Abstract
In the present work we reported a semiquantitative detection of messenger ribonucleic acids (mRNAs) encoding the human heat shock proteins Hsp70-1, the stress inducible member of the HSP70 family, and hsp90α, the inducible member of the HSP90 family. We investigated the change in the expression of these mRNAs in tissue samples taken from the right atrium of 48 pediatric patients, soon after the ischemic period during surgery to correct congenital heart diseases, in which a crystalloid cold cardioplegic solution was used. No significant variations were found for either hsp70-1 or hsp90α expressions. Moreover, we searched for an association between the hsp70-1 promoter region polymorphism and the expression of the hsp70-1 in a smaller group of these patients (n = 27). The −110AA genotype was on average significantly associated with a decrease in the hsp70-1 mRNA level (P < 0.05), whereas the other genotypes −110AC or −110CC did not seem to be associated with the hsp70-1 expression level. The lack of any observed increase in the hsp70-1 expression level may be due to the high basal level of the Hsp70 protein in the tissues examined.
INTRODUCTION
In response to adverse environmental changes, cells from many organisms increase the expression of a large class of proteins known as heat shock or stress proteins (Hsps), each named according to its molecular weight (Benjamin and McMillan 1998). Numerous studies have shown that synthesis of HSPs is enhanced to protect cells against various stress conditions, such as elevated temperature, exposure to heavy metals, as well as ischemia (Knowlton et al 1991). Indeed, HSPs act in protecting nascent or denaturated proteins from aggregation, assisting their folding or refolding into the correct conformation or their degradation after an irreversible damage, as well as in the translocation of damaged proteins.
The induction of hsp70 gene expression by ischemia as well as an inverse correlation between the gene expression of hsp70 and the infarct size have been previously demonstrated in some animal models, as recently reviewed (Benjamin and McMillan 1998). In a preliminary study, we investigated whether ischemic stress, induced by cardiopulmonary bypass (CPBP), can affect the expression of hsp70-1 in the right atria of pediatric patients undergoing surgery for congenital heart defect (CHD) (Storti et al 2001). Our results showed no significant changes in the hsp70-1 messenger ribonucleic acid (mRNA) expression, the stress-inducible gene of the HSP70 family, in atrial tissue after aortic cross-clamping (CC).
To better clarify whether the Hsps could have a cardioprotective role during surgical stress, we also measured the expression of hsp90α, the inducible member of the HSP90 family, together with that of hsp70-1 in human right atrium tissue samples, collected before and soon after the end of the aortic CC in 48 pediatric patients who had undergone surgery for CHD. Furthermore, we searched for an eventual association between the polymorphism of the hsp70-1 promoter region and the mRNA expression level to explain the lack of expression variations in the hsp70-1 gene during cardiac surgery. For this reason, we also genotyped 27 of these patients for this polymorphism.
MATERIALS AND METHODS
Patients
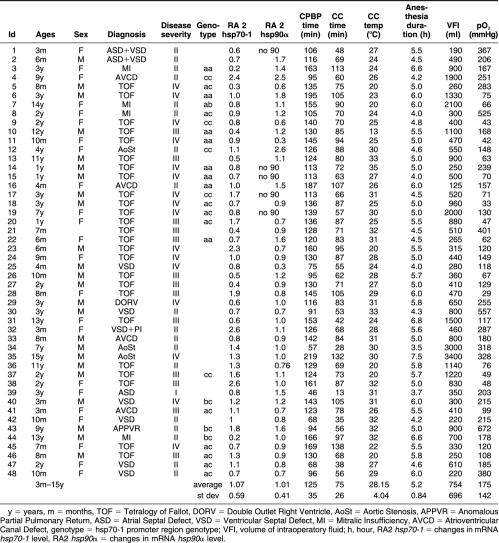
We examined 48 pediatric patients (ages ranging from 3 months to 15 years) undergoing elective surgical correction of CHD, 25 being affected by tetralogy of Fallot (TOF), 7 by ventricular septal defect (VSD), 1 by atrial septal defect (ASD), 4 by atrioventricular canal defect (AVCD), 4 by mitralic insufficiency (MI), 1 by double outlet right ventricle (DORV), 2 by ASD + VSD, 3 by aortic stenosis (AoSt), and 1 by anomalous partial pulmonary venous return (APPVR). Before the surgical procedure no patient was taking drugs nor was exposed to maneuvers known to increase blood pressure. CPBP was established with a crystalloid cold cardioplegic solution. During surgery, the mean CC temperature was 28.15°C ± 4.04 (average ± standard deviation). Mean CPBP time was 120 ± 33 minutes and mean CC time was 75 ± 26 minutes. Patients were divided into 4 groups according to their clinical condition and the gravity of their disease. These groups are shown in Table 1 along with the patients' ages, diagnoses, CPBP and CC times and temperatures, and pO2 (mmHg) at the beginning of surgery under ventilation.
Table 1.
Characterization of patients and changes in hsp70-1 and hsp90a mRNA expressiona
Two sequential samples were taken from a myocardial site in the right atrium discarded during the surgical intervention: the first specimen (right atrium 1 [RA1]) was obtained on average 20 minutes after the sternotomy, before any surgical manipulation of the heart. The second specimen (RA2) was obtained adjacent to the first one, outside the purse string, immediately after the end of the aortic CC. The duration of anesthesia up to the time that the RA1 specimen was taken as well as the total volume of the fluids administered to each patient are shown in Table 1 as a surrogate marker of anesthesia and intraoperative stress. The specimens were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Informed consent was obtained from parents because all children were under 18 years of age, and the study protocol was approved by the local Ethics Committee.
Semiquantitative reverse transcriptase–polymerase chain reaction
Total RNA was extracted from each specimen by the Tripure Isolation Reagent (Roche Molecular Biochemicals, Mannheim, Germany) in a procedure based on the guanidinum thiocyanide method (Chomczynski and Sacchi 1987). The integrity of each RNA preparation was determined by electrophoretic fractionation through an agarose-formaldehyde gel; 1 μg of RNA was treated with a DNase RQ1 (Promega, Madison, WI, USA) and 300 ng underwent reverse transcription by means of an oligo (dT)12–18 priming (Gibco BRL Life Technologies, MD, USA), in a mix containing also 2 μL of 5× Reverse Transcriptase buffer (Promega), 0.5 μL of deoxynucleoside triphosphates 1.25 mmol/L concentration of each, (Amersham Pharmacia Biotech, Piscataway, NJ, USA), 5 U of RNasin (Promega), and 20 U of Moloney Murine Leukemia Virus (M-MLV RT) (Promega) and water up to a final volume of 10 μL. Another contemporary reaction in which no RT was added (No RT) was performed. The complementary deoxyribonucleic acids (cDNA) of glyceraldehyde 3 phosphate dehydrogenase (GAPDH) and each hsp target were coamplified in a multiplex polymerase chain reaction (PCR), using 2 couples of specific primers. Primer sequences are summarized in Table 2 (Tang et al 1995). The PCR solution (final volume: 25 μL) contained: 5 μL of the first strand cDNA solution, 1 μL of 1.25 mmol/L dNTP, 2.5 μL of 10× PCR buffer, 0.8 μL of 50 mmol/L magnesium chloride, 0.3 μmol/L of forward and reverse GAPDH and hsp70-1 or hsp90α primers. The annealing temperature was 58°C for hsp70-1 and 63°C for hsp90α and 30 cycles were done in both cases. For each sample, and for each target gene, 2 separate PCRs were performed from the same RT. The PCR products were resolved on a 6% polyacrylamide gel (19:1 acrylamide–N,N-methyl-bis-acrylamide) stained by silver nitrate. The densities of the GAPDH and hsp70-1 or hsp90α bands were analyzed by the NIH Image 1.60 software (developed at the US National Institute of Health and available on line at http://rsb.info.nih.gov/nih-image/). The calculations were made as follows: first, the ratios between the hsp70-1 or hsp90α and GAPDH band densities were calculated; second, the variation of the hsp70-1 or hsp90α expression in RA2, referred to as RA1 expression taken as baseline, was evaluated for each sample and for the 2 different PCRs; third, the average of the 2 results was calculated.
Table 2.
Primer sequences and polymerase chain reaction product size
hsp70-1 Promoter polymorphism detection
DNA was extracted from myocardial tissue from 27 patients by the Tripure Isolation Reagent (Roche Molecular Biochemicals). The genotyping protocol for the promoter region polymorphism was taken from Cascino (Cascino et al 1993, Table 2), with some modifications. Briefly, 50 ng of DNA was amplified with 10 mol/L of each specific primer in 25 μL of total reaction volume, with an annealing temperature of 55°C. Five microliters of PCR product was loaded on a 10% polyacrylamide (29% acrylamide, 1% bis-acrylamide in Tris Boric EDTA-TBE-buffer (TBE buffer) gel vertical plate 20 cm in length, run at room temperature for 22 hours, at 18 mA in TBE buffer 1×, and then stained by silver nitrate.
RESULTS
DNase 1 treatment
DNase 1 treatment is usually done after total RNA extraction to assure that no genomic DNA is contaminating the RNA solution (Grillo and Margolis 1990). In the absence of genomic DNA contamination, the cDNA synthesis reactions, performed without RT (No RT), and the subsequent PCR gave no products (data not shown).
Expression of hsp70-1
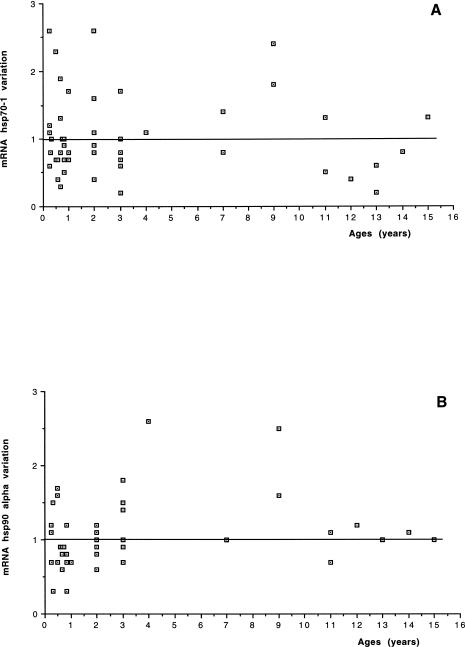
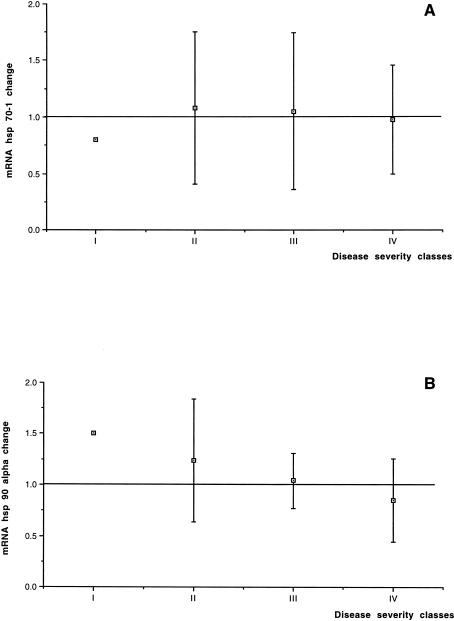
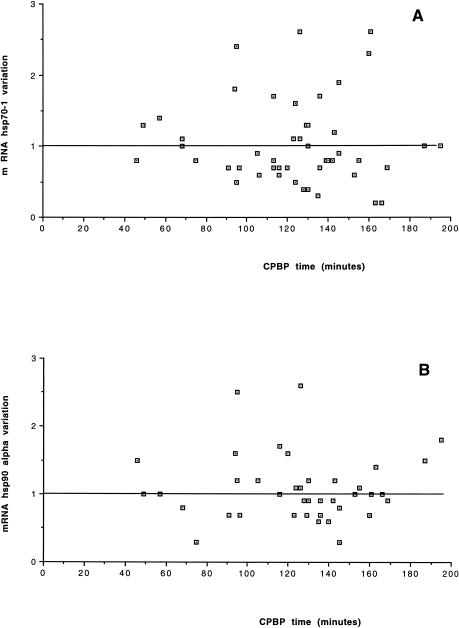
The results of hsp70-1 expression changes in the RA2 biopsy, compared with the mRNA expression in the RA1 biopsy considered as baseline in all 48 pediatric patients, are shown in Table 1. On average, no change in the mRNA level was found in hsp70-1 expression (1.07- ± 0.59-fold the RA1 baseline value). No relationship between changes in the hsp70-1 expression and patient age distribution, disease severity classes, or CPBP duration was found (Figs 1A, 2A, and 3A). Furthermore, no significant correlation was found between the expression changes and the CPBP time (R2 = 0.001, P = 0.8201) or CC time (R2 = 0.0001, P = 0.9435) or temperature (R2 = 0.004, P = 0.6886).
Fig 1.
Age distribution: relationship between age distribution of patients and changes and hsp70-1 (A) or hsp90α (B) expression
Fig 2.
Disease severity classes: relationship between the severity of patient disease , classified from I to IV according to the minor or major gravity of the congenital heart disease, and changes in hsp70-1 (A) or hsp90α (B) expression
Fig 3.
Cardiopulmonary bypass (CPBP) duration. Relationship between the duration of CPBP and changes and hsp70-1 (A) or hsp90α (B) expression
Expression of hsp90α
The results of the measurement of hsp90α mRNA expression in the 48 patients are shown in Table 1. On average, no variation in the mRNA level was found in hsp90α expression when the values obtained after the aortic CC were compared with those measured before CC (1.01- ± 0.41-fold the RA1 baseline value). No relationship was found between changes in hsp90α expression and patient age distribution, disease severity classes or CPBP duration (Figs 1B, 2B, and 3B). No significant correlation was found between the expression changes of hsp90α mRNA and the CPBP time (R2 = 0.001, P = 0.8680) or the CC time (R2 = 0.001, P = 0.8146) or temperature (R2 = 0.025, P = 0.3130).
Analysis of the hsp70-1 promoter region polymorphism
Using the primers described by Cascino (Cascino et al 1993), a single 496–base pair product was obtained. On the polyacrylamide gel, 3 bands of different mobility were interpreted as corresponding to different alleles and were named: a (slow), b (fast), c (intermediate). Variations among the 3 alleles were restricted to 2 sites: −110 and +120. At −110 the a allele differed from b and c for the presence of an adenine instead of a cytosine. At +120 the b allele differed from a and c for the presence of a cytosine instead of a thymine.
Twenty-seven patients were analyzed for this polymorphism, and the results are shown in Table 1. Individuals could exhibit either 1 or 2 bands; the combinations found were the following: “ab” (−110AC, +120TC) (1/27, fab = 0.037), “ac” (−110AC, +120TT) (10/27 fac = 0.370), “bc” (−110CC, +120CT) (3/27 fbc = 0.111), “aa” (−110AA, +120TT) (8/27 faa = 0.296), and “cc” (−110CC, +120TT) (5/27 fcc = 0.185). The “bb” (−110CC, +120CC) genotype has not been encountered, as previously reported by Cascino (Cascino et al 1993). The genotype assortment was as expected from the Hardy-Weinberg equilibrium in the 3 allelic system; the calculated frequencies of the 3 alleles being as follows: fa = 0.500, fb = 0.074, and fc = 0.426.
Relation between hsp70-1 promoter genotype and hsp70-1 mRNA expression
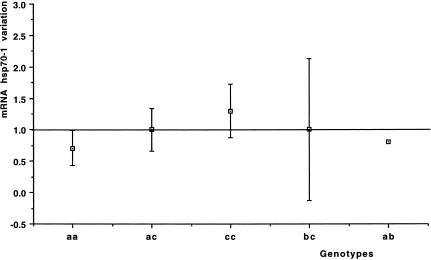
The “aa” genotype, found in 8 patients (3, 6, 10, 11, 14, 15, 16, 22), showed on average a significant decrease in the hsp70-1 mRNA level after the ischemic period (on average 0.71 ± 0.29, t-test P-value = 0.025, Table 1). Moreover, the only patient whose genotype was “ab” showed a decrease in the hsp70-1 mRNA level, whereas the other patients, characterized as “ac,” “bc,” or “cc,” had no significant changes of hsp70-1 mRNA expression before and after the ischemic period. Data from genotypic analysis are presented in Figure 4.
Fig 4.
hsp70-1 Promoter region polymorphism. Relationship between the mean change of mRNA hsp70-1 for each observed genotype
DISCUSSION
In our study we evaluated the change in hsp70-1 mRNA expression in biopsies from right atria of pediatric patients undergoing surgery for CHD correction. During surgery, CPBP was established by means of a cold crystalloid cardioplegic solution. Because of the young age of the patients, the samples collected were very small (8–20 mg). Consequently, a previously described semiquantitative method of quantifying mRNA expression from only 300 ng of total RNA (Storti et al 2001) was set up using the housekeeping GAPDH gene as an internal standard, on the assumption that its level would remain unchanged before and after the ischemic period. Before reverse-transcription, total RNA was treated with a DNase 1 and a No RT control was included (Grillo and Margolis 1990). The DNase I pretreatment is essential because hsp70-1 is an intronless gene (Wu et al 1985) and also because in genomic DNA there is a GAPDH pseudogene. Indeed, DNase I pretreatment eliminates the false positives that can result from any preexisting DNA and could confuse the interpretation of results.
In our study, on average no variation of hsp70-1 mRNA expression was found after the ischemic period (1.07- ± 0.59-fold the baseline value). However, a limitation of this study is that it has not been possible to have a truly baseline cardiac preparation with which to compare the intraoperative surgical samples because the samples were taken from individuals who had severe cardiac disease and who often experienced varying degrees of pathophysiologic stresses before surgery. In fact, the first sample represents a time point in which a patient has undergone an open sternotomy and this initial sample may reflect a significant degree of stress already experienced by the patient. However, this protocol of study was the best we could set up because it was not possible to obtain a completely unstressed human cardiac preparation to use as baseline.
In our opinion, among the hypotheses that could explain our result, 2 appear very interesting.
A first consideration may be done about the basal high level of the Hsp70 protein (Shi et al 1998) in patients before cardiosurgery. In fact, it has been noted that in higher eukaryotes, the stress signal leads to the elevated expression of heat shock genes; stress induced transcription requires activation of heat shock factors (HSFs) that bind to the heat shock promoter element. In unstressed cells, HSFs are maintained in an inert non–DNA-binding state. Upon exposure of cells to stress conditions, HSFs become activated to a DNA-binding, transcriptionally active state, which results in the preferential transcription of the heat shock genes. In stress conditions, Hsp70 stably associates with the heat shock factor 1 (HSF1), and so it functions as a repressor of transcriptional activity of the heat shock genes (Shi et al 1998). According to this hypothesis, a preexisting state of physiological stress, perhaps due in our patients to the congenital heart disease, can provoke an inhibition of the further transcription of all heat shock genes. In the present study, this hypothesis has been tested by the evaluation of the change in the expression of hsp90α, the inducible member of the HSP90 family. It has been shown, in fact, that in hsp70 overexpressing cells the transcription of the hsp90α gene was not induced after heat shock, whereas in cells uninduced for hsp70 there was a dramatic heat shock induction of the hsp90α gene transcription (Shi et al 1998). We found on average no induction of hsp90α mRNA too (1.01- ± 0.41-fold the baseline value). In conclusion, we can suggest that a negative feedback regulation by Hsp70 acting as an expression repressor on hsp genes transcription is possible.
A second hypothesis involves the hsp70-1 promoter region polymorphism. The method used for detecting the promoter polymorphism yields stereoscopic images of the DNA superhelical conformation, which can be directly related to the electrophoretic behavior. In effect, the introduction of an adenine at position −110 (ie, allele a) increases the curvature of the molecule and provides an adequate explanation of the electrophoretic retardation of this allele; the modification of the DNA curvature by introducing a cytosine at the +120 site (allele b) is much less marked. Moreover, the adenine-cytosine interchange at position −110, which gives the strongest effect on DNA curvature, is located between the first 2 of a series of 5 consecutive regulatory elements involved in the binding of HSF1 (Cascino et al 1993). It is possible that this interchange causes a variation of hsp70-1 expression after stress stimuli, owing to the intimate connection between the local curvature of a DNA segment and its function in regulatory processes. Indeed, our data indicate that the −110AA genotype seems to be significantly associated with an inactivation of the hsp70-1 expression, whereas the other genotypes do not seem to be associated with the hsp70-1 mRNA level. Unfortunately, we obtained the genotype data only from a minority of patients; our result, although suggestive, needs to be confirmed in a larger population, and so further studies are necessary to better clarify the pathophysiological importance of this association.
In conclusion, on average, no increase in the hsp70-1 mRNA expression was found in biopsies from human right atria taken soon after the ischemic period during heart surgery in the present study. It is possible that the lack of any observed increase in hsp70-1 expression may be due to the high basal level of Hsp70 protein because hsp90 failed to induce in the same tissue. Furthermore, our data revealed an association between the hsp70-1 promoter polymorphism and the expression of the hsp70-1: in fact, the −110AA genotype seems to be significantly associated with an inactivation of the hsp transcription.
REFERENCES
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- Cascino I, Sorrentino R, Tosi R. Strong genetic association between HLA-DR3 and a polymorphic variation in the regulatory region of the HSP70-1 gene. Immunogenetics. 1993;37:177–182. doi: 10.1007/BF00191882. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinum thiocyanite-phenolchloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Grillo M, Margolis FL. Use of reverse trancriptase polymerase chain reaction to monitor expression of intronless genes. Biotechniques. 1990;262:266–268. [PubMed] [Google Scholar]
- Knowlton AA, Brecher P, Apstein CS. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991;87:139–147. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti S, Vittorini S, Luisi VS, Sacchelli M, Collavoli A, Vanini V, Biagini A, Clerico A. No variation in Hsp70 mRNA level during cardiac surgery in pediatric patients evaluated by semiquantitative RT-PCR. Clin Chem Lab Med. 2001;39:1240–1243. doi: 10.1515/CCLM.2001.199. [DOI] [PubMed] [Google Scholar]
- Tang PZ, Gannon MJ, Andrew A, Miller D. Evidence for oestrogenic regulation of heat shock protein expression in human endometrium and steroid-responsive cell lines. Eur J Endocrinol. 1995;133:598–605. doi: 10.1530/eje.0.1330598. [DOI] [PubMed] [Google Scholar]
- Wu B, Hunt C, Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]