Abstract
In all species studied to date, the function of heat shock protein 90 (Hsp90), a ubiquitous and evolutionarily conserved molecular chaperone, is inhibited selectively by the natural product drugs geldanamycin (GA) and radicicol. Crystal structures of the N-terminal region of yeast and human Hsp90 have revealed that these compounds interact with the chaperone in a Bergerat-type adenine nucleotide–binding fold shared throughout the gyrase, Hsp90, histidine kinase mutL (GHKL) superfamily of adenosine triphosphatases. To better understand the consequences of disrupting Hsp90 function in a genetically tractable multicellular organism, we exposed the soil-dwelling nematode Caenorhabditis elegans to GA under a variety of conditions designed to optimize drug uptake. Mutations in the gene encoding C elegans Hsp90 affect larval viability, dauer development, fertility, and life span. However, exposure of worms to GA produced no discernable phenotypes, although the amino acid sequence of worm Hsp90 is 85% homologous to that of human Hsp90. Consistent with this observation, we found that solid phase–immobilized GA failed to bind worm Hsp90 from worm protein extracts or when translated in a rabbit reticulocyte lysate system. Further, affinity precipitation studies using chimeric worm-vertebrate fusion proteins or worm C-terminal truncations expressed in reticulocyte lysate revealed that the conserved nucleotide-binding fold of worm Hsp90 exhibits the novel ability to bind adenosine triphosphate but not GA. Despite its unusual GA resistance, worm Hsp90 appeared fully functional when expressed in a vertebrate background. It heterodimerized with its vertebrate counterpart and showed no evidence of compromising its essential cellular functions. Heterologous expression of worm Hsp90 in tumor cells, however, did not render them GA resistant. These findings provide new insights into the nature of unusual N-terminal nucleotide-binding fold of Hsp90 and suggest that target-related drug resistance is unlikely to emerge in patients receiving GA-like chemotherapeutic agents.
INTRODUCTION
Heat shock protein 90 (Hsp90) is an essential, ubiquitously expressed molecular chaperone, which modulates the maturation and function of many key regulatory proteins within the eukaryotic cell (Csermely et al 1998; Pratt 1998; Buchner 1999). Under physiological conditions, Hsp90 interacts with a select group of “client” proteins such as kinases and transcription factors to maintain them in a metastable state capable of activation in response to appropriate stimuli. As a result of this unique biochemical function, Hsp90, although possessing no intrinsic signaling activity of its own, plays a central role in regulating both normal developmental processes (Rutherford and Lindquist 1998; Queitsch et al 2002) and neoplastic transformation (Neckers et al 1999; Bagatell et al 2001). Because of the important role that Hsp90 plays in regulating cellular processes that are pathologically altered in various human diseases, especially cancer, much effort has been directed at the identification and development of drugs that can alter Hsp90 function. Although the clinical evaluation of such agents is at an early stage (Ivy et al 2002), Hsp90-binding drugs have already proven to be very helpful in the study of biology of Hsp90. In fact, much of our current understanding of cellular functions of Hsp90 has been achieved through the use of the highly selective natural product inhibitors geldanamycin (GA) and radicicol. These structurally unrelated compounds bind with high affinity to the chaperone in a Bergerat-type adenine nucleotide–binding fold shared throughout the GHKL superfamily of adenosine triphosphatases (Dutta and Inouye 2000). Drug binding disrupts cycling of the protein between its adenosine 5′-diphosphate– and adenosine triphosphate (ATP)–bound conformations, which appears to be essential for many, if not all, of its chaperoning functions (Panaretou et al 1998; Roe et al 1999).
To better understand the consequences of drug interaction with Hsp90 at a whole-animal level, we examined the effects of GA on the genetically tractable, free-living nematode Caenorhabditis elegans. This organism has provided many important molecular genetic insights into the highly conserved signaling pathways, which regulate growth, differentiation, and survival in higher animals. The sole Hsp90 ortholog in C elegans has been identified as daf-21, which encodes a protein sharing 74% and 76% amino acid identity to human Hsp90α and Hsp90β, respectively. There are no other C elegans genes with this degree of similarity to vertebrate Hsp90 (Fig 2), and this gene appears to be abundantly expressed based on the fact that it comprised 3% of the complementary deoxyribonucleic acid (cDNA) clones isolated in a library prepared from mixed-stage worms (Birnby et al 2000). Expression of the gene shows strong environmental and developmental regulation (Dalley and Golomb 1992; Cherkasova et al 2000). Deletion homozygotes are larval lethal, indicating that Hsp90 is essential in C elegans as it is in all eukaryotic organisms including yeast and Drosophila (Borkovich et al 1989; Cutforth and Rubin 1994). Consistent with its essential role in survival of this organism, DAF-21/Hsp90 protein has been shown to associate with UNC-45 as a cochaperone in regulating the stability and assembly of myosin, which is required for processes such as cell division, motility, and muscle contraction (Barral et al 2002). A temperature-sensitive missense mutation, daf-21(p673), caused abnormalities in a signal transduction pathway that regulates the formation of dauer larvae, an alternate third stage of development in C elegans, which enhances survival under harsh conditions (Riddle 1988). In light of this strong evidence for conservation of role of Hsp90 in regulating key cellular processes in C elegans, we were intrigued to find that exposure of worms to GA resulted in no discernable phenotype. We have subsequently examined the biochemical basis for this lack of drug activity and have provided data indicating that worm Hsp90 fails to bind GA, but its heterologous expression does not render human tumor cells GA resistant.
Fig 2.
Amino acid sequence alignment of heat shock protein 90 (Hsp90) orthologs. Identical and conservatively substituted residues in human Hsp90 (hHsp90, NCBI accession #NM 005348), C elegans DAF-21 (wHsp90, accession #074225), and S cerevisiae Hsp82 (yHsp90, accession #K01387) are highlighted in black. The highly conserved N-terminal nucleotide-binding domains are indicated by the dashed box
MATERIALS AND METHODS
Worms, cells, and reagents
Cultures of the C elegans N2 strain were obtained from the laboratory of S. Ward (University of Arizona) and from the Caenorhabditis Genetics Center (University of Minnesota). Worms were propagated on nematode growth medium (NGM) agar with Escherichia coli strain OP50 as food source. Mixed-stage cultures were incubated at 15°C for drug exposure experiments and at room temperature (20–25°C) for the preparation of lysates for affinity precipitation experiments. Human PC-3M prostate cancer cells were obtained from the National Cancer Institute, Developmental Therapeutics Program, and were grown in Roswell Park Memorial Institute 1640 medium under 6% CO2 in air. Human embryonic kidney cells transformed with adenovirus (HEK 293 cells) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in Dulbecco modified Eagle medium under 10% CO2 in air. All cell culture media were obtained from Invitrogen/Life Technologies (Carlsbad, CA, USA) and supplemented with 10% fetal calf serum (Irvine Scientific, Santa Ana, CA, USA), 2 mM l-glutamine, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid. Cells were confirmed negative for mycoplasma contamination by enzyme-linked immunosorbent assay. GA was obtained from the Drug Synthesis and Chemistry Branch (National Cancer Institute). All other biochemical reagents were obtained from Sigma (St Louis, MO, USA) unless otherwise specified. Stock drug solutions were formulated in dimethyl sulfoxide (DMSO) and maintained at −20°C in the dark.
Treatment of worms with GA
NGM plates first were spotted with E coli strain OP50 as a food source and then GA (178 μM final concentration) or adjuvant (or both) was added after overnight growth of the bacterial lawn. Approximately 100 embryos of wild-type C elegans were dispensed per plate (5 plates per treatment). Subsequent generations were maintained continuously on GA by transferring 5–10 young adult worms to fresh plates, allowing them to lay eggs overnight and then removing the adults the next morning. Parallel experiments were conducted at 15°C, 20°C, and 25°C. Larval viability and dauer formation were observed under a dissection microscope on each day until the worms reached adulthood. Fertility was determined by picking individual worms (50 per treatment) immediately before adulthood onto fresh plates, transferring them every day, and counting progeny until egg-laying ceased. These worms were further examined each day until death to determine life span.
Preparation of Hsp90 expression constructs
cDNA of wild-type worm encoding DAF-21/Hsp90 was generated by polymerase chain reaction (PCR) amplification from a worm cDNA library using the forward and reverse primers AAGGATCCATGTCCGAGAACGCCG and AAAGCGGCCGCAAACTGTGGAGGGGCTTTTG, respectively. Amplification (25 cycles of 30 seconds at 94°C, 30 seconds at 55°C, 150 seconds at 72°C) with high-fidelity Pfu DNA polymerase (Stratagene, La Jolla, CA, USA) produced a single band of the expected size derived from published daf-21 sequence data (NCBI accession #074225). Fidelity of the PCR product was confirmed by dideoxy sequencing (Arizona Research Labs, Core Facility). A 2.1-kb BamH1-Not1 fragment from this PCR product was subcloned into the expression vector pcDNA3.1(+) (Invitrogen), which encodes both a T7 ribonucleic acid (RNA) polymerase promoter for expression in reticulocyte lysate and cytomegalovirus enhancer-promoter sequence for high-level expression in mammalian cells. To create worm Hsp90 truncations, the pcDNA3.1 vector encoding full-length sequence was cut with HindIII and EcoRV to generate fragments encoding amino acids 1–210 and 1–272, respectively. These digests were then cut with XbaI, blunt ended, and religated. Full-length chick Hsp90α cDNA cloned into the vector pGEM4Z (Promega Corp, Madison, WI, USA) and a cDNA fragment encoding amino acids 1–222 cloned into the vector pBluescipt II SK (Stratagene) were provided by L. Neckers (National Cancer Institute). For expression in human cells, full-length chick cDNA was cut from the pGEM4Z vector as a SalI-SphI fragment and subcloned into pcDNA3.1(+). Chick-worm and worm-chick chimeras were constructed by taking advantage of a conserved EcoRI site present in the Hsp90 cDNA of both species. To create our chick-worm chimera, the pcDNA3.1 vector encoding worm Hsp90 was cut with BamH1 and EcoRI. The excised fragment was then replaced with a BamH1-EcoRI fragment previously isolated from the vector pGEM4Z encoding chick Hsp90. To create our worm-chick chimera, the entire coding sequence of chick Hsp90 was mobilized as a SalI-SphI fragment and subcloned into pSL1180 (Amersham Biosciences, Piscataway, NJ, USA) to add a NotI site to the end of its 3′ noncoding sequence. pcDNA3.1 vector encoding worm Hsp90 was then cut with EcoRI and NotI and the excised fragment replaced with an EcoRI-NotI fragment isolated from the pSL1180chick-Hsp90 vector.
In vitro transcription-translation reactions
Recombinant proteins were expressed from 1 μg of plasmid DNA using a TNT® rabbit reticulocyte lysate kit from Promega. Reactions (final volume 50 μL) were supplemented with translation grade [35S]l-methionine (1500 Ci/mmol, 15 mCi/mL; Amersham) and the appropriate RNA polymerase as per manufacturer's recommendations. Aliquots of each reaction (1 μL) were examined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and by autoradiography to check translation efficiency, and one half of a reaction was typically diluted into the appropriate buffer for affinity precipitations.
Solid-phase binding assays
For affinity precipitation from lysates of tissue culture cells and worms, extracts were prepared in cold TNES buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 1% Nonidet P-40) supplemented with protease inhibitors just before use (1 mM phenylmethylsulfonyl fluoride, 20 μg/mL leupeptin, 20 μg aprotinin/mL). Lysis buffer was added directly to frozen cell pellets, and incubation on ice with intermittent vortexing was continued for 10 minutes followed by centrifugation at 14 000 × g for 20 minutes at 4°C. To efficiently extract worms, frozen pellets were ground using a mortar and pestle over a dry ice–ethanol bath before addition of buffer. After centrifugation, protein determinations were performed on the clarified supernatants using bicinchoninic acid (BCA) reagent (Pierce, Rockford, IL, USA), and lysates were stored at −20°C until use. Affinity precipitation of Hsp90 was performed using 300–500 μg of protein per assay in a total volume of 300 μL and GA-coupled agarose beads (25 μL packed bead volume per assay) as previously described (Whitesell et al 1994). After gentle agitation for at least 2 hours at 4°C, beads were washed 4 times with TNES buffer and bound proteins eluted by heating in 1× reducing sample buffer. Samples were analyzed by SDS-PAGE followed by Coomassie blue staining or immunoblotting for Hsp90. For affinity precipitation of the [35S]methionine-labeled proteins generated in reticulocyte lysate, transcription-translation reactions were diluted 1:10 in TNES and rocked with GA-coupled beads. Alternatively, reactions were diluted 1:10 in incubation buffer as described by Grenert et al (1997) and added to Sepharose beads to which ATP was covalently bound by its γ-phosphate (Upstate Inc, Lake Placid, NY, USA). After 1–2 hours at 4°C with gentle agitation, bead pellets were washed 4 times with 1 mL of the relevant ice-cold buffer and bound proteins eluted by heating in 1× reducing sample buffer. Samples were then fractionated by SDS-PAGE and radioactive proteins observed by autoradiography of dried gels at room temperature using Kodak XAR-2 film. Multiple exposure times were evaluated for each gel to ensure that the band intensities observed were within the dynamic response range of the film.
Western blotting and immunoprecipitation
To detect the presence of Hsp90 in lysates and affinity precipitations, Western blotting was performed with the mouse monoclonal antibody AC88 (Stressgen Biotechnologies, Victoria, British Columbia, Canada), which recognizes both worm and vertebrate orthologs. Primary antibody was diluted 1:500 and incubated at 4°C overnight. Reactivity was detected using peroxidase-conjugated goat anti-mouse secondary antibody (1:100 000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and a chemiluminescent detection system as previously described (Whitesell L et al 1998). To examine the ability of worm Hsp90 to physically associate with vertebrate Hsp90, radiolabeled in vitro translation reactions were diluted 10-fold in TNES containing 0.5 M NaCl and then incubated overnight at 4°C with a polyclonal anti-Hsp90 antibody raised against an amino-terminal epitope in vertebrate Hsp90α that does not exist in the worm ortholog (SPA-771, Stressgen, 4 μg/reaction). Protein G Sepharose (Amersham) was added to all samples and incubation continued for 1 hour with rocking at 4°C. Beads were then washed 4 times with TNES and bound proteins analyzed by SDS-PAGE followed by autoradiography.
Flow cytometry
HEK 293 cells were transfected with Hsp90 expression vectors using 2 μg of DNA per 60-mm dish and cationic lipid reagent in serum-free medium as per manufacturer's recommendations (Lipofectamine Plus, Invitrogen). Forty-eight hours after transfection, the relative levels of cell surface insulin-like growth factor receptor type 1 (IGF-1R) were analyzed by indirect immunofluorescent staining and flow cytometry as previously described (Liu et al 1998). Briefly, cells were incubated with mouse monoclonal antibody αIR3 (Oncogene Research Products, Cambridge, MA, USA; Ab-1) in phosphate-buffered saline with 1% bovine serum albumin and 0.1% sodium azide for 1–2 hours at 4°C. After washing, cells were incubated in Alexa-488–conjugated goat anti-mouse antibody (Molecular Probes, Eugene, OR, USA) for an additional 60 minutes. After washing, cells were fixed in freshly prepared 1% paraformaldehyde overnight in cold and analyzed on a Becton Dickinson FACStar flow cytometer.
RESULTS
No apparent effect of Hsp90-binding drugs on C elegans growth and development
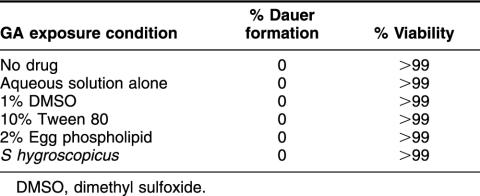
Previously reported genetic data demonstrated the involvement of the worm Hsp90 ortholog DAF-21/Hsp90 encoded by daf-21 in larval viability, dauer development, adult fertility, and life span. Surprisingly, we observed no discernible effects on any of these processes, despite continuous passage for 3 generations on plates saturated with GA (178 μM). This result was in marked contrast to the dramatic effects reported for these agents in organisms ranging from yeast (Bohen 1998) to flies (Rutherford and Lindquist 1998) to plants (Queitsch et al 2002). Suspecting a simple problem with absorption of the agents, we explored a variety of vehicles and techniques to enhance the uptake of GA into worms as summarized in Table 1. In these experiments, we focused on dauer formation and larval viability because marked effects on these parameters have been well described for the temperature-sensitive daf-21(p673) point mutation and daf-21 knockout mutation (12). Serial propagation for 3 generations was performed because a maternal rescue effect has been reported for some daf-21 mutations. Of note, even growing the worms on lawns of Streptomyces hygroscopicus, the organism that produces GA, instead of their usual food source E coli, resulted in no discernible phenotype. In contrast, shifting worms carrying temperature-sensitive daf-21 alleles to the nonpermissive temperature of 25°C resulted in 67% of the 500 animals evaluated being arrested in the dauer developmental stage as expected (Birnby et al 2000). This result confirms that if drug treatment had altered DAF-21/Hsp90 function significantly, we would have been able to detect the effect.
Table 1.
Effect of exposing age-synchronized populations of C elegans to geldanamycin (GA) (178 μM) plus the indicated adjuvants to enhance drug uptake. Alternatively, worms were cultured on lawns of GA-producing soil organism, S hygroscopicus, as a food source. Plates were examined by phase contrast microscopy to evaluate 500 animals per treatment group
Immobilized GA does not bind C elegans Hsp90
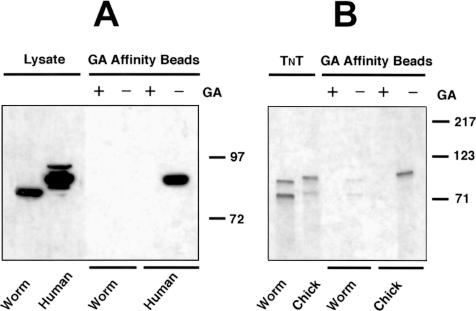
To pursue the puzzling lack of drug effects we observed in whole worms, we prepared nondenaturing protein extracts of mixed-stage worm cultures and examined the ability of the Hsp90 ortholog DAF-21 in these extracts to bind GA that had been immobilized on agarose beads as previously described (Whitesell et al 1994). For the data presented in Figure 1A, beads were incubated with lysates in the presence or absence of soluble GA (20 μM) as indicated. After 60 minutes at 4°C with gentle rocking, the beads were washed extensively and bound proteins fractionated by SDS-PAGE followed by Western blotting for Hsp90. As a control, aliquots of total lysate were also loaded on the gel to verify the migration position and immunoreactivity of the Hsp90 variants present in either the worm or human lysates. GA beads bound Hsp90 well in lysate prepared from the human cell line PC-3M, and this binding was effectively competed by the addition of soluble GA as expected. In contrast, GA beads failed to bind any of the readily detectable Hsp90 ortholog present in lysate prepared from mixed-stage worms (faster migrating band evident in Fig 1A, far left lane).
Fig 1.
Immobilized geldanamycin (GA) does not bind the heat shock protein 90 (Hsp90) ortholog expressed by C elegans. GA was derivatized and immobilized on agarose beads as described in Materials and Methods. (A) Tissue extracts. Beads were incubated in nonionic detergent lysates prepared from either mixed-stage worm cultures or the human cancer cell line PC-3M. Lysates were supplemented with soluble GA (18 μM) or an equal volume of dimethyl sulfoxide (DMSO) vehicle as indicated before addition of beads. Bound proteins were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted for Hsp90. As a control, total lysates (20 μg protein/lane) was also analyzed (lysate lanes) to verify the migration position and immunoreactivity of Hsp90 derived from the 2 sources. The migration positions of molecular size markers are indicated to the right of the panel. (B) Transcription-translation (TNT®) reactions. Constructs encoding either full-length chick or worm Hsp90 were translated in rabbit reticulocyte lysate supplemented with [35S]methionine. GA beads were then incubated in these translation reactions after addition of either soluble GA (18 μM) or DMSO vehicle as indicated. Bound proteins were fractionated by SDS-PAGE and the radioactive translation products observed by autoradiography. As a control, aliquots (1 μL) of each 50 μL reaction were also analyzed to verify the presence of approximately equivalent amounts of the relevant translation product in the lysate. Molecular sizes are indicated to the right of the panel. Data presented are representative of experiments performed on 3 independent occasions
To rule out the possibility that the failure of GA beads to bind Hsp90 in crude worm lysate was due to the presence of an unknown inhibitory or interfering substance in worms, we performed coupled transcription-translation reactions in reticulocyte lysate programmed with the full-length cDNA of either chicken or worm Hsp90. After 90-minute incubations at 30°C in the presence of [35S]methionine, an aliquot of the translation reactions was analyzed by SDS-PAGE and by autoradiography. As evident in Figure 1B, (TNT lanes) both cDNAs generated proteins of the expected molecular sizes (worm slightly smaller than chick) and smaller translation products that may have resulted from either premature termination or alternative start site utilization during the TNT reactions. Consistent with our findings in cell lysates, chick translation product bound well to GA affinity beads, and binding was competed by soluble GA, whereas the equivalent amount of worm product bound poorly. In fact, the small amount of signal detectable in the worm precipitation lane may have resulted from heterodimerization of the worm translation product with endogenous rabbit Hsp90 present in reticulocyte lysate and subsequent binding of rabbit Hsp90 to the GA beads (see below).
Despite extensive homology with human Hsp90, the N-terminal domain of worm Hsp90 fails to bind GA, whereas retaining the ability to bind ATP
The most obvious explanation for the surprising lack of GA binding by worm Hsp90, demonstrated in Figure 1, would be an alteration in the chaperone's N-terminal nucleotide-binding pocket. To examine this possibility, we performed an alignment of the known amino acid sequences of human Hsp90 (hHsp90) and its C elegans ortholog (wHsp90). We also included the sequence from S cerevisiae (yHsp90) because the Hsp90 ortholog in this lower eukaryote is known to bind GA (Bohen 1998). As seen in Figure 2, the human and worm sequences demonstrated 85% homology. Because the crystal structure of N-terminal fragments of human (Stebbins et al 1997) and yeast (Prodromou et al 1997) Hsp90 has been previously published, we also used the Swiss Protein Data Base viewer program to compare the known Hsp90 structure with that predicted for worm. Using the threading function, the 3-dimensional structures of the 2 proteins appeared to overlap completely (data not shown). No significant differences with respect to electrostatic profile or the composition and position of amino acid side chains within the drug-binding pocket were detected. A single amino acid (Ser52) that makes a hydrogen bond with a critical drug-contacting residue (Asp93) in the human protein is substituted by alanine in the worm protein. This change is probably not important, however, because the same substitution occurs in yeast Hsp90, which does bind GA.
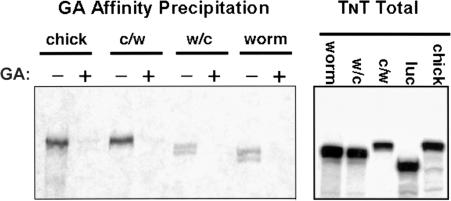
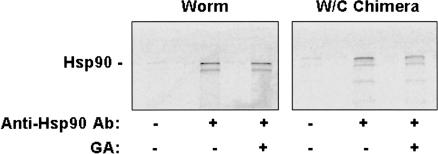
In light of the high degree of drug-binding pocket conservation that was predicted, we next considered the possibility that the failure of worm Hsp90 to bind GA could result from variation in the poorly understood interactions that are thought to occur between the N- and C-terminal regions of chaperone by a flexible, charged linker domain (Owen et al 2002; Soti et al 2002). This possibility seemed particularly attractive because this charged domain (human residues 210–272) shows the least amount of conservation between the human and worm sequences. Unfortunately, because of the intrinsic conformational flexibility of the full-length Hsp90 molecule, it has not been possible to resolve its structure using X-ray crystallography, only that of N-terminal fragments. As a result, we could not model potential differences in the interaction of the domains of chaperone based on primary sequence data. Instead, we generated chimeric fusion constructs for expression in reticulocyte lysate. In Figure 3, we compared the GA-binding ability of in vitro–translated, full-length chick and worm Hsp90 as well as that of chimeric proteins consisting of either chick N-terminus fused to worm C-terminus (c/w) or vice versa (w/c). The full-length chick and c/w chimera bound well to immobilized GA, and this binding was competed by soluble GA. In contrast, neither the full-length worm nor w/c chimera bound well. The weak signal that was detectable probably resulted from heterodimerization of the recombinant proteins with the abundant rabbit Hsp90 present in reticulocyte lysate and the binding of rabbit Hsp90 to immobilized GA (see below).
Fig 3.
Binding of heat shock protein 90 (Hsp90) chimeric fusion proteins to immobilized geldanamycin (GA). Transcription-translation reactions in reticulocyte lysate were programmed as indicated with plasmid constructs encoding either unmodified chick and worm proteins or chimeric proteins consisting of chick N-terminus fused to worm C-terminus (c/w) or worm N-terminus fused to chick C-terminus (w/c). The junction point for both fusions was located within the amino acid sequence QLFFRALL, which is identical in vertebrates and worms (see: Fig 2, wHsp90 residues 305–312). Left panel: After translation in the presence of [35S]methionine, reactions were supplemented with soluble GA (18 μM) or an equal volume of dimethyl sulfoxide. GA beads were then added and incubated at 4°C with gentle agitation for 60 minutes. After extensive washing, bound proteins were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and radioactive translation products observed by autoradiography. Right panel: Aliquots of each translation reaction were analyzed by SDS-PAGE and autoradiography to monitor the relative amounts of each translation product generated in the various reactions. The “luc” lane contained material from a reaction that was programmed with plasmid encoding firefly luciferase as a positive translation control. The same experimental design was repeated on 2 other occasions, with similar results.
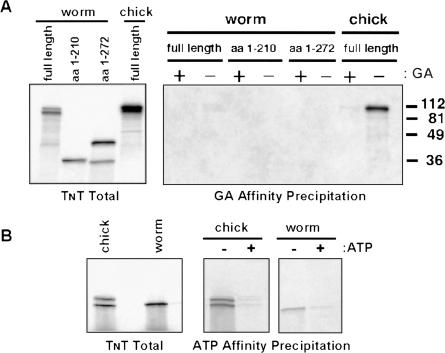
The results in Figure 3 indicated that the inability of worm Hsp90 to bind GA was unlikely to be a result of intramolecular interference by the worm's C-terminal domain. Thus, despite the high degree of sequence conservation between vertebrate and worm Hsp90 and the structural similarity predicted for their nucleotide-binding pockets, we were led to consider the possibility that the failure of worm Hsp90 to bind GA did reside within the N-terminus of chaperone. To address this issue, we expressed N-terminal fragments of worm Hsp90 in reticulate lysate and examined their ability to bind immobilized GA. As demonstrated in Figure 4A, neither the minimal N-terminal nucleotide-binding domain of worm Hsp90 nor a fragment containing the binding domain as well as the charged domain demonstrated detectable GA binding. In contrast, the full-length chick protein bound as expected. Note that these truncations lacking a C-terminal dimerization domain did not display the slight GA binding seen with chimeras and full-length worm Hsp90 and suggest that the low-level binding seen in Figure 3 was indeed due to heterodimerization with endogenous rabbit Hsp90. Because nucleotide binding is thought to play an essential role in chaperoning function of Hsp90, it was difficult to understand how the worm N-terminal fragment could fail to bind GA and yet the full-length protein could function effectively in C elegans. To address this issue, we compared the ability of chick and worm Hsp90 fragments to bind ATP using γ-phosphate–immobilized ATP as previously described (Grenert et al 1997). As demonstrated in Figure 4B, the N-terminal domains of both chick and worm Hsp90 bound well to immobilized ATP in a manner that could be competed by soluble ATP. This finding helps to explain how worm Hsp90 can still function as a chaperone and suggests that its nucleotide-binding pocket has evolved to distinguish nucleotide from drug. Until crystallographic data become available, however, the structural basis for this ability is likely to remain unknown.
Fig 4.
The N-terminus of worm heat shock protein 90 (Hsp90) binds immobilized adenosine triphosphate (ATP) but not geldanamycin (GA). Transcription-translation reactions were programmed with plasmid constructs encoding the indicated chick or worm proteins in the presence of [35S]methionine. (A) Binding to immobilized GA. Right panel: After translation, lysates were supplemented with soluble GA (18 μM) or dimethyl sulfoxide as indicated and the ability of GA beads to affinity precipitate translation products evaluated as in Figure 3. The migration positions of molecular size markers are indicated to the right of the panel. Left panel: Aliquots of each translation reaction were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography to verify the presence of the expected product in the lysate. (B) Binding to immobilized ATP. Reactions were programmed with plasmids encoding the N-terminus of either chick (amino acids 1–222) or worm (amino acids 1–210) Hsp90. Right panel: Reactions were supplemented with soluble ATP (10 mM) or an equal volume of water. Beads to which ATP was covalently bound by its γ-phosphate were then added and incubated at 4°C for 60 minutes with gentle agitation. Beads were washed and bound translation products observed by SDS-PAGE and autoradiography. Left panel: Aliquots of each translation reaction were analyzed on the same gel to verify the presence of the expected product in the lysate. Similar results were obtained when the same precipitations were performed on 2 other occasions.
Heterologous expression of worm Hsp90 in human cells does not render them GA resistant
Most of Hsp90 in the cytosol of vertebrate cells is known to exist as homodimers of either its α or β isoforms, and dimerization appears essential for its functions (Minami et al 1994; Chadli et al 2000). To begin assessing the ability of worm Hsp90 to function in a vertebrate background, we expressed the worm protein in reticulocyte lysate and examined its ability to dimerize with the endogenous rabbit Hsp90 present in this lysate. After coupled transcription-translation of [35S]-labeled worm Hsp90, immunoprecipitations were performed using an anti-Hsp90 antibody recognizing an amino terminal epitope consisting of the sequence PEETQTQDQPM that does not exist in the worm protein. As Figure 5 demonstrates, immunoprecipitation of rabbit Hsp90 with this antibody was able to bring down both full-length worm Hsp90 and our chimera consisting of worm amino-terminal sequence fused to chick C-terminal sequence. Interestingly, the presence of GA did not inhibit this coprecipitation, indicating that GA binding to Hsp90 does not disrupt its function by inhibiting dimerization.
Fig 5.
Evidence for dimerization of worm and vertebrate heat shock protein 90 (Hsp90). Full-length worm protein (left panel) or w/c chimera (right panel) was translated in the presence of [35S]methionine. After supplementation of the reaction mixtures with either geldanamycin (18 μM) or dimethyl sulfoxide, immunoprecipitation of endogenous rabbit Hsp90 was performed using antibody raised to an amino-terminal epitope in vertebrate Hsp90α that does not exist in the worm ortholog (SPA-771, Stressgen). In some precipitations, primary antibody was omitted as indicated to demonstrate specificity of the precipitation conditions used. Coprecipitation of radioactive worm translation products was observed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and autoradiography. This experiment was repeated once, with similar results.
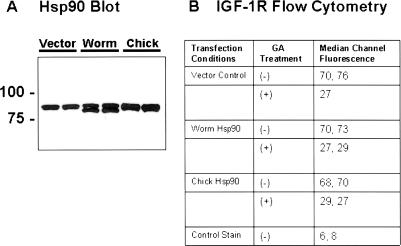
Knowing that worm Hsp90 could dimerize with its vertebrate ortholog, we next transfected a human cell line with eukaryotic expression constructs encoding either worm or chick Hsp90. Cultures were also transfected with vector backbone alone as a control for nonspecific effects. The HEK 293 cell line was used because we knew from previous work with a green fluorescent protein reporter construct that over 90% of these cells could be successfully transfected using cationic lipid (data not shown). Using this system, we were able to achieve robust expression of the 2 Hsp90 transgenes as demonstrated by Western blotting of total cell lysates 48 hours after transfection (Fig 6A). As expected, the protein product of the worm transgene was detected as a slightly faster migrating immunoreactive band that appeared to be of approximately equal intensity to that of the endogenous Hsp90. The product of the chick transgene comigrated with the endogenous cellular Hsp90 and resulted in a band of increased intensity relative to that observed in cells that were transfected with the control vector. Consistent with the ability to detect robust expression of both the worm and chick Hsp90 transgenes, we saw no significant vector-specific effects on cell viability 24–48 hours after transfection (data not shown). This finding suggests that worm Hsp90 was not acting as a dominant negative because Hsp90 knockout is lethal in eukaryotes, and a significant impairment in the function of Hsp90 in the vertebrate cells would have been expected to result in some toxicity, especially under the stress associated with plasmid transfection.
Fig 6.
Expression of worm heat shock protein 90 (Hsp90) in human cells. Replicate wells of human embryonic kidney cells (HEK 293 cells) were transfected with plasmids encoding either worm or chick Hsp90 under the transcriptional control of strong, constitutive cytomegalovirus promoter elements. As a control, wells were also transfected with the empty vector backbone, pcDNA 3.1(+). Forty-eight hours after transfection, cells were harvested by incubation in trypsin-ethylenediaminetetraacetic acid solution. Panel A: Cells from duplicate transfections with each plasmid construct were lysed in TNES buffer, and equal amounts of total cellular protein were analyzed by Western blotting with anti-Hsp90 antibody (clone AC88, Stressgen) followed by peroxidase-conjugated goat anti-mouse antibody and chemiluminescent detection. Panel B: Cells from duplicate transfections that had been refed 18 hours previously with medium containing geldanamycin (GA) (2 μM) or dimethyl sulfoxide were stained for flow cytometry to determine their relative levels of cell surface insulin-like growth factor receptor type 1. The data presented are the median channel fluorescence in arbitrary units of 10 000 events from the unimodal population of cells analyzed for each treatment condition. Only 1 value is reported for the “Vector control/(+) GA treatment” group in this experiment because the other sample for this condition was lost during processing. This experiment with minor modifications was repeated on 2 other occasions, with similar results.
Because we saw no impairment in viability associated with expression of worm Hsp90, we next examined the effect of transfection on the sensitivity of HEK 293 cells to GA exposure. Specifically, we quantified the ability of GA to deplete cellular levels of a well-known Hsp90 client protein, the receptor tyrosine kinase insulin-like growth factor receptor type 1 (IGF-1R). Twenty-four hours after transfection, cells were refed with medium containing either GA (2 μM) or an equal volume of the solvent vehicle DMSO. After an additional 18-hour incubation, cells were harvested, and an aliquot was used for determination of Hsp90 expression levels by western blotting as shown in Figure 6A. The cells remaining were stained with an antibody to the IGF-1R followed by fluorescent secondary antibody and analyzed by standard flow cytometry. The median channel fluorescence of independently transfected replicate cultures is presented in Figure 6B. Clearly, the ability of GA to destabilize the IGF-1R and markedly decrease its level on the surface of HEK 293 cells was not altered by expression of either worm or chick Hsp90 compared with vector controls. The same experiment was performed using a lower concentration of GA (100 nM) to be certain that exposure to a saturating drug concentration was not simply overwhelming any potential resistance afforded by expression of worm Hsp90. At lower drug concentration, depletion of cellular IGF-1R levels was not quite as profound, but again expression of worm Hsp90 afforded no protection (data not shown).
Last, we transfected HEK 293 cells with our expression vectors and attempted to isolate stable GA-resistant clones by passaging the cultures in the continuous presence of GA (either 1 μM or 100 nM) for 1 month. No resistant colonies could be isolated from duplicate transfections with the control vector, the chick Hsp90–, or the worm Hsp90–encoding constructs. This same experimental design with minor modifications was repeated on another occasion with the same negative outcome (data not shown).
DISCUSSION
Hsp90 plays a pervasive, fundamental role in basic processes controlling the growth and survival of both cells and whole organisms. As a result, it has proven very difficult to study this chaperone using the conventional molecular genetic techniques of targeted knockout and transgenesis. Instead, most of our current understanding of roles of Hsp90 in processes ranging from inhibition of protein aggregation (Young et al 1997; Scheibel et al 1998) to the buffering of genetic variation (Rutherford and Lindquist 1998; Queitsch et al 2002) has been achieved through the use of pharmacological agents that disrupt Hsp90 function by binding in an unusual, evolutionarily conserved nucleotide-binding pocket within the N-terminus of chaperone (Roe et al 1999). Despite its high degree of sequence similarity, however, we found that the C elegans ortholog of Hsp90 is insensitive to GA treatment in vivo and fails to bind the drug in vitro. Affinity precipitation experiments indicated that this behavior results from an intrinsic ability of the N-terminal binding site of worm Hsp90 to discriminate between ATP and GA. Despite its unusual resistance to GA, however, worm Hsp90 still appears capable of functioning in a vertebrate background. It heterodimerized with vertebrate Hsp90 and did not exert any apparent dominant negative effects, even when expressed at levels comparable with that of the endogenous protein. Heterologous expression of worm Hsp90 in human cells, however, did not render them resistant to the cytotoxicity of GA or the ability of the drug to destabilize membrane tyrosine kinases. These unexpected findings have several important implications for further development of Hsp90-binding drugs and the use of these agents to study the functions of chaperone.
First, the lack of a phenotype in worms exposed to GA demonstrates the high degree of selectivity that this drug possesses for Hsp90, its putative target of action. If GA interfered to a significant extent with other molecular targets in the intact worm, then toxicity or some effect on the growth or development (or both) of the animals would have been expected, although Hsp90 function was not affected. Second, it may not be so surprising that worm Hsp90 can apparently distinguish GA and ATP (Fig 4) and continue to function as a chaperone despite the exposure of intact worms to high concentrations of the drug (Table 1). C elegans is a free-living soil organism that inhabits the same ecological niche as the Streptomyces species that elaborates GA. The resistance of its Hsp90 ortholog to GA may well represent an example of adaptive evolution and would fit well with the observation that cultures of C elegans thrived on a pure diet of S hygroscopicus. This ecological explanation may also explain why the Hsp90 ortholog in worms is uniquely insensitive to GA, whereas those in lower (yeast) and higher (insect) organisms are sensitive. These organisms do not share the same niche as GA-producing bacteria.
The reason that heterologous expression of worm Hsp90 in human cells does not confer resistance to GA is unclear at this time. We were clearly able to generate high levels of the transgenic protein in transfected cells (Fig 6A). These levels approximated the level of endogenous Hsp90, one of the most abundant proteins in eukaryotic cytosol. Because we know that worm Hsp90 is capable of heterodimerizing with its vertebrate counterpart (Fig 5), it may be that the expression of roughly equivalent levels of worm and human Hsp90 led to the predominant formation of heterodimers. The function of such heterodimers may well have remained sensitive to disruption by GA because of the presence of the human Hsp90. It may be necessary to eliminate endogenous Hsp90 and replace it completely with worm Hsp90 before drug resistance can emerge. Such a hypothesis would best be tested in a GA-sensitive, but more genetically tractable, organism such as S cerevisiae. If correct, it would suggest that mutation of the GA-binding site would be an unlikely mechanism of drug resistance in the tumors of patients receiving experimental Hsp90-targeted agents. Both Hsp90 alleles would have to become mutated in the same cancer cell before resistance could emerge.
Finally, our findings highlight the limitations of predicting protein structure from primary sequence data. Our attempts to model the drug-binding pocket of worm Hsp90 based on its amino acid sequence and other known Hsp90 crystal structures could not explain why immobilized GA fails to bind the chaperone. When dealing with the interactions of a natural product drug with a protein, such difficulties may not be totally unexpected. Relatively subtle alterations in protein structure may have dramatic effects on drug-binding affinity and such alterations may not be predictable with current algorithms. Such considerations underlie the reality that although structure-based drug design methods are continuing to improve rapidly, combinations of rational design and combinatorial chemistry still provide the most effective approach to efficient drug discovery (Amzel 1998). If a crystal structure for the N-terminal domain of worm Hsp90 can be solved, it should provide valuable new insights into the nature of the unusual nucleotide-binding pocket of chaperone and aid in the synthesis of new, potentially more effective inhibitors of this exciting therapeutic target.
Acknowledgments
We thank Ms Marcie Fritz for secretarial assistance and Dr Sue Robertson (University of Arizona) for help with protein structure predictions. We thank Drs David Smith and Greg Nelson (Mayo Clinic, Scottsdale, AZ, USA) for helpful discussions and advice. The work was supported in part by NIH grant CA69537 and an award to E.J.P. from NIH Cancer Biology Training Grant CA09213.
Footnotes
Correspondence to: Luke Whitesell, Department of Pediatrics, Heme/Oncology Section, Room 5341, Arizona Health Sciences Center, 1501 N. Campbell Avenue, Tucson, AZ 85724, USA. Tel: 520-626-4851; Fax: 520-626-6986; whitelj@peds.arizona.edu
REFERENCES
- Amzel LM. Structure-based drug design. Curr Opin Biotechnol. 1998;9:366–369. doi: 10.1016/s0958-1669(98)80009-8. [DOI] [PubMed] [Google Scholar]
- Bagatell R, Khan O, Paine-Murrieta G, Taylor CW, Akinaga S, Whitesell L. Destabilization of steroid receptors by Hsp90-binding drugs: a ligand independent approach to hormonal therapy of breast cancer. Clin Cancer Res. 2001;7:2076–2084. [PubMed] [Google Scholar]
- Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. doi: 10.1126/science.1066648. [DOI] [PubMed] [Google Scholar]
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen SP. Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of hsp90-dependent signaling proteins. Mol Cell Biol. 1998;18:3330–3339. doi: 10.1128/mcb.18.6.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co—a holding for folding. TIBS. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Chadli A, Bouhouche I, Sullivan WP, Stensgard B, McMahon N, Catelli MG, Toft DO. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc Natl Acad Sci U S A. 2000;97:12525–12529. doi: 10.1073/pnas.220430297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasova V, Ayyadevara S, Egilmez N, Reis RS. Diverse Caenorhabditis elegans genes that are upregulated in dauer larvae also show elevated transcript levels in long-lived, aged, or starved adults. J Mol Biol. 2000;300:433–448. doi: 10.1006/jmbi.2000.3880. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Prohaskka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Rubin GM. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Dalley BK, Golomb M. Gene expression in the Caenorhabditis elegans dauer larva: developmental regulation of Hsp90 and other genes. Dev Biol. 1992;151:80–90. doi: 10.1016/0012-1606(92)90215-3. [DOI] [PubMed] [Google Scholar]
- Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25:24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- Grenert JP, Sullivan WP, and Fadden P. et al. 1997 The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 272:23843–23850. [DOI] [PubMed] [Google Scholar]
- Ivy SP, Lugo TG, Bernstein ML, and Smith MA 2002 Evolving molecular and targeted strategies. In: Principles and Practice of Pediatric Oncology, ed Pizzo PA, Poplack DG. Lippincott Williams and Wilkins, Philadelphia, PA, 309–349. [Google Scholar]
- Liu X, Turbyville T, Fritz A, Whitesell L. Inhibition of insulin-like growth factor I receptor expression in neuroblastoma cells induces the regression of established tumors in mice. Cancer Res. 1998;58:5432–5438. [PubMed] [Google Scholar]
- Minami Y, Kimura Y, Kawasaki H, Suzuki K, Yahara I. The carboxy-terminal region of mammalian Hsp90 is required for dimerization and function in vivo. Mol Cell Biol. 1994;14:1459–1464. doi: 10.1128/mcb.14.2.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L, Mimnaugh E, Schulte TW. Hsp90 as an anti-cancer target. Drug Resistance Updates. 1999;2:165–172. doi: 10.1054/drup.1999.0082. [DOI] [PubMed] [Google Scholar]
- Owen BAL, Sullivan WP, Felts SJ, Toft DO. Regulation of heat shock protein 90 (Hsp90) ATPase activity by sequences in the carboxyl terminus. J Biol Chem. 2002;277:7086–7091. doi: 10.1074/jbc.M111450200. [DOI] [PubMed] [Google Scholar]
- Panaretou B, Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB. The Hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–431. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Riddle DL 1988 The dauer larva. In: The Nematode Caenorhabditis Elegans, ed Wood EB. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 393–412. [Google Scholar]
- Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Scheibel T, Weikl T, Buchner J. Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc Natl Acad Sci U S A. 1998;95:1495–1499. doi: 10.1073/pnas.95.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soti C, Racz A, Csermely P. A nucleotide-dependent molecular switch controls ATP binding at the C-terminal domain of Hsp90. J Biol Chem. 2002;277:7066–7075. doi: 10.1074/jbc.M105568200. [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an hsp90-geldanamycin complex: targeting of a protein chaperone by an anti-tumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein Hsp90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Sutphin PD, Pulcini EJ, Martinez JD, Cook PH. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an Hsp90-binding agent. Mol Cell Biol. 1998;18:1517–1524. doi: 10.1128/mcb.18.3.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Schneider C, Hartl F. In vitro evidence that Hsp90 contains two independent chaperone sites. FEBS Lett. 1997;418:139–143. doi: 10.1016/s0014-5793(97)01363-x. [DOI] [PubMed] [Google Scholar]