Abstract
The 70-kDa heat shock protein (Hsp) family is composed of both environmentally inducible (Hsp) and constitutively expressed (Hsc) family members. We sequenced 2 genes encoding an Hsp70 and an Hsc70 in the Pacific oyster Crassostrea gigas. The Cghsc70 gene contained introns, whereas the Cghsp70 gene did not. Moreover, the corresponding amino acid sequences of the 2 genes presented all the characteristic motifs of the Hsp70 family. We also investigated the expression of Hsp70 in tissues of oysters experimentally exposed to metal. A recombinant Hsc72 was used as an antigen to produce a polyclonal antibody to quantify soluble Hsp70 by enzyme-linked immunosorbent assay in protein samples extracted from oysters. Our results showed that metals (copper and cadmium) induced a decrease in cytosolic Hsp70 level in gills and digestive gland of oysters experimentally exposed to metal. These data suggest that metals may inhibit stress protein synthesis.
INTRODUCTION
The cellular stress response is involved in protecting organisms from damage due to exposure to a variety of stressors, including temperature, heavy metals, and other xenobiotics. The stress response entails the rapid synthesis of heat shock proteins (Hsps) to protect cellular proteins against denaturation (Lindquist and Craig 1988; Sanders 1993; Feder and Hofmann 1999). Hsps were first described in Drosophila busckii (Ritossa 1962), and the genes encoding the Drosophila Hsps were among the first eukaryotic genes to be cloned (Livak et al 1978; Craig et al 1979). Molecular studies on Hsps indicated a high degree of conservation during evolution, especially in their protein-coding sequences (Lindquist 1986). The major, most highly conserved, and best studied of the Hsps is the 70-kDa protein family (Hsp70) because of its role in protein chaperoning (Gething and Sambrook 1992) and in acquired tolerance processes (Clegg et al 1998; Lindquist and Craig 1988). The genes encoding the Hsp70 are highly conserved and include both heat-inducible (Hsp) (Ingolia and Craig 1982; Craig et al 1983) and constitutive (Hsc: heat shock cognate) genes. The constitutive genes encode stress proteins under normal conditions (Craig et al 1983; Lindquist and Craig 1988; Hightower 1993).
The results of the studies on stress proteins in aquatic organisms are highly variable (Sanders 1993). In marine organisms, particularly in bivalves, molecular approaches, such as gene sequencing, are not greatly developed. To our knowledge, only 3 complete or partial sequences of hsp70 complementary deoxyribonucleic acid (cDNA) from marine bivalves are available: Crassostrea gigas (Gourdon et al 2000), Crassostrea virginica (Rathinam et al 2000), and Mytilus edulis (Luedeking et al, in preparation). Nevertheless, the stress response has been studied in bivalves (reviewed by Sanders 1993 and Gourdon et al 1998) where the synthesis of Hsp and induction of thermotolerance have been demonstrated in the Pacific oyster C gigas (Clegg et al 1998; Gourdon et al 2000) and in mussels M edulis and Mytilus galloprovincialis (Sanders 1988; Snyder et al 2001).
In the present study, the genes encoding an Hsc70 and an Hsp70 from a marine bivalve, the oyster C gigas, are described. We also quantified the expression of soluble stress proteins 70 in different organs of oysters exposed to several concentrations of metals under experimental conditions. For that purpose, we used an enzyme-linked immunosorbent assay (ELISA) with a polyclonal antibody developed in the laboratory against a recombinant C gigas Hsc72.
MATERIALS AND METHODS
Cloning and sequencing of hsc70 and hsp70 genes
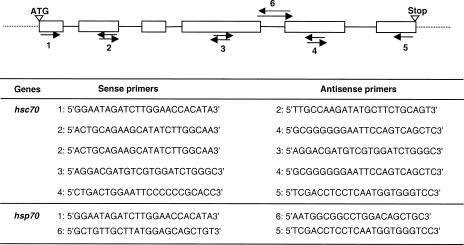
Total DNA was extracted, using standard procedures, from gills of 1 freshly opened C gigas. Polymerase chain reaction was performed using combinations of primers (Fig 1) designed from the C gigas hsc72 cDNA sequence (Gourdon et al 2000). Part of the hsc70 and hsp70 genes was coamplified with the combination of primers #2 and #4. Specific primers (#6 forward and reverse) were designed from the hsc70 gene sequence obtained to specifically amplify the remaining sequence of the hsp70 gene (Fig 1). The reaction mixture included 20 pmol of each primer, 20 ng of DNA template, 100 μM of dinucleotide Tri Phosphat (dNTPs), 2 mM MgCl2, 1× Taq polymerase buffer, and 1 unit of Taq polymerase (Promega, Madison, WI, USA) in 50 μL total volume. After an initial 5-minute denaturation at 94°C, 2-minute annealing at 57°C, and 2-minute elongation at 72°C, 35 amplification cycles were performed as follows: 30 seconds at 94°C, 40 seconds at 57°C, and 1 minute 30 at 72°C, and then a final 10 minutes at 72°C. Resulting products were isolated, gel purified using the QIAEX® II Gel Extraction Kit (Qiagen, Hilden, Germany), cloned in pGEM-T vector (Promega), and sequenced by extension from both ends using T7 and Sp6 universal primers (T7 sequencing kit; Amersham Pharmacia Biotech, Uppsala, Sweden).
Fig 1.
Combinations of primers (arrows) used to sequence hsc70 and hsp70 genes in the Pacific oyster C gigas. Specific primers used to amplify the Cghsc70 gene were designed from the hsc72 complementary deoxyribonucleic acid sequence of C gigas. Specific primers used to amplify the Cghsp70 gene were designed from the Cghsc70 sequence
Southern blot
DNA of C gigas was isolated as above. Ten micrograms of DNA was digested to completion with 3 restriction enzymes (BamHI, EcoRI, and HindIII; Promega) and separated by agarose gel electrophoresis (0.8% TAE gel). The fragments were then transferred onto a Hybond N+ nylon membrane (Amersham). Hybridization was carried out with a randomly primed Cghsc72 cDNA–labeled probe using the ECL kit (Amersham) according to the manufacturer's instructions.
Recombinant DNA manipulations
The hsc72 cDNA was amplified from the ATG start codon to the GAC terminal codon, and the resulting product was isolated, gel purified using the QIAEX® II Gel Extraction Kit (Qiagen), and ligated with the plasmid pBAD-TOPO® (pBAD-TOPO TA cloning® kit; Invitrogen, Groningen, Netherlands). The ligation product was then used to transform TOP10 One Shot® Escherichia coli competent cells (Invitrogen) according to the manufacturer's instructions. The transformation mixture was plated on Luria-Bertani medium (LB) with agar containing ampicillin at 100 μg/mL. Correct insert orientation was determined by sequencing alkaline lysis plasmid minipreparations (T7 sequencing kit; Amersham). One positive clone was then cultured in LB medium containing ampicillin at 100 μg/mL, and the expression of the recombinant protein was induced with the addition of l-arabinose at a final concentration of 0.02% for 4 hours, when the culture reached an optical density, OD600 = 0.5.
Recombinant CgHsc72 purification
The induced bacterial culture was centrifuged at 5000 × g, and the pellet was suspended in 100 μL of sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis sample buffer (0.5% β-mercaptoethanol and 1% SDS) per milliliter of culture. After a 5-minute denaturation at 100°C and a 30-minute centrifugation at 12 000 × g at 4°C, the supernatant fraction containing proteins was run through a denaturing acrylamide gel (12% and 4% total acrylamide for the running and the stacking gel, respectively, with 0.4% bisacrylamide in each) using the buffer system of Laemmli (1970). The recombinant protein CgHsc72 was then isolated using a modified protocol described by Amero et al (1996). After a brief staining with Coomassie® Brilliant Blue R-250 (Bio-Rad Laboratories, Hercules, CA, USA), the band corresponding to the recombinant CgHsc72 was collected and stored at 4°C before being used for rabbit immunization.
Production of a polyclonal antibody
Rabbits were immunized 2 times at 1-week intervals with about 250 μg of CgHsc72 homogenized in complete Freund's adjuvant (Sigma, St Louis, MO, USA) at 1 mL:1.5 mL protein-adjuvant ratio. A booster injection in incomplete Freund's adjuvant (Sigma) (about 250 μg of protein at the same protein to adjuvant ratio as above) was given after 2 months. On week 9, rabbit blood was collected by carotid puncture. Serum was collected and stored at −80°C.
Purification and biotin labeling of rabbit IgG
The immunoglobulin G (IgG) fraction from rabbit serum was purified by affinity chromatography on a protein-A column (HiTrap® 1 mL protein-A; Pharmacia, Uppsala, Sweden) using a fast protein liquid chromatography system pump (Pharmacia) as described by Page and Thorpe (1996). N-Hydroxysuccinimidobiotin (Sigma), dissolved in dimethyl sulfoxide (Sigma) at 1 mg/0.1 mL, was then added to purified IgG at 0.25 mg of biotin per milligram of IgG. The mixture was incubated at room temperature with gentle agitation for 2 hours before dialysis against 3 changes of phosphate-buffered saline (PBS) (pH 7.2) at 4°C. The conjugates were diluted in 50% glycerol and stored at −20°C until used.
Oyster conditioning
Oysters were collected from a non–metal-contaminated site, La Pointe du Château (Brittany, France), and maintained for 1 week in aerated, filtered seawater before experimentation. All experiments were carried out in a temperature-controlled room (15°C) at a salinity of 34‰. Groups of 20 oysters were exposed to 2 metals, one essential (Cu++ as CuCl2) and the other toxic (Cd++ as CdCl2). Each metal was applied from a stock solution (100 mM) at each of 2 final concentrations (0.4 and 4 μM) and also in a mixture (0.2 μM each) for 15 days. A group of 20 oysters was maintained in seawater, without metal, as a control. Seawater was renewed every day, and oysters were fed with microalgae every 2 days. The metals were reapplied at the appropriate concentrations after every water change.
Protein extraction and metal analysis in oyster tissues
On days 0, 1, 2, 3, 5, 7, and 15, the gills and digestive gland from exposed and control oysters (n = 3 for each sample) were collected and homogenized in protein extraction buffer according to the protocol described by Tendengren et al (1999) (150 mM NaCl, 10 mM NaH2PO4, 1 mM phenylmethanesulfonyl fluoride, pH 7.2). Samples were then centrifuged at 12 000 × g for 15 minutes at room temperature. Total soluble protein was quantified by the Dc Protein Assay kit (Bio-Rad) using dilutions of bovine serum albumin (BSA) (Sigma) as the standard. OD was measured at 620 nm using a microplate reader.
Insoluble proteins contained in the pellet were suspended in a SDS-containing buffer described by Buckley et al (2001) (50 mM Tris-HCl, pH 6.8, 4% SDS, 1 mM phenylmethanesulfonyl fluoride). The Hsp70 content was estimated by spectrophotometry according to the formula given by Gill and von Hippel (1989): A280 (1 mg/mL) = (5690 nw + 1280 ny + 120 nc)/M, where nw, ny, and nc are the number of tryptophan, tyrosin, and cystein residues in the protein of mass M, and 5690, 1280, and 120 are the respective extinction coefficients for these residues.
Pools of gills excised from 3 oysters were mineralized with suprapure nitric acid. Concentrations of cadmium and copper were measured in each tissue sample using the potentiometric stripping method (Riso et al 1997).
Western blot analysis
The cross-reactivity of the anti-CgHsc72 antibody developed in our laboratory was tested by Western blot as follows. Samples of C gigas (control and cadmium exposed) proteins were electrophoresed on SDS-polyacrylamide gel and electrotransferred onto polyvinylidine difluoride membrane (Bio-Rad). The membrane was blocked for 1 hour with blocking buffer (0.1 M Tris-HCl, 5% nonfat dry milk, pH 7.4) and then incubated with Tris-HCl (0.1 M, pH 7.4) buffer containing anti-CgHsc72 antibody (1:125 diluted) for 1 hour with gentle agitation at room temperature. The membrane was washed twice for 10 minutes with washing buffer (0.1 M Tris-HCl, 0.02% Tween 20, pH 7.4) and incubated with Tris-HCl buffer containing 1:1000 diluted anti-rabbit IgG horseradish peroxidase conjugated (Sigma) for 1 hour with gentle agitation at room temperature. Again the membrane was washed twice with washing buffer, and the reactive band was visualized by staining with 2.4 mM of 3-amino-9-ethyl-carbazole (Sigma) dissolved in 50 mM acetate buffer (0.2 M acetic acid, 0.2 M sodium acetate, pH 5) containing 5% of N,N-dimethyl formamide (Sigma) and 12‰ of H2O2.
Enzyme-linked immunosorbent assay
The recombinant Hsc72 was quantified by spectrophotometry in crude bacterial lysate according to the formula described above.
Microtitre plates were coated with 0.01, 0.05, 0.25, 0.5, 0.75, and 1 μg of recombinant Hsc72 by overnight incubation at 4°C. Active sites remaining on the plates were blocked with 200 μL per well of PBS (pH 7.2) containing 0.1% Tween 20 (PBS-T) and 1% BSA. After 3 washes with PBS-T, 120-, 160-, 240-, 480-, and 1200-ng quantities of biotin-labeled IgG in 100 μL of PBS-T were added and incubated for 1.5 hours at 37°C. All combinations of Hsc72 and labeled IgG doses were tested to determine the appropriate reaction concentrations and to obtain a standard curve. A volume of 100 μL of ExtrAvidine peroxidase conjugate (Sigma) diluted 1:1000 in PBS-T was added after 3 washes with PBS-T and incubated for 1.5 hours at 37°C. After 2 washes with PBS-T and 3 washes with PBS, 100 μL of phosphate-citrate buffer (0.045 M citric acid, 0.11 M Na2HPO4, pH 5.45) containing o-phenylenediamine (Sigma) and H2O2 at a final concentration of 0.06% and 0.001%, respectively, was added. The reaction was stopped after 20 minutes with 2 N H2SO4, and OD492 was measured with a microplate reader.
Hsps were then quantified in samples of 20 μg of total protein extracted from the digestive gland and gills of experimental oysters using the conditions determined above and the standard curve obtained (Hsc72μg = 11.873 × OD492 − 0.5367, R2 = 0.9953).
Nucleotide sequence accession number
The sequences of Cghsc70 and Cghsp70 genes reported in this study will appear in the DDJB/EMBL/GenBank database under accession numbers AJ305315 and AJ318882, respectively.
Statistical analysis
The variations in Hsp level during the experiment were analyzed by analysis of covariance (α = 0.05) using CSS Statistica (Statsoft, Tulsa, OK, USA).
RESULTS
Organization of the C gigas hsc70 and hsp70 genes
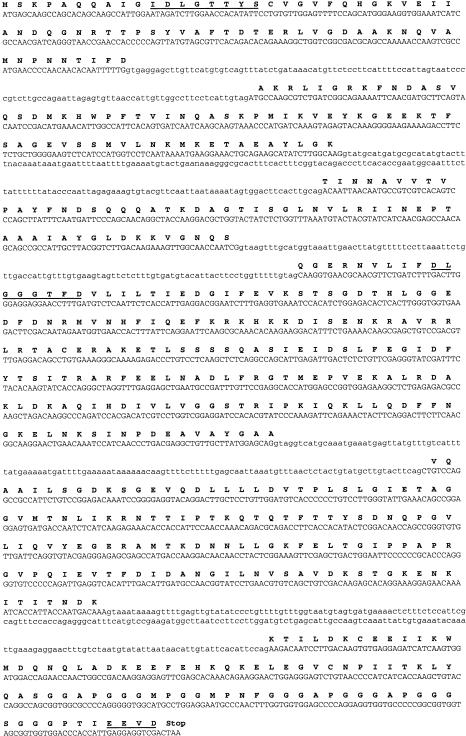
The sequence of the Cghsc70 gene contains 6 coding exons of 184, 206, 168, 553, 401, and 257 bp length. All the intron borders of Cghsc70 start and end with the consensus GT and AG splicing signals (Fig 2). The sequence of the Cghsp70 gene did not contain introns (Fig 3). The corresponding amino acid sequences of the 2 genes contained the 3 characteristic motifs of the Hsp70 family: IDLGTTYS, DLGGGTFD, and EEVD (Figs 2 and 3). The C-terminal region contained 2 repeats of the tetrapeptide and pentapeptide motifs, GGMP and GGGAP. A search for amino acid sequence homologies using the ALIGN program (Myers and Miller 1988) showed a degree of homology with other species of about 70%.
Fig 2.
The nucleotide sequence of the Cghsc70 gene and the predicted amino acid sequence: introns are in lowercase letters and exons are in uppercase, bold characters indicate the predicted amino acid sequence (at the first base of the codon), and characteristic motifs of the Hsp70 family are underlined
Fig 3.
The nucleotide sequence of the Cghsp70 gene and the predicted amino acid sequence (bold characters). The characteristic motifs of the Hsp70 family are underlined
Southern blot analysis
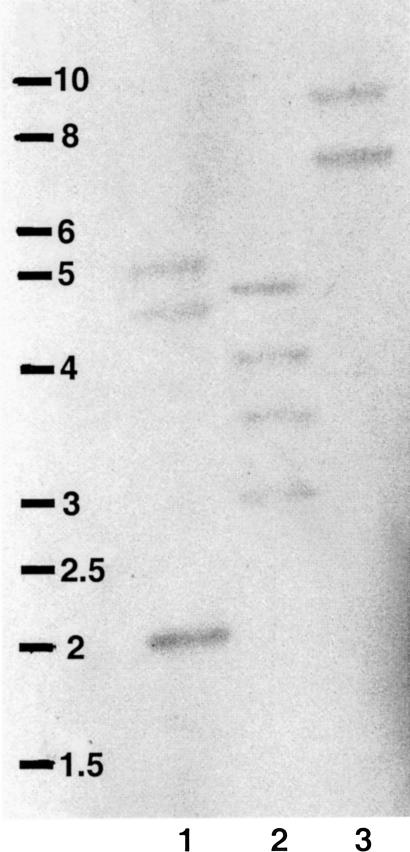
Southern blot analysis of C gigas DNA extracted from 1 oyster, digested with various restriction enzymes and probed with Cghsc72 cDNA, revealed several fragments with high- or low-intensity reactions, possibly reflecting differential degree of homology to the probe (Fig 4). Restriction analysis of our 2 sequences showed that only 2 enzymes (EcoRI and BamHI), among the 3 used, presented 1 or 2 restriction sites. Figure 4 shows that the digestion by HindIII (lane 3) revealed 2 bands, suggesting that 2 different Hsp70 genes are present in oyster genome.
Fig 4.
Southern blot of gill genomic deoxyribonucleic acid (DNA) from a single adult oyster, digested with BamHI (1), EcoRI (2), and HindIII (3), then electrophoresed and probed with Cghsc72 cDNA at high stringency. Marker is a 1-kb DNA Ladder (Eurogentec, Belgium)
Cross-reactivity of anti-CgHsc72 antibody with C gigas proteins
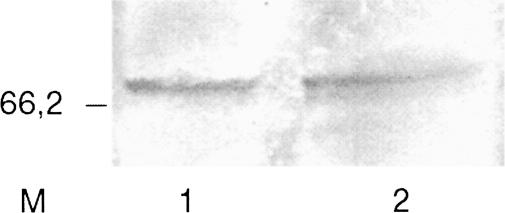
Western blot revealed a high cross-reactivity of our anti-CgHsc72 antibody with C gigas Hsp70 (Fig 5). One band appeared on the membrane at a molecular weight of 70 kDa, confirming the specificity of the antibody to Hsp70 of this oyster species.
Fig 5.
Western blot of digestive gland protein sample from control (1) and cadmium-exposed (2) oyster electrophoresed and probed with anti-Cghsc72 antibody. Marker (M) is sodium dodecyl sulfate–polyacrylamide gel electrophoresis standard broad range (Bio-Rad)
Quantification of Hsps by ELISA
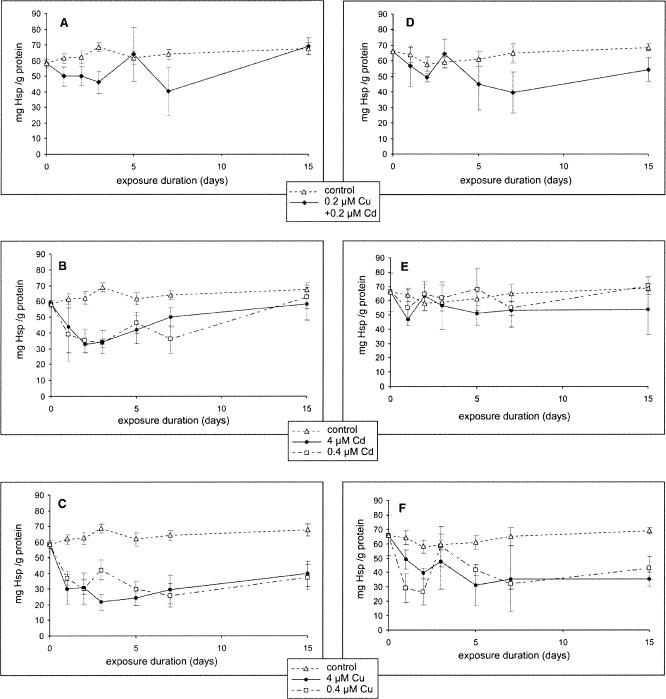
ELISA developed in this study was sufficiently sensitive to detect 50 ng of recombinant CgHsc72 and 21.7 mg Hsp/g protein using 480 ng of purified biotin-labeled IgG. Application of this assay to protein samples extracted from gills and digestive glands of experimentally exposed oysters showed that soluble Hsp levels decreased significantly (P < 0.05) in the gills and in the digestive gland (compared with control) in all treatments (Figs 6 A–F). The exception was with the exposure to 0.4 μM of cadmium (Fig 6E), which did not induce a decrease in Hsp in the digestive gland of oysters. No significant differences were observed between metal doses. The quantification by spectrophotometry of Hsp70 contained in the pellet showed that about 1% to 2% of these proteins remained in the pellet, this value being constant in the control and metal-exposed samples and in the 2 tissues tested.
Fig 6.
Quantification of soluble heat shock proteins 70 (mean ± SE) by enzyme-linked immunosorbent assay in the gills (A, B, and C) and in the digestive gland (D, E, and F) of C gigas exposed to copper and cadmium (A and D), cadmium (B and E), and copper (C and F)
Metal quantification in oyster tissues
Measurement of metal contents showed a significant increase of copper and cadmium in gills of oysters exposed to these 2 metals. Copper and cadmium concentrations in nonexposed individuals are 12 and 0.5 ng per gram weight of wet tissue (gwwt), respectively. An exposure to 4 and 0.4 μM of Cd++ entailed an increase of cadmium concentration in tissue from 0.4 to 60.5 ng Cd/gwwt and from 0.4 to 15.7 ng Cd/gwwt, respectively. Copper concentration varied from 14.5 to 68.6 ng Cu/gwwt and from 14.5 to 37 ng Cu/gwwt in oysters exposed to 0.4 and 4 μM of Cu++, respectively. In animals exposed to a mixture of these 2 metals, cadmium concentration increased from 0.4 to 5.6 ng Cd/gwwt, whereas copper concentration did not vary.
DISCUSSION
In this study, we report the sequence of 2 Hsp70 genes Cghsc70 and Cghsp70 from a marine bivalve, the oyster C gigas. The coding region of the Cghsc70 gene was interrupted by 5 introns, which is suggestive of constitutive expression (Günther and Walter 1994). It was suggested that the lack of introns in heat-induced hsp70 members may help to circumvent the block in ribonucleic acid (RNA) splicing by heat shock and allow the rapid synthesis of proteins (Kay et al 1987; Jie et al 1996). This characteristic enables preferential expression of these proteins during periods of stress, with the nuclear export signal probably being provided by the messenger RNA's secondary or tertiary structure (Huang et al 1999). The 2 genes have an open–reading frame coding for proteins possessing the same characteristics as those described in other species and contain the 3 characteristic motifs (IDLGTTYS, DLGGGTFD, and EEVD) of the Hsp70 family (Gupta and Singh 1994), confirming that the gene sequences obtained encode Hsps70. Moreover, our sequences present a high degree of homology with amino acid sequences of Hsp70 from other species. A notable feature of the protein is the presence of 2 consecutive repeats of the tetrapeptide motif GGMP (Demand et al 1998) and the pentapeptide motif GGGAP at its C-terminal region. Similar sequences have been observed in hsp70 genes from other organisms such as Rattus (O'Malley et al 1985), Homo sapiens (Dworniczak and Mirault 1987), Plasmodium (Sheppard et al 1989), and Setaria digitata (Jayasena et al 1999). Identification of GGMP repeats, as well as the presence of the cytosolic Hsp70–specific motif EEVD at the C-terminus (Freeman et al 1995; Demand et al 1998; Vayssier et al 1999) of the CgHsc70-Hsp70 sequences, strongly suggests that these proteins are cytosolic 70-kDa Hsp.
A recombinant Hsps70-CgHsc72 from the Pacific oyster C gigas was synthesized for use as an antigen in the development of antibodies and an immunochemical detection procedure. This protein was obtained by induction of Cghsc72 cDNA in a protein expression system. The cross-reactivity of the rabbit anti-CgHsc72 IgG with Hsp70 of C gigas supported the suitability of the antibodies as reagents to quantify soluble Hsp70 in samples collected from animals exposed to different stress. ELISA developed in this study permitted the specific and sensitive quantification of soluble Hsp70 in protein extracts from molluscs exposed to metals in the laboratory. Our results showed that the Hsp70 level is not significantly different in the 2 organs studied (gills and digestive gland). Quantities of 63.4 ± 3.6 mg Hsp/g and 63.1 ± 3.9 mg Hsp/g protein, about 6% of total protein, in the gills and the digestive gland, respectively, were measured in control oysters. Feige and Polla (1994) reported a figure of about 5% under normal conditions (without stress) in other organisms. We showed that the Hsp70 level decreased significantly in oysters during experimental metal exposure. We also observed that metal concentration increased significantly in oyster tissues during the exposure experiments. Several studies carried out in molluscs showed a significant Hsp70 induction in animals exposed to xenobiotics and heat for a short period (no longer than 24 hours and 2 hours, respectively). An increase in Hsp70 was observed in heat-shocked C gigas (Clegg et al 1998; Gourdon et al 2000) and in heat-shocked and oil-contaminated mussels, M galloprovincialis, and abalones, Haliotis rufescens (Snyder et al 2001). An increase in Hsp70 concentration was also observed in cadmium-contaminated mussels, M edulis, and limpets, Collisella pelta (Sanders 1988). Conversely, Veldhuizen-Tsoerkan et al (1991) found no variation in Hsp70 levels in sea mussels, M edulis, caged in seawater with various concentrations of cadmium. Our results showed an inverse relationship between contaminants and Hsp70, which contrasted with those described by Sanders (1988). The disparity may be attributed to our use of metal concentrations 4- to 40-fold greater than those used by Sanders (1988). A hypothesis could be made: it is possible that at these concentrations, the average degradation rate of Hsp70 will exceed its synthesis rate because of cytopathological damage, such as ruptured membranes in many cells (Pawert et al 1996; Triebskorn and Köhler 1996; Quig 1998). We previously described a similar phenomenon in C gigas collected from metal-contaminated sites, in which the metallothionein concentrations decreased in gills and digestive gland of oysters collected from the most contaminated site (Boutet et al 2002). A similar Hsp70 synthesis inhibition was observed in the earthworm Lumbricus terrestris exposed to a variety of metals (lead, cadmium, and copper) (Nadeau et al 2001). An inhibitory effect of metals, particularly copper, was also described in the seaweed Enteromorpha intestinalis (Lewis et al 2001). These authors observed that high copper concentrations appeared to damage protein synthesis, therefore impairing the Hsp70 response. The decline in Hsp70 levels was a result of a decreased total protein content of the algae. Such inhibition of protein metabolism by copper has been reported by other authors (Steinert and Pickwell 1988; Fernandes and Henriques 1991; Lage et al 1994). Steinert and Pickwell (1988) found that mussel tissues exposed to 150 μg/L copper before metabolic labeling failed to incorporate any radiolabel and suggested that it was the result of translational inhibition.
In this study, we characterized, for the first time, hsc70 and hsp70 genes in the oyster C gigas. These 2 genes exhibit the characteristic motifs of the Hsp70 family. ELISA developed in this study allowed us to specifically and rapidly quantify soluble Hsps70 in tissues from a marine mollusc. This immunological method has the advantage of quantifying the protein of interest, unlike the commonly used Western blot analysis (Clegg et al 1998; Nadeau et al 2001), which gives only a semiquantitative estimation of Hsp70 quantities. Finally, ELISA developed in this study showed that Hsp70 synthesis was inhibited by high metal contamination, as described earlier (Nadeau et al 2001).
Acknowledgments
This research program was supported by the Région Bretagne and the CE program FAIR CT98-4129: “Environmental factors and shellfish diseases.” The authors are grateful to Jean-Michel Escoubas for providing the C gigas hsc72 cDNA clone and to François Lamour for his help with purification of IgG. Thanks are also due to Professor Susan Ford for her useful English corrections, to Dr Ricardo Riso for metal quantification, to Dr Louis Quiniou for his help with use of the CSS Statistica Statsoft Software, and to Monique Briand for editing the figures.
REFERENCES
- Amero SA, James TC, and Elgin SCR 1996 Production of antibodies using proteins in gel bands. In: The Protein Protocols Handbook, ed Walker JM. Humana Press, Totowa, NJ, 717–720. [DOI] [PubMed] [Google Scholar]
- Boutet I, Tanguy A, Riso R, Auffret M, Moraga D. Immunochemical quantification of metallothioneins in the Pacific oyster Crassostrea gigas: characterisation of a metal exposure bioindicator. Environ Toxicol Chem. 2002;21:1009–1014. [PubMed] [Google Scholar]
- Buckley BA, Owen ME, Hofmann GE. Adjusting the thermostat: the threshold induction temperature for heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol. 2001;204:3571–3579. doi: 10.1242/jeb.204.20.3571. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Cher GN, Rifkin E, Friedman CS. Induced thermotolerance and the heat shock protein-70 family in the Pacific oyster Crassostrea gigas. Mol Mar Biol Biotechnol. 1998;7:21–30. [Google Scholar]
- Craig EA, Ingolia TD, Manseau LJ. Expression of Drosophila heat-shock cognate genes during heat-shock and development. Dev Biol. 1983;99:418–426. doi: 10.1016/0012-1606(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Craig EA, McCarthy BJ, Wadsworth SC. Sequence organization of two recombinant plasmids containing genes for the major heat shock-induced protein of D. melanogaster. Cell. 1979;16:575–588. doi: 10.1016/0092-8674(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Demand J, Lüders J, Höhfeld J. The carboxy-terminal domain of HSC70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol. 1998;18:2023–2028. doi: 10.1128/mcb.18.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworniczak B, Mirault ME. Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic Acids Res. 1987;15:5181–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feige U, Polla BS. Hsp70—a multi-gene, multi-function family with potential clinical applications. Experientia. 1994;50:979–986. doi: 10.1007/BF01923452. [DOI] [PubMed] [Google Scholar]
- Fernandes JC, Henriques FS. Biochemical, physiological and structural effects of excess copper in plants. Bot Rev. 1991;57:246–273. [Google Scholar]
- Freeman BC, Myers PM, Schumarer R, Morimoto RI. Identification of regulatory motif in HSP70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Gourdon I, Gricourt L, Kellner K, Roch P, Escoubas JM. Characterization of a cDNA encoding a 72 kDa heat shock cognate protein (Hsc72) from the Pacific oyster, Crassostrea gigas. DNA Seq. 2000;11:265–270. doi: 10.3109/10425170009033241. [DOI] [PubMed] [Google Scholar]
- Gourdon I, Guérin MC, Torreilles J. Cellular and molecular mechanisms of the stress response from marine bivalvia. CR Soc Biol. 1998;192:749–774. [PubMed] [Google Scholar]
- Günther E, Walter L. Genetic aspects of the hsp70 multi-family in vertebrates. Experientia. 1994;50:987–1001. doi: 10.1007/BF01923453. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Singh B. Phylogenetic analysis of 70 kD heat shock protein sequences suggests a chimeric origin for the eukaryotic cell nucleus. Curr Biol. 1994;4:1104–1114. doi: 10.1016/s0960-9822(00)00249-9. [DOI] [PubMed] [Google Scholar]
- Hightower LE. A brief perspective on the heat-shock response and stress proteins. Mar Environ Res. 1993;35:79–83. [Google Scholar]
- Huang Y, Wimler KM, Carmichael GG. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J. 1999;18:1642–1652. doi: 10.1093/emboj/18.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982;79:525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena SMT, Chandrasekharan NV, Karunanayake EH. Molecular characterisation of a hsp70 gene from the filarial parasite Setaria digitata. Int J Parasitol. 1999;29:581–591. doi: 10.1016/s0020-7519(99)00002-8. [DOI] [PubMed] [Google Scholar]
- Jie HB, Bailey CW, Ray BK, Estes DM, Kumar N, Carson CA. Single copy Babesia microti hsp70. Mol Biol Parasitol. 1996;83:241–246. doi: 10.1016/s0166-6851(96)02767-3. [DOI] [PubMed] [Google Scholar]
- Kay RJ, Russnak RH, Mathias C, Candido EPM. Expression of intron-containing C. elegans heat shock genes in mouse cells demonstrates divergence of 3′ splice recognition sequences between nematodes and vertebrates, and an inhibitory effect of heat shock on mammalian splicing apparatus. Nucleic Acids Res. 1987;15:3723–3741. doi: 10.1093/nar/15.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lage OM, Parente AM, Salema R. Electrophoretic analysis of polypeptides of Prorocentrum micans Ehrenberg exposed to toxic levels of copper. Rev Palaeobot Palynol. 1994;84:107–112. [Google Scholar]
- Lewis S, Donkin ME, Depledge MH. Hsp70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aquat Toxicol. 2001;51:277–291. doi: 10.1016/s0166-445x(00)00119-3. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Freund R, Schweber M, Wensink PC, Meselson M. Sequence organization and transcription at two heat shock loci in Drosophila. Proc Natl Acad Sci U S A. 1978;75:5613–5617. doi: 10.1073/pnas.75.11.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EW, Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988;4:11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Nadeau D, Corneau S, Plante I, Morrow G, Tanguay RM. Evaluation for Hsp70 as a biomarker of effect of pollutants on the earthworm Lumbricus terrestris. Cell Stress Chaperones. 2001;6:153–163. doi: 10.1379/1466-1268(2001)006<0153:efhaab>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley K, Mauron A, Barchas JD, Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat shock-like protein. Mol Cell Biol. 1985;5:3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M, Thorpe R 1996 Purification of IgG using protein A or protein G. In: The Protein Protocols Handbook, ed Walker JM. Humana Press, Totowa, NJ, 733–734. [Google Scholar]
- Pawert M, Triebskorn R, Gräff S, Berkus M, Schulz J, Köhler HR. Cellular alteration in collembolan midgut cells as a marker of heavy metal exposure: ultrastructure and intracellular metal distribution. Sci Tot Environ. 1996;181:187–200. doi: 10.1016/0048-9697(95)05009-4. [DOI] [PubMed] [Google Scholar]
- Quig D. Cystein metabolism and metal toxicity. Altern Med Rev. 1998;3:262–270. [PubMed] [Google Scholar]
- Rathinam AV, Chen TT, Grossfeld RM. Cloning and sequence analysis of a cDNA for an inducible 70 kDa heat shock protein (Hsp70) of the American oyster (Crassostrea virginica) DNA Seq. 2000;11:261. doi: 10.3109/10425170009033240. [DOI] [PubMed] [Google Scholar]
- Riso RD, Le Corre P, Chaumery CJ. Rapid and simultaneous analysis of trace metals (Cu, Pb and Cd) in seawater by potentiometric stripping analysis. Anal Chim Acta. 1997;351:83–89. [Google Scholar]
- Ritossa F. A new puffing pattern induced by heat shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- Sanders BM. The role of the stress proteins response in physiological adaptation of marine molluscs. Mar Environ Res. 1988;24:207–210. [Google Scholar]
- Sanders BM. Stress proteins in aquatic organisms: an environmental perspective. Crit Rev Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- Sheppard M, Kemp DJ, Anders RF, Lew AM. High level sequence homology between a Plasmodium chabaudi heat shock protein gene and its Plasmodium falciparum equivalent. Mol Biochem Parasitol. 1989;33:101–104. doi: 10.1016/0166-6851(89)90047-9. [DOI] [PubMed] [Google Scholar]
- Snyder MJ, Girvetz E, Mulder EP. Induction of marine mollusc stress proteins by chemical or physical stress. Arch Environ Contam Toxicol. 2001;41:22–29. doi: 10.1007/s002440010217. [DOI] [PubMed] [Google Scholar]
- Steinert SA, Pickwell GV. Expression of heat shock protein and metallothionein in mussels exposed to heat stress and metal ion challenge. Mar Environ Res. 1988;24:211–214. [Google Scholar]
- Tendengren M, Olsson B, Reimer O, Brown DC, Bradley BP. Heat pretreatment increases cadmium resistance and HSP70 levels in Baltic sea mussels. Aquat Toxicol. 1999;48:1–12. [Google Scholar]
- Triebskorn R, Köhler HR. The impact of heavy metals on the grey garden slug Deroceras reticulatum (Müller): metal storage, cellular effects and semi-quantitative evaluation of metal toxicity. Environ Pollut. 1996;93:327–343. doi: 10.1016/s0269-7491(96)00048-6. [DOI] [PubMed] [Google Scholar]
- Vayssier M, Leguerhier F, and Fabien JF. et al. 1999 Cloning and analysis of a Trichinella briotovi gene encoding a cytoplasmic heat shock protein of 72 kDa. Parasitology. 119:81–93. [DOI] [PubMed] [Google Scholar]
- Veldhuizen-Tsoerkan MB, Holwerda DA, de Bont AMT, Smaal AC, Zandee DI. A field study on stress indices in the sea mussel, Mytilus edulis: application of the stress approach in biomonitoring. Arch Environ Contam Toxicol. 1991;21:497–504. doi: 10.1007/BF01183870. [DOI] [PubMed] [Google Scholar]