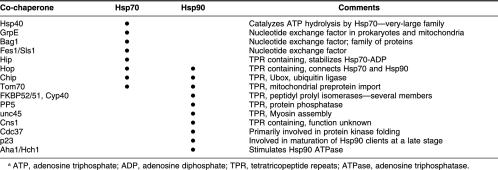
This and the following three issues of Cell Stress and Chaperones will contain several minireviews, each dedicated to a specific co-chaperone of Hsp70 or Hsp90. As regular readers of this journal may already know, there are many different nonclient proteins that bind to Hsp70 or Hsp90 (or both) (Table 1). However, it is generally unclear what they are all doing and how they fit into a pathway of chaperone action that facilitates the folding of a transcription factor or a protein kinase. Co-chaperones are nonclient-binding partners of Hsp90 or Hsp70 and may be loosely defined as proteins that participate in the function of other chaperones. They may themselves have chaperone activity, as judged by their ability to prevent polypeptide aggregation, and thus bind to Hsp70 or Hsp90 and the client simultaneously. Some co-chaperones do not interact with client polypeptides and have a regulatory function in chaperone action. This function may be catalyzing nucleotide binding or hydrolysis or physically linking Hsp90 with Hsp70, as in the case of Hop-Sti1. From a functional perspective, therefore, co-chaperones mediate the specificity of a chaperone reaction by choosing the client, presenting it to Hsp70 or Hsp90, and then coordinating the cycle of binding and release by Hsp70 or Hsp90 in a manner that facilitates polypeptide folding or protein disassembly. Individual co-chaperones can perform some or all of these tasks. Although it is likely that more than 100 different co-chaperones exist in mammals, most of them fall into 2 distinct classes based on their domain architecture. These are the “J”-domain found in Hsp40 co-chaperones of Hsp70 and the tetratricopeptide repeats (TPR) found in co-chaperones that interact with Hsp70 or Hsp90. In mammalian genomes, there are more than 70 different J-domain proteins and several hundred TPR domain proteins, although not all of these will interact with Hsp70 or Hsp90 because many TPRs also mediate protein-protein interactions independent of chaperones (Blatch and Lassle 1999). The evolution of these J-domain and TPR protein families in complex organisms reflects an increase in the range of biological processes that require Hsp70 or Hsp90 action. The co-chaperones having these more specialized functions appear to have adopted J-domains or TPR domains as a means of incorporating themselves into the established chaperone machineries of Hsp70 and Hsp90.
Table 1.
Hsp70 and Hsp90 co-chaperonesa
Initial characterization of Hsp70 co-chaperones as regulators of chaperone action was derived from studies in the Georgopoulos laboratory, whose analysis of DnaJ (a prokaryotic member of the Hsp40 family) and GrpE revealed that these act on the adenosine triphosphatase (ATPase) cycle of DnaK (as the major Escherichia coli Hsp70 is known). DnaJ had the ability to catalyze the hydrolysis of adenosine triphosphate (ATP) by DnaK, whereas GrpE catalyzed nucleotide exchange (Liberek et al 1991). The relevance of these findings remained largely unclear until the relationship between nucleotide dynamics in DnaK and the peptide binding and release cycles was understood. Furthermore, in a landmark paper from the Hartl laboratory, DnaJ was shown to have chaperone activity of its own (Langer et al 1992). These findings have been incorporated into a generally accepted model wherein polypeptides bind to DnaJ, which recruits DnaK. The polypeptide is then transferred to DnaK, and this interaction is stabilized by the ability of DnaJ to catalyze the formation of DnaK–adenosine diphosphate (ADP), which has a higher affinity for unfolded polypeptides than DnaK-ATP. The role of GrpE is to bind to DnaK and to effect nucleotide exchange, thereby weakening the DnaK-polypeptide interaction resulting in complex dissociation. This sequence of events, which has become a paradigm for co-chaperone action, is essential for Hsp70 function in protein folding (Frydman 2001). The finding that DnaJ (Hsp40) has evolved into an abundant and diverse protein family in eukaryotes underscores the generally accepted notion that these proteins provide the necessary specificity to the chaperone reaction by selecting the clients whose conformation is subsequently altered as a result of interaction with Hsp70. Furthermore, the Hsp70 ATPase cycle is regulated with greater complexity in higher eukaryotes; for example, the co-chaperone Hip stabilizes Hsp70-ADP in mammalian systems (Hohfeld et al 1995).
Those studying Hsp90 find the complement of co-chaperones to be quite different. This is undoubtedly because the Hsp90 mechanism of action is different from that of Hsp70 (Pearl and Prodromou 2001). Hsp90 only appears to function in the folding of a narrow range of protein families represented largely by transcription factors (such as nuclear receptors) and protein kinases.
Hsp90 co-chaperones can be loosely divided into those that contain TPR domains (which may also interact with Hsp70) and those that do not. TPR domain co-chaperones function in a wide variety of biological processes because they usually contain additional domains that catalyze reactions as diverse as ubiquitin ligation and peptidyl-prolyl isomerization. Non–TPR-containing co-chaperones include p23, Cdc37, and Aha1/Hch1. These co-chaperones are structurally diverse and are characterized as having few paralogs. For example, there are only 2 members of the p23 and Cdc37 families. Recent efforts to characterize the biochemical properties of Hsp90 co-chaperones have largely focused on 2 parameters: their abilities to bind to clients and their roles in regulating the relatively weak ATPase of Hsp90.
One unresolved paradox lies in the apparent absence of co-chaperones in the endoplasmic reticulum that interact with the luminal paralog of Hsp90, Grp94. No homologs of p23- or TPR-containing co-chaperones have been identified so far. Furthermore, Grp94 lacks the conserved EEVD motif at its C-terminus, which would mediate interaction with TPR-containing co-chaperones, and has instead an ER retention motif. Although it is possible that a completely different subset of co-chaperones perform functions similar to their cytosolic counterparts, these have yet to be identified. Alternatively, the organization of co-chaperones with respect to cytosolic and luminal Hsp90s might differ. For example, cyclophilin 40 has TPR motifs and binds directly to cytosolic Hsp90. On the other hand, the luminal cyclophilin B, which lacks TPR motifs, interacts with a large multichaperone complex containing Grp94, although this binding may be indirect (Meunier et al 2002). By contrast, several proteins with J-domains have been identified and shown to interact with the luminal Hsp70. So, are co-chaperones essential for Hsp90 function? For Grp94 at least, we shall have to wait and see.
The question of whether all co-chaperones have been identified is still open. Although it is possible to identify TPR domain proteins in genome databases, it is impossible to discern which will bind to Hsp90 or Hsp70 based on the sequence alone. Also, novel proteins that bind to Hsp70 and Hsp90 may await discovery. Finally, it is perhaps worth noting that some co-chaperones can act independently of Hsp90, with p23 being a prime example (Freeman and Yamamoto 2002). This behavior calls into question the view that co-chaperones are always subordinate to the function of Hsp90 or Hsp70. Whether this is a general property of co-chaperones remains to be determined.
Acknowledgments
I thank Drs J. Brodsky, M. Cheetham, and D. Cyr for their comments on the manuscript.
REFERENCES
- Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- Hohfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Langer T, Lu C, Echols H, Flanagan J, Hayer MK, Hartl FU. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L, Usherwood YK, Chung KT, Hendershot LM. A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol Biol Cell. 2002;13:4456–4469. doi: 10.1091/mbc.E02-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure, function, and mechanism of the Hsp90 molecular chaperone. Adv Protein Chem. 2001;59:157–186. doi: 10.1016/s0065-3233(01)59005-1. [DOI] [PubMed] [Google Scholar]