Abstract
Heat shock proteins (Hsps) or stress proteins, and, in particular, the inducible, cytosolic Hsp70, represent a highly conserved response to heat exposure and to a variety of noxious stimuli. Many investigations have shown correlations between the aberrant expression of Hsps and disease states. Whether the basal and inducible levels of Hsp70 are of any biological significance in patients with heat-induced diseases remains unknown. In the present study, we compared the basal and inducible levels of Hsp70 by flow cytometry in lymphocytes of patients with heat-induced diseases and after recovery from this disease, and in matched controls. Both groups comprised individuals who exercised by running in the same hot environment. The level of inducible Hsp70 was also measured after a heat treatment of lymphocytes in vitro. The results show that there is variation of basal and inducible Hsp70 levels among individuals. However, the group of patients suffering from heat-induced illnesses in May shows a significantly higher basal (P = 0.02) level of Hsp70 than does the control group. Individuals who have an increased level of Hsp70 may be more sensitive to heat or may respond differently. The level of Hsp70 may represent a biomarker to evaluate whether they are more susceptible to stresses than other individuals. Interestingly, the basal level of Hsp70 is higher in both the patient group and the control group in November than in May. In fact, the basal levels of Hsp70 in the patient and control groups are essentially the same in November, perhaps reflecting the successful stress conditioning of both groups.
INTRODUCTION
The heat shock or stress response is a highly conserved response of all cells, tissues, and organisms on exposure to elevated temperatures, to a variety of environmental stresses, and to pathological stimuli such as infections, fever, inflammation, malignancy, and autoimmunity (Lindquist and Craig 1988; Morimoto et al 1994). This response is characterized by a rapid induction of proteins, the heat shock proteins (Hsps). The Hsp that is studied the most is the highly inducible member of the Hsp70/Hsc70 family, with apparent molecular masses of 71 and 72 kDa in rat and human, respectively, and is referred to as Hsp70. Physiological stimuli such as rigorous exercise also can elicit a heat shock response (Locke et al 1995a; Noble 2002).
Members of the Hsp70 family can function as intracellular chaperones of naive, aberrantly folded, or mutated proteins and are involved in protein trafficking and transport within the cell (Morimoto et al 1994; Muchowski et al 2000). In cultured cells, organs, and whole animal models, Hsp70 has been shown to provide protection from further aggression, demonstrating its role in cytoprotection. This has been documented in whole organs by transient protection from ischemic injury in heart, brain, and kidney (Currie et al 1993; Locke et al 1995b; Marber et al 1995; Plumier et al 1995; Beck et al 2000), as well as from endotoxins in some animal models (Hotchkiss et al 1993; Paidas et al 2002).
Heat-induced and heat-related illnesses remain highly prevalent in many working and living environments, in particular, during a sudden increase of ambient temperature or a heat wave when acclimatization of the human body has not been established (reviewed in Bouchama and Knochel 2002). Such illnesses include heat syncope, heat rash, heat cramps, heat exhaustion, and heat stroke in order of increasing severity (Alzeer et al 1997). Previous observations have suggested that Hsps or antibodies against Hsps (or both) may be of significance in the pathogenesis or prognosis (or both) of some diseases (Welch 1992; Kaufmann and Schooel 1994; Minowada and Welch 1995; Schett et al 1995; Wu et al 1998; Xu et al 1999). Thus, we suggested recently that measurement of antibodies to Hsps may be useful in assessing how individuals respond to abnormal stress within their living and working environment and that they may be used as biomarkers to evaluate their susceptibility to heat-induced diseases and harsh working conditions (Wu et al 2001a, 2001b). Other observations also suggest that variation in Hsp gene expression and Hsp polymorphism can contribute to disease susceptibility and stress tolerance (Lyashko et al 1994; Favatier et al 1997; Boshoff et al 2000; Oehler et al 2001). So far, there have been few investigations on the expression of Hsp70 in patients with heat-induced diseases. This is surprising because Hsps were first discovered in organisms subjected to supraoptimal temperatures, and one of their important functions is to protect against thermal stress. In the present study, we investigated the differences in basal and inducible levels of Hsp70 in lymphocytes of patients with acute heat-induced illness resulting from intense physical training and after their recovery from this disease by using flow cytometry.
MATERIALS AND METHODS
Subjects and groups
The present study was conducted in May and November 2000 in the city of Nanjing, China. The first group consisted of 30 young male individuals aged from 20 years to 22 years who exercised in May by running 5 km in a hot environment (ambient temperature, 32–35°C; humidity, 59%; and wind velocity, 0.5–2.0 m/s) with a 17-kg load. The group was subdivided into a heat-induced illness group consisting of 14 individuals who suffered from heat-induced illness during or after the exercise, as diagnosed by clinical doctors, and a matched control group of 16 individuals who did not show any evidence of illnesses resulting from this intense exercise. All patients were diagnosed according to the diagnostic criteria and principles of management of occupational heat illness (GB11508-89; Petersdorf and Root 1987). This diagnosis is based mainly on heat exposure, oral temperature, and clinical characteristics such as headache, dizziness, nausea, vomiting, diarrhea, weakness, visual disturbance, painful spasms of the voluntary muscle of the abdomen and extremities, pale skin, tachycardia, hypotension, confusion, and hot and dry skin. Patients with fever resulting from other diseases were excluded. In November of the same year, 15 individuals who had recovered from acute heat-induced illness 6 months previously and 23 corresponding controls were reinvestigated. None of these 38 individuals had any symptoms or signs of acute and chronic heat-induced illness or other diseases as confirmed by a physical examination.
Evaluation of health status
The health status of all the individuals was evaluated using a questionnaire designed according to the diagnostic criteria and principles of management of occupational heat illness (GB11508-89; Petersdorf and Root 1987). Attention also was given to previous disease history, lifestyle, and the presence of possible inducing factors of acute heat-induced illness such as the condition of sleeping the day before testing and psychological factors such as nervousness. The activities of lactic dehydrogenase (LDH), creatine kinase (CK), and superoxide dismutase (SOD), and the contents of malondialdehyde (MDA) were determined with the appropriate kit assays (Najing Jiancheng Bioengineering Company, Nanjing, China).
Detection of basal and inducible expression of Hsp70 in lymphocytes
Approximately 8 mL of venous blood was collected in heparinized tubes. Lymphocytes were isolated using Ficoll-Hypaque (Biochemical Reagent Co., Shanghai, China). The lymphocytes collected were washed twice with phosphate-buffered saline (PBS) and counted, and the number of lymphocytes was adjusted to 5000/μL with PBS. Lymphocytes, 50 μL, were centrifuged and suspended in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal calf serum. They were then heat-shocked for 1 hour at 41°C in a hot water bath and allowed to recover in a 37°C incubator for 2 hours before measuring Hsp70. The basal level of expression of Hsp70 was determined in fresh lymphocytes without any heat treatment.
Cellular staining of Hsp70 for flow cytometry was performed according to Bachelet et al (1998), with slight modifications. Briefly, cells were centrifuged and washed in 0.5 mL PBS containing 1% bovine serum albumin (BSA). After centrifugation, cells were suspended in 0.3 mL of fixation solution containing 3% paraformaldehyde and fixed for 15 minutes at 4°C. Fixed lymphocytes were centrifuged for 5 minutes at 600 × g, suspended in 300 μL of a 1:500 dilution of rabbit–anti-human Hsp70 antibody specific for the inducible form of Hsp70 (antibody #799, Tanguay et al 1993) in a permeabilization solution, and incubated for 30 minutes at 4°C. After washing in 0.5 mL PBS containing 1% BSA, the cells were incubated with 300 μL of a fluorescein isothiocyanate–labeled (FITC) anti-rabbit immunoglobulin G (IgG) (diluted 1:300) for another 30 minutes. The cells were washed and resuspended in 0.5 mL cold PBS containing 1% BSA. Two negative controls were performed without anti-human Hsp70 antibody or without the secondary FITC-labeled anti-rabbit IgG. The stained cells were analyzed using a flow cytometer (FACS CALIBUR, Beckton Dickinson Company, San José, CA, USA). A total of 10 000 cells were counted, and the mean fluorescence intensity was measured at 525 nm.
Statistical analyses
All data were analyzed using Excel 2000 and SPSS software and present the mean ± standard deviation. Other analyses were carried out using the chi-square test and Student's t-test. Statistical inferences are based on the different levels of significance (P < 0.05 or P < 0.01). The percentage of induction was calculated by dividing (HS-Hsp70 level − basal Hsp70 level) by (basal level × 100).
RESULTS
General characteristics of patients with acute heat-induced illness (May 2000)
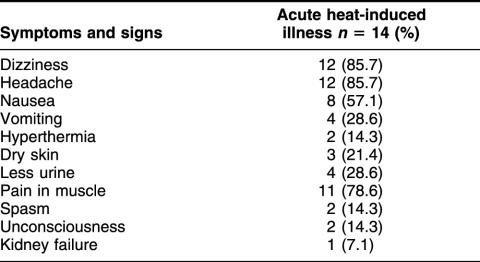
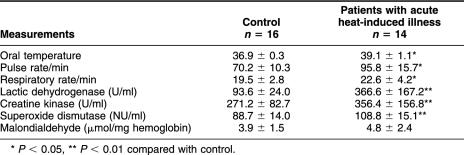
All the individuals in the study exercised in the same hot environment and with the same physical load. The symptoms noted in patients with heat-induced illness are listed in Table 1. The most common symptoms observed in over 75% of patients were dizziness, headache, and muscle pain. Other frequently observed symptoms were nausea (57%), vomiting and less urine (28%), and dry skin (21%). Severe hyperthermia, unconsciousness, and kidney failure were noted but were not common in those younger patients with acute heat-induced illness. Table 2 compares some physical and biochemical characteristics of these patients with those of the controls. The mean oral temperature was significantly higher in the patients (39.1 vs 36.9). Their pulse and respiratory rates also were higher than those of the control individuals. There was also a significant increase in the activities of LDH, CK, and SOD (P < 0.01) in patients. LDH and CK have been reported to be particularly good indicators of heat stroke severity in a study conducted in Saudi Arabia (Alzeer et al 1997). There was a slight increase in the contents of MDA, although it was not statistically significant.
Table 1.
Number of patients with the different symptoms of heat-induced illness (May)
Table 2.
Physical and biochemical characteristics of patients with acute heat-induced illness and of controls (mean ± SD)
Basal and inducible levels of Hsp70 in patients with acute heat-induced illness
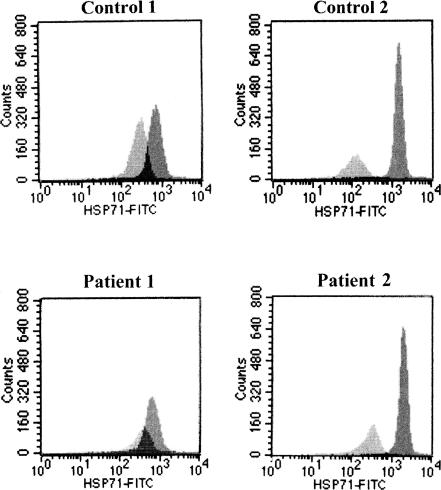
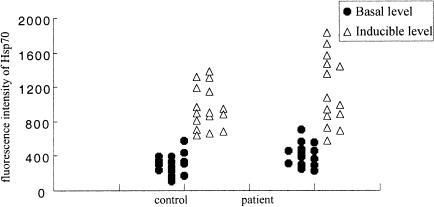
We next investigated the basal and inducible levels of Hsp70 in lymphocytes of patients with acute heat-induced illness resulting from intense physical training and in the matched controls. The level of Hsp70 was measured in fresh lymphocytes isolated after the exercise (basal level) or after a short heat shock on lymphocytes in vitro at 41°C for 1 hour followed by a 2-hour recuperation at 37°C (inducible level) using flow cytometry with an antibody specific for the inducible Hsp72. Figure 1 shows some examples of Hsp70 measurements in patients with disease and in controls. Table 3 summarizes the data of the mean basal and inducible levels of Hsp70 for both groups. The basal levels of Hsp70 in patients with acute heat-induced illness are significantly higher than those in the controls (P = 0.02). The increase in inducible Hsp70 after an in vitro heat shock appears to be slightly higher (225%) in the patient group than in the controls (180%), but this difference is not statistically significant (P = 0.12). The values of Hsp70 for individuals are plotted in Figure 2. The basal level and induced values differ among individuals within both groups.
Fig 1.
Examples of basal (light gray) and inducible (medium gray) levels of Hsp70 in 2 patients with acute heat-induced illness and in 2 controls (see Materials and Methods)
Table 3.
Basal and inducible levels of Hsp70 in lymphocytes of patients with acute heat-induced illness and of controls (May) (mean ± SD)
Fig 2.
Distribution of basal and inducible levels of Hsp70 measured in May in lymphocytes of individuals with acute heat-induced illness and in lymphocytes of controls
Basal and inducible levels of Hsp70 in individuals who had recovered from acute heat-induced illness and in controls
To understand the possible significance of basal and inducible expression of Hsp70 in heat injury and heat tolerance, we also analyzed the levels of Hsp70 in patients who had suffered acute heat-induced illness in May, after a recovery period of 6 months, and in controls under the more temperate climatic conditions of November. Figure 3 shows the distribution of basal and inducible levels of Hsp70 in individuals who had suffered from heat illness 6 months previously and in controls. Individuals from the heat illness group tended to show a higher content of Hsp70, but as shown in Table 4 there was no statistically significant difference in the basal levels of Hsp70 between those individuals who had recovered from acute heat-induced illness and controls (P = 0.66), and the small elevation in Hsp70 levels in heat-stressed lymphocytes from patients with heat-induced illness in May was of marginal significance (P = 0.06). Interestingly, both groups sampled in November had roughly twice the basal levels of Hsp70 of the groups that were sampled in May. The levels of Hsp70 induced by heating lymphocytes in vitro were the same for the samples of each group in May and November, indicating that the conditions that resulted in elevated basal levels did not affect the response of the lymphocytes in this assay.
Fig 3.
Distribution of basal and inducible levels of Hsp70 in lymphocytes of individuals who recovered from acute heat-induced illness in May and in lymphocytes of controls in November
Table 4.
Basal and inducible levels of Hsp70 in lymphocytes of individuals who recovered from acute heat-induced illness and of controls (November) (mean ± SD)
DISCUSSION
Whether the basal level of Hsp70 of an individual or his capacity to respond to heat shock by inducing this Hsp can predict his response to severe heat stress is presently unknown. We, therefore, examined the basal and inducible levels of the inducible member of the Hsp70 family, Hsp72, in lymphocytes from young males training under rather severe heat conditions in Nanjing. Hsps can be induced in response to various stresses including exposure to elevated temperature, and environmental and physiological stresses (reviewed in Lindquist and Craig 1988; Morimoto et al 1994). Hsps also can be induced by many pathological and physiological stimuli. Exercise is capable of inducing a heat shock response in a number of tissues in animals (Locke et al 1995a, 1995b; Milne and Noble 2002; Noble 2002) and in humans (Fehrenbach et al 2000).
Heat stress and heat stroke are generally more predominant in very young children and in elderly persons. The heat shock response seems to be impaired as a result of aging (Locke and Tanguay 1996). In the present study, we selected young individuals of the same sex without any disease who lived in a similar environment and received similar training, so that the effects of other stressful factors on the basal and the inducible levels of Hsp70 could be reduced as much as possible. This selection of young persons undergoing training has a further advantage in that their response to stress is not impaired by the aging factor.
Our data show that there is variation in the basal and inducible levels of Hsp70 among individuals from the heat-stressed group and the control group. The mean basal level of Hsp70 in patients with acute heat-induced illness is significantly higher than that of controls (P = 0.02). Interestingly, the levels of inducible Hsp70 in lymphocytes may be slightly higher in patients after 6 months of recovery from acute heat-induced illness than in controls (P = 0.06).
Another interesting point is that the mean level of Hsp70 after induction by a heat shock in vitro is similar in both groups, in the first experiment (May) and after recovery (November). The higher basal level of Hsp70 in both the groups observed in November may have resulted from a stress-conditioning effect because of continuous training and exposure to heat stress during the preceding 6 months. Experiments in rats have shown that acclimated rats have a higher level of Hsp72 and a faster response when placed under a heat stress (Maloyan et al 1999). However, this response is complex as the temperature of stress was of importance because rats stressed at higher temperature (43°C instead of 41°C) did not show the faster response to a severe heat shock observed at 41°C.
Is the basal level of Hsp70 important in heat-induced illness? Fehrenbach et al (2000) reported that the basal level of Hsp in leukocytes was lower in trained athletes than in untrained subjects, but the response of leukocytes to an in vitro heat shock was higher for trained individuals. It is possible that individuals who had higher basal levels of Hsp70 in May were more sensitive to exercise-induced heat stress because they were less fit physically. Thus, exercise regimens capable of inducing Hsp70 in this group may not have registered as an inducing condition for Hsp70 in the more fit group of individuals. The basal level of Hsp70 and Hsc70 also has been reported to be a determinant of inducibility in response to heat shock (Boshoff et al 2000). Unfortunately, we could not measure the level of Hsp70 before exercise in the present study. Nonetheless, our data show that individuals with a higher level of Hsp70 tend to have a slightly higher induced response. The response to the severe heat exercise–induced stress was variable among individuals who ran in the same hot environment. Whether individuals who suffered illness had higher endogenous levels of Hsp70 before exercise or responded more strongly to the severe training exercise remains to be determined.
We have preliminary evidence that individuals originating from regions with a hot climate respond differently to a training stress than do individuals from regions with colder climates. Most individuals in the present study came from Nanjing and the regions around this province (Jiansu Province), ie, from a hot climate region. Intraspecies variation in basal levels of Hsps has been reported previously in human populations (Lyashko et al 1994). Hsp expression is under complex regulatory control, operating at both the transcription and the translation levels (Morimoto 1998; Shi et al 1998). Members of the Hsp70 family also are polymorphic, and this polymorphism might affect the individual response to stress. It will be important to take such factors into account in future investigations on the susceptibility of persons to harsh exercise and their response to environmental aggressors. Previous investigations on fish and flies have suggested that a certain level of Hsp70 is needed to attain maximum acquired resistance. Interestingly, some individuals within an outbred population contain more than twice the normal level of Hsp70, but this additional Hsp70 does not result in higher levels of resistance, showing that various amounts of Hsp70 beyond a certain level do not necessarily imply a greater resistance (Norris et al 1995; Li and Nussenzweig 1996; Krebs and Feder 1997). The results obtained by Theodorakis et al (1999) also suggest that thermotolerant cells limit Hsp70 expression, perhaps to avoid the potential cytotoxic effect of these proteins.
Hot environments are the direct cause of acute heat-induced illness (Bouchama and Knochel 2002). Regulation of human body temperature and adaptation to heat involve the nervous, circulatory, endocrine, and urinary systems. Heat-induced illness occurs when the body temperature rises as a result of work and exercise in a hot environment and during dysfunctioning of these systems and organs. Although Hsps, and especially Hsp70, can protect many of these systems and organs from the damage of stresses (Hightower 1991), Hsps also can behave as danger markers under certain conditions (Moseley 2000). In our study, Hsp70 may be a danger marker for individuals at risk of heat-induced illnesses in May. However, the higher basal level of Hsp70 in both groups in November may indicate the presence of an altered physiological state brought on by the successful stress conditioning of both groups in November, and it may be a marker for the cytoprotected state under these conditions.
Acknowledgments
We are grateful to the individuals who voluntarily accepted to participate in the study and to the members of the medical personnel of Nanjing Wujing Hospital for their generous help in the examination and sampling of subjects. This work was supported by research funds from the National Natural Science Foundation of China (NNSFC), from the National Key Basic Research and Development Program, from the Fok Ying Tung Education Foundation, and from a Teaching and Research Award for Outstanding Young Teachers in Higher Education Institutions of the Ministry of Education (TRAPOYT, China) to T.W. T.W. and R.M.T. also acknowledge financial support from a Canadian Institute of Health Research (CIHR) and NNSFC exchange program and an operating CIHR grant (to R.M.T.).
REFERENCES
- Alzeer AH, El-Hazmi MA, Warsy AS, Ansari ZA, Yrkendi MS. Serum enzymes in heat stroke: prognostic implication. Clin Biochem. 1997;43:1182–1187. [PubMed] [Google Scholar]
- Bachelet M, Mariéthoz E, and Banzet N. et al. 1998 Flow cytometry is a rapid and reliable method for evaluating heat shock protein 70 expression in human monocytes. Cell Stress Chaperones. 3:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck FX, Neuhofer W, Müller E. Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol. 2000;279:F203–F215. doi: 10.1152/ajprenal.2000.279.2.F203. [DOI] [PubMed] [Google Scholar]
- Boshoff T, Lombard F, Eiselen R, Bornman JJ, Bachelet M, Polla BS, Mornman L. Differential basal synthesis of Hsp70/Hsc70 contributes to interindividual variation in Hsp70/Hsc70 inducibility. Cell Mol Life Sci. 2000;57:1317–1325. doi: 10.1007/PL00000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG. Heat-response and limitation of tissue necrosis during occusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Favatier F, Bornman L, Hightower LE, Günther E, Polla BS. Variation in Hsp gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2:141–155. doi: 10.1379/1466-1268(1997)002<0141:vihgea>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Schlotz E, Passek F, Dickhuth HH, Northoff H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol. 2000;89:704–710. doi: 10.1152/jappl.2000.89.2.704. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress protein, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE, Schooel B 1994 Heat shock proteins as antigens in immunity against infection and self. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 495–531. [Google Scholar]
- Krebs RA, Feder ME. Natural variation in the expression of heat-shock protein Hsp70 in a population of Drosophila melanogaster and its correlation with tolerance of ecologically relevant thermal stress. Evolution. 1997;51:173–179. doi: 10.1111/j.1558-5646.1997.tb02398.x. [DOI] [PubMed] [Google Scholar]
- Li GC, Nussenzweig A 1996 Thermotolerance and heat shock proteins: possible involvement of Ku autoantigen in regulating Hsp70 expression. In: Stress-Inducible Cellular Response, ed Feige U, Morimoto RI, Yahara I, Polla B. Birkhauser, Basel, Switzerland, 425–450. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG, Tanguay RM, Feild MR, Ianuzzo SE, Ianuzzo CD. Activation of heat-shock transcription factor in rat heart after heat shock and exercise. Am J Physiol. 1995a;268:C1387–C1394. doi: 10.1152/ajpcell.1995.268.6.C1387. [DOI] [PubMed] [Google Scholar]
- Locke M, Tanguay RM. Diminished heat shock response in the aged myocardium. Cell Stress Chaperones. 1996;1:251–260. doi: 10.1379/1466-1268(1996)001<0251:dhsrit>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M, Tanguay RM, Klabunde RE, Ianuzzo CD. Enhanced postischemic myocardial recovery following exercise induction of Hsp72. Am J Physiol. 1995b;269:H320–H325. doi: 10.1152/ajpheart.1995.269.1.H320. [DOI] [PubMed] [Google Scholar]
- Lyashko VN, Vikulova VN, Chernikov VG, Lvanov VI, Ulmasov KA, Evgen'ev MB. Comparison of the heat shock response in ethnically and ecologically different populations. Proc Natl Acad Sci U S A. 1994;91:12492–12495. doi: 10.1073/pnas.91.26.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloyan A, Palmon A, Horowitz M. Heat acclimation increases the basal Hsp72 level and alters its production dynamics during heat stress. Am J Physiol. 1999;276:R1506–R1515. doi: 10.1152/ajpregu.1999.276.5.R1506. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi S, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kDa heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;96:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne KJ, Noble EG. Exercise-induced elevation of Hsp70 is intensity dependent. J Appl Physiol. 2002;93:561–568. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C 1994 Progress and perspectives in the biology of heat shock proteins and molecular chaperones. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1–30. [Google Scholar]
- Moseley PL. Exercise, stress, and the immune conversation. Exerc Sport Sci Rev. 2000;28:128–132. [PubMed] [Google Scholar]
- Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and Hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci U S A. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EG 2002 Heat shock proteins and their induction with exercise. In Exercise and Stress Response: The Role of Stress Proteins, ed Locke M, Noble EG. CRC Press, Boca Raton, FL, R1374–R1381. [Google Scholar]
- Norris CE, Ditorio PT, Schultz RJ, Hightower LE. Variation in heat shock proteins within tropical and desert species of poeciliid fishes. Mol Biol Evol. 1995;12:1048–1062. doi: 10.1093/oxfordjournals.molbev.a040280. [DOI] [PubMed] [Google Scholar]
- Oehler R, Pusch E, and Zellner M. et al. 2001 Cell type-specific variations in the induction of Hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperones. 6:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidas CN, Mooney ML, Theodorakis NG, and De Maio A 2002 Accelerated recovery after endotoxin challenge in heat shock-pretreated mice. Am J Physiol R1374–R1381. [DOI] [PubMed] [Google Scholar]
- Petersdorf RG, Root RK 1987 Alterations in body temperature. In: Harrison's Principles of Internal Medicine, ed Braunwald E, Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, Fauci AS. McGraw Hill Book Company, New York, 43–49. [Google Scholar]
- Plumier C, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van Der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 65 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Gene Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock stress proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- Theodorakis NG, Drujan D, Maio AD. Thermotolerance cells show an attenuated expression of Hsp70 after heat shock. J Biol Chem. 1999;274:12081–12086. doi: 10.1074/jbc.274.17.12081. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Wu T, Chen S, and Sun Y. et al. 2001a Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones. 6:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ma J, and Chen S. et al. 2001b Association of plasma antibodies against heat stress protein Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones. 6:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones. 1998;3:161–167. doi: 10.1379/1466-1268(1998)003<0161:poaths>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.cir.100.11.1169. [DOI] [PubMed] [Google Scholar]