Abstract
Background: Since the introduction of the first atypical antipsychotics in the early 1990s, this class of medication has been increasingly relied upon for the treatment of a variety of patients with psychotic and mood disorders.
Data Sources: The following retrospective review was derived from the MEDLINE database using the search terms metabolic syndrome, insulin resistance, obesity, diabetes, severe mental illness, schizophrenia, bipolar disorder, mood disorders, depression, unipolar depression, and prevalence from 1966 to the present.
Literature Synthesis: Coincident with the growing usage of these agents, there have been a growing number of literature reports of changes in metabolic homeostasis among patients taking these medications. These changes have led to interest in evaluating whether there is a relationship among these mental illnesses, their psychiatric treatments, and certain physical comorbidities known collectively as the metabolic syndrome. This article reviews the existing literature around the metabolic syndrome in patients with severe mental illnesses.
Conclusion: Patients with severe mental illnesses, particularly schizophrenia and chronic mood disorders, demonstrate a higher prevalence of metabolic syndrome or its components compared with the general population. Based upon this increased risk in these patients, baseline and periodic medical evaluations should become a standard component in ongoing clinical assessment.
The connection between severe mental illness and the metabolic syndrome is emerging as a public health question of importance to both mental health and primary care practitioners. Originally identified by Reaven1,2 as syndrome X or the insulin resistance syndrome, the magnitude of public health impact of the metabolic syndrome is reflected by a recently estimated prevalence of approximately 24% in adults in the United States.3 Cardiovascular mortality and all-cause mortality are increased in men with the metabolic syndrome, and women have a heightened risk of coronary disease.4 The prevalence of 2 basic components of this syndrome, obesity and diabetes, has clearly increased over the past decade throughout the United States.5 Evidence is starting to accumulate that metabolic disturbances are common in patients with severe psychiatric illnesses.6,7 In this review, we discuss the pathophysiology of metabolic syndrome and the particular relevance of this disorder in patients with severe and persistent mental illness, with an emphasis on patients with psychotic and mood disorders.
DEFINITION OF METABOLIC SYNDROME
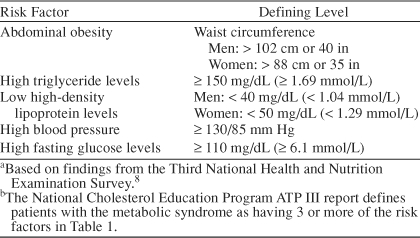
Metabolic syndrome is by definition a multisystem disorder. Metabolic syndrome, syndrome X, and the insulin resistance syndrome are all terms coined to describe the recognized clustering of metabolic and cardiovascular abnormalities including obesity, hypertension, dyslipidemia, hyperuricemia, and abnormalities of glucose homeostasis (i.e., insulin resistance, glucose intolerance, or diabetes mellitus). Along with competing names for this syndrome, different groups have suggested different diagnostic criteria.8,9 The criteria defined by the National Cholesterol Education Program8 are shown in Table 1. These diagnostic parameters are easily obtained in most clinical settings and provide valuable information about modifiable cardiovascular risk factors; furthermore, they also reflect some of the specific pathologic changes that occur in metabolic syndrome. As discussed below, central or upper body obesity is more closely associated with the syndrome than is lower body obesity, and waist circumference provides a surrogate measure for the visceral fat deposits that underlie this relationship. Likewise, high triglycerides and low high-density lipoprotein (HDL) cholesterol are specific blood lipid changes associated with metabolic syndrome. The pathophysiologic relationships between these multiple abnormalities have been the subject of great scientific interest.
Table 1.
Risk Factors for the Metabolic Syndrome Among U.S. Adultsa,b
PATHOPHYSIOLOGY OF METABOLIC SYNDROME
The biochemical perturbations observed in the metabolic syndrome include changes in glucose tolerance and lipoprotein levels as well as alterations in inflammatory mediators and procoagulant factors. Multiple organ systems are affected, including adipose, muscle, hepatic, nervous, and adrenal tissues, but from a clinical standpoint, the most important site of impact is the vasculature. Cumulative effects of classical risk factors such as glucose intolerance, dyslipidemia, and hypertension very likely contribute to increased risk of cardiovascular disease seen in individuals with the metabolic syndrome.10 Hyperinsulinemia, a surrogate for insulin resistance and a marker for metabolic syndrome, is, in itself, associated with a 2- to 3-fold increase in cardiovascular disease independent of classical risk factors.10,11 Other components of this syndrome that may contribute to a pro-atherogenic state include increased levels of plasminogen-activator inhibitor I (PAI-I), angiotensin II, interleukin-6 (IL-6), tumor necrosis factor α (TNFα, and others.12,13 The relative contribution of these various changes to cardiovascular risk in individuals with metabolic syndrome is unclear.
Impaired insulin responsiveness (i.e., insulin resistance) is presumed to be central to the metabolic syndrome and may provide the underlying process from which other abnormalities evolve.3,14 Insulin resistance is a major contributor to glucose intolerance, and the lipoprotein abnormalities seen in the metabolic syndrome are also predictable, at least in part, from the known effects of insulin to inhibit lipolysis in adipocytes.15,16 With resistance to insulin, unchecked lipolysis leads to increased delivery of free fatty acids to the liver for tri-glyceride synthesis and packaging into very low-density lipoprotein (VLDL) particles. Higher VLDL levels contribute to lower HDL levels because of the reciprocal exchanges between these lipoproteins mediated by cholesterol ester transfer protein.15 It has been shown that blood pressure is related to insulin resistance independent of differences in age, gender, and degree of obesity.17,18 The demonstration that insulin can stimulate endothelium-dependent vasodilation, and that this is blunted in insulin-resistant individuals, provides a plausible mechanism to explain the elevation of blood pressure in the metabolic syndrome.19 Evidence that insulin resistance underlies the metabolic syndrome is also provided by the fact that pharmacologic treatment with insulin-sensitizers (e.g., thiazolidinediones) can have beneficial effects not only on glucose and lipids, but also on blood pressure and on the inflammatory and pro-atherogenic derangements previously noted.20,21
Many suggest that visceral obesity is the primary determinant of insulin resistance and, as such, represents the fundamental pathophysiologic change leading to the metabolic syndrome.22–24 Adipocyte-derived humoral factors that are released in proportion to visceral fat stores and that may mediate effects on insulin sensitivity include free fatty acids (FFAs), TNFα, IL-6, resistin, and others.13,22 Perhaps the greatest support exists for FFAs, which have been shown to induce insulin resistance at both muscle and the liver.22 Adiponectin is another “adipokine” of great interest. Levels of this polypeptide hormone fall with rising adiposity, and adiponectin replacement has been shown to improve insulin sensitivity.25 The role of leptin in insulin resistance is unclear. Whereas some studies suggest that leptin may impair insulin action, leptin therapy dramatically improves insulin sensitivity in patients with lipodystrophy.21,26 Insulin resistance can also occur in lean individuals, which may be due to inherited insulin receptor and postreceptor defects.27 Despite this, the central role of visceral obesity in most cases of insulin resistance and the metabolic syndrome appears to be widely accepted.
The possible role of glucocorticoids in the pathogenesis of the metabolic syndrome is another area of active study. That cortisol excess can produce insulin resistance and the typical metabolic syndrome cluster is apparent from the clinical manifestation of Cushing's syndrome. However, it has been proposed that “subclinical Cushing's syndrome” may be a relatively common cause of visceral obesity and the insulin resistance syndrome.28 Some of these cases may be due to functioning adrenal adenomas,29 but physical stress or psychiatric stress have also been suggested as common causes of relative, and potentially relevant, hypercortisolemia.28,30 This mechanism is especially attractive as an explanation for the higher prevalence of the metabolic syndrome and type 2 diabetes mellitus among patients with severe mental illness in light of evidence for hypothalamic-pituitary-adrenal (HPA) axis overactivity and central adiposity discussed below.
METABOLIC SYNDROME AND PSYCHOTIC DISORDERS
Increasingly, physical disorders such as obesity, hyper-lipidemias, hypertension, and type 2 diabetes mellitus are becoming recognized as significant comorbidities in people with serious mental illnesses, including psychotic disorders such as schizophrenia. Whether these disorders are part of the disease process itself through increased stress and inflammatory responses, genetic vulnerabilities, or environmental factors versus sequelae of treatment of the disease has been a matter of debate. Only recently have clinicians and researchers in the field of psychiatry begun to evaluate these comorbidities in the context of the metabolic syndrome.
The prevalence of being overweight or obese in individuals with schizophrenia has generally been thought to be greater than in individuals without the disorder.31 Allison and colleagues32 found that patients with schizophrenia tended to be as or more obese than the general population. Some obese individuals with large amounts of body fat display few metabolic complications, while in other individuals who appear minimally overweight, the development of type 2 diabetes and cardiovascular diseases is increased.33–35 However, regarding an increased risk for metabolic syndrome, not just obesity itself but rather increased central obesity or visceral adiposity is thought to pose the greatest risk for development of type 2 diabetes, dyslipidemias, and other cardiovascular complications.36–42 Thakore and colleagues43 have shown that increased visceral fat distribution was present in individuals with schizophrenia, independent of any medication effects. The group used abdominal computed tomography scanning in a cross-sectional study of 15 schizophrenic subjects who were either drug-free or drug-naive to measure fat distribution compared with a matched control group. While the schizophrenic subjects were found to have a nonsignificantly higher degree of total body fat and subcutaneous fat compared with controls, schizophrenic patients had 3.4 times as much intra-abdominal fat as did the normal controls (p < .005). Visceral fat may be a common pathologic factor and may explain one reason why schizophrenic subjects are more likely to have an increased prevalence of metabolic complications associated with the metabolic syndrome.
Several recent studies looked at whether patients with serious mental illness have an increased prevalence of the metabolic syndrome in comparison to the general population. As stated earlier, the current age-adjusted prevalence for the metabolic syndrome among the general population of U.S. adults is approximately 24%.3 In 2 cross-sectional studies presented by Kato and colleagues in 2003, using the National Cholesterol Education Program ATP III criteria,8 a 60% prevalence rate of metabolic syndrome was estimated among 63 schizophrenic outpatients,44 and a 75% prevalence rate was estimated among 16 mood disorder patients of Hispanic origin.45
Littrell and colleagues,46 in a multicenter naturalistic study, examined 98 outpatients in the United States and 27 inpatients in Taiwan who had schizophrenia or schizo-affective disorder for the presence of insulin resistance and the metabolic syndrome using fasting laboratory and clinical assessments. They observed a 51% prevalence rate of metabolic syndrome in the U.S. outpatient cohort and a 22% prevalence rate in the Taiwanese inpatient cohort. Their data are consistent with those of Kato et al.,44 suggesting a need for an increased awareness of the metabolic syndrome among U.S. adults with schizophrenia. It is unknown how the observed rates in the Taiwanese psychiatric population compare with a general population estimate since a non–psychiatrically ill control group was not included in the study. This study looked further at the prevalence of insulin resistance using a homeostasis model assessment (HOMA-IR), which is based on fasting insulin and glucose levels ([Ins × Glu]/22.5). Using this calculation, the authors demonstrated that 70% of outpatients and 44% of inpatients in this study exhibited a clinically significant degree of insulin resistance.46 These data would support the suggestion by Reaven10 that increasing levels of insulin resistance are likely to be early predictors to the development of metabolic syndrome.
A study of metabolic syndrome within the Finnish general population47 demonstrated a prevalence ranging from 8% to 17%. Heiskanen and colleagues48 recently published a study examining the metabolic syndrome in 35 Finnish patients with schizophrenia and observed a 37% prevalence. Thus, as with the studies by Kato et al.44,45 and Littrell et al.,46 this study by Heiskanen and colleagues48 showed higher rates of the metabolic syndrome among patients with schizophrenia compared with background rates in the general population.
The studies by Littrell et al.46 and Heiskanen et al.48 included an examination of antipsychotic medication treatment and failed to observe any significant differences in metabolic syndrome prevalence across typical and atypical antipsychotic treatment groups. These studies seem to indicate that a significant part of the risk for metabolic syndrome parameters is inherent in the psychiatric disease process itself and that antipsychotic medication may be an indirect factor in contributing to metabolic syndrome risk. However, these conclusions are limited by the studies' cross-sectional design and the relatively small sample sizes.
Recent evidence49 has suggested that HPA axis dys-regulation also may play a significant role in the development of various components of the metabolic syndrome. While increased cortisol production is a normal response to acute stress, several studies43,50,51 have demonstrated a disruption in normal HPA axis activity and relative hypercortisolemia in patients with schizophrenia. Ryan and colleagues51 have recently shown that first-episode treatment-naive patients with schizophrenia demonstrated a significantly higher plasma cortisol level along with a higher percentage of patients having impaired fasting glucose, increased fasting blood glucose levels, and increased insulin resistance compared with a matched control group. Chronic elevation in plasma cortisol levels can lead to a pseudo-Cushing's syndrome characterized by increased visceral adiposity, hyperinsulinemia, insulin resistance, dyslipidemias, and hypertension, all hallmarks of the metabolic syndrome.42 Mück-Seler and colleagues52 have shown recently that, in a population of 86 patients with schizophrenia, 50% demonstrated an abnormal response to dexamethasone suppression. Both suppressors and nonsuppressors had significantly higher plasma cortisol levels compared with healthy controls (p < .001). There was also a significant difference (p < .01) in basal plasma cortisol levels between nonsuppressors and suppressors, which continued for up to 17 hours after dexa-methasone suppression testing.52
More recently, Shiloah and colleagues53 studied a group of 39 nondiabetic patients with acute psychotic stress admitted to an inpatient ward and examined the effects of the psychotic stress on glucose homeostasis. They demonstrated that patients undergoing an acute stress situation necessitating psychiatric emergency ward admission had disruptions in beta-cell function and insulin sensitivity that correlated inversely with their degree of stress, suggesting that severity of illness may have an increased impact on HPA axis disruption.
The role that HPA axis disruption may play on plasma cortisol levels in stable chronic patients with schizophrenia and ensuing metabolic parameters is less clear and needs further exploration.
METABOLIC SYNDROME AND MOOD DISORDERS
Formal clinical investigation of metabolic syndrome in patients with unipolar and bipolar mood disorders has been limited. However, associations of metabolic syndrome and psychological symptoms have been studied. Considerable information is also available on the interrelationships of mood disorders and components of metabolic syndrome, such as obesity, hyperglycemia, and diabetes mellitus.
In a recent prospective study by Räikkönen and coworkers,54 425 generally healthy women (aged 42–50 years at entry) were recruited and followed for up to 7.5 years. Three types of data were collected: markers of metabolic syndrome, psychological ratings (from separate scales for depression, anxiety, tension, anger, and stress), and lifestyle factors. Twenty-two women met criteria for metabolic syndrome at baseline, and 50 women developed metabolic syndrome during the study. At baseline, there were associations between psychological symptoms and metabolic syndrome, with the strongest associations for depressive symptoms. Psychological symptoms at baseline also increased risk of developing metabolic syndrome prospectively. Conversely, development of metabolic syndrome predicted increases in psychological symptoms, particularly anger and anxiety. Although this study did not examine incidence of DSM-IV mood disorders per se in patients who had or developed metabolic syndrome, its findings based on psychological rating scales support a reciprocal relationship between metabolic syndrome and mood disorders.
Several studies55–66 have examined associations between mood disorders and obesity. In general, these studies have looked at depression and have not carefully assessed historic polarity of mood. Furthermore, most have been designed to evaluate the purported effect of obesity on mood, rather than vice versa. The results have been mixed, with some studies showing positive or somewhat positive associations55–59 and others finding inverse correlations60,61 between obesity and depression. Roberts and colleagues62 conducted a prospective study that examined both directions of the relationship and found that obesity predicted depression, but that depression did not increase risk for obesity. The inability of depressed mood to serve as a robust predictor for obesity is not surprising given the differing patterns of weight change in depressed patients63–65 and the varied effects of antidepressant medications on weight.66 The relationship of obesity and bipolar disorder has been the focus of several more recent studies,69–73 which have found that patients with bipolar disorder are on average more obese than the general population; however, there is no evidence that patients with obesity have a higher risk for developing bipolar disorder. A confounding factor in all these studies is that many medications used as treatments in bipolar disorder, such as lithium,67 valproic acid,66 and several atypical antipsychotic agents68 such as olanzapine, are associated with weight gain.
Elmslie and coworkers69 estimated the prevalence of obesity in 89 patients with bipolar disorder, in comparison with 445 matched controls from the general population who had participated in a national health survey. They found patients with bipolar disorder were more likely to be overweight or obese, with a central pattern of obesity. In a follow-up analysis,70 these investigators found that these patients consumed more sugars and carbohydrates than controls.
McElroy and colleagues71 conducted a cross-sectional study in 644 bipolar patients and found that half were overweight and a quarter were obese. Obesity was associated with several factors, but by using adjusted multinomial logistic regression, the investigators determined that overweight and obesity were most strongly associated with hypertension, male gender, and arthritis.
Fagiolini and coworkers72 retrospectively analyzed data from 50 consecutively enrolled patients over a 10-year period in a bipolar maintenance pharmacotherapy protocol and found that one third of these patients were overweight and one third were obese at study baseline. Most weight gain occurred in patients who were not overweight, and it tended to occur early in treatment. During the course of 12 months of maintenance pharmaco-therapy, 26% of patients had weight gain of more than 5% of initial body weight, while 6% gained more than 10% of initial body weight. No patient had a 15% or greater weight gain, and the reported median weight gain over 12 months was 5 pounds. In a later report,73 these investigators reviewed data from 175 patients in the same study and again confirmed that slightly over a third of patients were obese. They discovered that obese patients had quicker relapses (significant clinical worsening of symptoms after remission) during treatment.
Beyond obesity, another important condition that is related to metabolic syndrome is diabetes mellitus. With regard to mood disorders, more is known about the relationship of diabetes and depression, compared with knowledge about the association between diabetes and bipolar disorder. Recently, Talbot and Nouwen74 reviewed the literature in this area and concluded that although there is an increased prevalence of depression in patients with diabetes, the evidence does not support a direct causative effect of type 2 diabetes on depression. For type 1 diabetes, which typically has a much earlier age at onset than depression in patients who suffer from both disorders concurrently, there may be a biological or psychosocial causative link to depression.75 Two studies76,77 have looked at the other side of the relationship and have found that depression may increase risk for diabetes.
Relatively few studies have looked at diabetes and bipolar disorder. Three retrospective chart reviews78–80 in psychiatric settings have found that patients with bipolar disorder have an increased prevalence of diabetes compared with the general population. In addition, there is some evidence81–84 that patients with bipolar disorder have abnormalities in oral glucose tolerance test. Two of these studies80,84 examining the possible association of medication effects found no significant relation between antipsychotic use and diabetes.
The common pathophysiologic link between obesity, diabetes, mood disorders, and metabolic syndrome may be hypercortisolemia. Hypercortisolemia is seen in patients with diabetes,85,86 as well as in patients with unipolar and bipolar disorders.87–89 Hypercortisolemia leads to visceral obesity, which has been observed in patients with major depression.90,91 Visceral obesity is associated with insulin resistance and diabetes.92 One hypothesis links environmental stress to hypothalamic overactivity and cortisol secretion, which initiates physiologic perturbations that cause metabolic syndrome.28,93
Many studies have elucidated reciprocal relationships between metabolic syndrome and its components and mood disorders. More information is available on the relationships of diabetes and obesity to unipolar mood disorder, although data are accumulating in bipolar disorder. Many confounds, particularly the potential effects of medications, have not been fully addressed. Nevertheless, in the comprehensive management of patients with mood disorders, the available data provide helpful information regarding the metabolic syndrome and its components.
CONCLUSIONS
Patients with severe mental illnesses, particularly schizophrenia and chronic mood disorders, have demonstrated a higher prevalence of metabolic syndrome or its components compared with the general population in several countries. Therefore, baseline and periodic medical evaluations should become a standard component in the ongoing assessment of these patients. Although individual risk factors associated with the metabolic syndrome are typically amenable to behavioral or pharmacologic treatment, management of these comorbidities in many patients with serious mental illness will require cooperation between psychiatrists and primary care physicians. Patient education and adequate control of psychiatric symptoms will also remain important parameters in achieving long-term treatment success.
Drug names: lithium (Eskalith, Lithobid, and others), olanzapine (Zyprexa), valproic acid (Depakene and others).
Footnotes
Mr. Toalson, Dr. Ahmed, and Dr. Hardy are employed by Eli Lilly and Company. Dr. Kabinoff has served on academic advisory boards for Eli Lilly and Company and has received honoraria from Eli Lilly, Pfizer, and Merck.
REFERENCES
- Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Syndrome X. Blood Press Suppl. 1992;4:13–16. [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, and Lakka TA. et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002 288:2709–2716. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, and Bowman BA. et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003 289:76–79. [DOI] [PubMed] [Google Scholar]
- Dixon LB, Wohlheiter K. Diabetes and mental illness: factors to keep in mind. Drug Benefit Trends. 2003;15:33–44. [Google Scholar]
- Bellnier TJ, Patil K, and Ortega T. et al. The prevalence of metabolic disturbances in schizophrenic and bipolar 1 patients prior to antipsychotic use. Presented at the 156th annual meeting of the American Psychiatric Association. 17–22May2003 San Francisco, Calif. [Google Scholar]
- National Institutes of Health. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel 3). Bethesda, Md: National Institutes of Health. 2001 [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications, pt 1: diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation. 2002;106:286–288. doi: 10.1161/01.cir.0000019884.36724.d9. [DOI] [PubMed] [Google Scholar]
- Howard G, O'Leary D, and Zaccaro D. et al. Insulin sensitivity and atherosclerosis. Circulation. 1996 93:1809–1817. [DOI] [PubMed] [Google Scholar]
- Das UN. Obesity, metabolic syndrome X, and inflammation. Nutrition. 2002;18:430–432. doi: 10.1016/s0899-9007(01)00747-x. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, and Chaudhuri A. et al. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetes. J Clin Endocrinol Metab. 2003 88:2422–2429. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich JE. Contributions of insulin-resistance and insulin-secretory defects to the pathogenesis of type 2 diabetes mellitus. Mayo Clin Proc. 2003;78:447–456. doi: 10.4065/78.4.447. [DOI] [PubMed] [Google Scholar]
- Zavaroni I, Mazza S, and Dall'Aglio E. et al. Prevalence of hyperinsulinaemia in patients with high blood pressure. J Intern Med. 1992 231:235–240. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Natali A, and Capaldo B. et al. Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. Hypertension. 1997 30:1144–1149. [DOI] [PubMed] [Google Scholar]
- Steinberg H, Chaker H, and Leaming R. et al. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996 97:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14:137–145. doi: 10.1016/s1043-2760(03)00024-9. [DOI] [PubMed] [Google Scholar]
- Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague C, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- Chandran M, Phillips S, and Ciaraldi T. et al. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003 26:2442–2450. [DOI] [PubMed] [Google Scholar]
- Peterson KF, Oral EA, and Dufour S. et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002 109:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SJ, Garvey WT. Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med. 1998;105:331–345. doi: 10.1016/s0002-9343(98)00300-3. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Hypothalamic origin of the metabolic syndrome X. Ann N Y Acad Sci. 1999;892:297–307. doi: 10.1111/j.1749-6632.1999.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Tauchmanova L, Rossi R, and Biondi B. et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002 87:4872–4878. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Hemingway H, and Walker BR. et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002 106:2659–2665. [DOI] [PubMed] [Google Scholar]
- Gopalaswamy AK, Morgan R. Too many chronic mentally disabled patients are too fat. Acta Psychiatr Scand. 1985;72:254–258. doi: 10.1111/j.1600-0447.1985.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, and Heo M. et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999 60:215–220. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes Metab Rev. 1988;4:615–622. doi: 10.1002/dmr.5610040607. [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Freedman DS, Peiris AN. Health risks of obesity. Med Clin North Am. 1989;73:111–138. doi: 10.1016/s0025-7125(16)30695-2. [DOI] [PubMed] [Google Scholar]
- Bray GA. Pathophysiology of obesity. Ann J Clin Nutr. 1992;55(suppl 2):488–494. doi: 10.1093/ajcn/55.2.488s. [DOI] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, and Larsson B. et al. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12-year follow up of participants in the population study of women in Gothenburg, Sweden. BMJ. 1984 289:1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson B, Svardsudd K, and Welin L. et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13-year follow up of participants in the study of men born in 1913. BMJ. 1984 288:1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson LO, Larsson B, and Svardsudd K. et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985 34:1055–1058. [DOI] [PubMed] [Google Scholar]
- Peiris AN, Sothmann MS, and Hennes MI. et al. Relative contribution of obesity and body fat distribution to alterations in glucose insulin homeostasis: predictive values of selected indices in premenopausal women. Am J Clin Nutr. 1989 49:758–764. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hua N, and Abbasi F. et al. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001 86:3574–3578. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, and Tuomi T. et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001 24:683–689. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Thakore JH. Physical consequences of schizophrenia and its treatment: the metabolic syndrome. Life Sci. 2002;71:239–257. doi: 10.1016/s0024-3205(02)01646-6. [DOI] [PubMed] [Google Scholar]
- Thakore JH, Mann JN, and Vlahos I. et al. Increased visceral fat distribution in drug-naive and drug-free patients with schizophrenia. Int J Obes Relat Metab Disord. 2002 26:137–141. [DOI] [PubMed] [Google Scholar]
- Kato M, Gonzalez-Blanco M, and Sotelo J. et al. Metabolic syndrome in schizophrenia: a pilot study. Presented at the 156th annual meeting of the American Psychiatric Association. 17–22May2003 San Francisco, Calif. [Google Scholar]
- Kato M, Sotelo J, and de Guia C. et al. Prevalence of the metabolic syndrome in Hispanic patients with mood disorder: a pilot study. Presented at the 156th annual meeting of the American Psychiatric Association. 17–22May2003 San Francisco, Calif. [Google Scholar]
- Littrell K, Perry R, and Hilligoss N. et al. Insulin resistance and syndrome X among schizophrenic patients. Presented at the 156th annual meeting of the American Psychiatric Association. 17–22May2003 San Francisco, Calif. [Google Scholar]
- Vanhala MJ, Kumpusalo EA, and Pitkajarvi TK. et al. Metabolic syndrome in a middle-aged Finnish population. J Cardiovasc Risk. 1997 4:291–295. [PubMed] [Google Scholar]
- Heiskanen T, Niskanen L, and Lyytikäinen R. et al. Metabolic syndrome in patients with schizophrenia. J Clin Psychiatry. 2003 64:575–579. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Jakovljevic M, Muck-Seler D, and Pivac N. et al. Platelet 5-HT and plasma cortisol concentrations after dexamethasone suppression test in patients with different time course of schizophrenia. Neuropsychobiology. 1998 37:142–145. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Mück-Seler D, Pivac N, and Jakovljevic M. et al. Platelet serotonin, plasma cortisol, and dexamethasone suppression test in schizophrenic patients. Biol Psychiatry. 1999 45:1433–1439. [DOI] [PubMed] [Google Scholar]
- Shiloah E, Witz S, and Abramovitch Y. et al. Effect of acute psychotic stress in nondiabetic subjects on beta-cell function and insulin sensitivity. Diabetes Care. 2003 26:1462–1467. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51:1573–1577. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord. 1992;16:999–1003. [PubMed] [Google Scholar]
- Ross CE. Overweight and depression. J Health Soc Behav. 1994;35:63–79. [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS, and Allison DB. et al. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000 90:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Kaplan GA, and Shema SJ. et al. Are the obese at greater risk for depression? Am J Epidemiol. 2000 152:163–170. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Strawbridge WJ, and Deleger S. et al. Are the fat more jolly? Ann Behav Med. 2002 24:169–180. [DOI] [PubMed] [Google Scholar]
- Crisp AH, McGuiness B. Jolly fat: relation between obesity and psychoneurosis in general population. BMJ. 1976;1:7–9. doi: 10.1136/bmj.1.6000.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinkas LA, Wingard DL, Barrett-Connor E. Depressive symptoms in overweight and obese older adults: a test of the ‘jolly fat’ hypothesis. J Psychosom Res. 1996;40:59–66. doi: 10.1016/0022-3999(95)00542-0. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Deleger S, and Strawbridge WJ. et al. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003 27:514–521. [DOI] [PubMed] [Google Scholar]
- Weissenburger JE, Rush AJ, and Giles DE. et al. Weight change in depression. Psychiatr Res. 1986 17:275–283. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Fernstrom MH, and Price A. et al. Direction of weight change in recurrent depression: consistency across episodes. Arch Gen Psychiatry. 1990 47:857–860. [DOI] [PubMed] [Google Scholar]
- DiPietro L, Anda RF, and Williamson DF. et al. Depressive symptoms and weight change in a national cohort of adults. Int J Obes Relat Metab Disord. 1992 16:745–753. [PubMed] [Google Scholar]
- Vanina Y, Podolskaya A, and Sedky K. et al. Body weight changes associated with psychopharmacology. Psychiatr Serv. 2002 53:842–847. [DOI] [PubMed] [Google Scholar]
- Baptista T, Teneud L, and Contreras Q. et al. Lithium and body weight gain. Pharmacopsychiatry. 1995 28:35–44. [DOI] [PubMed] [Google Scholar]
- Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(suppl 7):22–31. [PubMed] [Google Scholar]
- Elmslie JL, Silverstone JT, and Mann JI. et al. Prevalence of overweight and obesity in bipolar patients. J Clin Psychiatry. 2000 61:179–184. [DOI] [PubMed] [Google Scholar]
- Elmslie JL, Mann JI, and Silverstone JT. et al. Determinants of overweight and obesity in patients with bipolar disorder. J Clin Psychiatry. 2001 62:486–491. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Frye MA, and Suppes T. et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002 63:207–213. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Frank E, and Houck PR. et al. Prevalence of obesity and weight change during treatment in patients with bipolar 1 disorder. J Clin Psychiatry. 2002 63:528–533. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Kupfer DJ, and Houck PR. et al. Obesity as a correlate of outcome in patients with bipolar 1 disorder. Am J Psychiatry. 2003 160:112–117. [DOI] [PubMed] [Google Scholar]
- Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Cares. 2000;23:1556–1562. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. J Psychosom Res. 2002;53:903–906. doi: 10.1016/s0022-3999(02)00302-1. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Armenian H, and Gallo J. et al. Depression and risk for onset of type 2 diabetes: a prospective population-based study. Diabetes Care. 1996 19:1097–1102. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Takatsuka N, and Shimizu H. et al. Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care. 1999 22:1071–1076. [DOI] [PubMed] [Google Scholar]
- Lilliker SL. Prevalence of diabetes in a manic-depressive population. Compr Psychiatry. 1980;21:270–275. doi: 10.1016/0010-440x(80)90030-9. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Ahearn E, Carroll BJ. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry. 1999;156:1417–1420. doi: 10.1176/ajp.156.9.1417. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Thapar RK, and Marano C. et al. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord. 2002 70:19–26. [DOI] [PubMed] [Google Scholar]
- Gildea EF, McLean VL, Man EB. Oral and intravenous dextrose tolerance curves of patients with manic-depressive psychosis. Arch Neurol Psychiatry. 1943;49:852–859. [Google Scholar]
- van der Velde CD, Gordon MW. Manic-depressive illness, diabetes mellitus, and lithium carbonate. Arch Gen Psychiatry. 1969;21:478–485. doi: 10.1001/archpsyc.1969.01740220094011. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, and Fucetola R. et al. Glucose-induced increase in memory performance in patients with schizophrenia. Schizophr Bull. 1999 25:321–335. [DOI] [PubMed] [Google Scholar]
- Ruzickova M, Slaney C, and Garnham J. et al. Clinical features of bipolar disorder with and without comorbid diabetes mellitus. Can J Psychiatry. 2003 48:458–461. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Kronfol Z, and Greden JF. et al. Hypothalamic-pituitary-adrenocortical activity in patients with diabetes mellitus. Arch Gen Psychiatry. 1984 41:1090–1095. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hudson MS, and Rothschild AJ. et al. Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch Gen Psychiatry. 1984 41:1086–1089. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, and Schlesser MA. et al. The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatry. 1996 57:470–484. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Ritchie JC, Carroll BJ. Plasma dexamethasone concentration and cortisol response during manic episodes. Biol Psychiatry. 1998;43:747–754. doi: 10.1016/s0006-3223(97)00274-6. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Thakore JH, Richards PJ, and Reznek RH. et al. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry. 1997 41:1140–1142. [DOI] [PubMed] [Google Scholar]
- Weber-Hamann B, Hentschel F, and Kniest A. et al. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med. 2002 64:274–277. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002;90:3G–10G. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Stress induced disturbances of the HPA axis: a pathway to type 2 diabetes? Med Sci Monit. 2003;9:RA35–RA39. [PubMed] [Google Scholar]


