Abstract
Historically, sodium azide has been used to anesthetize the nematode Caenorhabditis elegans; however, the mechanism by which it survives this exposure is not understood. In this study, we report that exposure of wild-type C elegans to 10 mM sodium azide for up to 90 minutes confers thermotolerance (defined as significantly increased survival probability [SP] at 37°C) on the animal. In addition, sodium dodecyl sulfate–polyacrylamide gel electrophoresis revealed enhanced Hsp70 expression, whereas Western blot analysis revealed the induction of Hsp16. We also tested the only known C elegans Hsp mutant daf-21 (codes for Hsp90), which constitutively enters the stress-resistant state known as the dauer larvae. Daf-21 mutants also acquire sodium azide–induced thermotolerance, whereas 3 non-Hsp, constitutive dauer-forming mutants exhibited a variable response to azide exposure. We conclude that the ability of C elegans to survive exposure to azide is associated with the induction of at least 2 stress proteins.
INTRODUCTION
Caenorhabditis elegans is a free-living soil nematode, which is used as a model biological system to investigate issues in genetics, developmental, cellular, and molecular biology (Brenner 1974). In addition, C elegans has served as a model organism for various toxicological studies (for examples, see Williams and Dusenberry 1988; Stringham and Candido 1994; Candido and Jones 1996; Tatara et al 1997; Power and de Pomerai 1999; Dhawan et al 2000; Williams et al 2000; Hoss et al 2001). One interesting, but overlooked, fact concerning C elegans biology is that it can be anesthetized with 10 mM (0.7%) sodium azide (Sulston and Hodgkin 1988). The fact that sodium azide inhibits both cytochrome c oxidase (Duncan and Mackler 1966) and adenosine triphosphate (ATP) synthase (Herweijer et al 1985; Van der Bend et al 1985) makes the ability of C elegans to survive this chemically induced hypoxic state quite remarkable. Although other anesthetics have been tested (for examples see Kayser et al 1998; van Swinderen et al 1998), the question of the mechanism by which C elegans survives azide exposure remains unanswered.
The response to environmental stress, including energy-related stress, has been extensively studied (Morimoto et al 1997; Moseley 1997; Nollen and Morimoto 2002). A common feature of the stress response is the induction of stress proteins, which were first discovered in cells exposed to slight hyperthermia (Ritossa 1962, 1996; Tissieres et al 1974; see Gabai and Sherman 2002 for review). The presence of these proteins confers resistance to further stresses such as additional hyperthermia (Li and Werb 1982); although, experiments with Drosophila melanogaster demonstrate that the induction of Hsp70 by hyperthermia is neither uniform nor without cost to the organism (Krebs and Feder 1997a, 1997b). Of particular interest to our laboratory is the fact that ischemic stress can induce stress proteins (Myrmel et al 1994).
Two factors make C elegans an excellent model for studying the linkage between disruption of energy metabolism and stress protein induction: first, the ability of C elegans to survive chemically induced hypoxia; and second, the availability of a variety of mutant strains of this organism, at least 1 of which involves a mutation to a hsp gene. Regarding stress response studies with C elegans, its response to stresses such as chemically induced hypoxia has not been fully characterized. Snutch and Baillie (1983) demonstrated that exposure of C elegans to elevated temperatures leads to the induction of several heat-inducible proteins. However, the majority of C elegans stress response research has focused on the formation of the highly stress-resistant dauer larval state (for examples, see Wadsworth and Riddle 1989; Riddle and Albert 1997; Braeckman et al 2000).
We hypothesized that disruption of energy metabolism by azide induces a stress response in C elegans and that this response would be characterized at the organismal level by the development of thermotolerance. Furthermore, we hypothesized that this thermotolerant state could be correlated with the induction of stress proteins. To test these hypotheses, we pretreated populations of wild-type nematodes by exposing them either to sodium azide or to a slight thermal stress. We then compared survival of these pretreated populations after exposure to a high-temperature stress (37°C) with the survival of unexposed control populations after the same high-temperature stress and demonstrated that sodium azide exposure leads to the acquisition of thermotolerance and is associated with Hsp70 and Hsp16 induction.
RESULTS
Exposure to sodium azide induces thermotolerance in C elegans
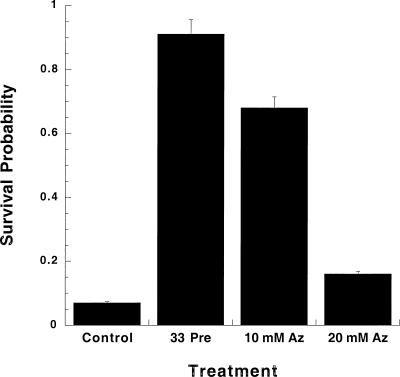
To test the hypothesis that sodium azide exposure would induce thermotolerance in C elegans, N2 Bristol (wild type) animals were exposed to several concentrations of sodium azide: 10 mM (the concentration normally used as an anesthetic [Sulston and Hodgkin 1988]), 20, 50, and 100 mM for 60 minutes. The results of 3 independent trials are shown in Figure 1. Control nematodes, which received no pretreatments, as expected, had a very low SP: 0.07 ± 0.01 (all data presented as the mean ± standard error of the mean) at 37°C. However, nematodes exposed to either pretreatment demonstrated significantly increased SPs at 37°C: 0.91 ± 0.04 (33°C) and 0.68 ± 0.06 (10 mM sodium azide), P < 0.01 for both pretreatments when compared with the control. Exposure to sodium azide concentrations greater than 20 mM, or for longer than 90 minutes, proved lethal. A concentration response study, using sodium azide concentrations from 2.5 to 20 mM in 2.5 mM increments, indicated that 10 mM sodium azide produced maximal SP (data not shown). These results demonstrate that low concentrations of sodium azide induce thermotolerance in C elegans.
Fig 1.
Sodium azide is capable of inducing thermotolerance in C elegans. Control N2s (wild-type strain) were exposed to 37°C for 2 hours, whereas the 33°C and 10 mM sodium azide N2s were pretreated for 1 hour and then allowed to recover for 4 hours before exposure to 37°C. All animals were exposed to the elevated temperatures simultaneously. Animals receiving a 50 or 100 mM sodium azide pretreatment did not survive that treatment. Statistical analysis (ANOVA) of the data indicates that the differences between N2 controls and each pretreatment give values of P < 0.01. Nematode strains N2 Bristol (wild-type strain) and the constitutive dauer-forming strains described in Table 1 (DR77 [daf-14], DR62 [daf-7], and DR1564 [daf-2]) were obtained from the Caenorhabditis Genetics Center at the University of Minnesota. Nematodes were grown using standard culture methods (see Lewis and Fleming 1995) and were harvested by the sucrose flotation method (Sulston and Hodgkin 1988). Three nematode growth media (NGM) petri dishes without a bacterial lawn were prepared, and asynchronous populations of approximately 200–300 N2 animals were placed on each plate. The control plate received no pretreatment and remained at 22°C during the exposure period. The thermal pretreated nematodes received a 1-hour exposure to 33°C in a Chicago Surgical and Electrical Company incubator. The azide pretreatment plates received a 1-hour exposure to sodium azide (Sigma, St Louis, MO, USA) at the following concentrations: 10, 20, 50, and 100 mM. Azide plates were always prepared on the day of the experiment by dilution of a 200 mM sodium azide stock solution into liquid NGM before solidification. After pretreatment, azide-exposed nematodes were harvested and washed 3 times before being transferred to clean NGM plates. The thermal pretreated and azide-exposed nematodes were given a 4-hour recovery at 22°C. Examination of pretreated plates revealed actively migrating nematodes for the 33°C and 10 and 20 mM azide–treated animals. Control, 33°C, and azide-exposed plates were heat shocked simultaneously at 37°C for 2 hours and then allowed to recover for a minimum of 18 hours at 22°C. After recovery from heat shock, the survivability of animals was assessed. A small drop of water was placed on each petri dish, allowing surviving animals to float in water and begin moving. Actively swimming animals were scored as living, whereas stiff, nonmoving animals were scored as dead. It was observed that the animals that were not moving at 18-hour post-37°C exposure never regained motility, never reproduced, did not respond to touch, and as such, were presumed dead. Animals were counted on a grid system using a Nikon Stereo Zoom Dissecting Microscope. Survival probability (SP) values were calculated for each plate (SP = total living animals/total animals in experiment). We found that temperature regulation by the incubator is critical for the 37°C exposure, with an increase of 1.5°C resulting in a SP of 0 and a decrease of 1.5°C resulting in a SP of 1.0. A minimum of 3 trials were performed, with the total number of animals examined for each experimental group ranging between 600 and 1000
Heat shock proteins in C elegans are induced with heat or azide exposure
The next question we addressed was whether exposure of C elegans to sodium azide induced heat shock proteins. Using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis, we observed induction of Hsp70 and Hsp16 (Fig 2 A,B). In the thermal pretreated animals, SDS-PAGE analysis showed significant induction of Hsp70, which is consistent with the observations of Snutch and Baillie (1983) and Snutch et al (1988). In addition, the azide-exposed animals induced Hsp70 but to a much smaller extent.
Fig 2.
Hsp70 and Hsp16 are induced in C elegans exposed to 33°C or 10 mM sodium azide. Animals were treated as described in Fig 1. Polyacrylamide gels (7.5% and 12.5%) with 3.6% stacking gels were prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (as described by Laemmli [1970]) and Western blotting. (A) Laemmli gel (7.5%). Three myofibrillar proteins, actin, paramyosin, and myosin, are identified for reference as per Schachat et al (1978) and Mackenzie and Epstein (1980). In the 33°C lane, there is significant induction of a protein consistent with the size of Hsp70, as evidenced by the bovine serum albumin (66 kDa) standard. Quantitative densitometry indicates a 2.6-fold (±0.2) increase in Hsp70 levels after this treatment. C elegans exposed to 10 mM sodium azide had a smaller (1.4-fold [±0.1]) increase in Hsp70 levels. Con = controls; 33 = 33°C pretreatment; Az = 10 mM sodium azide pretreatment, and n = 2. (B) Western blots of heat shock proteins demonstrate that Hsp70 and Hsp16 are induced by azide exposure. Monoclonal antibodies against Hsp90, Hsp70, and Hsp60 were used to assay for the induction of these heat shock proteins. Western blot analysis did not reveal any significant induction of Hsp90 or Hsp60, whereas Hsp70 did show induction on both the Laemmli gel and the Western blot. Each blot represents a separate assay and was done using 7.5% Laemmli gels. A 12.5% Laemmli gel used for Western blotting with a polyclonal α-Hsp16 antibody demonstrated that Hsp16 is induced by exposure to 33°C or 10 mM sodium azide. Control N2s did not exhibit any significant level of Hsp16 expression, whereas both the 33°C and 10 mM sodium azide–treated animals did show induction of Hsp16. Because of the extremely low levels of Hsp16 in control animals, quantitative densitometry was difficult. Nevertheless, consistent with the results for Hsp70, the 33°C treatment caused a significantly greater induction of Hsp16 as compared with the 10 mM sodium azide–treated animals. (C) The time course for Hsp16 induction in the 10 mM sodium azide–treated animals indicates that it takes 3–4 hours after exposure to the azide before Hsp16 can be detected. For all gels, experimental animals were harvested by the sucrose flotation method. Pelleted animals were resuspended in 95°C Laemmli gel sample buffer for 10 minutes. Mini slab gels (Idea Scientific, Corvallis, OR, USA) were used, and these whole-animal homogenates were observed with Coomassie Brilliant Blue G-250. For the Western blots, a first gel was run to standardize total cellular actin levels in each sample. A second gel was run and the proteins transferred using a Mini-Genie Electroblotter (Idea Scientific) and Immobilon polyvinylidene fluoride transfer membranes (Millipore, Bedford, MA, USA). Amido black staining of duplicate loadings verified equivalent amounts of actin in each lane. Standard Western blotting techniques were then used. Blots were incubated separately with monoclonal antibodies directed against Hsp90, Hsp70, and Hsp60 (StressGen, San Diego, CA, USA, catalog nos SPA-830, SPA-810, and SPA-807, respectively) and a polyclonal rabbit α-Hsp16 antibody (Hockertz et al 1991). The appropriate alkaline phosphatase conjugate (α-mouse IgG [Sigma] for Hsp90, Hsp70, Hsp60 and α-rabbit IgG [Sigma] for Hsp16) was used for the secondary antibody that were preabsorbed with a nematode acetone powder. Colorimetric detection was performed with SIGMA FAST BCIP/NBT tablets. All images were obtained using a Photoshop-driven Arcus II scanner (Agfa). A calibrated gray scale tablet (Eastman Kodak, Rochester, NY, USA) was scanned with the gels or Western blots for calibration of the digitized image. Densitometric profiles were obtained using MacIntosh computers and NIH Image, v1.49
Quantitative densitometry—using total cellular actin as the internal standard—performed on Laemmli gel samples indicated a 2.6-fold (±0.2) increase in the Hsp70 in 33°C pretreated animals. Induction of Hsp70 with azide exposure was lower with a 1.4-fold (±0.1) increase (P < 0.01). These results indicate that both stressors induce Hsp70, but the degree of the response is different. SDS-PAGE did not reveal induction of any other heat shock proteins including Hsp90, Hsp60, or Hsp16. As such, Western blots were used to assay for the induction of these Hsps. Using commercial antibodies against Hsp90 and Hsp60, only constitutive expression was observed, whereas Hsp16 is induced after exposure to the different stressors (Fig 2B). Although expression of Hsp16 in these animals was lower in azide-exposed animals—consistent with the Hsp70 results—induction still occurred when compared with the control population (Fig 2B).
A time course experiment was performed to assess Hsp16 induction after azide exposure. Samples were collected after 0, 1, 2, 3, and 4 hours of recovery after a 1-hour exposure to 10 mM azide. The data indicate that detectable levels of Hsp16 expression did not occur until 3–4 hours after azide exposure (Fig 2C).
The role of Hsp90 in the acquisition of thermotolerance
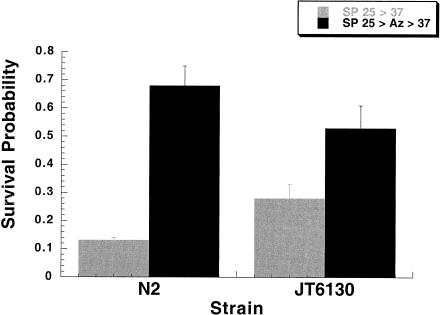
Currently, although there are many thousands of C elegans mutants, there is only 1 nonlethal stress protein mutant of C elegans. The mutant gene daf-21 (strain JT6130) encodes Hsp90 and has been characterized as a conserved glutamate → lysine substitution in the primary sequence (Birnby et al 2000). Although our data demonstrate that Hsp90 is not induced, because this animal is expressing a mutant Hsp90, we examined this strain's ability to undergo sodium azide–induced thermotolerance.
JT6130 grown at the restrictive temperature of 25°C and then exposed directly to 37°C demonstrate a slightly higher, but statistically insignificant, SP than do N2 controls. JT6130 exposed to 10 mM sodium azide had a slight increase in thermotolerance when compared with their untreated controls and a statistically insignificant decrease in thermotolerance when compared with the N2 controls treated similarly (Fig 3). The JT6130 data suggest that Hsp90 does not play an essential role in response of C elegans to exposure to sodium azide.
Fig 3.
JT6130 (daf-21, a Hsp90 mutant) shows a slight intrinsic thermotolerance when grown at the restrictive temperature. This constitutive dauer-forming mutant when exposed to 10 mM sodium azide does acquire additional thermotolerance. This argues against a critical role for Hsp90 in the animal's stress response to sodium azide. JT6130 (daf-21) was obtained from Dr James Thomas at the University of Washington
Testing of other dauer mutants
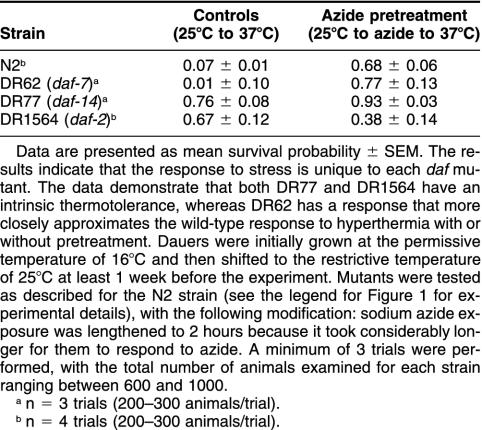
The dauer larval state in C elegans represents a strong organismal response to severe external conditions and is characterized by the arrest of growth at the third larval stage, cessation of pharyngeal pumping, the development of a very thick cuticle, and significantly increased life span (see Riddle 1988; Riddle and Albert 1997 for review). Interestingly, Golden and Riddle (1984) reported that elevated temperatures can induce dauer formation. Given these facts, we were concerned that the daf-21 results might result from the dauer state itself and not from the Hsp90 mutation. Therefore, we tested other dauer-forming mutants for their ability to acquire azide-induced thermotolerance. Given the diversity of genes involved in the dauer larvae formation pathway (Riddle and Albert 1997), we tested 3 other known mutants: daf-2, daf-7, and daf-14 that are involved in signaling pathways and lead to constitutive entry into the dauer state at 25°C.
The results of these studies are summarized in Table 1 and indicate that there is a considerable variation in the ability of different dauer mutants to respond to azide exposure. DR62 (daf-7, encoding a transforming growth factor-β receptor [Schackwitz et al 1996]) grown at the restrictive temperature had a highly variable response. Animals grown at 25°C and exposed directly to 37°C were generally unable to survive the heat shock. In contrast, however, DR62s exposed to sodium azide acquired thermotolerance when compared with untreated DR62 and the N2 controls (P < 0.01).
Table 1.
Survival probabilities of constitutive dauer-forming mutants exposed to sodium azide
DR77 (daf-14, encoding a Smad protein [Inoue and Thomas 2000]) responded significantly differently. DR77 grown at the restrictive temperature and exposed directly to 37°C demonstrated a high level of intrinsic thermotolerance when compared with the N2 controls (P < 0.01), whereas their counterparts exposed to azide had slightly increased thermotolerance when compared with untreated DR77. As such, in this particular mutant, there is already a significant level of intrinsic thermotolerance that can be slightly enhanced by azide exposure.
Finally, DR1564 (daf-2, encoding an insulin or insulin-like growth factor receptor [Gottlieb and Ruvkun 1994; Kimura et al 1997]) also demonstrated an intrinsic thermotolerance to elevated temperatures when grown at the restrictive temperature (P < 0.01 when compared with N2 controls). Interestingly, when exposed to sodium azide, these animals demonstrated reduced thermotolerance when compared with the animals exposed directly to 37°C.
Therefore, for the 3 additional daf mutants we tested, their response to direct exposure to 37°C, and the ability to acquire azide-induced thermotolerance was unique to each mutant. This suggests that it is not the dauer state alone that is responsible for the results we observed with daf-21.
DISCUSSION
The acquisition of thermotolerance is defined as the ability of an organism to survive an otherwise lethal exposure to elevated temperatures by prior exposure to a slightly elevated temperature. The results reported in this study indicate that under our conditions, the classic acquisition of the thermotolerant state can be demonstrated in C elegans. In addition, we demonstrate that exposure of C elegans to low levels of the metabolic inhibitor sodium azide induces thermotolerance as evidenced by increased survival after exposure to 37°C. Several concentrations of sodium azide were tested, and it was determined that 10 mM (its concentration as an anesthetic) was the optimal concentration for inducing thermotolerance.
The results also indicate that the acquisition of thermotolerance is associated with the induction of Hsp70 and Hsp16. Unlike the multigene Hsp70 family, where there are both constitutive and inducible Hsp70s, Hsp16 must be stress induced (Russnak and Candido 1985). The reduced levels of azide-induced Hsp expression when compared with the thermally pretreated controls is not surprising, given the necessity of ATP for transcription and the expectation that exposure to sodium azide may well reduce ATP content in the animal.
A review of the literature suggests that the response to azide exposure must be considered for each individual organism examined. Cochrane et al (1991) reported that in the rotifer Brachionus plicatilis, exposure to azide does not induce synthesis of a heat-inducible mitochondrial Hsp. Boutibonnes et al (1995), however, reported that azide could induce transient thermotolerance in the bacteria Lactococcus lactis subsp. lactis; although, Western blot analysis did not reveal Hsp induction.
Given the important biological functions of the stress proteins, it is not surprising that mutant screens failed to isolate stress protein mutants, and it seems highly likely that mutations in these genes would result in a lethal phenotype. The JT6130 (daf-21) strain is a Hsp90 mutant and is the only nonembryonic lethal Hsp mutant strain isolated to date. Dalley and Golomb (1992) and Cherkasova et al (2000) reported that N2 induced to form dauer larvae are enriched for Hsp90 messenger ribonucleic acid, which suggests that Hsp90 can be induced under certain conditions. In our experiments, however, repeated Western blot analysis using a commercially prepared α-Hsp90 monoclonal antibody failed to demonstrate induction of Hsp90 in our azide-exposed N2s. Furthermore, our thermotolerance assays with a mutated Hsp90—which is enough to induce constitutive dauer formation—are not enough to prevent the acquisition of azide-induced thermotolerance and suggest that Hsp90 is not an essential part of the response of C elegans to azide exposure.
The data from studies on other dauer mutants demonstrate that the Hsp90 mutant data did not result from the dauer state alone. The data indicate that the response to azide exposure is unique for each dauer mutant tested and can vary considerably, suggesting that it is not simply the dauer state itself that brings about these results. Two of the dauer mutants that we tested (DR77 and DR1564) demonstrated a clear intrinsic thermotolerance, which is consistent with previously reported results (Lithgow et al 1995; Gems et al 1998).
When considering the possible explanations for the sodium azide results reported in this study, it is interesting to consider that plant cells contain a cyanide-resistant alternative oxidase. Activation of this enzyme results in increased heat production within the plant cell (Sluse and Jarmuszkiewicz 2000). Although no equivalent gene has been identified in C elegans, it may be that sodium azide exposure also results in a slight hyperthermic state being produced.
Given the fairly severe constraints put on energy production by sodium azide, it is remarkable that a multicellular organism could survive prolonged exposure to this metabolic inhibitor at such a high concentration. Our results demonstrate that exposure to sodium azide does induce physiological changes within C elegans, including the induction of 2 stress proteins and the acquisition of thermotolerance. As sodium azide continues to be the anesthetic of choice for this organism, it is important to note that its use is not without immediate physiological consequences for the animal.
Acknowledgments
The authors thank the Academic Honors Program for partial support of this project and Dr Gordon Swain of the Mathematics Department for help with the statistical analyses. The authors thank Dr Peter Candido for the α-Hsp16 antibody and Dr James Thomas for the JT6130 strain. G.E.W. dedicates this paper to the memory of Hattori Hisatsugu, Harold David White, and Virginia Burns Spivey. Portions of this work were presented at the American Society for Cell Biology meeting (1997 and 1999), the 12th International C elegans Meeting at the University of Wisconsin (1999), and the Society of Toxicology meeting (2000).
REFERENCES
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (Daf-11) and Hsp90 (Daf-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutibonnes P, Bisson V, Thammavongs B, Hartke A, Panoff JM, Benachour A, Auffray Y. Induction of thermotolerance by chemical agents in Lactococcus lactis subsp. lactis IL1403. Int J Food Microbiol. 1995;25:83–94. doi: 10.1016/0168-1605(94)00149-z. [DOI] [PubMed] [Google Scholar]
- Braeckman BP, Houthoofd K, Vanfleteren JR. Patterns of metabolic activity during aging of the wild type and longevity mutants of Caenorhabditis elegans. Age. 2000;23:55–73. doi: 10.1007/s11357-000-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido EP, Jones D. Transgenic Caenorhabditis elegans strains as biosensors. Trends Biotechnol. 1996;14:125–129. doi: 10.1016/0167-7799(96)10016-0. [DOI] [PubMed] [Google Scholar]
- Cherkasova V, Ayyadevara S, Egilmez N, Shmookler-Reis R. Diverse Caenorhabditis elegans genes that are upregulated in dauer larvae also show elevated transcript levels in long-lived, aged, or starved adults. J Mol Biol. 2000;300:433–438. doi: 10.1006/jmbi.2000.3880. [DOI] [PubMed] [Google Scholar]
- Cochrane BJ, Irby RB, Snell TW. Effects of copper and tributyltin on stress protein abundance in the rotifer Brachionus plicatilis. Comp Biochem Physiol C. 1991;98:385–390. doi: 10.1016/0742-8413(91)90221-e. [DOI] [PubMed] [Google Scholar]
- Dalley BK, Golomb M. Gene expression in the Caenorhabditis elegans dauer larva: developmental regulation of Hsp90 and other genes. Dev Biol. 1992;151:80–90. doi: 10.1016/0012-1606(92)90215-3. [DOI] [PubMed] [Google Scholar]
- Dhawan R, Dusenbery DB, Williams PL. A comparison of metal-induced lethality and behavioral responses in the nematode Caenorhabditis elegans. Environ Toxicol Chem. 2000;19:3061–3067. [Google Scholar]
- Duncan HM, Mackler B. Electron transport systems of yeast. 3. Preparation and properties of cytochrome oxidase. J Biol Chem. 1966;241:1694–1697. [PubMed] [Google Scholar]
- Gabai VL, Sherman MY. Invited review: interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol. 2002;92(4):1743–1748. doi: 10.1152/japplphysiol.01101.2001. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb S, Ruvkun G. Daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweijer MA, Berden JA, Kemp A, Slater EC. Inhibition of energy-transducing reactions by 8-nitreno-ATP covalently bound to bovine heart submitochondrial particles: direct interaction between ATPase and redox enzymes. Biochim Biophys Acta. 1985;809:81–89. doi: 10.1016/0005-2728(85)90170-7. [DOI] [PubMed] [Google Scholar]
- Hockertz MK, Clark-Lewis I, Candido EP. Studies of the small heat shock proteins of Caenorhabditis elegans using anti-peptide antibodies. FEBS Lett. 1991;280:375–378. doi: 10.1016/0014-5793(91)80335-z. [DOI] [PubMed] [Google Scholar]
- Hoss S, Henschel T, Haitzer M, Traunspurger W, Steinberg CE. Toxicity of cadmium to Caenorhabditis elegans (Nematoda) in whole sediment and pore water—the ambiguous role of organic matter. Environ Toxicol Chem. 2001;20:2794–2801. [PubMed] [Google Scholar]
- Inoue T, Thomas JH. Targets of TGF-beta signaling in Caenorhabditis elegans dauer formation. Dev Biol. 2000;217:192–204. doi: 10.1006/dbio.1999.9545. [DOI] [PubMed] [Google Scholar]
- Kayser B, Rajaram S, Thomas S, Morgan PG, Sedensky MM. Control of anesthetic response in C. elegans. Toxicol Lett. 1998;101:339–346. doi: 10.1016/s0378-4274(98)00204-5. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones. 1997a;2:60–71. doi: 10.1379/1466-1268(1997)002<0060:dcohoi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Tissue-specific variation in Hsp70 expression and thermal damage in Drosophila melanogaster larvae. J Exp Biol. 1997b;200:2007–2015. doi: 10.1242/jeb.200.14.2007. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Fleming JT 1995 Basic culture methods. In: Caenorhabditis elegans. Modern Biological Analysis of an Organism. Methods In Cell Biology, vol 48, ed Epstein HR, Shakes DC. Academic Press, San Francisco, CA, 3–29. [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie JM Jr, Epstein HF. Paramyosin is necessary for determination of nematode thick filament length in vivo. Cell. 1980;22:747–755. doi: 10.1016/0092-8674(80)90551-6. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413–1417. doi: 10.1152/jappl.1997.83.5.1413. [DOI] [PubMed] [Google Scholar]
- Myrmel T, McCully JD, Malikin L, Krukenkamp IB, and Levitsky S 1994 Heat-shock protein 70 mRNA is induced by anaerobic metabolism in rat hearts. Circulation. 90(II). 299–305. [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock' proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- Power RS, de Pomerai DI. Effect of single and paired metal inputs in soil on a stress-inducible transgenic nematode. Arch Environ Contam Toxicol. 1999;37:503–511. doi: 10.1007/s002449900545. [DOI] [PubMed] [Google Scholar]
- Riddle DL 1988 The dauer larvae. In: The Nematode Caenorhabditis elegans, ed Wood WB. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 393–412. [Google Scholar]
- Riddle DL, Albert PS 1997 Genetic and environmental regulation of dauer larva development. In: C. elegans II, ed Riddle DL, Blumenthal T, Meyer BJ, Priess, JR. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 739–768. [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–98. doi: 10.1379/1466-1268(1996)001<0097:dothsr>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russnak RH, Candido EP. Locus encoding a family of small heat shock genes in Caenorhabditis elegans: two genes duplicated to form a 3.8-kilobase inverted repeat. Mol Cell Biol. 1985;5:1268–1278. doi: 10.1128/mcb.5.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachat FH, Garcea RL, Epstein HF. Myosins exist as homodimers of heavy chains: demonstration with specific antibody purified by nematode mutant myosin affinity chromatography. Cell. 1978;15:405–411. doi: 10.1016/0092-8674(78)90009-0. [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Sluse FE, Jarmuszkiewicz W. Activity and functional interaction of alternative oxidase and uncoupling protein in mitochondria from tomato fruit. Braz J Med Biol Res. 2000;33:259–268. doi: 10.1590/s0100-879x2000000300002. [DOI] [PubMed] [Google Scholar]
- Snutch TP, Baillie DL. Alterations in the pattern of gene expression following heat shock in the nematode Caenorhabditis elegans. Can J Biochem Cell Biol. 1983;61:480–487. doi: 10.1139/o83-064. [DOI] [PubMed] [Google Scholar]
- Snutch TP, Heschl MF, Baillie DL. The Caenorhabditis elegans hsp70 gene family: a molecular genetic characterization. Gene. 1988;64:241–255. doi: 10.1016/0378-1119(88)90339-3. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Candido EPM. Transgenic hsp16-lacZ strains of the soil nematode Caenorhabditis elegans as biological monitors of environmental stress. Environ Toxicol Chem. 1994;13:1211–1220. [Google Scholar]
- Sulston JE, Hodgkin J 1988 Methods. In: The Nematode Caenorhabditis elegans, ed Wood WB. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 587–606. [Google Scholar]
- Tatara CP, Newman MC, McCloskey JT, Williams PL. Predicting relative metal toxicity with ion characteristics: Caenorhabditis elegans LC50. Aquat Toxicol. 1997;39:279–290. [Google Scholar]
- Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;85:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Van der Bend RL, Duetz W, Colen AM, Van Dam K, Berden JA. Differential effects of triphenyltin and 8-azido-ATP on the ATP synthesis, ATP-Pi exchange, and ATP hydrolysis in liposomes containing ATP synthase and bacteriorhodopsin. Arch Biochem Biophys. 1985;241:461–471. doi: 10.1016/0003-9861(85)90571-5. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Galifianakis A, Crowder CM. A quantitative genetic approach towards volatile anesthetic mechanisms in C. elegans. Toxicol Lett. 1998;101:309–317. doi: 10.1016/s0378-4274(98)00200-8. [DOI] [PubMed] [Google Scholar]
- Wadsworth WG, Riddle DL. Developmental regulation of energy metabolism in Caenorhabditis elegans. Dev Biol. 1989;132:167–173. doi: 10.1016/0012-1606(89)90214-5. [DOI] [PubMed] [Google Scholar]
- Williams PL, Anderson GL, Johnstone JL, Nunn AD, Tweedle MF, Wedeking P. Caenorhabditis elegans as an alternative animal species. J Toxicol Environ Health A. 2000;61:641–647. doi: 10.1080/00984100050195125. [DOI] [PubMed] [Google Scholar]
- Williams PL, Dusenbery DB. Using the nematode C. elegans to predict mammalian acute lethality to metallic salts. Toxicol Ind Health. 1988;4:469–478. doi: 10.1177/074823378800400406. [DOI] [PubMed] [Google Scholar]