Abstract
p23 is a small but important cochaperone for the Hsp90 chaperoning pathway. It appears to facilitate the adenosine triphosphate–driven cycle of Hsp90 binding to client proteins. It enters at a late stage of the cycle and enhances the maturation of client proteins. Although this role of p23 is fairly well established, recent studies suggest that it may have additional functions in the cell that merit further exploration.
INTRODUCTION
Early studies to characterize Hsp90 chaperone complexes revealed a number of associated proteins. Two of these proteins, Hop and p23, were thought to participate directly in the chaperoning process and have often been referred to as cochaperones of Hsp90. These plus Hsp70 and its cochaperone, Hsp40, join with Hsp90 to make a minimal in vitro system that is capable of chaperoning client proteins as diverse as steroid receptors and hepatitis B virus polymerase to their active states (Richter and Buchner 2001; Young et al 2001; Pratt and Toft 2003).
p23 is the smallest protein in the Hsp90 machine (Mr = 18 000–25 000), with a relatively simple structure (Weikl et al 1999; Weaver et al 2000). However, after several years of study, its functions and significance are still not totally clear. p23 is a ubiquitous and highly conserved protein from yeast to humans, with probable homologs in plants (Garcia-Ranea et al 2002). It was first discovered as part of the complex of Hsp90 with the progesterone receptor (Johnson et al 1994). However, it has been found in complexes with a variety of Hsp90 clients, including the Fes tyrosine kinase (Nair et al 1996), the heme-regulated kinase HRI (Xu et al 1997), the transcription factor Hsf1 (Nair et al 1996), the Ah receptor (Nair et al 1996), and polymerases such as telomerase (Holt et al 1999) and hepadnavirus reverse transcriptase (Hu et al 2002). Whether p23 itself interacts with these client proteins or only with Hsp90 is not clear. In vitro, p23 is able to bind specifically to 1 conformational state of Hsp90, but its actions may not stop there. It has passive chaperoning activity in that it can suppress the aggregation of denatured proteins (Bose et al 1996; Freeman et al 1996), and some studies indicate that it may continue to act on client proteins that have been released from Hsp90 (Freeman et al 2000; Freeman and Yamamoto 2002). Recently, p23 has been claimed to possess a prostaglandin synthase activity (Tanioka et al 2000), and the core-folding motif of p23 has been shown in a number of proteins that lack any known relationship to chaperoning (Garcia-Ranea et al 2002). Thus, the range of possible activities for this simple protein is surprisingly large. In this review, we will assess our present state of knowledge on the structure and functions of this Hsp90 cochaperone.
STRUCTURE
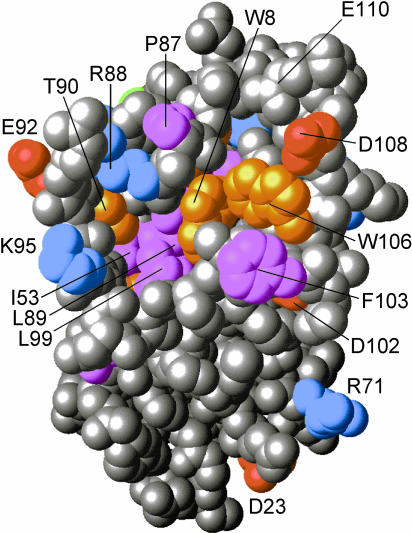
A crystal structure has been solved for human p23 lacking 35 amino acids at the carboxyl terminus (Weaver et al 2000). Although p23 is likely a monomer in solution, a disulfide-linked dimer was characterized, in which each subunit contained 8 β-strands in a compact, antiparallel sandwich encompassing residues 1–110 of the protein. Alternating hydrophobic side chains from the opposing sheets formed a hydrophobic core centered on the ring stacking of Phe19 and Phe38. Residues 86–92 (WPRLTKE), previously recognized as a p23 signature, were found to be contained in an extensive but well-defined loop between β strands 7 and 8. Stabilization of this loop and the β8-strand asymmetrically distributed the conserved residues to 1 face of the p23 molecule (see Fig 1). Conserved residues Phe103 and Trp106 were exposed on this face, as was a solvent-accessible cavity surrounded by conserved residues Lys95, Thr90, Arg88, Pro87, and Trp8 and having an apolar floor of conserved residues Ile53, Leu89, and Leu99. Mutation studies indicate that this conserved face of p23 is involved in its binding to Hsp90 (W. Sullivan and D. Toft, personal communication). The region C-terminal to residue 110 (ie, 110–160) appeared to be unstructured, and this was consistent with the biochemical studies by Weikl et al (1999). Based on protease sensitivity and circular dichroism (CD) spectrum analysis, these investigators also determined p23 to have a very stable β-sheet structure with a flexible tail at the C-terminus.
Fig 1.
A model of the conserved face of human p23 (residues 1–110) based on its crystal structure. Highly conserved residues are numbered and in color. The opposing face (not shown) contains few conserved residues. The unstructured C-terminal tail (not shown) extends from residue 110 at the top of the model
When p23 was first discovered, it seemed quite possible that it was a member of the small heat shock proteins that are chaperones of similar size. However, the linear p23 sequence showed little similarity to those of the small heat shock proteins. Surprisingly, a comparison of crystal structures has since revealed highly homologous β-sandwich structures for p23 and the Hsp20-like proteins MjHsp16.5 from Methanococcus jannischii and Hsp16.9B from wheat (Weaver et al 2000; van Montfort et al 2001). These molecules have more recently been suggested by multiple sequence alignment to contain the most ancient conserved residues of what may be a larger family of proteins that vary in size and in purpose but contain this folding motif as part of their structure (Garcia-Ranea et al 2002). The highly conserved loop of p23 aligns well with the β6-containing loop in the small heat shock proteins that, along with a flexible C-terminus, forms “arms” that interact with the neighboring dimer to form the dodecameric structure of MiHsp16.5. It has been postulated that heat shock causes disassembly of the dodecamer into dimers and thus releases these arms for chaperone functions. Like p23, the flexible C-terminus of the small heat shock proteins contains many charged residues, and these have been suggested to be important in promoting solubility when complexed with denatured proteins (Pasta et al 2002). We have found that the C-terminal tail of p23 is phosphorylated at 3 serine residues, which would enhance its ionic character (W. Sullivan and D. Toft, personal communication). Removal of the C-terminal tail of p23 blunts its chaperoning activity in either the passive chaperoning of denatured proteins or in the cooperative chaperoning of the progesterone receptor (Weikl et al 1999; Weaver et al 2000). However, the core structure of p23 is sufficient for binding to Hsp90 (Weikl et al 1999; Weaver et al 2000). Thus, an Hsp20-like model might be drawn for p23, in which the face of p23 containing the β7-β8 loop might cooperate with the mobile C-terminus to work with (and bind to) Hsp90 in order to chaperone a client protein.
FUNCTIONAL STUDIES IN VITRO
Binding to Hsp90
Although p23 is clearly a component of steroid receptor complexes with Hsp90, it has also been shown to exist in cell lysates in a relatively abundant complex with Hsp90 that is apparently not bound to clients (Johnson and Toft 1994). A key toward understanding the interactions of p23 was the observation that its presence in Hsp90 complexes was dependent on adenosine triphosphate (ATP) and was inhibited by the addition of the Hsp90 inhibitor, geldanamycin (Johnson and Toft 1994). Later, with purified proteins p23 was shown to bind directly to Hsp90 but only when Hsp90 was in a conformational state dictated by bound ATP (Sullivan et al 1997). This provided an in vitro assay for p23-Hsp90 interactions, and, more importantly, it indicated a role for nucleotides in the regulation of Hsp90 structure and function, thus stimulating a number of studies on the binding and hydrolysis of ATP by Hsp90, activities that have been shown to be essential for some Hsp90 functions (reviewed in Young et al 2001). Recent studies have confirmed that Hsp90 binds ATP and undergoes a conformational change where additional dimer contacts form near the N-terminus (Prodromou et al 2000). At this stage, the ATP becomes trapped by Hsp90 and committed toward hydrolysis (Weikl et al 2000; Sullivan et al 2002). p23 binds Hsp90 somewhere in this step and clearly stabilizes this conformational state (see Fig 2). The exact site of p23 binding on Hsp90 is unknown. p23 binding requires the ATP-binding domain of Hsp90 (residues 1–220) but not the adjacent highly charged region. However, an additional 200 residues beyond the charged region are needed for p23 binding, and this must be in a dimer configuration (Chadli et al 2000). Smaller fragments of Hsp90 do not bind p23, presumably because they cannot assume the proper conformational state. In a series of washout experiments, Sullivan et al (2002) showed that after the removal of nucleotide, the ATP-induced state of Hsp90 slowly dissipated with a half-life of ∼45 minutes at 30°C. This half-life was extended markedly by p23 even though p23 bound Hsp90 with rapid on and off rates (t1/2 ∼ 2 minutes). Within the context of this simplified system, it is still unclear whether p23 affects Hsp90 ATPase activity. Recent work by Panaretou et al (2002) showed that yeast p23 (Sba1) could inhibit yeast Hsp90, albeit weakly. Interestingly, yeast or human Hsp90 ATPase was stimulated by the activator Aha1, and the stimulated level of hydrolysis was inhibited quite effectively by yeast p23. In a somewhat parallel fashion, human p23 has been shown to suppress human Hsp90 ATPase activity, particularly when that activity has been stimulated by the addition of client protein (McLaughlin et al 2002). Obviously, there are many details yet to be elucidated, and a better understanding of these dynamic events and their biologic significance will likely require the integration of data from experiments using the client protein and including other cochaperones.
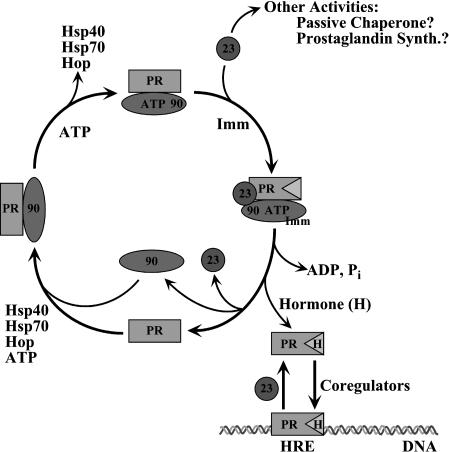
Fig 2.
Multiple roles of p23. The central model shows the assembly of chaperones with the progesterone receptor (PR), emphasizing the adenosine triphosphate–dependent cycle of Hsp90 interactions. This leads to the formation of the hormone-binding site on the PR, a step that is enhanced by p23 and accompanied by the binding of immunophilins (Imm). p23 may also dissociate or mobilize receptors that are bound to hormone response elements (HRE) and active in transcriptional regulation (lower right). Other possible activities of p23 are passive chaperoning and a role in prostaglandin synthesis
Chaperoning of client proteins
Only a few client proteins have been studied in vitro regarding their chaperoning by Hsp90 (and thus p23). Early studies on steroid receptor complexes indicated that p23 stabilized the mature complex with Hsp90, where the receptor was active and able to bind hormones (Richter and Buchner 2001; Young et al 2001; Pratt and Toft 2003). As outlined above, this model became clearer when studies showed that p23 stabilizes the ATP-induced conformational state of Hsp90. A purified system of 5 proteins (Hsp40, Hsp70, Hop, Hsp90, and p23) is able to chaperone steroid receptors to their active state (Pratt and Toft 2003). All these proteins are needed for efficient chaperoning; however, some chaperoning activity remains in the absence of p23 (Dittmar et al 1997). This is consistent with genetic studies in yeast demonstrating that p23 is important but not essential for the activity of Hsp90 clients (Bohen 1998; Fang et al 1998; Knoblauch and Garabedian 1999). More recently, studies have demonstrated chaperoning of hepadnavirus reverse transcriptase with this system (Hu et al 2002). In what appears to be a situation similar to that of steroid receptors, p23 was not absolutely essential for reconstitution of in vitro transcriptase activity (protein priming), but p23 enhanced the kinetics of in vitro reconstitution. Curiously, it is still possible that not all Hsp90 client proteins will require p23 chaperoning. For example, the in vitro folding of heat-denatured firefly luciferase can be accomplished with the help of Hsp40 and Hsp70. Although luciferase is not known to be an Hsp90 client, in this system Hsp90 and Hop enhance the chaperoning process (Johnson et al 2000), but p23 is without effect (B. Johnson and D. Toft, personal communication).
In the example of steroid receptors, p23 enhances the abundance of complexes that are able to bind hormone. This enhancing activity is consistent with the stabilization effects of p23 on the ATP-charged state of Hsp90. Mutants of Hsp90 that are unable to bind ATP, hydrolyze ATP, and/or bind p23 are also poor at chaperoning client proteins (Grenert et al 1999; Young and Hartl 2000). But p23 probably also contributes to the chaperoning process in other ways. C-terminal truncations of p23 that can still bind Hsp90 are less able to confer full hormone-binding activity to the receptor (Weaver et al 2000). These truncated p23 molecules are also less active as passive chaperones (see below), suggesting that the C-terminus of p23 may facilitate receptor folding. However, it is equally likely that p23 may facilitate structural movements in Hsp90. Young and Hartl (2000) found that the dissociation of stable receptor-Hsp90 complexes could be promoted by the addition of ATP and p23, and this release of client protein required the ATPase activity of Hsp90. These authors thus suggested that p23 couples the ATPase activity of Hsp90 to the release of client proteins.
Passive chaperoning
Several molecular chaperones, including Hsp90 and some of its cochaperones, are able to bind denatured proteins and suppress their aggregation in a passive or ATP-independent manner. p23 has been shown to suppress the aggregation of heat-denatured citrate synthase (Bose et al 1996) and to hold denatured β-galactosidase in a folding-competent state (Freeman et al 1996). This passive chaperoning activity requires both the core structure and the flexible tail of p23 (Weikl et al 1999; Weaver et al 2000). These results suggest that p23 may function to interact with the client proteins in Hsp90 complexes. However, one should be cautious about this conclusion. Although passive chaperoning assays are used frequently as a measure of chaperoning capacity, in each case supporting evidence should be sought to verify the biological significance of such assay results. Direct binding of p23 to Hsp90 client proteins has not yet been clearly demonstrated. At the late stage of chaperoning, when p23 enters the Hsp90 complex, most client proteins are probably in a near-native state and may not require such passive chaperoning. Nevertheless, as discussed above, it is possible that the ability of p23 to interact with unfolded proteins might be crucial to its potential function during conformational transitions of either Hsp90 or the client protein at the latter stages of client protein folding.
CELLULAR STUDIES
Although many details of the role of p23 in the Hsp90 chaperone pathway remain to be elucidated, cellular studies confirm that p23 increases the effectiveness of at least a few key client proteins. The role of p23 in cell-signaling kinase pathways has not been well studied, and the available data are not very clear. Yeast lacking p23 are also compromised in regulating the amino acid biosynthetic pathway because the regulatory kinase Gcn2 is an Hsp90 client protein (Donzé and Picard 1999). Yet, p23 disruption in yeast only has a modest effect on v-src activity (Fang et al 1998). In parallel to in vitro studies with steroid receptors, a model for p23 function in the regulation of steroid receptors dominates the in vivo literature. Observations that geldanamycin treatment of transfected COS cells decreased the amount of Hsp90-p23–bound progesterone receptors in favor of Hsp90-Hop-Hsp70 intermediate complexes confirmed the stepwise nature of receptor complex formation suggested by studies in reticulocyte lysate (Smith et al 1995). All these studies pointed toward the hypothesis that p23 functioned late in steroid receptor chaperoning.
Cloning of the Saccharomyces cerevisiae ortholog of p23 (SBA1) put into play a number of key experiments that have since revealed many subtleties of p23 function. Although the function of p23 in yeast biology remains poorly characterized, the use of various yeast strains expressing mammalian steroid receptors set the stage for some interesting studies of this class of client proteins and, thus, developed the chaperone pathway model even further. In yeast cells expressing a mammalian steroid receptor, treatment with the geldanamycin-related drug, macbecin, caused an inhibition of 3H-dexamethasone binding (Bohen 1998). Deletion of the yeast p23 gene was not lethal to the cells and caused gene expression dependent on glucocorticoid receptor (GR) (Bohen 1998) or androgen receptor (Fang et al 1998) to be lowered only slightly. Yet, in the p23-deleted strain, hormone-inducible lacZ reporter gene expression was more sensitive to macbecin treatment, again pointing toward p23 functioning late in receptor action (Bohen 1998). In a third yeast study, Knoblauch and Garabedian (1999) showed that overexpression of p23 increased estradiol binding and transcriptional activation by the estrogen receptor (ER). Green fluorescent protein (GFP) tagged p23 colocalized to the nucleus with ER. Interestingly, a mutant ER with decreased hormone-binding activity was made much more active and was localized to the yeast nucleus when p23 was overexpressed. Treatment of the yeast with estradiol caused the GFP-p23 to be redistributed to the cytoplasm, presumably as a consequence of its release from ER complexes. In total, these yeast studies reiterated the hypothesis that p23 acts late in receptor function, regulating hormone binding and/or transcriptional activation.
The most recent studies of p23 function have tested the hypothesis that p23 may have an Hsp90-independent role in the regulation of gene expression by intracellular receptors. In a very complex series of experiments, Freeman et al (2000) showed that (1) the activities of various intracellular receptors (in both yeast and mammalian cells) were affected differently by coexpression of p23: the activities of GR and progesterone receptor (PR) were enhanced by p23, whereas the activities of mineralocorticoid receptor, ER, and thyroid hormone receptor (TR) were suppressed; (2) nonetheless, p23 exerted its effect on each of the receptors at the level of ligand efficacy (ie, the level of maximal transcriptional activation); and (3) p23 stimulated hormone binding (ie, ligand potency) of only PR and ER. It is difficult to explain these selective results on the basis of only p23 activity as a cochaperone for Hsp90. Freeman et al (2000) suggested that p23 has a second, more sustained, role in modulating the activity of receptors in the nucleus. This was supported by observations that in vitro, p23 could inhibit the binding of TR to DNA and that this inhibition was relieved by a peptide representing an interaction site of the receptor coactivator GRIP1.
In follow-up studies in mammalian cells, Freeman and Yamamoto (2002) presented data that suggest that p23 (and, to a lesser extent, Hsp90) may be localized to the chromatin of hormone-responsive genes and there function, in a very dynamic way, to facilitate the disassembly of transcriptional regulatory complexes. This localization of p23 may enhance the mobility of functional receptors, allowing the cell to respond quickly to changes in transcriptional needs. The authors suggested that this chaperone function may apply to other signal transduction systems where a rapid response to changes in external stimuli is required (see also Freeman and Yamamoto 2001; Morimoto 2002).
SUMMARY
Studies to date clearly show p23 to have a more universally applicable structure than was initially appreciated, and many experimental approaches have firmly established p23 to be an important modulator of Hsp90 activity. In this review we have focused on the chaperone-related activities of p23, particularly as a cochaperone for Hsp90. However, a recent study indicates that p23, surprisingly, may participate in prostaglandin synthesis (Tanioka et al 2000). In this study both cellular and purified recombinant p23 were shown to have glutathione-dependent prostaglandin E2 synthase activity. This enzyme activity is difficult to understand within the background of the more established functions of p23, but further studies may reveal a new dimension to the cellular functions of p23. The role of p23 as a cochaperone for Hsp90 has been tested only with a few Hsp90 client proteins. Although it seems likely that p23 is a general cochaperone for most Hsp90 activities, the studies of Freeman et al (2000) and Freeman and Yamamoto (2002) indicate that its actions may be more complex and in some ways selective. Thus, there is growing evidence that p23 may have biological functions on its own. It may assist in handling the cell's load of partially unfolded proteins. It may participate in the disassembly of some transcription complexes. Further investigations are clearly needed to realize the full significance of p23 in cellular processes.
REFERENCES
- Bohen SP. Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins. Mol Cell Biol. 1998;18:3330–3339. doi: 10.1128/mcb.18.6.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Weikl T, Bügl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. doi: 10.1126/science.274.5293.1715. [DOI] [PubMed] [Google Scholar]
- Chadli A, Bouhouche I, Sullivan W, Stensgard B, McMahon N, Catelli MG, Toft DO. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc Natl Acad Sci U S A. 2000;97:12524–12529. doi: 10.1073/pnas.220430297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. J Biol Chem. 1997;272:21213–21220. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- Donzé O, Picard D. Hsp90 binds and regulates GCN2, the ligand-inducible kinase of α subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 1999;19:8422–8432. doi: 10.1128/mcb.19.12.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Fliss AE, Rao J, Caplan A. SBA1 encodes a yeast Hsp90 cochaperone that is homologous to vertebrate p23 proteins. Mol Cell Biol. 1998;18:3727–3734. doi: 10.1128/mcb.18.7.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Felts SJ, Toft DO, Yamamoto KR. The molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 2000;14:422–434. [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Continuous recycling: a mechanism for modulatory signal transduction. Trends Biochem Sci. 2001;26:285–290. doi: 10.1016/s0968-0004(01)01834-5. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- Garcia-Ranea JA, Mirey G, Camonis J, Valencia A. p23 and Hsp20/α-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett. 2002;529:162–167. doi: 10.1016/s0014-5793(02)03321-5. [DOI] [PubMed] [Google Scholar]
- Grenert JP, Johnson BD, Toft DO. The importance of ATP binding and hydrolysis by Hsp90 in formation and function of protein heterocomplexes. J Biol Chem. 1999;274:17525–17533. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
- Holt SE, Aisner DL, and Baur J. et al. 1999 Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 13:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Toft DO, Anselmo D, Wang X. In vitro reconstitution of functional hepadnavirus reverse transcriptase with cellular chaperone proteins. J Virol. 2002;76:269–279. doi: 10.1128/JVI.76.1.269-279.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Chadli A, Felts SJ, Bouhouche I, Catelli MG, Toft DO. Hsp90 chaperone activity requires the full-length protein and interaction among its multiple domains. J Biol Chem. 2000;275:32499–32507. doi: 10.1074/jbc.M005195200. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Beito TG, Krco CJ, Toft DO. Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol Cell Biol. 1994;14:1956–1963. doi: 10.1128/mcb.14.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Toft DO. A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J Biol Chem. 1994;269:24989–24993. [PubMed] [Google Scholar]
- Knoblauch R, Garabedian MJ. Role for Hsp90-associated cochaperone p23 in estrogen receptor signal transduction. Mol Cell Biol. 1999;19:3748–3759. doi: 10.1128/mcb.19.5.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SH, Smith HW, Jackson SE. Stimulation of the weak ATPase activity of human Hsp90 by a client protein. J Mol Biol. 2002;315:787–798. doi: 10.1006/jmbi.2001.5245. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Dynamic remodeling of transcription complexes by molecular chaperones. Cell. 2002;110:281–284. doi: 10.1016/s0092-8674(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgall TE, Smith DF. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretou B, Siligardi G, and Meyer P. et al. 2002 Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol Cell. 10:1307–1318. [DOI] [PubMed] [Google Scholar]
- Pasta SY, Raman B, Ramakrishna T, Rao CM. Role of the C-terminal extensions of α-crystallins. J Biol Chem. 2002;277:45821–45828. doi: 10.1074/jbc.M206499200. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the Hsp90/Hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Panaretou B, and Chohan S. et al. 2000 The ATPase cycle of hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 19:4383–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimerman RA. Progesterone receptor structure and function altered by geldanamycin, an Hsp90-binding agent. Mol Cell Biol. 1995;15:6894–6912. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan WP, Owen BAL, Toft DO. The influence of ATP and p23 on the conformation of Hsp90. J Biol Chem. 2002;277:45942–45948. doi: 10.1074/jbc.M207754200. [DOI] [PubMed] [Google Scholar]
- Sullivan WP, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri ES, Litwack G, Toft D. Nucleotides and two functional states of hsp90. J Biol Chem. 1997;272:8007–8012. doi: 10.1074/jbc.272.12.8007. [DOI] [PubMed] [Google Scholar]
- Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Weaver AJ, Sullivan WP, Felts SJ, Owen BAL, Toft DO. Crystal structure and activity of human p23, a heat shock protein 90 co-chaperone. J Biol Chem. 2000;275:23045–23052. doi: 10.1074/jbc.M003410200. [DOI] [PubMed] [Google Scholar]
- Weikl T, Abelmann K, Buchner J. An unstructured C-terminal region of the Hsp90 co-chaperone p23 is important for its chaperone function. J Mol Biol. 1999;293:685–691. doi: 10.1006/jmbi.1999.3172. [DOI] [PubMed] [Google Scholar]
- Weikl T, Muschler P, Richter K, Veit T, Reinstein J, Buchner J. C-Terminal regions of Hsp90 are important for trapping the nucleotide during the ATPase cycle. J Mol Biochem. 2000;303:583–592. doi: 10.1006/jmbi.2000.4157. [DOI] [PubMed] [Google Scholar]
- Xu Z, Pal JK, Thulsairaman V, Hahn HP, Chen JJ, Matts RL. The role of the 90-kDa heat shock protein and its associated cohorts in stabilizing the heme-regulated eIF-2α kinases in reticulocyte lysates during heat stress. Eur J Biochem. 1997;246:461–470. doi: 10.1111/j.1432-1033.1997.t01-1-00461.x. [DOI] [PubMed] [Google Scholar]
- Young JC, Hartl FU. Polypeptide release by Hsp90 involves ATP hydrolysis and is enhanced by the co-chaperone p23. EMBO J. 2000;19:5930–5940. doi: 10.1093/emboj/19.21.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]