Abstract
Heat shock proteins (Hsps) have been reported to protect cells, tissues, and organisms against damage from a wide variety of stressful stimuli. Whether they protect against deoxyribonucleic acid (DNA) damage in individuals exposed to environmental stresses and chemical carcinogens is unknown. In the study, we investigated the association between Hsp70 levels (the most abundant mammalian Hsp) and genotoxic damage in lymphocytes of workers exposed to coke-oven emission using Western dot blot and 2 DNA damage assays, the comet assay and the micronucleus test. The data show that there is a significant increase in Hsp70 levels, DNA damage score, and micronucleus rates in lymphocytes of workers exposed to coke-oven emission as compared with the control subjects. Furthermore, there was a significant negative correlation of Hsp70 levels with DNA damage scores in the comet assay (r = −0.663, P < 0.01) and with micronucleus rates (r = −0.461, P < 0.01) in the exposed group. In the control group, there was also a light negative correlation between Hsp70 with DNA damage and micronuclei rate (r = −0.236 and r = 0.242, respectively), but it did not reach a statistically significant level (P > 0.05). Our results show that individuals who had high Hsp70 levels generally showed lower genotoxic damage than others. These results suggest a role of Hsp70 in the protection of DNA from genotoxic damage induced by coke-oven emission.
INTRODUCTION
Heat shock proteins (Hsps) are highly conserved proteins found in all prokaryotes and eukaryotes. Under physiological conditions, Hsps are expressed at low levels, but a wide variety of stressful stimuli can induce a substantial increase in intracellular levels of Hsps. This cellular response is referred to as the stress response (reviewed in Lindquist and Craig 1988; Morimoto et al 1994). These stimuli include many environmental stresses that are common in the workplace or living environment, such as exposure to extreme heat, ultraviolet radiation, heavy metals, and carbon monoxide (CO). Pathological stimuli such as viral, bacterial, and parasitic infections, fever, inflammation, malignancy and autoimmunity, and physiological stimuli encountered during cell growth, cell differentiation, hormonal stimulation, or tissue development can also induce a complete or partial stress response. The best-known Hsp is the inducible member of the Hsp/Hsc70 family, with an apparent molecular mass of 71 kDa and 72 kDa in rat and human, respectively, and is referred to in this study as Hsp70. Many Hsps function as intracellular chaperones of naive, aberrantly folded, or mutated proteins. In addition, Hsps have also been shown to be involved in cytoprotection against many of the stress stimuli described above (Hightower 1991; Morimoto et al 1994; Muchowski et al 2000). These protective functions seem to be related to the development of tolerance to heat or toxins (or both), but the underlying molecular mechanisms responsible for the protection and the possible biomedical significance in humans are still unknown.
Workers of coal gas production plants are exposed to many different harmful factors such as extreme heat, high CO, and coke-oven emission, the latter being most harmful. These emissions, in addition to heavy dust, contain many toxic chemicals like benzene, methylbenzene, and polycyclic aryl hydrocarbons (PAHs, formed during combustion of fossil fuels and typified by the ubiquitous pollutant benzo[a]pyrene [BaP]). PAHs are potent procarcinogens and mutagens that can induce transformation of normal cells and can cause malignancies in experimental animals (reviewed in Heidelberger 1975). Epidemiological data have also shown an association between BaP exposure (including exposure to coke-oven emission) and increased incidence of certain cancers such as lung, skin, and colon cancer in humans (International Agency for Research on Cancer 1984; Mastrangelo et al 1996). Whether Hsp70 is associated with genotoxic damage in workers exposed to environmental toxins is presently unknown. In this study, we investigated if there was any relation between Hsp70 levels and genotoxic damages in lymphocytes of workers exposed to coke-oven emission by using Western dot blot, comet assay, and micronucleus test.
MATERIALS AND METHODS
Subjects and environmental conditions
Eighty-three male workers were divided into control and exposed groups on the basis of the environmental monitoring of their workplaces and some general criteria such as age and employment period. None of the workers had obvious diseases as assessed by physical examination and answers to a questionnaire. The exposed group comprised 43 individuals working on top of coke ovens at a coal gas production unit; they were exposed to heat, CO, and coke-oven emission on a regular basis. The control group consisted of 40 office workers at the mechanical plant of the same factory who were not exposed on a regular basis to the stressful environmental factors described above. The 2 groups were matched in age and employment period and were similar in their use of tobacco (Table 1). Temperatures were determined with dry- and wet-ventilated and globe bulb thermometers. The concentrations of CO, benzene, and methylbenzene were measured using a gas chromatograph (Shanghai Analytical Instrument General Factory, Model 106, Shanghai, People's Republic of China). The concentration of dust in the air was determined by collecting dust onto a filter with an air-sampling pump and by weighing the dust accumulated on the filter. The concentrations of CO, benzene-soluble matter (BSM), which represents coal tar pitch volatiles at coke ovens (Jongeneelen 1992), and BaP were determined by high-pressure liquid chromatography. Temperature, sampling of dust, and measurements for CO, benzene, methylbenzene, BSM, and BaP were performed 9 times, ie, at 10 AM, 3 PM, and 5 PM for 3 consecutive days. Blood samples were collected at the end of these days. The study was approved by the Tongji Medical College Ethics Committee, by Wuhan Steel & Iron Limited, and by workers group committees.
Table 1.
General condition of workers in the 2 groups (x̄ ± SD)
Determination of Hsp70 in lymphocytes of workers
Approximately 5 mL venous blood was collected into a heparinized tube and used to isolate lymphocytes using a standard separation medium (Biochemical Reagent Co, Shanghai, People's Republic of China). The cell viability was >95%, and cell numbers were within normal range for all groups. The collected lymphocytes were washed twice with phosphate-buffered saline (PBS) and counted. The concentration of lymphocytes was adjusted to 5000/μL with PBS. Two hundred microliters of PBS buffer containing lymphocytes was centrifuged and the entire buffer removed as soon as possible. Two hundred microliters of cold lysis buffer containing 0.05 M Tris-Cl, 0.15 M NaCl, 0.02% NaN3, 100 μg/mL phenylmethylsulfonyl fluoride, and 1% NP40 was then added to each sample and mixed well. Twenty microliters of the lysate (about 105 lymphocytes) was loaded onto nitrocellulose membranes by pipetting on a small piece of precut membrane using a vacuum system. The load was monitored by staining with Ponceau S (Sigma, St. Louis, MO, USA ). Nitrocellulose membranes were then saturated with blocking buffer (PBS containing 5% skim milk powder) for 1 hour at 37°C with gentle agitation and washed with PBS–0.05% Tween 80 for 5 minutes. A rabbit anti-human Hsp70 antibody specific for the inducible Hsp71 (#799 in Tanguay et al 1993) was added at a dilution of 1:1000 in PBS containing 5% skim milk powder, and the nitrocellulose membranes were incubated at 37°C for 1 hour with gentle agitation. After washing the membranes 6 times (10 minutes each time) with 200 μL PBS–0.05% Tween 80, horseradish peroxidase–labeled goat anti-rabbit IgG in blocking buffer (1:1000) was added and membranes incubated at 37°C for another hour. Membranes were washed 4 times with 200 μL PBS–0.05% Tween 80. The presence of Hsp70 on nitrocellulose membranes was revealed using DAB (3,3-diaminobenzidine tetrahydrochloride) buffer for 3–5 minutes, as described previously (Wu et al 2001). The levels of Hsp70 were quantified using an imaging densitometer (Shimadzu model CS930, Shimadzu, Japan) at 460 nm, and the value presented is the integrated optical density.
Detection of genotoxic damage in lymphocytes of workers
Genotoxic damage of lymphocytes in workers was assessed by both the alkaline single-cell gel test (comet assay) and a micronuclei test because these can measure with different sensitivities the genotoxicity induced by different deoxyribonucleic acid (DNA)-damaging compounds. DNA damage to lymphocytes was assayed by the alkaline single-cell gel technique (Singh et al 1989; Mckelvey-Martin et al 1993). Images of 25 randomly selected lymphocytes were analyzed for each sample. In this test, the damage to cells was graded visually into 4 categories, corresponding to the distance of DNA in the tail: no damage, <5 μm (value of 0 given for evaluation of DNA damage); low-level damage, 5–25 μm (value of 1); medium-level damage, 25–45 μm (value of 2); and high-level damage, >45 μm (value of 4) (Wu et al 1998). The determination of micronuclei in lymphocytes was performed as described by Fenech and Morley (1985). Two thousand cells per sample were counted and scored according to the criteria of Fenech (1996) by a well-trained research assistant under double blindness throughout the study. The data are reported as the occurrence rate of micronucleated cells per 106 cells.
Statistical analyses
The analyses were carried out using Statistical Package for Social Sciences (SPSS) in univariate and multivariate logistic regression models or by the Chi-square test and Student's t-test. Statistical inferences are based on the different levels of significance (P < 0.05 or P < 0.01).
RESULTS
Environmental conditions of workplaces
The environmental conditions of the workplace for each group of workers are summarized in Table 2. As can be seen, the concentrations of dust, BSM, BaP, benzene, methylbenzene, and CO and the working temperatures at the workplace of the exposed group are significantly higher than those at the workplace of the control group (P < 0.01). This table also shows that the workers of the exposed group are experiencing a rather complex mixture of chemical and environmental stresses.
Table 2.
Environmental conditions for the 2 groups of workers (x̄ ± SD)
Hsp70, DNA damage, and micronuclei frequency in lymphocytes of workers
The levels of inducible Hsp70 in lymphocytes of workers were next determined by a Western dot blot assay using an antibody specific to this Hsp. We first examined whether the exposure to the complex environmental stresses induced the synthesis of Hsp70 in workers. The results show that such exposure does induce a low but significant increase of Hsp70 in lymphocytes of exposed workers as compared with workers of the control group (Table 3, P < 0.05). This table also shows that exposure to these complex stresses resulted in a 2-fold increase in DNA damages in the exposed workers when compared with the controls (P < 0.05), as measured by both the comet assay and the micronuclei test.
Table 3.
Hsp70 level, DNA damage, and micronuclei rate in the 2 groups (x̄ ± SD)a
Association of Hsp70 with DNA damage and micronuclei frequency in lymphocytes
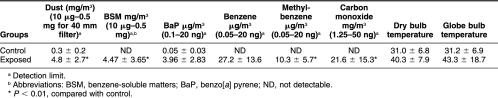
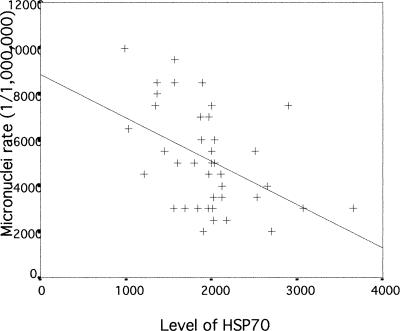
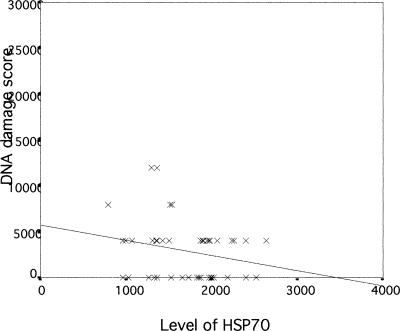
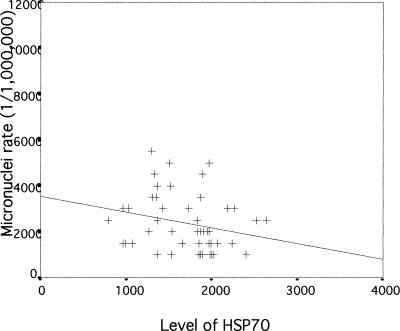
We next examined if there was any association between Hsp70 levels, DNA damage, and micronuclei rate in lymphocytes of exposed workers to further evaluate a possible role and a potent biomedical significance of Hsp70 levels in genotoxicity. The correlation between Hsp70 and DNA damage measured by the comet assay in lymphocytes of workers exposed to coke-oven emission is plotted in Figure 1. As can be seen, there is a significant negative correlation between Hsp70 level and DNA damage in exposed workers (r = −0.663, P < 0.01). A similar significant negative correlation between Hsp70 levels and micronuclei rate in exposed workers (r = −0.461, P < 0.01) was observed, as shown in Figure 2. In the control group, Hsp70 levels were not significantly correlated with DNA damage and micronuclei rate (r = −0.236; r = 0.242, P > 0.05; respectively) (Figs 3 and 4). Interestingly, although most individuals with low Hsp70 tended to have more DNA damage, some with high Hsp70 also had high DNA damage as can be seen particularly in the micronuclei test (see Fig 2).
Fig. 1.
Correlation between Hsp70 level and deoxyribonucleic acid damage assessed with the comet assay in lymphocytes of workers exposed to coke-oven emission (r = −0664, P = 0.00000306)
Fig. 2.
Correlation between Hsp70 level and micronuclei rates in lymphocytes of workers exposed to coke-oven emission (r = −0.461, P = 0.003)
Fig. 3.
Correlation between Hsp70 level and deoxyribonucleic acid damage assessed with the comet assay in lymphocytes of workers in control group (r = −0.236, P = 0.111)
Fig. 4.
Correlation between Hsp70 level and micronuclei rates in lymphocytes of workers in control group (r = −0.242, P = 0.101)
DISCUSSION
Hsps are generally induced in all organisms as a response to elevated temperature and to other forms of stress such as exposure to harmful chemicals. Many of these (heavy metals, CO, photochemical air pollutants, and urban acid air pollutants such as ozone and nitric acid) are common to both our working and living environments (Wu et al 1996). The main functions of Hsps include an intracellular chaperone role in protein folding and transport through membranes and in cytoprotection against various stresses (Hightower 1991; Muchowski et al 2000). This was first documented by the demonstrations of the role of various Hsps in the acquisition of transitory thermotolerance in cultured cells (Landry et al 1989; Angelidis et al 1991; Li et al 1991; Rollet et al 1992; Parsell and Lindquist 1994) and in transient protection from ischemic injury in whole organs such as the heart, brain, and kidney (Currie et al 1993; Marber et al 1995; Plumier et al 1995, 1997). In humans, many observations have shown links between an aberrant expression of Hsps and disease states (reviewed in Welch 1992; Minowada and Welch 1995). However, very little is known about the expression of Hsp70, its relation with genotoxicity caused by environmental stresses, and its possible biomedical significance in pathogenesis processes in humans.
Results from the present study show that exposure to coke-oven emission in workers can induce a significant increase in the levels of Hsp70, DNA damage, and micronucleus rates when compared with a control nonexposed group of workers (Table 3). Previous investigations have shown that many of the complex environmental factors of workplaces can induce the synthesis of Hsp70 and regulate the heat shock response (Wu et al 1996; Bartosiewicz et al 2001). Furthermore, it has been shown that exposure to environmental factors such as BaP, benzene, and their metabolites can result in genotoxicity (Smith et al 1989; Beland et al 1994; Drouin et al 1995; Speit et al 1996; Hanelt et al 1997; Wu et al 1998; Vayssier-Taussat et al 2001).
The present data also show a significant negative correlation of Hsp70 levels with DNA damage and with micronucleus rates in the exposed group. A similar relation was observed in controls but did not reach statistical significance. There are only few studies on a possible relation between Hsp70 levels and DNA damage. In a study on the effects of the heavy metal cadmium on a marine sponge, Schröder et al (1999) reported that the increase in the constitutive form (but not the inducible form) of Hsp70 paralleled the increase in DNA damage at high cadmium dose. At low dose, the increase in DNA damage preceded the increase in Hsp70, suggesting that DNA damage was responsible for Hsp induction. In the case of fumonisin B1, a group 2 carcinogen metabolite of the fungi Fusarium that interferes with ceramide synthesis, Hsp70 of treated astrocytes was more elevated at lower dose, which induced lower DNA damage as measured by the comet assay. The highest level of Hsp70 occurred earlier than that of DNA damage, suggesting that Hsp induction was an early response to cell stress and was not strictly related to DNA damage (Galvano et al 2002). Finally, after treatment of cells with the anticancer drug doxorubicin, cells overexpressing Hsp70 were found to reenter the cell cycle and proliferate better than the control cells not expressing Hsp70. However, the DNA damages were comparable in both cell lines (Karlseder et al 1996). Thus, the relation between Hsp70 and DNA damage remains controversial. It must also be pointed out that the 3 compounds used in these reports use different pathways to induce a stress response.
Is the negative relation between Hsp70 levels and DNA damage observed here in lymphocytes of workers physiologically significant? The environmental toxic factors present in coke-oven emission could induce the synthesis of Hsp70 or increase DNA damages (or both). The workers from the top of coke ovens clearly show an increase in both. Because Hsp70 is a chaperone and can confer cytoprotection, its induction could protect cells from damages such as those measured in this study. Thus, individuals with a higher amount of Hsp70 would suffer less damage than the others, and this was the trend generally observed in the present study. This is consistent with numerous observations reporting that Hsp70 can confer transient protection against the adverse effects of subsequent heat and chemical or abnormal stresses, as discussed above. Alternatively, Hsp70 might act as a danger signal in stressed cells (Moseley 2000; Vayssier-Taussat et al 2001). In this scenario, DNA damage would trigger an increased need of Hsp70 to counter the deleterious effects of the coke-oven emissions. Although it is not possible in a study such as the present one to assess which event occurred first, we note that workers exposed to the toxic coke-oven emissions, who had higher levels of Hsp70, had less DNA damage.
Finally, a close examination of individual results shows that some workers with high Hsp70 levels still showed high DNA damage (see Figs 1 and 2). Variations in hsp gene expression and Hsp polymorphism may contribute to differential disease susceptibility and stress tolerance (Favatier et al 1997). This could explain individual differences in sensitivity and tolerance to environmental stressors.
Acknowledgments
We are particularly grateful to all individuals who volunteered to participate in this study and to the many members of the medical personnel of Wugang Hospital and Wuhan Steel and Iron Limited Co for their generous help in the examination and sampling of subjects. This work was supported by research funds from the National Natural Science Foundation of China (NNSFC), a Teaching and Research Award for Outstanding Young Teachers in Higher Education Institutions of the Ministry of Education, People's Republic of China, and the Scientific committee of Wuhan to T.W. We also acknowledge the help of the NNSFC of China and the Canadian Institute of Health Research of Canada for a research exchange program to T.W. and R.M.T.
REFERENCES
- Angelidis CE, Lazaridis I, Pagoulatos GN. Constitutive expression of heat shock protein 70 in mammalian cells confers thermotolerance. Eur J Biochem. 1991;199:35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Bartosiewicz M, Peen S, Buckpitt A. Application of gene arrays in environmental toxicology: fingerprints of gene regulation associated with cadmium chloride, benzo (a) pyrene, and trichloroethylene. Environ Health Perspect. 2001;109:71–74. doi: 10.1289/ehp.0110971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beland FA, Poirier MC 1994 DNA adducts and their consequences. In: Methods to Assess DNA Damage and Repair: Interspecies Comparisons, ed Tardiff RG, Lohmann PHM, Wogan GN. John Wiley and Sons, Chichester, UK, 29–55. [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG. Heat-response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Drouin EE, Lech J, Loechler EL. The major N2-Gua adduct of the (+)-anti-benzo(a)pyrene diol epoxide can be unstable in double-stranded DNA. Biochemistry. 1995;34:2251–2259. doi: 10.1021/bi00007a020. [DOI] [PubMed] [Google Scholar]
- Favatier F, Bornman L, Hightower LE, Gunther E, Polla BS. Variation in hsp gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2:141–155. doi: 10.1379/1466-1268(1997)002<0141:vihgea>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M 1996 The cytokinesis block micronucleus techniques. In: Technologies for Detection of DNA Damage and Mutations, ed Pfeifer GP. Plenum Press, New York, 25–36. [Google Scholar]
- Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Galvano F, Campisi A, and Russo A. et al. 2002 DNA damage in astrocytes exposed to fumonisin B1. Neurochem Res. 27:345–351. [DOI] [PubMed] [Google Scholar]
- Hanelt S, Helbig R, Hartmann A, Lang M, Seidel A, Speit G. A comparative investigation of DNA adducts, DNA strand breaks and gene mutations induced by benzo(a)pyrene and (±)-anti-benzo(a)pyrene-7,8-diol 9,10-oxide in cultured human cells. Mutat Res. 1997;390:179–188. doi: 10.1016/s0165-1218(97)00019-0. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress protein, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. 1984 Polynuclear aromatic compounds. Part 3: industrial exposure in aluminium production, coal gasification, coke production and iron and steel founding. In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, no 34. International Agency for Research on Cancer, Lyon, France, 101–131. [Google Scholar]
- Jongeneelen FJ. Biological exposure limit for occupational exposure to coal tar pitch volatiles at cokeovens. Int Arch Occup Environ Health. 1992;63:511–516. doi: 10.1007/BF00386338. [DOI] [PubMed] [Google Scholar]
- Karlseder J, Wissing D, Holzer G, Orel L, Sliutz G, Auer H, Jäättelä M, Simon MM. HSP70 overexpression mediates the escape of a doxorubicin-induced G2 cell cycle arrest. Biochem Biophys Res Commun. 1996;220:153–159. doi: 10.1006/bbrc.1996.0373. [DOI] [PubMed] [Google Scholar]
- Landry J, Chrétien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Li LY, Liu K, Mak JK, Chen L, Lee WMF. Thermal response of rat fibroblasts stably transfected with the human 70 kDa heat shock protein encoding gene. Proc Natl Acad Sci U S A. 1991;88:1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi S, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kDa heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Investig. 1995;96:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo G, Fadda E, Marzia V. Polycyclic aromatic hydrocarbon and cancer in man. Environ Health Perspect. 1996;104:1166–1170. doi: 10.1289/ehp.961041166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckelvey-Martin VJ, Green MHL, Schmezer P, Pool-Zobel BL, Demo MP, Collins A. The single cell gel electrophoresis assay (comet assay): a European review. Mutat Res. 1993;288:47–63. doi: 10.1016/0027-5107(93)90207-v. [DOI] [PubMed] [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Investig. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C. ed. 1994 Progress and perspectives in the biology of heat shock proteins and molecular chaperones. In: The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1–30. [Google Scholar]
- Moseley PL. Exercise, stress, and the immune conversation. Exerc Sport Sci Rev. 2000;28:128–132. [PubMed] [Google Scholar]
- Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci U S A. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S 1994 Heat shock proteins and stress tolerance. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 457–494. [DOI] [PubMed] [Google Scholar]
- Plumier C, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier C, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expression of the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Investig. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollet E, Lavoie JN, Landry J, Tanguay RM. Expression of Drosophila 27kDa heat shock protein in rodent cell confers thermal resistance. Biochem Biophys Res Commun. 1992;185:116–120. doi: 10.1016/s0006-291x(05)80963-5. [DOI] [PubMed] [Google Scholar]
- Schröder HC, Hassanein HMA, and Lauenroth S. et al. 1999 Induction of DNA strand breaks and expression of HSP70 and GRP78 homolog by cadmium in the marine sponge Suberities domuncula. Arch Environ Contam Toxicol. 36:47–55. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR. A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res. 1989;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Smith MT, Yager JW, Steinmetz KL, Eastmond DA. Peroxidase-dependent metabolism of benzene's phenolic metabolites and its potential role in benzene toxicity and carcinogenicity. Environ Health Perspect. 1989;82:23–29. doi: 10.1289/ehp.898223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speit G, Hanelt S, Helbig R, Seidel A, Hartmann A. Detection of DNA effects in human cells with the comet assay and their relevance for mutagenesis. Toxicol Lett. 1996;88:91–98. doi: 10.1016/0378-4274(96)03723-x. [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock stress proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- Vayssier-Taussat M, Camilli T, Aron Y, Meplan C, Hainaut P, Polla BS, and Weksler B 2001 Effects of tobacco smoke and benzo(a)pyrene on human endothelial cell and monocyte stress responses. Am J Physiol. 280 H. 1293–H1300. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Wu T, Chen S, and Sun Y. et al. 2001 Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones. 6:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Tanguay RM, Wu Y, He H, Xu D, Feng J, Shi W, Zhang G. Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci. 1996;9:370–379. [PubMed] [Google Scholar]
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones. 1998;3:161–167. doi: 10.1379/1466-1268(1998)003<0161:poaths>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]