− tumor cells always remained unaltered (data not shown). Figure 1 represents the mean data derived from cytotoxicity assays, using PBMNC derived from 4 different donors, which had been used for in vivo mouse assays. PBMNC had been stimulated for 4 days with either IL-2 (100 IU/mL) plus Hsp70-peptide TKD (2 μg/mL) or IL-2 alone. The amount of NK cells in PBMNC, as determined by phenotype characterization using CD56 and CD94 mAbs, in these experiments was 13% (9–18%) for IL-2 plus TKD stimulation and 11% (8–13%) for IL-2 stimulation. To understand which effector cell population mediates the Hsp70 reactivity, PBMNC stimulated with low-dose IL-2 plus TKD for 4 days were separated using CD94 mAb in a CD94 positive, CD3 negative (CD94+/CD3−) NK population and a CD94 negative, CD3 positive (CD94−/CD3+) T cell population. A phenotypic characterization of both subpopulations revealed costaining of CD94+ cells with the NK cell–specific markers CD56 (89%); only 11% of the CD94+ cells were found to be CD3+ T cells. Except 3%, all CD94− cells were positive for the T cell marker CD3 and negative for CD56 (97%). As shown in Figure 2, the cytolytic activity against CX+ (left panel) and CX− (right panel) tumor cells was selectively mediated by CD94+/CD3− NK cells (filled circles), and not by CD94−/CD3+ T cells (open circles), at effector to target cell (E:T) ratios ranging from 10:1 to 1:1. Furthermore, differences in the lytic activity against Hsp70 membrane-positive CX+ tumor cells (left panel) and membrane-negative CX− tumor cells (right panel) were only observed with CD94+/CD3− NK cells (filled circles) and not with CD94−/CD3+ T cells (open circles). To exclude a nonspecific stimulation of NK cells by positive selection using CD94 mAb, the experiment was repeated by negative selection using mAbs against CD14, CD3, and CD19 to deplete monocytes, T cells, and B cells. Negatively selected NK cells were also found to be positive for CD94 and CD56. They exhibited an identical cytolytic activity as shown for the CD94 positive NK cell population (data not shown).
Fig. 1.
In vitro cytolytic activity of interleukin (IL)-2 plus Hsp70-peptide TKDNNLLGRFELSG450–463 (TKD) or IL-2–stimulated peripheral blood mononuclear cells derived from 4 healthy human donors against Hsp70-expressing CX+ and nonexpressing CX− tumor cells. IL-2 was added to the medium at a final concentration of 100 IU/mL and TKD at a concentration of 2 μg/mL. The amount of CD56 positive cells ranged between 9% and 18% for IL-2 plus TKD stimulation and between 8% and 13% for IL-2 stimulation. The effector cell populations were used for adoptive transfer experiments in severe combined immunodeficiency/beige mice. The spontaneous release for each target cell was less than 20%. Effector to target cell ratios ranged from 80:1 to 5:1
Fig. 2.
Comparative cytolytic activity of CD94 positive, CD3 negative (filled circles, CD94+) and CD94 negative, CD3 positive (open circles, CD94−) cells derived by cell separation of peripheral blood mononuclear cells stimulated with interleukin-2 plus Hsp70-peptide TKDNNLLGRFELSG450–463 for 4 days. As target cells, Hsp70-expressing CX+ and nonexpressing CX− tumor cells were used. The spontaneous release for each target cell was less than 20%. Effector to target cell ratios ranged from 10:1 to 1:1
Adoptive transfer of Hsp70-peptide TKD-activated PBMNC after ip implantation of CX2 tumor cells into SCID/beige mice
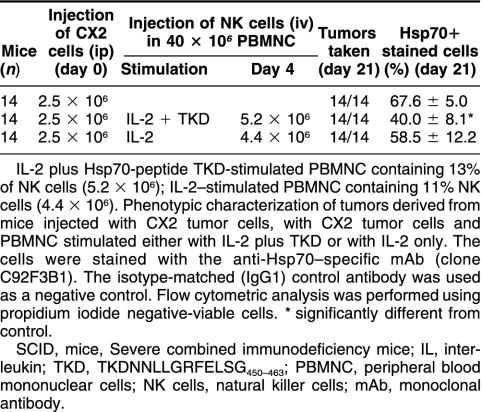
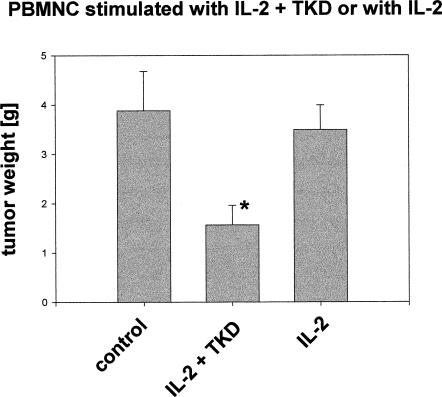
The cytolytic activity of the effector cell populations used for adoptive transfer experiments was determined by an in vitro cytotoxicity assay. As shown in Figure 1, only IL-2 plus TKD-stimulated PBMNC exhibited an increased cytolytic response against Hsp70-positive tumor cells. Previously, we reported that after ip injection of 2.5 × 106 Hsp70-positive CX+ tumor cells, an iv injection of 10 × 106 NK cells on day 4 completely abrogates tumor growth (Multhoff et al 2000). Here, we study the capacity of unseparated PBMNC, stimulated either with IL-2 alone or with IL-2 plus Hsp70-peptide TKD, in the eradication of CX2 tumors in vivo. The experimental details of the tumor inoculation and the adoptive transfer of the effector cells are summarized in Table 2. On day 0, SCID/beige mice were injected with 2.5 × 106 CX2 tumor cells (ip) that exhibit an Hsp70-positive phenotype on 65% of the cells. As determined earlier, the growth kinetics of the human colon carcinoma cell lines CX+, CX−, and CX2 in vitro and in SCID/beige mice were comparable (Botzler et al 1998). On day 4 after tumor cell inoculation, tumor-bearing SCID/beige mice were immunoreconstituted by an iv injection of 40 × 106 IL-2 plus TKD or IL-2–activated PBMNC. PBMNC stimulated with IL-2 plus TKD contained 5.2 × 106 NK cells; PBMNC stimulated with IL-2 alone contained 4.4 × 106 NK cells, as determined by flow cytometry. The control group was injected with CX2 tumor cells only. On day 21, all mice were sacrificed. A macroscopic analysis revealed that all mice developed ip-localized tumors. As expected for ip injection, local tumor growth, but no spread of distant metastases, was detectable (Botzler et al 1998). In Figure 2, we have shown that the cytolytic response is mediated by CD94+/CD3− NK cells and not by CD94−/CD3+ T cells. Because only 5.2 × 106 and 4.4 × 106 NK cells had been injected per mouse, a complete tumor regression was not expected. As previously shown, at least 10 × 106 isolated NK cells are necessary for a complete tumor regression (Multhoff et al 2000). On day 21, tumors were collected, and tumor weight was determined separately. As summarized in Figure 3, compared with control group (first column, 3.89 g), a significant reduction in tumor weight was observed in mice that had been adoptively transferred with Hsp70-peptide TKD-activated PBMNC (second column, 1.56 g). In mice that had been immunoreconstituted with IL-2–stimulated PBMNC, tumor reduction was less than 10% (third column, 3.64 g).
Table 2.
Injection protocol of tumor and effector cells into SCID/beige mice and phenotypic characterization of tumors
Fig. 3.
Comparative inhibition of tumor growth in mice engrafted with CX2 tumor cells, followed by an iv injection of interleukin (IL)-2 plus Hsp70-peptide TKDNNLLGRFELSG450–463 (TKD) or IL-2–stimulated peripheral blood mononuclear cells (PBMNC). The experimental details are summarized in Table 2. The first column indicates the total weight of primary tumors (3.89 g) derived from mice engrafted with CX2 cells, the second column the weight of tumors after immunoreconstitution with IL-2 plus TKD-stimulated PBMNC (1.56 g), and third column the weight of tumors after immunoreconstitution with IL-2–stimulated PBMNC (3.64 g). * significantly different from control
Phenotypic characterization of tumors collected from SCID/beige mice
A phenotypic characterization of the injected CX2 tumor cell line revealed that 65% of the cells were Hsp70 positive. After determination of the weight of each tumor, single-cell suspensions were generated, as described in Materials and Methods. Briefly, after cutting and meshing through a sterile mesh, the Hsp70 cell-surface expression pattern was determined on propidium iodide–negative, viable cells by flow cytometry. As summarized in Table 2, tumors derived from control mice revealed Hsp70 membrane expression on 67.6%. This result was not significantly different from the value obtained with the injected CX2 cells (65%). On tumors of mice that were immunoreconstituted with IL-2 plus TKD-activated PBMNC, the Hsp70 membrane expression was significantly decreased (40.0%), whereas in mice that had been injected with IL-2–stimulated PBMNC, the Hsp70 expression was not significantly different from that of the control tumors (58.9%). The human origin of the tumor material was further confirmed by a positive staining using the anti-human major histocompatibility complex (MHC) class I–specific mAb W6/32 (data not shown).
Immunoreconstitution of tumor-bearing SCID/beige mice with Hsp70-peptide TKD-activated PBMNC on different days after tumor inoculation
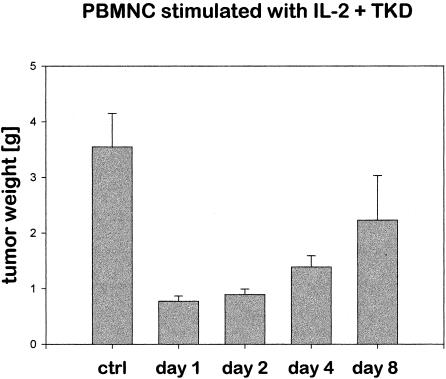
To evaluate whether tumor reduction is dependent on the E:T ratio, in vivo, SCID/beige mice were injected intraperitoneally on day 0 with 2.5 × 106 CX2 tumor cells; on day 1, 2, 4, or 8, an iv injection of 40 × 106 IL-2 plus TKD-activated PBMNC was given. PBMNC were derived from the leukapheresis product of 2 different donors, each containing 10% NK cells. After Ficoll separation, the cells were frozen in aliquots, sequentially thawed, and stimulated. As in the previous experiment, on day 21, all mice were sacrificed. The tumor of each mouse was collected and weighed separately. As summarized in Figure 4, depending on the day of immunoreconstitution using Hsp70-peptide TKD-stimulated PBMNC, the tumor size was significantly different. On day 21, the weight of the tumors of control mice that were injected with CX2 tumor cells alone was 3.55 g. If the tumor-bearing mice were injected with Hsp70-peptide TKD-activated PBMNC on day 1 or day 2 after tumor inoculation, the tumor weight was 0.77 g or 0.89 g, respectively. As compared with untreated control, tumor size was reduced 79% and 75%, respectively. An immunoreconstitution on day 4 results in a tumor weight of 1.39 g (61% reduction in tumor size as compared with untreated control mice) and on day 8 in a tumor weight of 2.23 g (37% reduction in tumor size as compared with untreated control mice).
Fig. 4.
Growth of CX2 tumors in mice that were immunoreconstituted with peripheral blood mononuclear cells (intravenous, 40 × 106 cells/mouse) stimulated with interleukin (IL)-2 plus Hsp70-peptide TKDNNLLGRFELSG450–463 on days 1, 2, 4, and 8 after tumor cell inoculation. The amount of CD94 positive natural killer (NK) cells was 10%, indicating that 4 × 106 NK cells were injected per mouse. The weight of each tumor was determined separately on day 21 after tumor cell inoculation. The data represent the mean of 3 independent experiments, using 6 mice for each day and treatment
DISCUSSION
Although Hsps are ubiquitously distributed and among the most highly conserved proteins, they have been found to elicit a specific tumor immune response (Tamura et al 1993; Suto and Srivastava 1995). Three possibilities are discussed as to how Hsps mediate immunity against cancer: (1) they act as carriers for tumor-derived peptides that are re-presented by antigen presenting cells (APC) by MHC class I molecules and, thus, stimulate CD8 positive cytotoxic T lymphocytes (Tamura et al 1997). On APC, several receptors, including α2-macroglobulin receptor CD91, Toll-like receptor 4 (TLR4), or CD14 (or all the three), are discussed to be involved in the uptake of Hsp-peptide immune complexes (Schild et al 1999; Binder et al 2000), (2) after contact of Hsp with Hsp-receptors on APCs, the secretion of immunostimulatory cytokines, including IL-12, granulocyte-monocyte colony-stimulating factor, and tumor necrosis factor-α, is induced by APC. For this “chaperokine effect,” Hsp-chaperoned, immunogenic peptides are not required (Asea et al 2000). (3) We identified an NK cell–mediated immune response associated with an unusual plasma membrane localization of Hsp70. A cell-surface expression of cytoplasmic Hsp with a molecular weight of 70 kDa and 90 kDa has been determined on several tumor cell lines but not on normal cells (Ferrarini et al 1992; Multhoff et al 1995b; Piselli et al 1995). The amount of membrane-bound Hsp70 correlates with an increased sensitivity to lysis mediated by NK cells (Multhoff et al 1995a; Botzler et al 1996). The importance of Hsp70 as the target structure for NK cells was demonstrated by Hsp70-antibody and Hsp70-protein blocking studies (Multhoff et al 1999).
Binding assays using the fluorescein-conjugated Hsp70-protein or the Hsp70-peptide TKD revealed that similar to APC, NK cells might express Hsp70-specific receptors that are responsible for immune functions. An incubation of isolated NK cells with Hsp70-protein (Multhoff et al 1999) or Hsp70-peptide TKD (Multhoff et al 2001) results in an up-regulation of CD57, CD56, and C type lectin KIR CD94 (Multhoff et al 1999) that acts as an inhibitory and activatory KIR (Cantoni et al 1998). Concomitantly, an increased proliferation and cytolytic activity against Hsp70-expressing tumors have been determined (Multhoff et al 2001). Because NK cell purification results in loss of cells, we asked the question whether NK cell stimulation is also possible using unseparated PBMNC. We have shown that incubation of unseparated PBMNC with low-dose IL-2 plus Hsp70-peptide TKD stimulates the cytolytic activity against Hsp70-positive tumors, although not as pronounced as it has been shown for isolated NK cells (Multhoff et al 1997). This finding might be the result of suboptimal peptide concentrations; it is possible that part of the peptide is internalized by macrophages. In accordance with Hsp70-peptide–stimulated NK cells, an incubation of PBMNC with TKD also results in a downshift of the CD16 mean fluorescence values. In contrast, the mfi of the C type lectin receptor CD94 and CD56 was up-regulated. Depending on the coreceptor, CD94 acts as an activating or inhibitory receptor. Because flow cytometric analysis using CD94-specific mAb does not describe the functional status of NK cells, the cytolytic activity was determined with CD94 positively and negatively sorted NK cells by a standard cytotoxicity assay. We could show that the cytolytic activity against Hsp70-positive tumor cells was mediated by CD94+/CD3− NK cells; the CD94−/CD3+ T cell population did not exhibit any significant cytolytic activity, neither against Hsp70 membrane-positive nor against Hsp70 membrane-negative tumor cells. These data provide evidence that low-dose IL-2 plus TKD selectively stimulate an NK cell–mediated cytolytic response against Hsp70 positive tumor cells.
In a SCID/beige mouse tumor model, we compared the efficiency of IL-2 plus Hsp70-peptide TKD and IL-2–stimulated PBMNC against CX2 colon carcinoma cells. A single injection of low-dose IL-2 plus Hsp70-peptide TKD-stimulated PBMNC results in a significant tumor reduction. In contrast, stimulation of PBMNC with low-dose IL-2 (100 IU/mL) only marginally suppresses tumor growth. Higher IL-2 concentrations ranging from 1000 IU/mL to 10 000 IU/mL predominantly stimulate nonspecific lymphokine activated killer cells (LAK) activity (Bradly et al 1998; Whiteside et al 1998). Although elevated IL-2 concentrations result in an increased proliferation of NK cells, on the long run, high-dose IL-2 induces apoptotic cell death in NK cells (data not shown). Our data implicate that for the induction of an NK-mediated Hsp70 reactivity, low-dose IL-2 in combination with Hsp70-peptide TKD or with Hsp70-protein is optimal (Multhoff et al 1999). A phenotypic characterization of the remaining tumor material derived from the mice that had been immunoreconstituted with PBMNC incubated with IL-2 plus TKD revealed that predominantly Hsp70-positive tumors have been eliminated. These results are in line with the previously published data using purified NK cells for the adoptive transfer in mice with Hsp70-positive tumors (Multhoff et al 2000). In contrast, the phenotype of tumors derived from mice that had been injected with IL-2–stimulated PBMNC remained unaltered as compared with that of tumors derived from control mice. Taken together, these data indicate that the 14-mer peptide TKD plus low-dose IL-2 selectively stimulate NK cells with target specificity for Hsp70 membrane-positive tumors. It is known that resistance to chemotherapy often correlates with elevated Hsp70 levels (Li et al 2000). A Hsp70 membrane expression is frequently found on acute myeloid leukemia (AML) blasts of patients with unfavorable karyotype (Gehrmann et al, personal communication). Furthermore, metastases exhibit enhanced levels of membrane-bound Hsp70 as compared with primary tumors (Hantschel et al 2000). Therefore, an immunological approach that exploits Hsp70 as a target recognition structure for the induction of a specific anti-Hsp70 immune response might provide a promising adjuvant immunotherapy to overcome therapy-resistant tumor clones and metastases. In an attempt to evaluate the optimal E:T ratio, kinetic studies have been performed. These data suggest that in vivo tumor eradication is highly dependent on a low tumor load. With respect to the development of an immunotherapeutical approach, based on Hsp70-peptide TKD-activated NK cells, major goals will be the identification of tumor patients with minimal residual disease and a high risk for developing Hsp70 membrane-positive metastases. To achieve an optimal effector to target ratio in vivo, an ex vivo enrichment and expansion of Hsp70-specific CD94+/CD3− NK cells might be desirable. An improvement of the antitumor effects might also be achieved by repeated treatment cycles using TKD-activated NK cells. Such experiments using tumor-bearing SCID/beige mice are currently under investigation.
Acknowledgments
Mathias Gehrmann and Gerald Thonigs are funded by the Wilhelm-Sander Stiftung, Catharina Gross is funded by multimmune GmbH. The animal facilities were funded by the Bartling Stiftung. The work was supported by BMBF and multimmune GmbH.
REFERENCES
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo CG, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:434–439. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- Blunt T, Finnie NJ, and Taccioli GE. et al. 1995 Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 80:813–823. [DOI] [PubMed] [Google Scholar]
- Botzler C, Kolb H-J, Issels R, Multhoff G. Noncytotoxic alkyl-lysophospholipid treatment increases sensitivity of leukemic K562 cells to lysis by natural killer cells. Int J Cancer. 1996;65:633–638. doi: 10.1002/(SICI)1097-0215(19960301)65:5<633::AID-IJC13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Botzler C, Schmidt J, Luz A, Jennen L, Issels R, Multhoff G. Differential Hsp70 plasma membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int J Cancer. 1998;77:942–948. doi: 10.1002/(sici)1097-0215(19980911)77:6<942::aid-ijc25>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bradly M, Zeytun A, Rafi-Janrjreh A, Nagarkatti PS, Nagarkatti M. Role of spontaneous and IL-2 induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas− tumor cells. Blood. 1998;92:4248–4255. [PubMed] [Google Scholar]
- Cantoni C, Biassoni R, and Pende D. et al. 1998 The activating form of CD94 receptor complex: CD94 covalently associates with the Kp30 protein that represents the product of the NKG2-C gene. Eur J Immunol. 28:327–338. [DOI] [PubMed] [Google Scholar]
- Clark PR, Menoret A. The inducible Hsp70 as a marker of tumor immunogenicity. Cell Stress Chaperones. 2001;6:121–125. doi: 10.1379/1466-1268(2001)006<0121:tihaam>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel R, Elsner L, Quentin T, Walter L, Günther E. Heat shock protein 70 is able to prevent heat shock-induced resistance of target cells to CTL. J Immunol. 2000;164:2362–2371. doi: 10.4049/jimmunol.164.5.2362. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Hantschel M, Pfister K, and Jordan A. et al. 2000 Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones. 5:438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–581. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Li CY, Lee YG, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase 3 activation. J Biol Chem. 2000;275:25665–25672. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- MacDonald HR, Engers HD, Cerottini JC, Brunner KT. Generation of cytotoxic T lymphocytes in vitro. J Exp Med. 1974;140:718–722. doi: 10.1084/jem.140.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells. A recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD3− large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood. 1995a;86:1374–1382. [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995b;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Kampinga HH, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of NK cells. Exp Hematol. 1999;27:1627–1636. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Pfister K, Botzler C, Jordan A, Scholz R, Schmetzer H, Burgstahler R, Hiddemann W. Adoptive transfer of human natural killer cells in mice with sever combined immunodeficiency inhibits growth of Hsp70-expressing tumors. Int J Cancer. 2000;88:791–797. doi: 10.1002/1097-0215(20001201)88:5<791::aid-ijc17>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W. A 14-mer Hsp70-peptide stimulates natural killer cell activity. Cell Stress Chaperones. 2001;6:3–9. doi: 10.1379/1466-1268(2001)006<0337:amhpsn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piselli P, Vendetti S, Poccia F, Cicconi R, Mattei M, Bolognesi A, Stripe F, Colizzi V. In vitro and in vivo efficacy of heat shock protein specific immunotoxins on human tumor cells. J Biol Regul Homeost Agents. 1995;9:55–62. [PubMed] [Google Scholar]
- Rabinovich B, Shannon J, Su R-C, Miller R. Stress renders T cell blasts sensitive to killing by activated syngeneic NK cells. J Immunol. 2000;165:2390–2397. doi: 10.4049/jimmunol.165.5.2390. [DOI] [PubMed] [Google Scholar]
- Schild H-J, Arnold-Schild A, Lammert E, Rammensee H-G. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr Opin Immunol. 1999;11:109–114. doi: 10.1016/s0952-7915(99)80019-3. [DOI] [PubMed] [Google Scholar]
- Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1589. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of NK cells in tumor growth and metastasis in beige mice. Nature. 1980;284:622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Tsuboi N, Sato N, Kikuchi K. 70 kDa heat shock cognate protein is a transformation-associated antigen and a possible target for the host's antitumor immunity. J Immunol. 1993;151:5516–5524. [PubMed] [Google Scholar]
- Vujanovic NL, Rabinovich H, Lee YJ, Herbermann RB, Whiteside TL. Distinct phenotypic and functional characteristics of human natural killer cells obtained by rapid IL-2 adherence to plastic. Cell Immunol. 1993;151:133–137. doi: 10.1006/cimm.1993.1227. [DOI] [PubMed] [Google Scholar]
- Whiteside TL, Vujanovic NL, Herbermann RB. Natural killer cells and tumor therapy. Curr Top Microbiol Immunol. 1998;230:221–244. doi: 10.1007/978-3-642-46859-9_13. [DOI] [PubMed] [Google Scholar]