Abstract
In this study, we demonstrate by a variety of approaches (ie, morphological analysis, Western blots, immunolocalization, and the use of specific antibodies) that hyperosmotic deciliation stress of sea urchin embryos induces a thermotolerant response. Deciliation is also able to activate a phosphorylation signaling cascade the effector of which might be the p38 stress-activated protein kinase because we found that the administration of the p38 inhibitor SB203580 to sea urchin deciliated gastrula embryos makes the hyperosmotic deciliation stress lethal.
INTRODUCTION
The stress response involves the synthesis of a set of highly conserved proteins, called Hsps. The induction of Hsps has also been shown to bring about stress tolerance. Typically, stress tolerance is defined by measuring cell survival after each of 2 consecutive stresses. Initially this phenomenon was called the adaptive response of cells exposed to elevated temperatures because a mild heat shock treatment allows cells to withstand the effect of a second, more intense temperature insult, which would otherwise be lethal.
This acquired stress tolerance is recognized as a general and highly conserved strategy that protects cells from many other stress-inducing agents (Samali and Orrenius 1998).
It has been previously demonstrated that in sea urchin, Paracentrotus lividus, embryos heated at 31°C and only after the stage of hatching blastula, the bulk proteins synthesis is inhibited, whereas a large group of Hsps is induced (Roccheri et al 1986, 1993).
It has also been demonstrated that P lividus embryos degenerate when heated at 35°C. On the other hand, they survive and develop if this lethal heat treatment is preceded by heating at 31°C (Sconzo et al 1986).
Some other nonthermal stress can induce thermotolerance; thus, for example, sea urchin embryos treated with ethylene glycol-bis(aminoethylether)-tetraacetic acid (EGTA) (which inhibits the bulk protein synthesis and induces the synthesis of the complete set of Hsps) survive the otherwise lethal temperature of 35°C (Roccheri et al 2000). On the contrary, Zn+ treatment of embryos, which also induces synthesis of Hsps but not the complete repertoire of them, does not inhibit the synthesis of bulk proteins nor induces thermotolerance (Roccheri et al 1988).
Thermotolerance can also be achieved through phosphorylation of some Hsps, such as those of 38–40 kDa, induced by tetradecanoylphorbol-13 acetate (TPA) treatment or heat shock (Roccheri et al 1995).
In the last few years it has become clear that the expression of Hsps in response to mild stress, in various biological systems, could induce thermotolerance through inhibition of apoptosis: after phosphorylation, Hsp27 preferentially blocks mitochondrial cytochrome C release, whereas Hsp72 interferes with apoptosomal caspase activation (Arrigo and Orrenius 2001; Beere and Green 2001; Kato et al 2001; Samali et al 2001).
Two different signal transduction cascades may be responsible for phosphorylation of Hsp27: the p38 mitogen-activated protein kinase (MAPk) pathway and the protein kinase C cascade (Kato et al 2001).
Various forms of cellular stress (hyperosmotic stress, heat shock, deoxyribonucleic acid damage, anysomicin, sodium arsenite, inflammatory cytokines, lipopolysaccharides, ultraviolet irradiation) and also immune response are known to activate p38 stress-activated protein kinase (SAPk). Activation of p38 requires dual phosphorylation of both threonine and tyrosine residues in the activation domain. Once activated, p38 can phosphorylate both nuclear transcription factors, such as ATF2, ELK1, cJun, and cytoplasmic substrates, such as IkB or the small Hsp27 (Kyriakis and Avruch 1996; Han et al 1998; Blanco 2000).
The HOG/p38SAPk route is an important osmostress-activated signal transduction pathway, well conserved in all eukaryotes. In yeast, the existence of 2 different activation mechanisms, which depend on osmotic conditions, has been demonstrated for this pathway (Van Wuytswinkel et al 2000).
We have previously demonstrated that sea urchin embryos, planktonic life of which could depend on swimmer and sensorial ciliary functions, when deciliated by hypertonic shock, develop a stress response that transiently upregulates the expression and phosphorylation of a 40-kDa stress protein (Casano et al 1998, and unpublished data).
In this study, we show that hyperosmotic deciliation of sea urchin embryos induces thermotolerance, activates a phosphorylation pathway, and finally activates p38SAPk.
MATERIALS AND METHODS
Embryo culture and treatments
Adult sea urchins of Mediterranean P lividus species, collected along the Sicily's western coast, were used in this study as previously described (Casano et al 1998). Briefly, eggs were fertilized and embryos, at a concentration of 5000/mL, were grown at 18°C in Millipore filtered seawater containing antibiotics under continuous rotation, until the gastrula stage (at earlier stages, sea urchin embryos are unable to respond to stress). Embryo cultures were exposed to nonlethal and lethal heat shock stress and to deciliation as already described (Roccheri et al 1995; Casano et al 1998). The SB203580 p38 inhibitor was added at concentrations of 30 and 60 μM in seawater.
Electrophoretic analysis
Control and treated embryos were Dounce homogenized in O'Farrel lysis buffer including the protease inhibitor cocktail (complete, Mini, ethylenediaminetetraacetic acid–free protease inhibitor cocktail tablets; Roche, Mannheim, Germany). Equal amounts of total proteins (40 μg) were analyzed by monodimensional sodium dodecyl sulfate (SDS)–10% polyacrylamide slab gels, as previously described (Giudice et al 1980).
Molecular weights were evaluated by comparing with a set of standard proteins (Rainbow protein molecular weight markers; Amersham, Buckinghamshire, UK).
Western blotting
After electrophoresis, the proteins were electroblotted onto nitrocellulose filters (Hybond C or ECL; Amersham) with a semidry apparatus (Novablot; Pharmacia, Peapack, NJ, USA) at 0.8 mA/cm2 for 2 hours.
The filters were then preincubated for 3 hours with blocking solution (3% bovine serum albumin, 5% horse serum, 0.02% sodium azide in phosphate-buffered saline [PBS]), washed 3 times with PBS, and finally incubated overnight with the specific antibody.
The following antibodies were used at the dilutions indicated in parentheses:
Monoclonal (mouse) antiphosphoserine, clone PSR-45 (Sigma, St Louis, MO, USA; 1:500).
Monoclonal (mouse) anti-nonactivated p38MAPk, clone p38-YNP, which reacts specifically with the nonphosphorylated, nonactivated form of p38MAPk (Sigma; 1:2000).
Monoclonal (mouse) antiactivated p38MAPk, (diphosphorylated p38), clone p38-TY, which reacts specifically with the active, doubly phosphorylated form of p38MAPk and its related isoforms. It does not recognize the nonphosphorylated and monophosphorylated forms of p38 molecules (Sigma; 1:500).
After removal of nonspecific complexes by several washings with PBS, the filters were incubated with anti-mouse IgG alkaline phosphatase (AP) conjugate (1:7500) and finally stained with 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium (BCIP-NBT) (Sigma).
Embryo fixation and immunocytochemistry
Control and deciliated embryos at the gastrula stage were fixed with Bouin fixative, and immunocytochemistry was carried out as previously described (Roccheri et al 2001). Monoclonal (mouse) anti-p38MAPk–activated (diphosphorylated p38) antibody was used at a dilution of 1:250. Anti-IgG AP conjugated (1:1000, Promega, Madison, WI, USA) was added, as secondary antibody, and staining was carried out with NBT-BCIP (Roche).
RESULTS
Deciliation and thermotolerance acquisition
It has been previously shown that TPA treatment of P lividus embryos does not induce synthesis of Hsps, although it does induce thermotolerance. In addition, it has been suggested that thermotolerance could be achieved through phosphorylation of the constitutive Hsps and that both TPA and heat shock treatments were shown to induce phosphorylation of a constitutive protein of 38–40 kDa (Roccheri et al 1995).
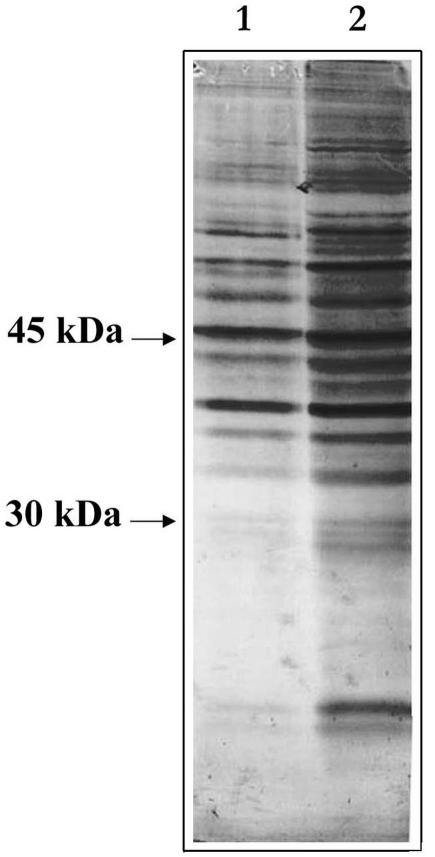
To establish whether deciliation also confers thermotolerance to sea urchin embryos, if yes, through which mechanism, we initially looked at the general phosphorylation pattern induced by deciliation stress, by monodimensional Western blot analysis of proteins extracted from control gastrula and from 4-fold deciliated gastrula embryos, probed with antiphosphoserine antibody. As shown in Figure 1, the deciliation stress activates a complex phosphorylation pattern.
Fig. 1.
Deciliation activates a serine phosphorylation pathway. Equal amounts (40 μg) of total proteins extracted from control gastrula (1) and 4-fold deciliated embryos (2) were run on sodium dodecyl sulfate–polyacrylamide gel and blotted onto nitrocellulose filter for Western analysis. The phosphorylation status of the samples was investigated by using a monoclonal antiphosphoserine antibody
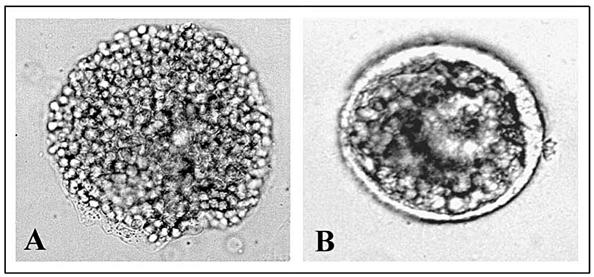
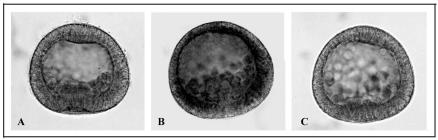
This observation led us to examine the effects of deciliation on thermotolerance. Figure 2 shows the result of the morphological analysis, which demonstrates that deciliation induces thermotolerance. In fact, the embryos heated directly at 35°C (A) degenerate, whereas those deciliated (B) before incubation at 35°C survive the otherwise lethal treatment, although with a delay in development.
Fig. 2.
Thermotolerance achievement after deciliation. (1) Gastrula embryos subjected to heat shock treatment of 35°C for 1 hour. (2) Four-fold deciliated gastrula embryos were incubated in normal seawater for 1 hour and then incubated at the temperature of 35°C for 1 hour. Morphological observations were made 24 hours after the end of 35°C treatment with an Axioskop 20 photomicroscope equipped with an automatic MC80 exposure system at 40× magnification
Deciliation and p38SAPk activation
Cells, from yeasts to humans, use multiple MAPk cascades to transduce diverse extracellular stimuli to the nucleus. MAPks dedicated to stress signaling, generically called SAPks, include the highly homologous Hog1p of budding yeast, Spc1 of fission yeast, and Drosophila-mammalian p38 (Blanco 2000; Nebreda and Porras 2000).
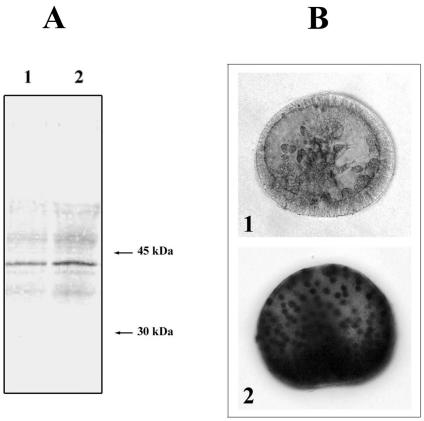
To establish whether sea urchin deciliation, as other stresses, activates p38SAPk, we performed Western blot analysis of total proteins extracted from both control and 4-fold deciliated P lividus gastrula embryos. The results shown in Figure 3A clearly demonstrate that the antiactivated p38 monoclonal antibody identifies a p38-activated form that, even if already present in normal embryos, increases after deciliation stress. Whole-mount in situ immunoreaction analysis with the same antiactivated p38 monoclonal antibody on control gastrula and 4-fold deciliated gastrula embryos (Fig 3B) further confirms the increase in the p38-activated form in 4-fold deciliated embryos and, in addition, suggests its nuclear localization. Strikingly, a staining reaction of only 10 minutes is sufficient to highlight the p38 nuclear localization, whereas the staining of the control embryos is very less.
Fig. 3.
Identification of the p38-activated form. (A) Equal amounts (40 μg) of total proteins extracted either from control (1) or from 4-fold deciliated (2) gastrula embryos were run on sodium dodecyl sulfate–polyacrylamide gel and blotted on nitrocellulose filter for Western analysis. (B) Whole-mount in situ immunoreaction of control gastrula embryos (1) and 4-fold deciliated embryos (2). The presence of a p38-activated form was revealed, in both cases, with a monoclonal anti-p38 mitogen-activated protein kinase antibody that was able to react specifically with the active, doubly phosphorylated form of p38. Note that the nuclear localization of p38 is already visible after a 10-minute staining reaction
In Drosophila, activation of p38 (ie, the concentration of the double-phosphorylated form) by osmotic shock is proportional to NaCl concentration, with a maximum at 300 μM, a very high osmotic stress for Drosophila. It has also been shown that activation reached a maximum after 5 minutes and returned to the basal level within 60 minutes, suggesting that p38 activation is one of the earliest events after osmotic shock in Drosophila (Han et al 1998).
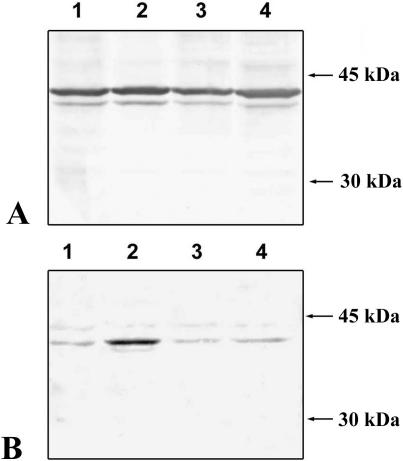
To verify whether p38 activation after deciliation in sea urchin embryos follows a similar time course, we carried out a Western blot analysis of the total proteins extracted from either control or deciliated embryos at different times after deciliation. As shown in Figure 4, although the amount of nonactivated p38 does not change at any time after deciliation (Fig 4A), p38 activation—as revealed by the monoclonal, specific antibody—achieves a maximum within 5 minutes after deciliation and returns to basal level within 60 minutes (Fig 4B), thus reproducing the behavior described in Drosophila.
Fig. 4.
Time course of p38 activation after deciliation. Forty milligrams of proteins was subjected to monodimensional electrophoresis and then electroblotted on nitrocellulose filters. The filters were then probed with 2 different antibodies. (A) Monoclonal anti-p38 mitogen-activated protein kinase (MAPk) nonactivated form, which is able to react specifically with the nonphosphorylated, nonactivated form of p38MAPk. (B) Monoclonal anti-p38MAPk activated form, which reacts specifically with the active, doubly phosphorylated form of p38MAPk. (1) Gastrula control embryos. (2) Four-fold deciliated gastrula embryos, 5 minutes after the last deciliation treatment. (3) Four-fold deciliated gastrula embryos, 1 hour after the last deciliation treatment. (4) Four-fold deciliated gastrula embryos, 3 hours after the last deciliation treatment
SB203580 treatment and inhibition of p38
SB203580 is a well-known, specific p38 inhibitor (Cuenda et al 1995); with respect to the use of this inhibitor in the sea urchin embryonal system, the only report concerns the Litechinus pictus species in which SB203580 was used to determine the role of p38 in sea urchin development. In particular, it has been shown that “SB” treatment selectively inhibits skeletogenesis if added before the hatched blastula stage (Bradham and McClay 2000).
First of all, we determined the optimal of use of SB203580 inhibitor in P lividus (we tried 2 different concentrations: 30 and 60 μM in seawater). In particular, we found that only the concentration of 60 μM of SB203580 has an inhibitory effect but only in early stages of development. In fact, 8 of 16 blastomeres of treated embryos do not survive, whereas late embryos (gastrula embryos) treated with the same concentration of “SB” show a rather normal morphologic development (see Table 1).
Table 1.
Summary of morphological observation on development of “normal and deciliated” Paracentrotus lividus embryos either not treated (−) or treated (+) with 60 mM of the p38 inhibitor SB203580. Note that SB inhibitor must be added during deciliation and also during the regeneration of the cilia (the p38 activation in fact occurs during this time)
Once established that the development of P lividus gastrula embryos does not suffer as a result of the SB203580 treatment, we deciliated them in the presence of 60 μM of SB203580.
We highlight that it was not possible to carry out more than 2 consecutive cycles of deciliation in the presence of SB203580 because, after the third deciliation, the embryos degenerate.
The results obtained are the following (see also Table 1). Two-fold deciliated control embryos regenerate the cilia, develop slowly but normally, and exhibit thermotolerance; on the contrary, 2-fold deciliated embryos treated with 60 μM of SB203580 do not regenerate the cilia, develop malformations, and do not survive the “lethal” stress of 35°C.
The inhibition of p38 phosphorylation in the SB-treated and deciliated embryos was confirmed by whole-mount immunolocalization with monoclonal antiactivated p38MAPk. Although 2-fold deciliated embryos show a remarkable increase in the phosphorylated form of p38MAPk (Fig 5B) compared with normal embryos (Fig 5A), no increase is detected in 2-fold deciliated embryos treated with 60 μM SB203580 (Fig 5C). These results, in our opinion, directly correlate deciliation stress with p38 activation and, in turn, with the acquisition of thermotolerance.
Fig. 5.
Inhibition of mitogen-activated protein kinase (p38MAPk) by SB203580. Whole-mount in situ immunoreaction of control gastrula embryos. (A) Two-fold deciliated embryos. (B) Two-fold deciliated embryos treated with 60 μM of SB203580, probed with a monoclonal antibody directed against the activated, doubly phosphorylated form of p38MAPk
CONCLUSIONS
In sea urchin embryos, different stressors (heat shock, Zn+, EGTA, TPA) induce different stress responses and not all induce a thermotolerant response.
In this study, we demonstrate that a particular hyperosmotic stress, a NaCl concentration that is twice that of seawater (Casano et al 1998), induces a thermotolerant stress response probably by activating a phosphorylation signaling cascade, such as the TPA treatment (Roccheri et al 1995). We suggest that p38SAPk is the effector of this pathway because we found that SB203580 treatment inhibits the regeneration of the cilia, causes malformations, and does not give rise to acquired thermotolerance.
Interestingly, the brief exposition to NaCl double concentration is the only hyperosmotic stress that causes deciliation in sea urchin embryos, whereas inducing a specific stress response (ie, it induces synthesis of a 40-kDa stress protein but not the synthesis of the main Hsps). We now plan to ascertain whether the p38 activation is a specific deciliation response or whether it is also induced by other stressors such as heat shock or EGTA, which also induces thermotolerance.
We underline that this result is the first evidence, in sea urchin embryos, of p38SAPk induction by stress and that it adds further evidence for the involvement of the SAPks in the hyperosmotic stress response in invertebrates (Drosophila, Cenorabditis elegans, and Suberites domuncula). Moreover, the result suggests that in P lividus, as in the sponge S domuncula (another marine organism), there is a complete conserved p38 protein kinase cascade pathway that is able to specifically sense hyperosmolarity conditions with respect to seawater (Bohm et al 2000). Finally, in light of the present results, it will be interesting to study the mechanism of transcriptional activation of specific tubulin genes (Gianguzza et al 1995; Casano et al 1996) involved in the ciliogenesis of P lividus as one of the possible final targets of this signaling pathway.
Acknowledgments
We thank I. Di Liegro and I. Albanese for critical reading of the manuscript, and F.G. in particular thank his friend A. Cestelli for all the general and scientific discussions in the past and for his ability to stimulate him even at present. This work was supported by grants from MIUR and CNR (60% and Stress 99.02499)
REFERENCES
- Arrigo AP, Orrenius S. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:hpmotc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Green DR. Stress management—heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- Blanco EM. p38MAPK signalling cascades: ancient roles and new functions. Bioessays. 2000;22:637–645. doi: 10.1002/1521-1878(200007)22:7<637::AID-BIES6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bohm M, Schroder HC, Muller IM, Muller WEG, Gamulin V. The mitogen-activated protein kinase pathway is conserved in metazoans: cloning and activation of the SAPK2 subfamily from the sponge Suberites domuncula. Biol Cell. 2000;92:95–104. doi: 10.1016/s0248-4900(00)89017-6. [DOI] [PubMed] [Google Scholar]
- Bradham C, McClay D 2000 p38 Activity is required for embryonic skeletal patterning in the sea urchin. Communication at 14th International Congress of Developmental Biology. [Google Scholar]
- Casano C, Ragusa M, Cutrera M, Costa S, Gianguzza F. Spatial expression of a and b tubulin genes in the late embryogenesis of the sea urchin P. lividus. Int J Dev Biol. 1996;40:1033–1041. [PubMed] [Google Scholar]
- Casano C, Roccheri MC, Onorato K, Cascino D, Gianguzza F. Deciliation: a stressful event for Paracentrotus lividus embryos. Biochem Biophys Res Commun. 1998;248:628–634. doi: 10.1006/bbrc.1998.9032. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Gianguzza F, Casano C, Ragusa M. a-Tubulin gene of neural territory of sea urchin embryos detected by whole mount in situ hybridization. Int J Dev Biol. 1995;39:477–483. [PubMed] [Google Scholar]
- Giudice G, Roccheri MC, DiBernardo MG. Synthesis of “heat shock” proteins in sea urchin embryos. Cell Biol Int Rep. 1980;4:69–74. doi: 10.1016/0309-1651(80)90011-9. [DOI] [PubMed] [Google Scholar]
- Han SJ, Choi KY, Brey PT, Lee WJ. Molecular cloning and characterization of Drosophila p38 mitogen activated protein kinase. J Biol Chem. 1998;273:369–374. doi: 10.1074/jbc.273.1.369. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Iwamoto I, Iida K, Inaguma Y. Protein kinase inhibitors can suppress stress-induced dissociation of Hsp27. Cell Stress Chaperones. 2001;6:16–20. doi: 10.1379/1466-1268(2001)006<0016:pkicss>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Porras A. p38MAP kinases: beyond the stress response. Trends Biochem Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Cascino D, Giudice G. Two-dimensional electrophoretic analysis of stress proteins in Paracentrotus lividus. J Submicrosc Cytol Pathol. 1993;25:173–179. [PubMed] [Google Scholar]
- Roccheri MC, Isola MG, Bosco L, Cascino D, Giudice G. Achievement of thermotolerance through Hsps phosphorylation in sea urchin embryos. Cell Biol Int. 1995;19:137–141. doi: 10.1006/cbir.1995.1054. [DOI] [PubMed] [Google Scholar]
- Roccheri MC, La Rosa M, Ferraro MG, Cantone M, Cascino D, Giudice G, Sconzo G. Stress proteins by zinc ions in sea urchin embryos. Cell Differ. 1988;24:209–214. doi: 10.1016/0045-6039(88)90052-8. [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Onorato K, Tipa C, Casano C. EGTA treatment causes the synthesis of heat shock proteins in sea urchin embryos. Mol Cell Biol Res Commun. 2000;3:306–311. doi: 10.1006/mcbr.2000.0230. [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Patti M, Agnello M, Gianguzza F, Carra E, Rinaldi AM. Localization of mitochondrial HSP56 chaperonin during sea urchin development. B B R C. 2001;287:1093–1098. doi: 10.1006/bbrc.2001.5503. [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Sconzo G, LaRosa ML, Oliva D, Abrignani A, Giudice G. Response to heat shock of different sea urchin species. Cell Differ. 1986;18:131–135. doi: 10.1016/0045-6039(86)90007-2. [DOI] [PubMed] [Google Scholar]
- Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998;3:228–236. doi: 10.1379/1466-1268(1998)003<0228:hspros>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Robertson JD, and Peterson E. et al. 2001 Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 6:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sconzo G, Roccheri MC, La Rosa M, Oliva D, Abrignani A, Giudice G. Acquisition of thermotolerance in sea urchin embryos correlates with the synthesis and age of the heat shock proteins. Cell Differ. 1986;19:173–177. doi: 10.1016/0045-6039(86)90093-x. [DOI] [PubMed] [Google Scholar]
- Van Wuytswinkel O, Reiser V, Siderius M, Kelders MC, Ammerer G, Ruis H, Mager WH. Response of Saccharomyces cerevisiae to severe osmotic stress: evidence for a novel activation mechanism of the HOG MAP kinase pathway. Mol Microbiol. 2000;37:382–397. doi: 10.1046/j.1365-2958.2000.02002.x. [DOI] [PubMed] [Google Scholar]