Abstract
Endothelial cell migration, a key process in angiogenesis, requires the coordinated integration of motogenic signals elicited by the adhesion of endothelial cells to extracellular matrices and by angiogenic cytokines such as the vascular endothelial growth factor (VEGF). In this study, we found that addition of VEGF to human umbilical vein endothelial cells cultivated on vitronectin triggers a synergistic interaction between the VEGF receptor VEGFR2 and the clustered integrin receptor αvβ3. The interaction between VEGFR2 and αvβ3 is required for full phosphorylation of VEGFR2 and to drive the activation of motogenic pathways involving focal adhesion kinase (FAK) and stress-activated protein kinase-2/p38 (SAPK2/p38). The signal emanating from the VEGFR2 and αvβ3 interaction and leading to SAPK2/p38 activation proceeds directly from VEGFR2. The chaperone Hsp90 is found in a complex that coprecipitates with inactivated VEGFR2, and the association is increased by VEGF and decreased by geldanamycin, a specific inhibitor of Hsp90-mediated events. Geldanamycin also impairs the phosphorylation of FAK that results from the interaction between VEGFR2 and αvβ3, and this is accompanied by an inhibition of the recruitment of vinculin to VEGFR2. We conclude that a necessary cross talk should occur between VEGFR2 and the integrin αvβ3 to transduce the VEGF signals to SAPK2/p38 and FAK and that Hsp90 is instrumental in the building up of focal adhesions by allowing the phosphorylation of FAK and the recruitment of vinculin to VEGFR2.
INTRODUCTION
Vascular endothelial growth factor-A (VEGF) is a major angiogenic agent that regulates key steps of the angiogenic process. It promotes endothelial cell proliferation and migration, and it increases the production of plasminogen activators and the permeability of the vascular endothelial layer (Neufeld et al 1999). VEGF exerts its effects after binding to 2 homologous membrane tyrosine kinase receptors present on the surface of human endothelial cells: VEGFR1, encoded by Flt1, and VEGFR2, encoded by KDR/Flk1 (Mustonen and Alitalo 1995; Petrova et al 1999). VEGFR1 and VEGFR2 belong to the platelet-derived growth factor (PDGF) receptor subfamily, which consists of 7 immunoglobulin (Ig) homology domains in its extracellular part and of an intracellular tyrosine kinase domain that is split by a kinase insert of 65–97 hydrophobic residues. This kinase insert is important for substrate recognition (Petrova et al 1999). On endothelial cells, VEGF also binds to neuropilin, a coreceptor that increases the affinity of VEGFR2 for VEGF (Soker et al 1998). VEGFR1 and VEGFR2 are essential for vasculogenesis and angiogenesis, and knockout mice for both receptors are embryonic lethal (Fong et al 1995; Shalaby et al 1995).
Like other tyrosine kinase receptors, VEGFRs undergo ligand-induced homodimerization and oligomerization, which activates their intrinsic tyrosine kinase activity. The formation of heterodimers between VEGFR1 and VEGFR2 has recently been reported, but their role is still ambiguous (Rousseau et al 2000a; Sato et al 2000b). VEGFR1 is poorly autophosphorylated in response to VEGF in endothelial cells. Moreover, mice expressing a truncated form of VEGFR1, which lacks the tyrosine kinase domain, possess normal vasculature conversely to the full-VEGFR1 knockout animals. This indicates that VEGFR1 is weakly involved in transducing the VEGF angiogenic signals and supports the concept that it might act as a decoy receptor rather than as a signal-transducing molecule (Petrova et al 1999). In contrast, ligand-induced homodimerization of VEGFR2 leads to a strong autophosphorylation of VEGFR2 on tyrosine residues, which drives the activation of major signaling pathways that include extracellular regulated kinase (ERK) and stress-activated protein kinase-2/p38 (SAPK2/p38) mitogen-activated protein (MAP) kinases (Seetharam et al 1995; Rousseau et al 1997). Activation of ERK leads to increased proliferation of endothelial cells, whereas activation of SAPK2/p38 triggers the cytoskeleton remodeling that is required to drive actin-based motility (Huttenlocher et al 1995; Rousseau et al 2000b). Major autophosphorylation sites on VEGFR2 have been ascribed as Y1175 and Y1214 (Takahashi et al 2001). Other putatively important phosphorylated sites include Y951 and Y996 in the kinase insert domain and Y1054 and Y1059 in the tyrosine kinase catalytic domain. These tyrosine residues, when phosphorylated, are involved as docking sites to recruit molecules containing src homology 2(SH2) and 3(SH3) domains, or phosphotyrosine-binding (PTB) domain and to convey signals to downstream pathways (Petrova et al 1999). In particular, phosphorylation of Y1175 by VEGF is crucial to initiate the activation of phospholipase C-γ (PLCγ) as well as to convey ERK-mediated signal to deoxyribonucleic acid (DNA) synthesis (Takahashi et al 2001). The adapter protein VEGF receptor–associated protein is possibly involved in connecting phosphoinositide 3-kinase and PLCγ to VEGFR2, presumably through interaction with Y951 (Wu et al 2000). Shc is recruited to a yet unknown tyrosine on the phosphorylated VEGFR2, which triggers connection to the adapter protein Grb2 and then transmits the mitogenic signals to Sos, Ras, and ERK (Kroll and Waltenberger 2000). The adapter molecules and the tyrosine residues that couple VEGFR2 to SAPK2/p38 or FAK remain to be identified. In the case of FAK, its association with VEGFR2 required the last 125 amino acids of the C terminal tail but seems to be independent of the Y residues present in this region (Qi and Claesson-Welsh 2001).
Integrins are transmembrane adhesion receptors consisting of α and β chains, which associate into dimers on binding with their respective extracellular matrix (ECM) ligands. Integrins are involved in an active bidirectional signaling between the cells and the ECM, and they contribute to the linkage between ECM and cells (Schoenwaelder and Burridge 1999). Notably, activation of the integrins allows the functional connection between focal contacts and actin cytoskeleton that is required to drive cell migration (Klemke et al 1997; Giancotti 2000). There is a wealth of evidence that supports that integrins and growth factor signaling pathways interact to coordinately integrate the message initiated by both types of receptors. For example, αvβ1 integrin mediates fibronectin-induced epithelial cell proliferation through activation of the epidermal growth factor (EGF) receptors (Kuwada and Li 2000). Similarly, αvβ3 integrin associates with PDGFβ and VEGFR2 to potentiate their activity (Soldi et al 1999; Borges et al 2000). Little is known, however, on how the signals initiated by growth factor–integrin receptor complexes are integrated by the cells to activate the appropriate targets. Focal adhesion kinase (FAK) appears to integrate the signal generated by integrins and the EGF and PDGF receptors, and integrin-linked kinase (ILK) integrates the insulin and fibronectin-dependent signals (Dedhar et al 1999; Sieg et al 2000).
Hsp90 is an abundant and highly conserved chaperone protein, which is involved in a vast array of cellular response. It acts as a scaffolding protein that maintains the nuclear receptors for steroids, the ErbB2 receptors, and the signaling molecules such as Src, Raf, Akt in a conformational state, which allows signaling (Pratt 1997; Sato et al 2000a; Xu et al 2001, 2002). The in vivo functions of Hsp90 require adenosine triphosphate (ATP) binding and ATP hydrolysis by intrinsic adenosine 5′-triphosphatase (ATPase) activity (Obermann et al 1998). Inhibiting ATP binding with geldanamycin, an ansamycin derivative, specifically suppresses the ATPase activity of Hsp90 and disrupts its functions (Obermann et al 1998). In particular, inhibition of Hsp90 by geldanamycin blocks the assembly of Hsp90 heterocomplexes and destabilizes preformed heterocomplexes (Pratt 1998). Hsp90 is importantly involved in VEGF signaling, being required for VEGFR2-mediated activation of endothelial NO synthase and FAK tyrosine phosphorylation in endothelial cells stimulated with VEGF (Garcia-Cardena and Folkman 1998; Rousseau et al 2000b). Interestingly, recent studies indicated that α2β1 integrin–mediated adhesion of platelet to collagen is involved in modulating the disassembly of a signaling modulatory complex that contains Hsp90 and phosphatase-1 (Polanowska-Grabowska et al 1997; Polanowska-Grabowska and Gear 2000).
In this study, we show that integrin αvβ3 synergistically interacts with VEGFR2 and demonstrate for the first time that this leads to the activation of SAPK2/p38 and tyrosine phosphorylation of FAK and to endothelial cell migration in response to VEGF. We also report for the first time that Hsp90 is present in a complex that coprecipitates with VEGFR2 and that it has a crucial role in regulating the recruitment of vinculin to VEGFR2, which then initiates the building up of the nascent focal adhesions.
MATERIALS AND METHODS
Reagents
VEGF165, endothelial cell growth supplement (ECGS), endotoxin-free cell culture–tested bovine serum albumin (BSA), and geldanamycin were obtained from Sigma, St. Louis, MO, USA. Contortrostatin was a generous gift from Dr Frank Markland (USC Keck School of Medicine). Chemicals for electrophoresis were purchased from Bio-Rad, Mississauga, ON, Canada and Fisher, Montreal, Quebec, Canada. Plasma bovine vitronectin was from Sigma.
Antibodies, peptides, and adenovirus
Anti-FAK used for immunoprecipitation is a rabbit polyclonal antibody obtained from Pharmingen (La Jolla, CA, USA), and anti-FAK used for Western blotting is a mouse monoclonal antibody from Transduction Laboratories, Mississauga, ON, Canada. Anti-VEGFR2 blocking (KF1-6.64) and nonblocking (KF2-5.52) antibodies were gifts from ImClone Systems (New York, NY, USA) (Witte et al 1998; Zhu et al 1998). Other anti-VEGFR2 antibodies were from Santa Cruz, Santa Cruz, CA, USA, (Flk-1, clone 1158) and Sigma (Kdr, clone 2). Function-blocking antibody against integrin β1 subunit (clone P4C10) was from Life Technology, Burlington, ON, Canada. Neutralizing anti-integrin αvβ3 (clone LM609) and αvβ5 (clone P1F6) antibodies were obtained from Chemicon (San Diego, CA, USA). Anti-mouse IgG-horseradish peroxidase (HRP) and anti-rabbit IgG-HRP were from Jackson Laboratories, West Grove, PA, USA. Monoclonal antiphosphotyrosine antibody (clone PY20) and anti-Hsp90 mouse were from Transduction Laboratories. Antiphospho p38 was from New England Biolabs, Beverly, MA, USA. Anti-p38 antibody was given by Dr Jacques Landry (Laval University, Québec). Anti-vinculin antibody was from Sigma. Anti-mouse IgG-Alexa 568 antibody was purchased from Molecular Probes, Eugene, OR, USA. pEGFP-C1 was purchased from Clonetech, Mississauga, ON, Canada and FAK-related nonkinase protein (FRNK) construct was given by Dr Jun-Lin Guan (Cornell University). The adenovirus expressing a dominant negative mutant of p38 was prepared as follows: the kinase-inactive p38α:Arg-Gly-Phe (AGF) complementary DNA (Raingeaud et al 1995) was cloned in an adenovirus transfer plasmid and made into a replication-incompetent Ad-5 (delE1, E3) virus as described (Valerie 1999; Rosenberg et al 2001). The virus expresses p38α (T180GY → AGF) with a Flag-tag at its amino terminus. Infection of human keratinocytes with this virus completely inhibits the activation of p38 by ultraviolet B (Chouinard et al 2002).
Cells
HUVEC were isolated by collagenase digestion of umbilical veins from undamaged sections of fresh cords. The umbilical vein was cannulated, washed with Earle's basic salt solution (EBSS), and perfused for 10 minutes with collagenase (1 mg/mL) in EBSS at 37°C. After perfusion, the detached cells were collected, the vein was washed with 199 medium, and the wash-off pooled with the perfusate. After washing, cells were plated on gelatin-coated 75-cm2 culture dishes in MXV medium (199 medium containing 20% heat-inactivated fetal bovine serum [FBS], ECGS [60 μg/mL], glutamine, heparin, and antibiotics). Subcultures were obtained by trypsination and were used at passages <4. The identity of HUVEC as endothelial cells was confirmed by their polygonal shape or by detecting their immunoreactivity for factor VIII–related antigens. For all experiments, treatments were done on HUVEC that were cultivated on 3 μg/mL of vitronectin and that were made quiescent after incubation for 16–20 hours in ECGS-free medium containing 5% FBS, glutamine, and antibiotics. Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Gene transfer
Gene transfer was achieved by using adenoviral constructs containing β-galactosidase or p38 AGF, a dominant negative form of SAPK2/p38α, given by Drs Claude Gravel (Laval University, Québec) and Kristoffer Valerie (Virginia Commonwealth University, Richmond, VA), respectively. Briefly, HUVEC were plated for 24 hours in 35-mm petri dishes. Five microliters of adenoviral vector suspension was then added for 8 hours to the monolayers of HUVEC cultures. The infection media were then changed for fresh media. Twenty-four hours later, the cells, 80% of which expressed p38 AGF, were treated and processed for migration assays as described below. Electroporated gene transfer was obtained with 30 μg of DNA construct per 1 × 106 HUVEC. Suspended cells were left on ice in minimal media with DNA for 3 minutes and then were electroporated at 25 μF and 300 V. Cells were then left on ice for an additional 10 minutes and seeded with complete media in 60-mm petri dishes. Four hours later, media were changed for fresh media. Twenty-four hours later, the cells were treated and processed for migration assays as described below. Cotransfection of a green fluorescent protein (GFP) construct allows an estimation of the transfection efficiency as 30%. This has been done in fluorescence microscopy by evaluating the percentage of cells that express GFP.
Immunoprecipitation
For tyrosine phosphorylation of FAK, the cells plated were scraped and extracted in 75 μL of boiled denaturation buffer containing 1% sodium dodecyl sulfate (SDS), 1 mM Na3VO4, and 10 mM Tris (pH 7.4). The further steps were done at 4°C. For assays in suspension cultures, cells were trypsinized, kept in suspension in 1.5 mL of ECGS-free medium for 20 minutes at 37°C and 5% CO2. Incubation in the presence of the blocking antibodies lasted for all the suspension time. VEGF was added for the last 5 minutes. Cells were centrifuged at 200 × g for 5 minutes at 4°C, washed with phosphate-buffered saline (PBS), and lysed in 75 μL of boiled denaturation buffer. Extracts were boiled twice for 5 minutes. Samples were immediately used for immunoprecipitation or were stored at −80°C. Proteins were quantified according to Bradford assay, and 65 μg of proteins was diluted 10 times in B buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 0.1% sodium deoxycholate, 2 mM ethylenediaminetetraacetic acid, 2 mM ethylene glycol-bis(aminoethylether)-tetraacetic acid, 1 mM Na3VO4, 1 mM leupeptin, 50 μg/mL pepstatin, and 1 mM phenylmethane sulfonyl fluoride and was incubated for 2 hours at 4°C with 2 μL of FAK antibody preincubated with 12 μL of protein A Sepharose (50% v/v). Immunoprecipitated proteins were separated through 7.5% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose as previously described (Rousseau et al 2000b) and processed for Western blotting. For the detection of VEGFR2 and proteins associated with VEGFR2, cells were extracted as for FAK immunoprecipitation, except that they were lysed in 250 μL of buffer B containing 0.1% Triton X-100. After protein quantification, 250 μg of proteins was incubated with 20 μL of anti-VEGFR2 antibody preincubated with 20 μL of protein A Sepharose (50% v/v). Proteins were separated through 7.5% SDS-PAGE, transferred onto nitrocellulose, and processed for Western blotting using antiphosphotyrosine, anti-VEGFR2, anti-Hsp90, or antivinculin. For detection of SAPK2/p38, cells were extracted as for VEGFR2 immunoprecipitation, except that they were lysed in 100 μL of SDS-PAGE loading buffer. After protein quantification, 30 μg of proteins were separated through 10% SDS-PAGE, transferred onto nitrocellulose, and processed for Western blotting.
Western blotting
After reacting membranes with appropriate primary antibody, antigen-antibody complexes were detected with anti-IgG HRP antibody and then proteins were revealed using an enhanced chemiluminescence kit. SAPK2/p38 activity was evaluated using antibody that recognizes the phosphorylated form of SAPK2/p38 (New England Biolabs). Anti-VEGFR2 was detected using anti-Kdr (clone 2 from Sigma). For stripping, nitrocellulose membranes were first washed in Tris-buffered saline (TBS) 1× containing 0.1% Tween. Then, they were incubated for 30 minutes at 68°C in stripping buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, and 42 mM fresh β-mercaptoethanol) and washed again in TBS containing 0.1% Tween. In some experiments, the immunoreactive bands were quantitated by scanning densitometry using the NIH Image Software.
Adhesion assays
Ninety-six–well plastic dishes (Nunc) were coated with 150 ng of bovine vitronectin contained in 50 μL of PBS for 1 hour at 37°C and then were saturated with 1% BSA in PBS. After detachment, cells (3 × 104) were resuspended in 100 μL of MXV medium in the presence or absence of blocking antibodies and then were seeded in 96-well dishes precoated with vitronectin. Cells were left to adhere at 37°C for 1 hour. After 2 washes with medium, attached cells were fixed with glutaraldehyde 1% for 30 minutes, stained with 50 μL of 0.1% (w/v) crystal violet, and lysed with 0.1 mL of 1% SDS. Optical density was measured at 550 nm.
Cell migration assay
Cell migration was assayed using a modified Boyden chamber assay (Waltenberger et al 1994; Rousseau et al 1997). Exponentially growing cells were harvested with trypsin, counted, centrifuged, and resuspended at 0.5 × 106 cells/mL in migration buffer (199 medium, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid [pH 7.4], 1 mM MgCl2, 0.5% BSA). Cells were either not preincubated or preincubated for 20 minutes in suspension with blocking antibodies against integrins, and they were then added on an 8.0-μm–pore size polycarbonate membrane that was coated on both sides with vitronectin (3 μg/mL in PBS) and that separated the upper and lower chambers of a 6.5-mm transwell. VEGF was added to the lower chamber. Four hours later, cells on the upper face of the membrane were scraped using a cotton swab, and cells on the lower face were fixed with 3.7% formaldehyde and stained with Mayer's hematoxylin solution. The number of migrating cells on the lower face of the filter was counted in 5 fields under 100× magnification. In transfected cells expressing FRNK or GFP (or both), the number of fluorescent cells that have crossed the membrane was determined using an inverted fluorescence microscope. Assays were done in triplicates and repeated at least 3 times.
Immunofluorescence microscopy
LabTek (Nunc) wells were coated with 600 ng of bovine vitronectin contained in 200 μL for 1 hour at 37°C. Cells (2 × 104) were seeded in each chamber in 300 μL of MXV medium and then were made quiescent. After treatments, quiescent cells were washed twice with PBS (pH 7.5), fixed with formaldehyde 1% for 20 minutes at 37°C, and permeabilized with 0.2% Triton X-100 in PBS for 3 minutes. Vinculin was detected using the hVIN-1 monoclonal antibody. Vinculin antigen-antibody complexes were detected with anti-mouse IgG coupled to Alexia 568. Antibodies were incubated in 3% BSA, 0.05% Tween 20, and 0.08% sodium azide. Slides were observed by confocal microscopy with a Nikon Diaphot-TDM equipped with a 60× objective lens with a 1.4 numerical aperture.
RESULTS
Endothelial cell migration induced by VEGF on vitronectin depends on VEGFR2 and αvβ3 integrin and requires the activation of SAPK2/p38 and FAK
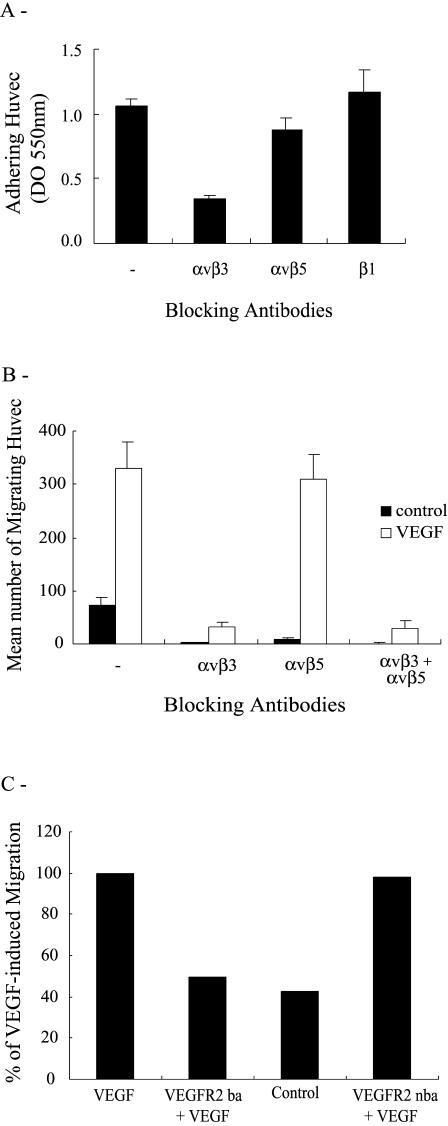
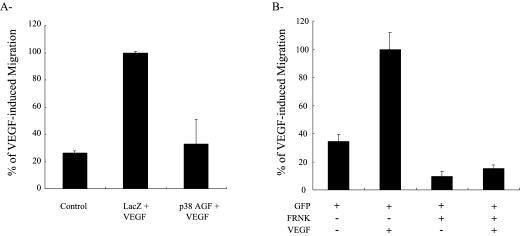
Endothelial cell migration is driven by the coordinated integration of the motogenic messages elicited by the interaction of adhesive molecules and angiogenic cytokines with their respective receptors. In this study, we investigated the role of integrins and VEGF receptors in mediating the motogenic signals induced by VEGF in endothelial cells cultivated on vitronectin, a major constituent of the basal membrane, which separates endothelial cells from vascular smooth muscle cells. Four integrins recognize vitronectin: αvβ3, αvβ5, αIIbβ3, and αvβ1. Of these, αvβ3, αvβ5, and αvβ1 are expressed by endothelial cells (Felding-Habermann et al 1997; Erdreich-Epstein et al 2000). To understand the role of these integrins in cell migration, we first verified their involvement in mediating the adhesion of HUVEC on vitronectin. Cells in suspension were either not pretreated or pretreated for 20 minutes with various concentrations of anti-αvβ3, anti-αvβ5, and anti-β1 blocking antibodies and then were added to a vitronectin matrix. Adhesion was assessed after 60 minutes. Results showed, for the maximally effective concentrations of the antibodies, that the anti-αvβ3 and anti-αvβ5 reduced the adhesion of HUVEC to vitronectin 75% and 25%, respectively, whereas the anti-β1 antibody did not affect cell adhesion (Fig 1A, data not shown). Consistent with their role in cell adhesion, both αvβ3 and αvβ5 integrins were involved in regulating the matrix-associated cell migration because both the anti-αvβ3 and anti-αvβ5 inhibited basal cell migration (Fig 1B). However, only αvβ3 was required to mediate the VEGF-induced increase in migration of HUVEC. This is illustrated in Figure 1B, which shows that the neutralizing anti-αvβ3 integrin antibody completely inhibited chemotactic cell migration induced by VEGF. The anti-αvβ5 neutralizing antibody did not affect chemotactic cell migration excluding the participation of αvβ5. Integrin αvβ1 or other integrins were not involved either because no migration was observed when both αvβ3 and αvβ5 were concomitantly blocked. The αvβ3-mediated, increased chemotactic cell migration required the activation of the VEGF receptor VEGFR2 because it was reduced down to basal level by pretreating the cells with an anti-VEGFR2 neutralizing antibody (Fig 1C). In contrast, a binding but not neutralizing anti-VEGFR2 antibody had no effect on VEGF-induced endothelial cell migration. We previously reported that the VEGF-induced, increased migration of HUVEC cultivated on gelatin, another matrix that binds integrin αvβ3, required an enhanced SAPK2/p38 MAP kinase activity and FAK activation (Petitclerc et al 1999; Rousseau et al 2000b). In accordance with these findings, we found that adenovirus-mediated overexpression of a dominant negative form of SAPK2/p38 (p38 AGF) almost completely blocked the VEGF-induced increase of cell migration (Fig 2A). Similarly, transfection of a dominant negative regulator of FAK (FRNK) blocked the VEGF-induced, increased endothelial cell migration (Fig 2B). The major conclusion drawn from Figures 1 and 2 is that the VEGF-induced endothelial cell migration on vitronectin depends on both VEGFR2 and integrin αvβ3 and requires the activation of SAPK2/p38 and FAK.
Fig 1.
VEGF-induced migration of HUVEC on vitronectin is mediated by VEGFR2 and integrin αvβ3. (A) HUVEC in suspension were either not incubated or incubated for 20 minutes with optimal concentrations or dilutions of anti-αvβ3 (1 μg/mL), anti-αvβ5 (1 μg/mL), or anti-β1 (1:1000) integrin antibodies. Cells were then plated in 96-well culture dishes precoated with 3 μg/mL vitronectin. After 60 minutes at 37°C, adhering cells were stained with crystal violet and quantified by optical density (550 nm). Data points represent means ± SD of triplicate samples from 3 different experiments. (B) HUVEC in suspension were either not preincubated or preincubated for 20 minutes with anti-αvβ3 (1 μg/mL), anti-αvβ5 (1 μg/mL), or anti-β1 (1:1000) integrin antibodies. (C) HUVEC in suspension were preincubated in the presence or absence of a VEGFR2 blocking (VEGFR2-ba 10 μg/mL, 20 minutes) or nonblocking antibody (VEGFR2-nba 10 μg/mL, 20 minutes). After treatments, in both B and C, cells were seeded on the upper part of a vitronectin-coated membrane in a modified Boyden chamber either containing or not containing VEGF (5 ng/mL) in the lower part. After 4 hours of migration, cells on the upper part of the membrane were scraped, and the cells on the lower part were stained with Mayer's hematoxylin. The cells of each well were counted in 5 fields at 100× magnification. Data points represent means from triplicate samples taken from at least 3 different experiments. VEGF, vascular endothelial growth factor; HUVEC, human umbilical vein endothelial cells; VEGFR2, VEGF receptor 2
Fig 2.
VEGF-induced migration of HUVEC on vitronectin is mediated by activation of SAPK2/p38 and FAK. (A) HUVEC were infected with adenoviral vectors expressing Lacz or p38 AGF, a dominant negative form of p38α. (B) HUVEC were electroporated at 25 μF and 300 V with a total of 30 μg of a GFP construct with or without a FRNK construct. In both A and B, infected and transfected cells were seeded on the upper part of a vitronectin-coated membrane in a modified Boyden chamber either containing or not containing VEGF (5 ng/mL) in the lower part. After 4 hours of migration, cells on the upper part of the membrane were scraped. In A, the cells on the lower part were stained with Mayer's hematoxylin and counted in 5 fields at 100× magnification. In B, fluorescent cells, on the lower part, expressing FRNK or GFP (or both) were counted in 7 fields using an inverted fluorescence microscope at 200×. Data points represent means of triplicate samples. VEGF, vascular endothelial growth factor; HUVEC, human umbilical vein endothelial cells; SAPK2/p38, stress-activated protein kinase-2/p38; FAK, focal adhesion kinase; GFP, green fluorescent protein; FRNK, FAK-related nonkinase protein
Activation of SAPK2/p38 and FAK by VEGF requires a synergistic interaction between VEGFR2 and αvβ3 integrin
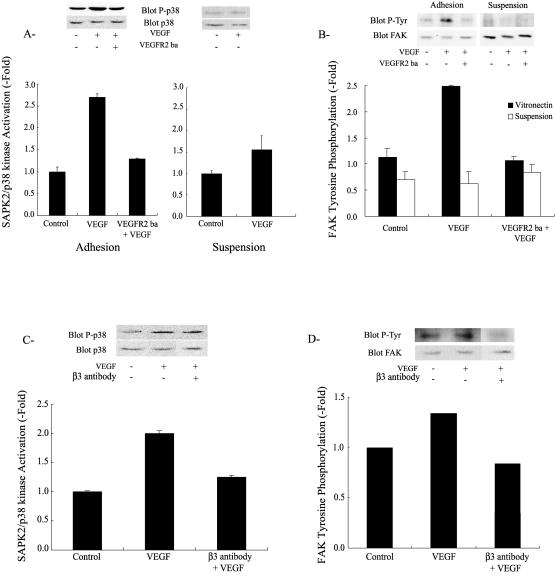
We next ascertained the role of both VEGFR2 and αvβ3 integrin receptors in activating SAPK2/p38 and FAK. Untreated HUVEC adhering on vitronectin have a low level of SAPK2/p38 activity as illustrated by the weak basal phosphorylation level of SAPK2/p38 (Fig 3A). However, addition of VEGF (5 ng/mL) induced, within 5 minutes, a 3-fold increase in SAPK2/p38 activation (Fig 3A). Inhibiting VEGFR2 with an anti-VEGFR2 antibody inhibited the activation of SAPK2/p38 (Fig 3A). This indicated that activation of VEGFR2 was required to trigger the VEGF-induced activation of SAPK2/p38 in endothelial cells cultivated on vitronectin. Similarly, we found that VEGF in concentration (5 ng/mL) that maximally enhanced activation of SAPK2/p38 and cell migration induced, within 5 minutes, a 2.5-fold increase in FAK tyrosine phosphorylation. The effect was also mediated by VEGFR2, being totally inhibited by the anti-VEGFR2 blocking antibody (Fig 3B). Intriguingly, the activities of SAPK2/p38 and FAK in the presence of VEGF remained near basal level in suspension cultures of HUVEC (Fig 3 A,B). However, both kinases were still activable by Il-1β (data not shown). This suggested that binding of VEGF to VEGFR2 was not sufficient by itself to initiate activation of SAPK2/p38 and FAK. Because activation of integrin αvβ3 was required to trigger the VEGF-induced chemotactic cell migration and because cell suspension is a condition that precludes activation and clustering of integrins, the findings strongly suggested that αvβ3 integrin was involved in mediating activation of SAPK2/p38 and FAK on HUVEC maintained on vitronectin. We next confirmed the role of αvβ3 in activating SAPK2/p38 and FAK in response to VEGF. HUVEC in suspension were either not treated or treated for 20 minutes in the presence of the anti-αvβ3 neutralizing integrin antibody. Then, they were put on vitronectin and left to adhere for 55 minutes before addition of 5 ng/mL VEGF for 5 minutes. Using this special suspension/55 minutes readhesion protocol, VEGF induced the activation of SAPK2/p38 and FAK but to a lesser level than in HUVEC that were maintained for longer periods on vitronectin (Fig 3 C,D vs 3 A,B). In both cases, the anti-αvβ3 neutralizing integrin antibody inhibited the activation of SAPK2/p38 and the tyrosine phosphorylation of FAK (Fig 3 C,D), which supported the point that αvβ3 was specifically involved in modulating the VEGF signal associated with VEGFR2 and that led to activation of SAPK2/p38 and phosphorylation of FAK.
Fig 3.
VEGF-induced SAPK2/p38 activation and FAK tyrosine phosphorylation require both VEGFR2 and integrin αvβ3. (A) Quiescent HUVEC on vitronectin or in suspension were either not pretreated or pretreated with a VEGFR2 blocking antibody (VEGFR2-ba 10 μg/mL, 20 minutes) and then were either treated or not treated with VEGF (5 ng/mL, 5 minutes). Cells were then extracted and subjected to SAPK2/p38 assay. Extracts were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed by Western blot for phospho p38 detection. The membrane was stripped and reprobed for total p38 to ensure equal protein loading. Data points represent means ± SD of duplicate samples from 2 separate experiments. Representative blots are shown. (B) Quiescent HUVEC were maintained on vitronectin or put in suspension for 20 minutes. Adhering cells were either not pretreated or pretreated with a VEGFR2 blocking antibody (VEGFR2-ba 10 μg/mL, 20 minutes) and then were treated with VEGF (5 ng/mL, 5 minutes). Cells in suspension were either not pretreated or pretreated with the VEGFR2 blocking antibody (VEGFR2-ba 10 μg/mL, 20 minutes), and VEGF (5 ng/mL) was added for the last 5 minutes of suspension. Cells were then extracted and subjected to FAK immunoprecipitation, separated on SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was processed for phosphotyrosine detection. The membrane was stripped and reprobed for total FAK to ensure equal protein loading. Data points represent means ± SD of duplicate samples from 2 separate experiments. Representative blots are shown. (C, D) Cells in suspension were either not pretreated or pretreated with anti-integrin αvβ3 antibody (1 μg/mL, 20 minutes) and then were plated on vitronectin for 60 minutes. After 55 minutes, cells were either not treated or treated with VEGF (5 ng/mL, 5 minutes). In C, cells were extracted and subjected to p38 assay as in A. In D, cells were extracted and subjected to FAK assay as in B. Data points represent means of triplicate samples. Representative blots are shown. VEGF, vascular endothelial growth factor; SAPK2/p38, stress-activated protein kinase-2/p38; FAK, focal adhesion kinase; HUVEC, human umbilical vein endothelial cells; VEGFR2, VEGF receptor 2; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis
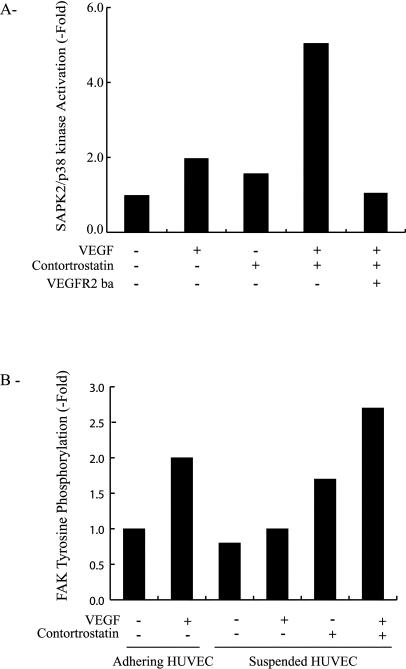
Signaling from integrins depends on their clustering after their binding to appropriate binding sites on the ligand protein. To ascertain the role of integrin αvβ3 clustering or activation as a prerequisite to the stimulation of SAPK2/p38 by VEGF/VEGFR2, we used contortrostatin, a snake venom disintegrin that specifically induced clustering of integrin αvβ3 (Schmitmeier et al 2000). HUVEC were pretreated in suspension with 10 nM contortrostatin in the presence or absence of VEGF and the neutralizing anti-VEGFR2. Results showed that contortrostatin induced activation of SAPK2/p38 in the presence of VEGF but not in its absence. Moreover, contortrostatin failed to activate SAPK2/p38, even in the presence of VEGF, when VEGFR2 was blocked by the anti-VEGFR2 neutralizing antibody (Fig 4A). In contrast, treating the cells in suspension with an arginine-glycine-aspartic acid (RGD) peptide to induce the engagement but not the clustering of integrin did not contribute to the activatation of SAPK2/p38 (data not shown). This suggests that clustering or activation of αvβ3 was required to modulate the VEGF signal to SAPK2/p38 but was not sufficient by itself to activate the kinase. In corollary, this suggests that the VEGF signal to SAPK2/p38 does not emanate directly from integrin but rather proceeds from VEGFR2 or molecules associated with VEGFR2. Tyrosine phosphorylation of FAK is a typical event that derives from integrin clustering. Accordingly, contortrostatin induced the tyrosine phosphorylation of FAK in suspension, which confirms its reported efficacy in clustering integrin αvβ3 (Fig 4B; Schmitmeier et al 2000). Interestingly, the tyrosine phosphorylation of FAK by contortrostatin was enhanced by VEGF. Taken together, the results indicate that both VEGFR2 and αvβ3 are required to activate SAPK2/p38 and FAK in response to VEGF. They also suggest that a synergistic interaction between VEGFR2 and αvβ3 drives the VEGF-induced activation of SAPK2 and FAK. This is consistent with the findings that both receptors interact in untreated HUVEC and that the interaction is markedly increased on stimulation with VEGF (Soldi et al 1999; Borges et al 2000; Byzova et al 2000).
Fig 4.
VEGF-induced SAPK2/p38 activation and FAK tyrosine phosphorylation require clustering of integrin αvβ3. (A) Quiescent cells were put in suspension and were either not pretreated or pretreated for the last 10 minutes of suspension with 10 nM contortrostatin. Then, they were either not treated or treated with VEGF (5 ng/mL, last 5 minutes of suspension) in the presence or absence of the VEGFR2 blocking antibody (VEGFR2-ba 10 μg/mL). Cells were then extracted and processed for SAPK2/p38 assay as in Figure 3A. Data points represent means of triplicate samples for each condition. Results are representative of 2 different experiments. (B) Quiescent HUVEC maintained on vitronectin were left to adhere or were put in suspension for 20 minutes. Adhering cells were either not treated or treated with VEGF (5 ng/mL, 5 minutes). Cells in suspension were either not pretreated or pretreated with 10 nM contortrostatin for the last 10 minutes of suspension and were either not treated or treated with VEGF (5 ng/mL, last 5 minutes of suspension). Cells were then extracted and subjected to FAK phosphorylation assay as in Figure 3B. Data points represent means of duplicate samples. Results are representative of 2 different experiments. VEGF, vascular endothelial growth factor; SAPK2/p38, stress-activated protein kinase-2/p38; FAK, focal adhesion kinase; VEGFR2, VEGF receptor 2; HUVEC, human umbilical vein endothelial cells
Integrin αvβ3 enhances the phosphorylation of VEGFR2 in response to VEGF
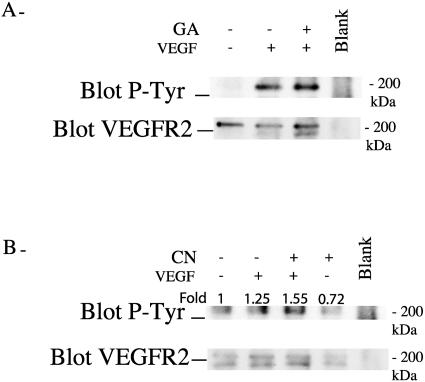
We investigated next how the association of integrin αvβ3 with VEGFR2 modulates the activation of VEGFR2. Signals elicited from tyrosine kinase receptor such as VEGFR2 are initiated after activation of their intrinsic tyrosine kinase activity. This results in the auto- and transphosphorylation of the receptor on specific tyrosine residues. The phosphorylated residues then serve as docking sites for signaling molecules involved in transducing further the signal to downstream pathways such as SAPK2/p38. As shown in Figure 5A, VEGF increased the level of tyrosine phosphorylation of VEGFR2 in HUVEC maintained on vitronectin. In contrast, VEGF did not increase the tyrosine phosphorylation of VEGFR2 under a condition, such as suspension, that impairs clustering of αvβ3. However, inducing the clustering of αvβ3 with contortrostatin resulted in a 1.5-fold increase in the phosphorylation of VEGFR2 in suspension cultures of HUVEC treated with VEGF (Fig 5B). These results suggested that integrin clustering was essential to allow the synergistic interaction between αvβ3 and VEGFR2, which then contribute to phosphorylation of VEGFR2. Hence, one of the first events that result from the binding of VEGF to VEGFR2 in monolayer culture might be to increase the clustering of integrin αvβ3, which in turn facilitate the interaction with VEGFR2 and trigger full phosphorylation of VEGFR2 and the activation of downstream targets.
Fig 5.
Integrin αvβ3 mediates tyrosine phosphorylation of VEGFR2 in response to VEGF. (A, B) Quiescent HUVEC were maintained on vitronectin or put in suspension for 20 minutes. Adhering cells (A) were either not pretreated or pretreated with geldanamycin (GA, 1 μg/mL, 60 minutes) and then were either not treated or treated with VEGF (5 ng/mL, 5 minutes). For cells in suspension (B), contortrostatin (CN, 10 nM) was either not added or added for the last 10 minutes of suspension, and VEGF (5 ng/mL) was either not added or added for the last 5 minutes. Cells were then extracted and subjected to VEGFR2 immunoprecipitation. Thereafter, they were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane to detect phosphotyrosinated VEGFR2. The membrane was stripped and reprobed for total VEGFR2 to ensure equal protein loading. In the blank track (A, B), no cellular extract was added. Results are representative of 2 different experiments. VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; HUVEC, human umbilical vein endothelial cells
Role of Hsp90 in VEGFR2- and αvβ3 integrin–mediated VEGF signaling
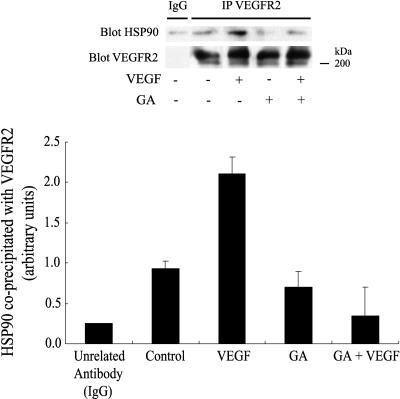
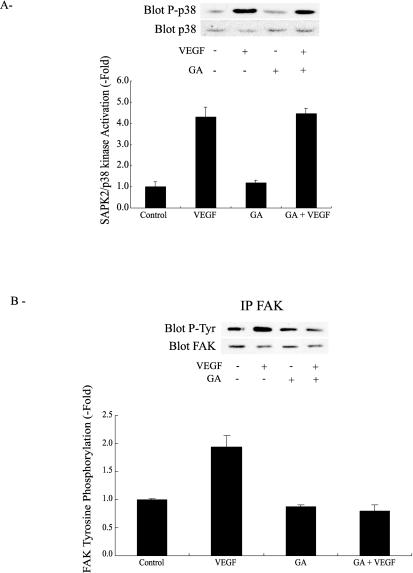
The chaperone Hsp90 is essential for VEGF-induced endothelial cell migration because geldanamycin and radicicol, 2 inhibitors of Hsp90-mediated events, inhibit endothelial cell migration by VEGF and because overexpression of Hsp90 reverts this inhibition (Rousseau et al 2000b). In the present study, we show that Hsp90 coprecipitated with unstimulated VEGFR2 in HUVEC maintained on vitronectin (Fig 6). Exposure to VEGF increased 2-fold the amount of Hsp90 associated with VEGFR2. Inhibiting Hsp90 by geldanamycin impaired the VEGF-induced increased association between Hsp90 and VEGFR2 (Fig 6). However, it did not blunt tyrosine phosphorylation level of the receptor (Fig 5). This is consistent with the fact that geldanamycin did not impair the activation of SAPK2/p38 and ERK, 2 events associated with phosphorylation of VEGFR2 (Fig 7A; Rousseau et al 2000b). In contrast, geldanamycin markedly inhibited the tyrosine phosphorylation of FAK induced by VEGF, confirming the role for Hsp90 in the process (Fig 7B). As expected, geldanamycin did not inhibit the integrin αvβ3–mediated tyrosine phosphorylation of FAK by contortrostatin in HUVEC in suspension, which suggested that the direct activation of FAK by integrin is independent of Hsp90 (data not shown).
Fig 6.
Hsp90 coprecipitates with VEGFR2. Quiescent HUVEC maintained on vitronectin were either not pretreated or pretreated for 60 minutes with geldanamycin (1 μg/mL) and then they were either not treated or treated with VEGF (5 ng/mL, 5 minutes). Cells were then extracted and subjected to VEGFR2 immunoprecipitation and were processed for immunodetection of Hsp90, as described in Materials and Methods. Data points are means ± SD of duplicate samples from 2 different experiments. Representative blots are shown. VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; HUVEC, human umbilical vein endothelial cells
Fig 7.
Geldanamycin does not inhibit SAPK2/p38 activation induced by VEGF, but it does block tyrosine phosphorylation of FAK. (A, B) Quiescent HUVEC on vitronectin were either not pretreated or pretreated for 60 minutes with geldanamycin (1 μg/mL) and then they were subsequently either not treated or treated with VEGF (5 ng/mL, 5 minutes). Cells were then extracted, separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and SAPK2/p38 (A) and tyrosine phosphorylation of FAK (B) were assessed as described in Figure 3. Data points represent means ± SD of triplicate samples from 2 different experiments. Representative blots are shown. SAPK2/p38, stress-activated protein kinase-2/p38; VEGF, vascular endothelial growth factor; FAK, focal adhesion kinase; HUVEC, human umbilical vein endothelial cells
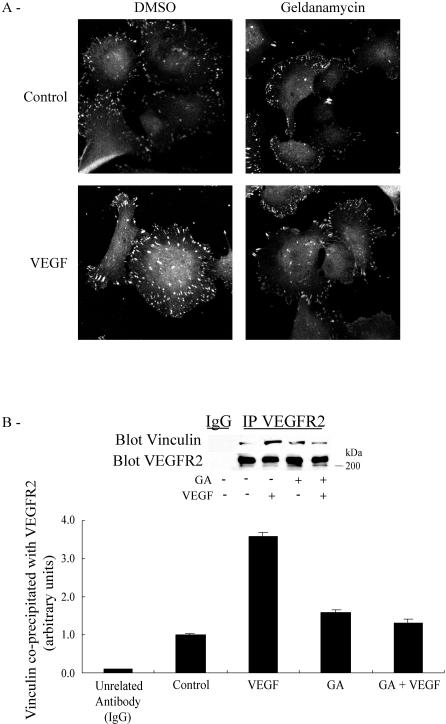
Tyrosine phosphorylation of FAK is an important determinant of focal adhesion assembly, being involved in the recruitment of proteins such as vinculin to the nascent focal adhesions plaques. We thus verified next whether the Hsp90-dependent phosphorylation of FAK was associated with the assembly of focal contacts. We found that vinculin was quickly recruited to ventral focal adhesions after activation of HUVEC by VEGF and that geldanamycin did inhibit the recruitment of vinculin to the plaques (Fig 8A). Interestingly, vinculin was recruited to VEGFR2 in response to VEGF, and geldanamycin also inhibited this recruitment (Fig 8B). These results are consistent with the possibility that Hsp90, by facilitating the phosphorylation of FAK, allows the recruitment of vinculin to VEGFR2 and raises the possibility that a VEGFR2-Hsp90-αvβ3 complex is formed and acts as an anchorage point to the building up of the nascent focal contacts in response to VEGF.
Fig 8.
VEGF-mediated recruitment of vinculin to VEGFR2 and focal adhesion assembly are inhibited by geldanamycin. (A) Quiescent HUVEC plated on vitronectin-coated labtek chamber were either not pretreated or pretreated with geldanamycin (1 μg/mL, 60 minutes) and then were either not exposed or exposed for 15 minutes to VEGF (5 ng/mL). Cells were fixed, and vinculin was detected with vinculin antibody and then revealed with anti-mouse IgG coupled to Alexa 568. Cells were examined by fluorescence microscopy. Representative fields are shown. (B) Quiescent HUVEC on vitronectin were either not pretreated or pretreated for 60 minutes with geldanamycin (1 μg/mL), and they were subsequently either treated or treated with VEGF (5 ng/mL, 5 minutes). Cells were then extracted and subjected to VEGFR2 immunoprecipitation, separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. The membrane was then processed by Western blot for immunodetection of vinculin. Data points represent means ± SD of triplicate samples from 3 different experiments. Representative blots are shown. VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; HUVEC, human umbilical vein endothelial cells
DISCUSSION
Angiogenesis, the formation of new blood vessels from preexisting ones, is closely associated with endothelial cell migration. The process requires the coordinated integration of motogenic signals elicited by the adhesion of endothelial cells with ECM and by angiogenic cytokines such as VEGF (Rousseau et al 2000a). In the present study, we found that cell migration induced by VEGF in HUVEC maintained on vitronectin required a synergistic interaction between the VEGF receptor VEGFR2 and the integrin αvβ3. This leads to activation of motogenic pathways involving SAPK2/p38 and FAK.
HUVEC have a low basal capacity to migrate across a polycarbonate membrane coated with vitronectin, a major component of the basement membrane matrix, which separates endothelial cells from vascular smooth muscle cells. This matrix-driven cell migration was abolished by inhibiting integrins αvβ3 and αvβ5 with appropriate neutralizing antibodies, which suggested that binding of both integrins to their ligands was able to elicit motogenic signals. In contrast, basal cell migration was not inhibited by an anti-β1 neutralizing antibody, indicating that this integrin, which is also expressed by HUVEC and recognized by vitronectin, was not involved in the process. Little is known on how integrins can by themselves initiate motogenic signals. The matrix-derived activation of motogenic pathways downstream of integrins can differ between cell types. In HUVEC, αvβ3 integrin–driven cell migration on vitronectin is associated with a sustained activation of ERK MAP kinase (Eliceiri et al 1998). In mouse embryonic fibroblasts on fibronectin, αvβ3 integrin requires functional MAP kinase ERK kinase 1 (MEK1) to trigger haptotaxis, a form of chemotaxis induced by insoluble component of the ECM, but is independent of the activation of ERK downstream of MEK1 (Giroux et al 1999). In HT29 colon carcinoma cells, activation of protein kinase C mediates haptotaxis induced by several integrins including αvβ3 (Rigot et al 1998). The capacity of a given integrin to activate diverse signaling pathways depending on cell types may well explain why the importance of a given integrin to drive haptotaxis and matrix-driven migration also varies with cell types. For instance, we found in HUVEC that αvβ5 was as important as αvβ3 in driving basal cell migration, whereas it was not involved in regulating the migration of mouse embryonic fibroblasts (Giroux et al 1999). In keratinocytes, αvβ5 mediates the locomotion on vitronectin but not on fibronectin (Kim et al 1994; Koivisto et al 1999). The functional role of the low basal level of haptotaxis and matrix-associated cell migration of endothelial cells is still unclear. Mathematical models have been developed, suggesting that haptotaxis has a role to play during the early phases of angiogenesis (Orme and Chaplain 1996, 1997).
Addition of VEGF enhanced the migration of endothelial cells on vitronectin. This effect was inhibited by impairing the binding of αvβ3 to vitronectin with an anti–αvβ3 integrin. In contrast, the anti-αvβ5 integrin antibody did not inhibit the VEGF-induced cell migration. Thus, whereas αvβ3 and αvβ5 are both involved in modulating matrix-associated increase in cell migration, only αvβ3 integrin is able to sustain chemotactic migration induced by VEGF. This suggests that the VEGF receptors should interact with αvβ3 integrin to initiate VEGF signaling to cell migration. VEGF binds to 2 tyrosine kinase receptors on endothelial cells, VEGFR1 and VEGFR2. It also binds to neuropilin, a coreceptor that increases the affinity of VEGFR2 for VEGF (Petrova et al 1999). In this study, we found that activation of VEGFR2 was required to drive migration of endothelial cell because an anti-VEGFR2 neutralizing antibody totally inhibited migration of HUVEC across a polycarbonate membrane coated with vitronectin. This confirms the findings from a previous study in which we showed, in porcine aortic endothelial cells stably expressing VEGFR1 or VEGFR2, that VEGF induces cell migration only in the cells that express VEGFR2 (Rousseau et al 2000b). Similarly, VEGFR2-selective binding mutants of VEGF are as effective as wild type VEGF to increase migration of HUVEC, whereas VEGFR1-selective binding VEGF mutants are unable to increase cell migration over background level (Gille et al 2001). Overall, these results are consistent with the fact that endothelial cell migration induced by VEGF requires a synergistic interaction between VEGFR2 and integrin αvβ3.
The findings that inhibiting SAPK2/p38- and FAK-mediated events by overexpression of dominant negative regulators of the kinases (p38 AGF for SAPK2/p38 and FRNK for FAK) impaired cell migration are conclusive indications that activation of the SAPK2/p38 and FAK pathways is essential to transduce the motogenic signals initiated by VEGFR2 and αvβ3 in response to VEGF. Activation of cell migration by stimulation of SAPK2/p38 results from an increased phosphorylation of Hsp27 that triggers the actin polymerization and remodeling that is required for cell migration (Rousseau et al 2000a; Laferrière et al 2001). In particular, SAPK2/p38-Hsp27–mediated increased actin polymerization contributes to the formation of lamellipodiae (Lavoie et al 1993; Piotrowicz et al 1998) and stress fibers (Rousseau et al 1997), which allows both the protruding (lamellipodiae) and contracting (stress fibers) forces required for cell migration. The role of SAPK2/p38 in cell migration is in line with the finding that SAPK2/p38 null mice present variable deficiencies in vascularization of the embryo (Ihle 2000). In the case of FAK, its involvement in triggering cell migration has been ascribed to its role in the assembly/disassembly process of focal adhesions (Aplin et al 1998; Rousseau et al 2000a). For example, tyrosine phosphorylation of FAK is increased in migrating HUVEC, and both FAK localization to focal adhesion sites and cell migration are impaired by overexpression of FRNK (Romer et al 1994; Gilmore and Romer 1996). By regulating the turnover of focal adhesions assembly, activation of FAK contributes, in concert with increased actin polymerization generated through the SAPK2/p38 pathway, to the induction of the actin remodeling, which is necessary to drive cell migration.
Because VEGFR2 and αvβ3 are both essential to VEGF-induced chemotaxis, this suggests that both receptors are involved in activating SAPK2/p38 and FAK. The fact that VEGFR2 is required to activate SAPK2/p38 and FAK is directly supported by the finding that the neutralizing antibody against VEGFR2 specifically inhibits the activation of both kinases. The fact that αvβ3 is required to activate SAPK2/p38 and FAK is directly supported by the finding that activation of VEGFR2 by VEGF cannot by itself trigger cell migration when αvβ3 integrin is blocked. It is also indirectly supported by the observation that the VEGFR2-mediated activation of SAPK2/p38 and FAK was reduced to near basal level in endothelial cells maintained in suspension, a condition that precludes clustering of integrins (Germer et al 1998; Stupack et al 1999). The requirement of integrin αvβ3 clustering to transduce the VEGF signal to SAPK2/p38 is further supported by our findings that VEGF activates SAPK2/p38 in suspended cells when they are treated with contortrostatin. Indeed, contortrostatin is a snake venom disintegrin that specifically induces the engagement and clustering of αvβ3 (Ritter et al 2000), just as it occurs after addition of VEGF to HUVEC on vitronectin. The fact that contortrostatin by itself cannot activate SAPK2/38 in suspended cells indicates that activation of this kinase is not directly downstream of clustered αvβ3, which suggests that the VEGF signal that leads to the activation of SAPK2/p38 emanates directly from VEGFR2. Of note, a previous report showed that the MAP kinase ERK was not directly downstream of αvβ3 either because it could be activated by contortrostatin, even in cells that do not express αvβ3 (Ritter and Markland 2000). The findings that contortrostatin does activate ERK but not SAPK2/p38 in suspension, however, are intriguing and indicate that the cell signaling induced by this disintegrin is multiple.
How VEGFR2 and αvβ3 integrins interact to synergistically transduce signals that converge to SAPK2/p38 and FAK remains to be elucidated. On binding with their ligands, receptors like VEGFR2 dimerize and oligomerize, which contribute to activate tyrosine kinase activities intrinsically associated with the receptors. This leads to auto- and transphosphorylation of the receptor on specific tyrosine residues, which triggers recruitment, to the newly phosphorylated receptors, of adapter proteins that contain SH2 or PTB domains (or both). These adapter proteins become themselves phosphorylated on tyrosine residues and then recruit other signaling molecules that transmit the signals further downstream. An important finding in our study was that VEGFR2 from HUVEC in suspension was not phosphorylated in response to VEGF in comparison with the cells maintained on vitronectin. Most importantly, inducing the clustering of αvβ3 with contortrostatin allows tyrosine phosphorylation of VEGFR2 by VEGF in suspension cultures. This suggests that engagement and clustering of αvβ3 integrin, as in VEGF-treated HUVEC maintained on vitronectin, are required for the phosphorylation of VEGFR2. Intriguingly, both receptors are known to associate in unstimulated condition, and exposures to VEGF increased the amount of αvβ3 that associates with VEGFR2. It is thus plausible that the increased recruitment of αvβ3 integrin to VEGFR2 is required to trigger phosphorylation and activation of VEGFR2 (Soldi et al 1999; Byzova et al 2000). Whereas the constitutive association of VEGFR2 and αvβ3 takes place outside the cell (Borges et al 2000), it is not clear whether the recruited association or localization also takes place outside the cells and whether it involves the α or the β chain of αvβ3. The basal amount of the αvβ3 constitutively associated with VEGFR2 by the external portion of the receptors might help to maintain VEGFR2 in a conformation that favors binding of VEGF to VEGFR2. Upon VEGF binding, this will allow the quick initiation of the signals needed to trigger the recruitment of the additional αvβ3 molecules required for full phosphorylation of VEGFR2. How the additional αvβ3 integrin is recruited to VEGFR2 is still unclear. The simplest possibility is that binding of VEGF to VEGFR2 first induces lateral movement in the membrane, allowing VEGFR2 to interact with αvβ3. Then, the αvβ3 integrin newly recruited to VEGFR2 bound to VEGF might induce a configuration change in VEGFR2 that will allow full phosphorylation and productive signaling. It is possible that the association of αvβ3 integrin with VEGFR2 might protect VEGFR2 against the activity of phosphatase, thus contributing to maintain the phosphorylation of VEGFR2 (Soldi et al 1999). The finding that activation of SAPK2/p38 follows the same pattern as the tyrosine phosphorylation of VEGFR2 and the fact that, in suspension, both events are not induced by contortrostatin except in the presence of VEGF bring further support to the point that the signal to SAPK2/p38 proceeds from phosphorylated VEGFR2. Interestingly, phosphorylation of FAK is enhanced by inducing clustering of integrin αvβ3 even in the absence of VEGF, which indicates that activation of αvβ3 is signaling to FAK. Incidentally, a recent study has shown that the formation of the VEGFR2–αvβ3 integrin complex activates αvβ3 and then triggers signaling from this integrin (Byzova et al 2000).
Hsp90 is a molecular chaperone that forms heterocomplexes with multiple signaling molecules that include the nuclear receptor for steroid hormones, the tyrosine kinase receptor ErbB2, and the signaling molecules Raf, Src, and Sch (Pratt 1997; Xu et al 2001). The role of Hsp90 is to maintain the associated proteins in an activable form (Young et al 2001). Notably, Hsp90 is essential to maintain the stability of both newly synthesized and mature Erb2 (Xu et al 2002). A major contribution of our study was to have shown that Hsp90 coprecipitates with VEGFR2, which indicates that both proteins are associated. We further obtained evidence that the association of Hsp90 with VEGFR2 is functionally important because specifically inhibiting Hsp90 with geldanamycin quickly abolished the association of Hsp90 with VEGFR2, thereby decreasing the VEGF-induced tyrosine phosphorylation of FAK, the increased association of vinculin with VEGFR2, and the recruitment of vinculin to focal adhesions. We propose that the Hsp90 associated with VEGFR2, by assuring the proper phosphorylation of FAK, is required for the building up of the focal contacts at the receptor site. The fact that geldanamycin did not reduce the quick tyrosine phosphorylation increase of VEGFR2 that followed exposure to VEGF nor the activation of SAPK2/p38 indicates that Hsp90 has a specific role in facilitating the transduction of the VEGF signal to FAK. Moreover, this suggests that the quick inhibition of FAK phosphorylation by geldanamycin may occur independently of a possible long-lasting effect of Hsp90 on maintaining the stability of VEGFR2 in contrast to what has been reported for Erb2 (Xu et al 2002). In fact, short exposures (1 hour) to geldanamycin do not affect the cellular level of FAK even if these decrease its tyrosine phosphorylation (Rousseau et al 2000b). Of note, long exposures (>12 hours) to geldanamycin increase the breakdown of FAK, which raises the possibility that FAK might be a client protein for Hsp90 (Ochel et al 1999). However, we were unable to see a direct association between Hsp90 and FAK in immunoprecipitation experiments (LeBoeuf et al, in preparation). Interestingly, geldanamycin decreases the level of the c-Met receptor even if c-Met is not associated with Hsp90 (Maulik et al 2002). A possible explanation is that FAK or c-Met becomes degraded by ubiquitination in response to prolonged exposure to geldanamycin. Src is an important regulator of FAK by VEGF, and Hsp90 is known to be involved in Src-mediated events (Pratt 1997; Garcia-Cardena et al 1998; Takahashi et al 1999). A challenging possibility raised by finding that VEGF increased the association of Hsp90 with VEGFR2 is that Hsp90 is instrumental in recruiting Src and then FAK to VEGFR2.
In summary, we confirm in the present study that VEGFR2 and αvβ3 integrin synergistically interact and we demonstrate, for the first time, that this interaction initiates the motogenic signals that lead to activation of SAPK2/p38 and FAK in endothelial cells exposed to VEGF. Activation of SAPK2/p38 proceeds from phosphorylated VEGFR2, whereas activation of FAK results from αvβ3 associated with VEGFR2. We also report, for the first time, that Hsp90 associated with VEGFR2 plays crucial roles in FAK phosphorylation in response to VEGF and in recruiting vinculin to VEGFR2 and focal contacts. These novel findings add important new concepts that help to better understand the complex regulation of endothelial cell migration induced by VEGF.
Acknowledgments
We thank Dr Jacques Landry for providing anti-p38, Dr Frank Markland for giving contortrostatin, and Josée N. Lavoie for supplying anti-E4orf4. We are also indebted to ImClone Systems for giving the anti-VEGFR2 antibodies and to Drs Kristopher Valerie and Claude Gravel for giving adenoviral constructs containing CMV p38 AGF and β-galactosidase. We thank Nadine Chouinard for amplifying adenovirus and André Lévesques for his help in microscopy. We are grateful to the staff of the Pathology Department of the Hôpital St-François d'Assise, Québec, for providing the human umbilical cord samples. This work was supported by a grant from the Canadian Institutes for Health Research (MT-15402). B.M.G. holds postdoctoral fellowships from the Association de Recherche contre le Cancer (France) and FCAR-Québec and J.L. owns studentships for the Cancer Research Society of Canada Inc and from Le Fonds de Recherche en Santé du Québec.
REFERENCES
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- Borges E, Jan Y, Ruoslahti E. PDGF-receptor-(beta) and EGF-receptor-2 bind to the (beta)3 integrin through its extracellular domain. J Biol Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- Chouinard N, Valerie K, Rouabhia M, Huot J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing p53. Biochem J. 2002;365:133–145. doi: 10.1042/BJ20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdreich-Epstein A, Shimada H, and Groshen S. et al. 2000 Integrins alpha(v)beta3 and alpha(v)beta5 are expressed by endothelium of high-risk neuroblastoma and their inhibition is associated with increased endogenous ceramide. Cancer Res. 60:712–721. [PubMed] [Google Scholar]
- Felding-Habermann B, Silletti S, and Mei F. et al. 1997 A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J Cell Biol. 139:1567–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Folkman J. Is there a role for nitric oxide in tumor angiogenesis? J Natl Cancer Inst. 1998;90:560–561. doi: 10.1093/jnci/90.8.560. [DOI] [PubMed] [Google Scholar]
- Germer M, Kanse SM, Kirkegaard T, Kjoller L, Felding-Habermann B, Goodman S, Preissner KT. Kinetic analysis of integrin-dependent cell adhesion on vitronectin—the inhibitory potential of plasminogen activator inhibitor-1 and RGD peptides. Eur J Biochem. 1998;253:669–674. doi: 10.1046/j.1432-1327.1998.2530669.x. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol. 2000;2:E13–E14. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux S, Tremblay M, and Bernard D. et al. 1999 Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr Biol. 9:369–372. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Ihle JN. The challenges of translating knockout phenotypes into gene function. Cell. 2000;102:131–134. doi: 10.1016/s0092-8674(00)00017-9. [DOI] [PubMed] [Google Scholar]
- Kim JP, Zhang K, Chen JD, Kramer RH, Woodley DT. Vitronectin-driven human keratinocyte locomotion is mediated by the alpha v beta 5 integrin receptor. J Biol Chem. 1994;269:26926–26932. [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto L, Larjava K, Hakkinen L, Uitto VJ, Heino J, Larjava H. Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhes Commun. 1999;7:245–257. doi: 10.3109/15419069909010806. [DOI] [PubMed] [Google Scholar]
- Kroll J, Waltenberger J. Regulation of the endothelial function and angiogenesis by vascular endothelial growth factor-A (VEGF-A) Z Kardiol. 2000;89:206–218. doi: 10.1007/s003920050472. [DOI] [PubMed] [Google Scholar]
- Kuwada SK, Li X. Integrin alpha5/beta1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol Biol Cell. 2000;11:2485–2496. doi: 10.1091/mbc.11.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrière J, Houle F, Taher MM, Valerie K, Huot J. Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. J Biol Chem. 2001;276:33762–33772. doi: 10.1074/jbc.M008564200. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LE, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Maulik G, Kijima T, and Ma PC. et al. 2002 Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 8:620–627. [PubMed] [Google Scholar]
- Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143:901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochel H, Schulte J, Nguyen TW, Trepel J, Neckers L. The benzoquinone ansamycin geldanamycin stimulates proteolytic degradation of focal adhesion kinase. Mol Genet Metab. 1999;66:24–30. doi: 10.1006/mgme.1998.2774. [DOI] [PubMed] [Google Scholar]
- Orme ME, Chaplain MA. A mathematical model of the first steps of tumour-related angiogenesis: capillary sprout formation and secondary branching. IMA J Math Appl Med Biol. 1996;13:73–98. [PubMed] [Google Scholar]
- Orme ME, Chaplain MA. Two-dimensional models of tumour angiogenesis and anti-angiogenesis strategies. IMA J Math Appl Med Biol. 1997;14:189–205. [PubMed] [Google Scholar]
- Petitclerc E, Stromblad S, von Schalscha TL, Mitjans F, Piulats J, Montgomery AM, Cheresh DA, Brooks PC. Integrin alpha(v)beta3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 1999;59:2724–2730. [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res. 1999;253:117–130. doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- Polanowska-Grabowska R, Gear AR. Heat-shock proteins and platelet function. Platelets. 2000;11:6–22. doi: 10.1080/09537100075742. [DOI] [PubMed] [Google Scholar]
- Polanowska-Grabowska R, Simon CG, Falchetto F, Shabanowitz J, Hunt DF, Gear AR. Platelet adhesion to collagen under flow causes dissociation of a phosphoprotein complex of heat-shock proteins and protein phosphatase 1. Blood. 1997;90:1516–1526. [PubMed] [Google Scholar]
- Pratt WB. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326. doi: 10.1146/annurev.pharmtox.37.1.297. [DOI] [PubMed] [Google Scholar]
- Pratt WB. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–434. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- Qi JH, Claesson-Welsh L. VEGF-induced activation of phosphoinositide 3-kinase is dependent on focal adhesion kinase. Exp Cell Res. 2001;263:173–182. doi: 10.1006/excr.2000.5102. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Rigot V, Lehman M, Andre F, Daemi N, Marvaldi J, Luis J. Integrin ligation and PKC activation are required for migration of colon carcinoma cells. J Cell Sci. 1998;111:3119–3127. doi: 10.1242/jcs.111.20.3119. [DOI] [PubMed] [Google Scholar]
- Ritter MR, Markland FS Jr.. Contortrostatin activates ERK2 and tyrosine phosphorylation events via distinct pathways. Biochem Biophys Res Commun. 2000;274:142–148. doi: 10.1006/bbrc.2000.3111. [DOI] [PubMed] [Google Scholar]
- Ritter MR, Zhou Q, Markland FS Jr.. Contortrostatin, a snake venom disintegrin, induces avb-mediated tyrosine phosphorylation of CAS and FAK in tumor cells. J Cell Biochem. 2000;79:28–37. [PubMed] [Google Scholar]
- Romer LH, McLean N, Turner CE, Burridge K. Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. Mol Biol Cell. 1994;5:349–361. doi: 10.1091/mbc.5.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E, Taher MM, Kuemmerle NB, Farnsworth J, Valerie K. A truncated human xeroderma pigmentosum complementation group A protein expressed from an adenovirus sensitizes human tumor cells to ultraviolet light and cisplatin. Cancer Res. 2001;61:764–770. [PubMed] [Google Scholar]
- Rousseau S, Houle F, Huot J. Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc Med. 2000a;10:321–327. doi: 10.1016/s1050-1738(01)00072-x. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem. 2000b;275:10661–10672. doi: 10.1074/jbc.275.14.10661. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of akt kinase activity by binding to hsp90. Proc Natl Acad Sci U S A. 2000a;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, Shibuya M. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci. 2000b;902:201–205. doi: 10.1111/j.1749-6632.2000.tb06314.x. [DOI] [PubMed] [Google Scholar]
- Schmitmeier S, Markland FS, Chen TC. Anti-invasive effect of contortrostatin, a snake venom disintegrin, and TNF-alpha on malignant glioma cells. Anticancer Res. 2000;20:4227–4233. [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Li E, Silletti SA, Kehler JA, Geahlen RL, Hahn K, Nemerow GR, Cheresh DA. Matrix valency regulates integrin-mediated lymphoid adhesion via Syk kinase. J Cell Biol. 1999;144:777–788. doi: 10.1083/jcb.144.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Seko Y, Noiri E, Tobe K, Kadowaki T, Sabe H, Yazaki Y. Vascular endothelial growth factor induces activation and subcellular translocation of focal adhesion kinase (p125FAK) in cultured rat cardiac myocytes. Circ Res. 1999;84:1194–1202. doi: 10.1161/01.res.84.10.1194. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K 1999 Viral vectors for gene delivery. In: Biopharmaceutical Drug Design and Development, ed Wu-Pong S, Rojanasakul Y. Humana Press, Inc., Totowa, NJ, USA. 69–42. [Google Scholar]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, Bohlen P. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17:155–161. doi: 10.1023/a:1006094117427. [DOI] [PubMed] [Google Scholar]
- Wu LW, Mayo LD, Dunbar JD, Kessler KM, Ozes ON, Warren RS, Donner DB. VRAP is an adaptor protein that binds KDR, a receptor for vascular endothelial cell growth factor. J Biol Chem. 2000;275:6059–6062. doi: 10.1074/jbc.275.9.6059. [DOI] [PubMed] [Google Scholar]
- Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, Neckers L. Sensitivity of mature ErbB2 to geldanamycin is conferred by its kinase domain and is mediated by chaperone protein Hsp90. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- Xu W, Mimnaugh EG, Kim JS, Trepel JB, Neckers LM. Hsp90, not Grp94, regulates the intracellular trafficking and stability of nascent ErbB2. Cell Stress Chaperones. 2002;7:91–96. doi: 10.1379/1466-1268(2002)007<0091:hngrti>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Rockwell P, Lu D, Kotanides H, Pytowski B, Hicklin DJ, Bohlen P, Witte L. Inhibition of vascular endothelial growth factor-induced receptor activation with anti-kinase insert domain-containing receptor single-chain antibodies from a phage display library. Cancer Res. 1998;58:3209–3214. [PubMed] [Google Scholar]