Summary
Alopecia areata (AA) development is associated with both innate and adaptive immune cell activation, migration to peri-and intra-follicular regions, and hair follicle disruption. Both CD4+ and CD8+ lymphocytes are abundant in AA lesions; however, CD8+ cytotoxic T lymphocytes are more likely to enter inside hair follicles, circumstantially suggesting that they have a significant role to play in AA development. Several rodent models recapitulate important features of the human autoimmune disease and demonstrate that CD8+ cytotoxic T lymphocytes are fundamentally required for AA induction and perpetuation. However, the initiating events, the self-antigens involved, and the molecular signaling pathways, all need further exploration. Studying CD8+ cytotoxic T lymphocytes and their fate decisions in AA development may reveal new and improved treatment approaches.
Keywords: Alopecia areata, Pathogenesis, Lymphocytes, Hair follicles, Autoimmune inflammation, Treatment
Introduction
Alopecia areata (AA) is a common, non-scarring hair disorder involving sudden loss of hair with spontaneous remission, reoccurrence, and exacerbation [1]. Human AA typically presents as patchy hair loss in the early acute phase, but with time, it can progress to the loss of all scalp hair (alopecia totalis) or body hair (alopecia universalis) in subsets of affected individuals [1]. Hair loss affected areas of skin are smooth with natural color, or occasionally slightly pink. The hair fibers at the border of AA patches may be short, broken “exclamation mark” hair fibers with a broader distal end compared to the proximal end [1,2]. Nail pitting and longitudinal ridging abnormalities can also be observed in around 17% of AA patients [3]. The lifetime incidence of AA is greater than 1% in most populations; in a recent analysis the rate in North America was calculated at 2.1% [4]. It is one of the more common hair loss diseases accounting for 25% of all dermatology clinic alopecia cases [5,6]. The distribution of AA across both sexes and different races is believed to be generally comparable [6]. The course of the disease is unpredictable and no treatment is curative or preventive [7]. Consequently, the onset of disease can be emotionally disturbing, despite it being non-life threatening, and mental health comorbid conditions (depression, anxiety) are common in AA patients [8]. AA is likely associated with other autoimmune diseases including thyroid disorders, anemia, and psoriasis, though the true correlative statistical significance remains somewhat controversial [9–12]. More consistently demonstrated in epidemiology studies is a high prevalence of atopy (allergic rhinitis, asthma, and/or eczema) among individuals with AA [13,14].
Substantial progress in basic and clinical immunology research suggests that AA is a CD8+ cell, Th1-type autoimmune reaction against anagen stage hair follicles [15]. Many other cell types, including keratinocytes [16], fibroblasts [17], mast cells [18] and dendritic cells [19–21] also contribute to AA pathogenesis, coordinating with T cells. Immune activity is particularly enriched in the skin [22] and discrete populations of resident and recirculating memory T cells with differing territories of migration and distinct functional activities have been identified [23]. In this review we set out to establish the principle mechanisms by which lymphocytes, particularly CD8+ cytotoxic T lymphocytes (CTLs), may contribute to AA pathogenesis and propose potential approaches through which lymphocytes can be modulated to curb the development of this common autoimmune disease.
Basic evidence in support of a lymphocyte mediated disease mechanism
Genetic studies, including single-nucleotide polymorphisms (SNPs) and genome-wide association (GWAS) investigations, demonstrate that AA is a polygenic disorder involving a complex interaction of environmental and genetic factors [24]. Most of the identified GWAS loci implicate genes with innate and adaptive immune function and several have previously been linked to other autoimmune diseases [25]. While there is no obvious, clinically visible inflammation in most AA patients’ skin, histopathological examination reveals anagen stage hair follicles to be affected by a peri-and intra-follicular inflammatory cell infiltrate of primarily CD4+ and CD8+ cells [26]. In association with active disease, there is increased expression of major histocompatibility complex (MHC) class I and II along with increased numbers of antigen presenting cells (APCs) in AA lesions [27]. CD8+ cells readily penetrate to intra-follicular regions while CD4+ cells are located almost exclusively in a peri-follicular location in AA [26,28,29]. As considerable cell destruction in inflammation typically occurs through close association between lymphocytes and target cells, the CD8+ cells are in the most suitable location to disrupt hair follicle growth in AA. Factors expressed by activated CTLs, perforin, granzymes, granulysin and Fas-ligand that trigger programmed cell-death, are all elevated in AA affected hair follicles [30–33]. CTLs in AA can also release tumour necrosis factor alpha (TNFα) and interferon-gamma (IFNγ) which may potentiate cell killing [34]. CD4+ T-cells, the T helper (Th) cells, likely play a critical supporting role for CTLs by means of the cytokines they produce, particularly IL-2 and IFNγ [34,35]. Surprisingly, while follicular inflammation can be quite intense, there is typically no scar tissue formation in AA [36]. Anagen stage hair follicles may enter a telogen resting phase to avoid CTL aggression and permanent destruction. Subsequently, hair follicles may return to anagen if the inflammation subsides [37]. Counteracting follicular inflammation in AA with immunosuppressive drugs can enable hair regrowth [38][8].
The role of lymphocytes in alopecia areata pathogenesis
Hair follicle immune privilege
The current popular hypothesis to explain AA pathogenesis focuses on “immune privilege” (IP) collapse in hair follicles and an associated activation of autoreactive lymphocyte cells [39]. Anagen stage hair follicles have a thick proteoglycan-rich basement membrane that may block the penetration of the hair follicle by immune cells [40]. The lower one third of hair follicles are normally devoid of MHC class I and Class II which are required for the presentation of antigens to the immune system [40–42]. Research also suggests hair follicles may have some ability to block lymphocyte activity through the Fas – Fas ligand system as identified in eye graft models [33,43,44]. Recent studies indicate that the connective tissue sheath cells of hair follilces express PDL1 [45]. PDL1 is significant in feto-maternal tolerance [46–48]; it delivers an inhibitory signal through PD1 on T cells to induce cell anergy [49,50]. In addition, one of the first lines of defense in hair follicle IP likely involves secretory factors. Secreted immune suppressors αMSH, IGF-1, and TGFβ are all expressed in hair follicles [40,51]. Studies also suggest pleiotropic secretory factors such as somatostatin may further help support hair follicle IP [52]. These factors could allow hair follicles to regulate immune cells in the surrounding vicinity in advance of actual contact with hair follicle tissues.
Immune privilege collapse in alopecia areata
Antigenic challenge or activated lymphocyte cell transfer can induce autoimmune disease against most immune privileged sites including the testes [53,54], cornea [55], and pregnant uterus [56]. Any putative hair follicle IP may also similarly be broken by activating the immune system against hair follicle autoantigens. Potentially, focal infection, microtrauma, neurogenic inflammation, or endocrine dysfunction, disrupts hair follicle IP. A deficiency of some immunoregulatory factors in the hair follicle such as red/IK, an antagonist of IFNγ-induced expression of MHC class II antigens [57], may further facilitate IP breakdown. With loss of immune suppressor functions, any ectopic upregulation of MHC class I expression may be recognized by autoreactive CD8+ cytotoxic cells which are intrinsically able to perceive and be activated by very few peptide -MHC class I complexes [58]. The activation of CD8+ cells may potentially lead to a more extensive immune response. Cells disrupted by CD8+ cytotoxic cells release nucleic acids and self-antigens, these complexes can activate APCs promoting T cell priming, additional lymphocyte recruitment, and effector differentiation [31,39,59,60]. Peri-and intra-follicular inflammatory cell infiltration promotes formation of dystrophic anagen stage hair follicles in early AA and later may enforce a chronic inactive telogen state [61].
More fundamental evidence has been amassed in support of an IP collapse mechanism: CD8+ cells, CD4+ cells, and CD56+ cells (NK cells) are rarely seen around anagen hair follicles in healthy skin, however, these cells prominently infiltrate in and around hair follicles in AA lesions [62]. MHC class I is strongly expressed in AA lesions, along with MHC II. Classical upregulators of MHC I expression like interferon (IFN)-γ [31], tumor necrosis factor (TNF)-α, and interleukin (IL)-1β [63] are enhanced in AA lesions and down-modulators of MHC I expression (IL-10, αMSH, IGF-1, TGFβ) are modified in AA lesions as well [18,31,64]. Specific genetic polymorphisms of HLA-DQB1 and HLA-DRB1, which belong to the HLA class II beta chain paralogs, strongly correlate to increased probability of AA onset [65–67].
Furthermore, IFNγ can be used to induce AA in C3H/HeJ mice [68], though this observation has been difficult to replicate [69]. However, mice genetically deficient in IFNγ are resistant to the development of AA [70]. It has also been shown that αMSH, IGF-1, and TGFβ can downregulate IFNγ induced ectopic MHC class I expression in human anagen hair follicles in vitro [71]. A failure of normal MHC suppression in the proximal anagen hair bulbs may render these hair follicle cells susceptible to recognition by CD8+ cytotoxic cells and/or APCs. Loss of hair follicle IP might be an early key event in the pathogenesis of AA. Currently, AA research is challenged with identifying the key inducers of hair follicle IP collapse and “hair follicle -IP guardians” that prevent and/or can restore IP collapse [39].
T cell-mediated immune responses in alopecia areata
Lymphocyte balance between self-tolerance and autoimmunity
T cell-mediated immunity includes priming of naïve T cells, effector functions of activated T CD4+ T helper cells and cytotoxic CD8+ T cells and later, long-term persistence of memory T cells [72]. A predominance of inhibitory over stimulatory signals is required for the maintenance of self-tolerance and conversely, a predominance of stimulatory signals over inhibitory signals is required for effective immune responses to pathogens or for autoimmunity to develop. Autoimmunity results from failure to maintain peripheral tolerance to self-antigens [73]. T cells can oppose or promote autoimmune disease through regulatory and suppressor cells activities, or as helper and cytotoxic effectors, respectively [74]. CTLs can help prevent autoimmune disease by assisting with the elimination of self-reactive cells and self-antigen sources [75]. Breakdown of immune regulatory mechanisms may enable the onset of autoimmunity [76].CTLs can promote autoimmune disease by dysregulated secretion of pro-inflammatory cytokines to skew lymphocyte differentiation profiles and induce inappropriate apoptosis induction of target cells.
Activation of lymphocytes in alopecia areata
Scalp immunohistochemistry reveals that activated CD4+ and CD8+ T cells, APCs and a few neutrophils and mature mast cells, accumulate around anagen stage hair follicles [18]. The CD8+ cells localize to intra-follicular regions in the proximal hair bulb early in acute AA [31,77,78]. CD8+ cells are less numerous than CD4+ T cells, but their ability to penetrate to intra-follicular locations likely enables greater disruption and destruction by CD8+ CTLs. The changes in lymphocyte subsets are not just localized to the skin, but are also reflected at the systemic level in draining lymph nodes and spleens of AA affected mice, and peripheral blood mononuclear cell (PBMC) populations of AA patients [31,35]. With loss of hair follicle IP, infiltrating CTLs can recognize antigenic peptides presented in the context of MHC class I via their TCR [79].
Using a skin graft-induced mouse model and a sequential time course study, rapid changes occur in the immune system several weeks in advance of visible hair loss. APCs are depleted from the skin and accumulate in draining lymph nodes, while proinflammatory cytokine expression increases [80]. Presumably, upon recognition of autoantigens (in this case, hair follicle-associated proteins) presented by APCs, naïve CD8+ T cells differentiate into CTLs and undergo clonal expansion in secondary lymphoid organs; primarily skin draining lymph nodes. As activated effector cells, they migrate to peripheral tissues. Subsequently, but still in advance of overt hair loss, a diffuse lymphocyte infiltrate accumulates in mouse skin, and over time exhibits greater focus on hair follicles [29]. Similarly, the earliest observations on human AA reveal an extravasation from dermal capillaries and diffuse accumulation of lymphocytes around the upper regions of follicles, swiftly followed by a more focused inflammation of hair follicle bulbs as hair loss occurs [81].
CD8+ cytotoxic lymphocyte mediated hair follicle disruption
Mounting evidence, primarily derived from studies with animal disease models, has demonstrated that CD8+ CTLs are fundamentally required for AA induction and perpetuation [82]. By microarray analyses, several key effector CTL specific transcripts have been identified in mouse and human AA skin [16]. Depletion of CD4+ or CD8+ cells using monoclonal antibodies (mAb) enables hair regrowth in mouse and rat models [83–85]. Transfer of CD8+ T cells in conjunction with CD4+ T cells can induce extensive AA lesions in mouse models [16,77,86–88]. Subcutaneous injection of CD8+ cells alone induces localized hair loss and CD4+ cells alone promotes systemic AA [86], highlighting the different characteristics and roles of CD8+ T cells and CD4+ T cells in skin disease, with CD8+ T cells as executors and CD4+ T cells as a helper cells [89]. Further, it has been shown that clonal class I MHC-restricted CD8+ lymphocytes can independently mediate AA after intravenous transfer into mice [77]. The transfer of human CD8+ cells alone suffices to induce AA in AA patients’ scalp skin transplanted to severe combined immunodeficient (SCID) mice [87]. Therefore, it has been proposed that CD8+ cells promote AA pathogenesis, acting as cytolytic effectors responsible for the autoimmune attack on hair follicles [16].
However, much less is known about how the inflammatory infiltrate elicits hair loss. Destruction of hair follicle cells by CTLs may be mediated directly through the Fas or perforin pathways and/or indirectly by the release of cytokines to cause cellular damage and tissue destruction [74]. The elevation of pro-inflammatory mediators such as IFNγ and IL-6 family cytokines in AA affected skin could directly adversely affect hair growth [51,90]. Notably, while apoptosis is significantly increased in inflamed, AA affected, anagen stage hair follicles, and the pathology of the follicles is severely disrupted, the follicles are not completely destroyed [91]. Hair follicles have superior regenerative ability [92], and it may also be possible that the CTL action is somewhat restrained by any residual IP activity.
TH17 cells may contribute to alopecia areata development
TH17 cells are a subset of T helper cells producing interleukin 17 (IL-17) that are developmentally distinct from TH1 and TH2 cell types [93]. The proinflammatory activity of TH17 cells can be beneficial during infection [94]. However, the production of IL-17 by TH17 cells is strongly implicated in the development of autoimmune disorders including psoriasis, rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and multiple sclerosis (MS). There is a reciprocal developmental pathway for the generation of pathogenic TH17 cells and protective T regulatory (Treg) cells in the immune system. TH17 and Treg subsets may therefore have evolved to induce or regulate tissue inflammation, analogous to the dichotomy of TH1 and TH2 T-cell subsets [95]. Cytokines TGFβ, IL-6 and IL-1β are essential for initiating TH17 cell development, but they inhibit Treg differentiation [96]. IL-23, a cytokine produced by dendritic cells and other antigen-presenting cells, serves as a pivotal factor that drives both the differentiation and inflammatory functions of pathogenic TH17 cells [97]. It has been revealed that SNPs of the IL17RA gene are associated with AA onset age in Korean patients [98]. Serum cytokine levels of IL-17A are significantly increased, and TGFβ1 is significantly decreased, in patients with AA compared with controls [99]. In AA lesions, infiltration of CD4+IL-17A+ TH cells have been found in the dermis, particularly around hair follicles [100][101]. Recently, a decrease in the number of skin infiltrating IL-17+ T cells was observed after topical immunotherapy with squaric acid dibutylester (SADBE) [102]. The data suggests that IL-17 is involved in the pathogenesis of AA, though the full significance remains unclear.
Deficient function of regulatory T cells in alopecia areata
Regulatory T cells are subpopulations of T cells which modulate the immune system, maintain tolerance to self-antigens, and are involved in preventing autoimmunity [103]. Tregs come in many forms with the most well-understood being those that are CD4+CD25+ and Foxp3+ (natural Tregs) [104]. The cells manifest their regulatory function through cell contact mediated regulation via TCR, and other molecules such as CTLA-4, GITR, and LAG-3 [105]. Foxp3+ T cells also produce a number of regulatory cytokines, including TGFβ, IL-10 and IL-35 [106–109]. Of these, TGFβ appears to have a crucial role in Foxp3 maintenance of Treg cells and the ability of Treg cells to induce non-Tregs to develop regulatory properties, a feature known as infectious tolerance [110,111]. Evidence from murine studies suggests that suppression mediated by cytokines is of particular importance at environmental interfaces, such as the gut, skin and lungs [106,112,113]. In autoimmunity, a lower frequency of Tregs is often observed in the active disease state [114,115]. Genetic deficiency in Treg production significantly increases the risk of autoimmune disease development [116]. Also of note, some CD8+ T-cells can express Foxp3. These cells have been shown to be induced in vitro through TCR-dependent stimulation and exhibit immunosuppressive activity [117].
Research on Tregs in AA is limited. There is a decrease in the number of Tregs in AA affected C3H/HeJ mice [86]. Transfer of unexpanded Treg cells from AA mice to healthy recipients was less likely to induce an AA phenotype. Treg transfer was also able to prohibit systemic AA development induced by CD4+CD25− cells as well as prevent injection site-specific hair loss induced by CD8+ cells [86]. Mice resistant to the onset of AA exhibit high levels of regulatory cytokines such as IL-10 [79]. These observations indicate that deficiency in the potential regulatory properties of CD4+CD25+ Treg cells may further enable AA development. In humans with AA, significant defects in the activity of Treg cells have been identified. Circulating Treg cells from AA patients are incapable of restraining activated lymphocytes while pathogenic cells exhibit apoptosis resistance [34,35]. Although overall Foxp3+ Treg numbers seem to be normal in PBMC populations of AA patients, their function is impaired [99,118]. SNPs in the promoter regions of gene Foxp3 and gene ICOSLG (inducible T-cell co-stimulator ligand) which affects Treg functions have been associated with a reduced expression of the FOXP3 and ICOSLG genes in AA patients [119]. It has recently been shown that cutaneous memory Tregs (mTregs) reside in human skin which have unique cell surface marker expression and cytokine production [120]. Interestingly, mTregs preferentially localize to hair follicles and are more abundant in skin with high hair density [120]. The potential role of mTregs in AA has not so far been investigated.
Dysregulation of cytotoxic T lymphocyte activation in alopecia areata
Naïve T cells are produced in the thymus and released into the bloodstream in low numbers until they encounter foreign antigens in secondary lymphoid tissues. The cells are maintained in a quiescent and non-dividing state by interleukin-7 (IL-7) and TCR signaling, which sustain expression of antiapoptotic molecules and allow the cells to survive in interphase [121]. TCR-peptide-MHC ligation and costimulatory signals on the surface of APCs are a prerequisite for naïve T cell activation [122,123]. With antigenic peptides engaged by TCR and costimulatory signals from APCs, classically first encountered in draining lymph nodes [88], naïve T-cells proliferate and differentiate into effector cells (Figure 1). Subsequently, 90–95% of the effector cells undergo apoptosis following the peak of the expansion phase during which effector cells exhibit significant cytokine production and cytolosis functions. The remaining 5–10% of T cells survive and give rise to long-lived memory populations [124]. The peripheral maturation of naïve T cells is regulated by cytokine and TCR signaling [125]. In contrast to naïve T cells, the homeostatic proliferation of memory T cells is not dependent on MHC molecules; only IL-2, IL-15 and IL-7 are required for induction of memory CD8+ T cell proliferation [125,126]. Expression of genes involved in the IL-7 receptor (IL-7r) pathway and TCR signaling correlates with poor a prognosis in some autoimmune diseases [127].
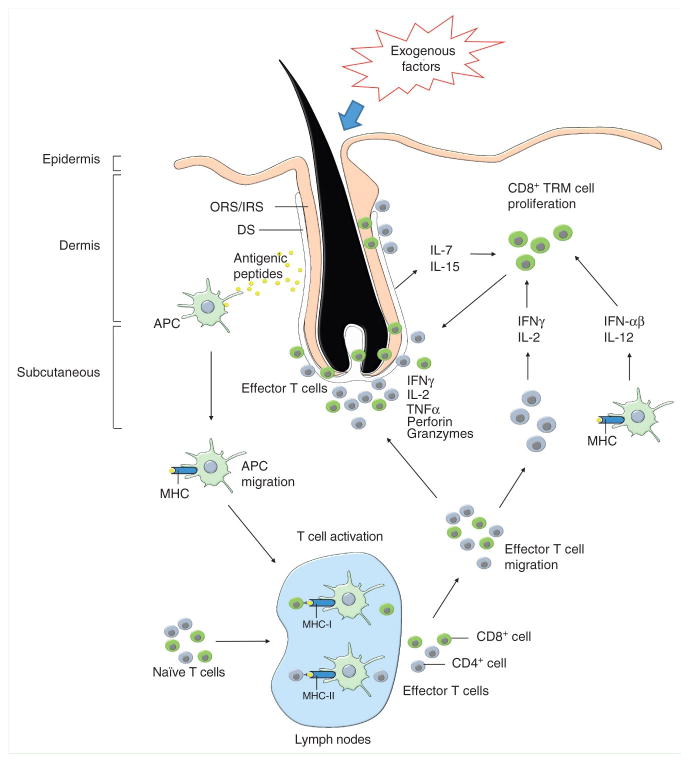
Figure 1. Effector CD8+ T cells plays a critical role in initiating alopecia areata.
Changes in normal hair follicle parameters in response to exogenous factors, such as infectious agents and/or trauma, in conjunction with endogenous factors, such as elevated stress hormones and/or genetic susceptibility, elicits activation of skin resident antigen presenting cells (APCs). APCs process hair follicle specific antigen epitopes in the presence of proinflammatory signals. Activated APCs migrate to skin draining lymph nodes to mature and present the pathogenic peptides to naïve T cells. Upon recognition of peptide-major histocompatibility complexes (MHC), in association with proinflamatory costimulatory signals on the surface of APCs, naïve T-cells proliferate and differentiate into effector T cells. Then, the effector T cells migrate to the skin and accumulate around anagen stage hair follicles. Primarily CD8+ T cells penetrate to intrafollicular locations to initiate the autoimmune attack in response to autoantigens in anagen stage hair follicles, while CD4+ cells remain in perifollicular locations. Hair follicle disruption may be mediated via pro-apoptotic signals such as perforin and granzymes, and/or hair follicle regression may be induced via inflammatory cytokines. In addition to the inflammatory lymphocyte infiltrate, inflamed hair follicle keratinocytes in dystrophic hair follicles release cytokines, including IL-15 and IL-7, and may induce tissue resident memory T cell proliferation. The activated memory T cells recruit new autoantigen-specific memory T cells and bystander cells from the circulation and further support direct disruption of hair follicle cells.
In a transgenic model of AA, mice with dual TCR expression develop spontaneous AA at nearly 100% incidence [77]. Notably MHC class I restricted CD8+ T cells are capable of independently mediating alopecia development and progression, while MHC class II are not required for disease development in this model [77,86]. Similarly in a humanized mouse model, CD8+ cells promote AA [88]. The data identify the key role of MHC class I restricted antigen presentation to CD8+ T cells in AA. Surprisingly, only a few genome studies indicate MHC class I loci are associated with AA susceptibility, whereas several studies demonstrate strong linkage between MHC class II loci and AA in both humans and rodent models [24,128].
Notably, there is an unusually high frequency of AA in polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) patients [129]. Mutations of the autoimmune regulator (AIRE) gene, causes the monogenic autoimmune disease APECED [130]. APECED patients have significantly decreased expression of IL-7r, as well as a decreased expression of the homing factors CCR7 and CD62L on CD8+ T cells. The, dysregulation induces a naïve CD8+ T cell population skew towards an effector CD8+ CTL phenotype [131]. Gene expression profiles from lesions of AA patients show modulation of IL7r expression [132], and upregulation of IL-15 and IL-15Rβ on infiltrating CD8+ T cells have been found in human AA [16]. The data suggest there is dysregulation of CD8+ cell activation in AA.
Lymphocyte homeostasis and migration in alopecia areata
T cell homeostatic mechanisms involve maintenance of a diverse repertoire of naïve cells, rapid elimination of effector cells after pathogen clearance, and long-term survival of memory cells [121]. Naïve T cells, effector T cells, and memory T cells, are characterized by different phenotypes and anatomical locations [133]. Lymph node homing molecules L-selectin (CD62L), CCR7 and lymphocyte function-associated antigen (LFA-1) are highly expressed on naïve CD8+ T cells and are critical for the homing of the cells. Effector CD8+ T cells lose expression of CD62L and CCR7, while gaining the expression of P-and E-selectin ligands, inflammatory chemokine receptors (e.g., CXCR3 and CCR5) and additional integrins, resulting in efficient recruitment of these cells to inflamed tissues [124]. Memory CD8+ T cells re-express CCR7 and CD62L and memory CD8+ T cells heterogeneously express higher levels of β1 (CD29, CD49d and CD49e) and β2 (CD11a, CD11b and CD18) integrins, CD2, CD44, CD54, CD58 and other cell surface molecules [134]. Compared to naïve cells, memory T cells express the IL-2R β-chain (CD122), Ly-6C and the common leukocyte antigen (CD45) [133].
The selective migration of T cells is regulated by the combined expression of selectins, integrins and chemokine receptors [135]. CCL17/CCR4 and CCL20/CCR6 which are important for effector T cells to reach to inflamed tissues are increased in AA lesions [21]. CD8+ T cells in AA development have low expression of CD62L and high expression of CD44 and CD44 variants which are important for the migration of leukocytes to inflamed tissue [21,80]. Transfer of CD8αβ+NKG2D+ T cells with an activated T effector memory cell phenotype (CD62lowCD44hi) induced AA in healthy recipients [16,36]. The studies suggest that AA is associated with markedly increased proportions of memory effector cells as defined by diminished levels of CD45Rb and CD62L and increased expression of CD44, CD69 and CD25 [77]. Taken together, circulating effector memory CD8+ T cells with skin homing properties are present in AA development.
Potential role of skin resident T cells in alopecia areata
The circulating memory T-cell population is divided into three subsets: effector memory (TEM), central memory (TCM) and tissue-resident memory (TRM) cells. These subsets can be distinguished by their localization, as TCM home to secondary lymphoid organs and provide spontaneous help to B cells [121], TEM circulate through non-lymphoid tissues, and TRM are found in peripheral tissues where they exert reactive memory functions [136]. Since the majority of TRM cells are CD8+ T cells, tissue-resident CD8+ T cells are now commonly referred to as TRM [137]. The origins of the TRM subset and how this subset relates to TCM and TEM remains unclear, it is possible that TRM arises directly from the effector T cell pool by unique signals from the microenvironment of the peripheral tissue [137].
There are distinct differences in the skin localization of CD4+ and CD8+ T cells. It has been shown that slow-moving CD8+ T cells actively patrol the epidermis, isolated from the circulating pool. In contrast, dynamic CD4+ T cells traffic rapidly through the dermis as part of a wider recirculation pattern [89,138]. Generation of TRM cells in skin requires CD69, TGFβ and IL-15 signals [139,140]. Skin resident T cells are able to respond to antigen exposure more rapidly than circulating memory CD8+ T cells [139] and potentially, they could effect a range of peripheral immune phenomena [89]. They can recruit new antigen-specific memory CD8+ T cells and bystander cells from the circulation, activate neighboring NK cells and B cells by providing a local source of IL-2, IFNγ, and TNFα, and directly kill local cells [140].
Skin resident T cells have recently been characterized [141,142]. There are a significant number of memory T cells in normal skin; nearly twice the number of T cells observed in the circulation [136]. Skin resident T cells uniformly express high levels of cutaneous lymphocyte function-associated antigen (CLA), CCR4, and CCR6. CLA is a skin-homing receptor uniquely expressed by skin T cells [141]. Also, CCR4, and CCR6 have been implicated in cutaneous T cell homing under homeostatic as well as inflammatory conditions [143,144]. Theoretically, skin resident T cells may play initial roles in AA progression. Infiltrating T cells in AA lesions strongly express CLA, CCR4, and CCR6, suggesting skin resident T cells may be active in AA pathogenesis [21,145].
Other cell types involved in alopecia areata pathogenesis
Dendritic cells as specialized sentinel cells in alopecia areata
Dendritic cells (DCs) control both steady-state T cell tolerance and the development of autoreactive T cells, serving as a bridge between the innate and adaptive immune systems without engaging directly in effector functions [146]. There are three main cutaneous DC populations: epidermal Langerhans cells, plasmacytoid DCs (pDCs) and conventional or classical DCs (cDCs) (previously called Myeloid DCs, mDCs) [147][148]. In healthy human skin the majority of cDCs express CD1c and CD11c [149]. During steady-state conditions, pDCs are absent from the skin, they have only been identified in inflamed skin where they promote wound repair and mediate the systemic pro-inflammatory response [150]. DCs are dedicated APCs that have a characteristic dendritic morphology and express high levels of MHC class II molecules and are highly effective at antigen presentation and T-cell stimulation [59]. DCs recognize pathogen-associated molecular patterns (PAMPs) using pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), and then they migrate to T cell areas of lymphoid organs (such as initial afferent lymph vessels of the skin) to present pathogen-derived antigens to antigen-specific T cells [151]. Antimicrobial peptides may lead to pDC activation and protective immune responses to skin injury, however overexpression of antimicrobial peptides in psoriasis drives excessive sensing of self-nucleic acids by pDCs resulting in IFN-driven autoimmunity. Therefore type I IFN production by pDCs, might represent a common mechanism that leads to pathogenesis in autoimmune diseases such as psoriasis, systematic lupus erythema (SLE) and diabetes mellitus type 1 (T1D) [148].
In AA, immunohistochemical detection of pDCs using anti-BDCA-2 (a marker highly specific for DCs) and anti-myxovirus resistance protein A (MxA) (for detection of IFN-β neutralizing antibodies) respectively showed that pDCs were present in peri-bulbar locations in all AA lesions and in an active state producing type I IFNs [19]. The number of epidermal LCs in the epidermis of the AA patients do not differ from healthy controls, whereas diphenylcyclopropenone (DPC) induced contact sensitization leads to an increased number of MMP-9+ CD1a+ dermal cells during the initial phase of sensitization, suggesting the migration of LCs from the epidermis, through the dermis, and later to the draining lymph nodes [20]. The limited data suggest that DCs could contribute to AA pathogenesis, though the underlying significance needs further research.
Activation of mast cells in alopecia areata initiation and progression
Mast cells are the lead effector cells in the immediate responses that can occur when sensitized individuals come into contact with allergens in peripheral tissues [152][153]. Significant mast cell activity is elicited by allergens complexed to immunoglobulin-E (IgE) molecules, as well as many other non-allergic triggers including anaphylatoxins (C3a and C5a), aggregated IgG, certain drugs, venoms, and physical stimuli (pressure and temperature changes), cytokines and neuropeptides [154,155]. Activation of mast cells leads to degranulation and release of a broad array of mediators including histamine, serotonin, proteases, and tumor necrosis factor (TNF) [152,154]. Mast cells can also selectively release pro-inflammatory mediators without degranulation, particularly IL-6 and vascular endothelial growth factor (VEGF) [156]. Abnormal interactions between mast cells and lymphocytes has been demonstrated in multiple sclerosis (MS), rheumatoid arthritis (RA), insulin-dependent diabetes mellitus (IDDM), bullous pemphigoid, chronic idiopathic urticaria, atopic dermatitis and experimental vasculitis [157]. In AA, significantly more physical mast cell/CD8+ T cell contact is observed compared to healthy or non-lesional human skin. During the interaction with CD8+ T cells, mast cells prominently express MHC class I and OX40L, suggesting that mast cells may potentially present antigens and/or co-stimulatory signals to CD8+ T-cells [18]. Treatments targeting mast cells have been investigated for use in some autoimmue diseases [156,157 {Theoharides, 2015 #233], drugs considered to ‘stabilize’ mast cells (for example, cromolyn sodium) have been showed ameliorating disease severity in EAE.[158]. Use of anti-histamines as an adjunct treatment in AA has been suggested [15].
Alopecia areata treatments and treatment development
Current common treatments for alopecia areata
Among the various therapies presently available for AA, the most common approach is the use of intralesional and topical steroids [159]. Though rarely used in North America, systemic corticosteroid regimens are also effective [7]. Intralesional injections of corticosteroid suspensions, primarily triamcinolone acetonide, have been used for the treatment of AA for over 50 years [160]. Several clinical studies demonstrate hair regrowth at the site of injection [161]. Although widely used for AA, there are few placebo controlled studies on corticosteroid efficacy for AA and none fully satisfy the requirements of evidence based medicine (EBM). However, comparative intra-control investigations demonstrate a significant beneficial effect [162].
Use of skin irritants to treat AA have been recorded in literature since ancient times and AA has been treated with contact sensitizing agents for more than 30 years [163–165]. Today, diphenylcyclopropenone (DCP) or squaric acid dibutylester (SADBE) are widely used in countries outside of the USA for the treatment of more extensive AA presentations [7]. Though the mode of action is not fully understood, inducing a mild inflammatory response to contact sensitizing agents enables hair regrowth in some individuals. Treatment with DCP and SADBE have been shown to be effective according to the rules of EBM [160].
The discovery of the underlying mechanisms of current therapies may shed light on the future treatment development. Rodent AA models have been used to screen experimental and approved drugs for efficacy [166]. In SADBE-treated mice, the number of perifollicular FasL positive cells is strikingly increased [167]; leukocyte traffic to the skin is hampered [168], and the emigration of APCs into the draining lymph nodes is hindered [21]. Studies suggest that the therapeutic efficacy of contact sensitizers in AA may also involve driving autoreactive T cells into activation-induced cell death [167], reversing a disturbed chemokine balance that interferes with effector T-cell homing into AA-affected skin, reducing antigen presentation in draining lymph nodes [21,168], and possibly, expansion of myeloid suppressor cells that contribute to autoreactive T cell silencing [169].
Immunoregulatory drug treatments for alopecia areata investigated with rodent models
Differences between rodents and humans mean that not all treatments can be screened or investigated with disease models, particularly biologics specifically designed to target human molecules. Further, research data from rodents is not always directly translatable to the human state. However, a potential significant advantage with animal models of human disease is for rapid screening of new treatment candidates and/or investigation of their mode of action [166].
Previously, C3H/HeJ mice and DEBR rats have been treated successfully with tacrolimus (FK506). The treated rodents had reduced perifollicular infiltrates of CD4+ and CD8+ cells and a decreased expression of MHC class I and II and ICAM-1 on hair follicle epithelium, providing evidence that suppressing the T cell mediated immune response and reversing hair follicle IP deficiencies are effective approaches for AA therapy [170]. However, studies with topical tacrolimus formulations in humans have proven less effective, possibly due to a lack of skin dermis penetration [171,172]. Similar rodent studies with cyclosporin in topical liposome formulations have also been conducted [173].
More recently, the mouse model has been used to study Janus kinase (JAK) inhibitors. IFNγ produced by CD8+ effector T cells signals via JAK1 and JAK2 intracellular pathways to produce IL-15 and IL-15r. The binding of IL-15 and IL-15r on CD8+ T cells mediates activation and further enhances the production of IFNγ by CD8+ T cells via JAK1 and JAK3 pathways [16]. Ruxolitinib and tofacitinib are US Food and Drug Administration (FDA)–approved small-molecule JAK inhibitors. While ruxolitinib inhibits JAK1 and JAK2, tofacitinib inhibits JAK3 [36]. Systemic ruxolitinib and tofacitinib prevents the onset of AA in mice, and topical ruxolitinib and topical tofacitinib result in complete hair regrowth in AA mice, associated with a markedly reduced number of CD8+NKG2D+ T cells in the treated skin and lymph nodes [16]. Notably, three AA patients treated with oral ruxolitinib achieved near-complete hair regrowth within 5 months of treatment, suggesting the potential clinical utility of JAK inhibition in human AA [16]. Currently, there are several promising investigations into the use of these and other JAK inhibitors for treating AA (unpublished data).
Design of effective immunotherapies for alopecia areata
Taken together, from treatments currently in use and treatment investigations in rodent models, plus the research into hair follicle IP and its loss in AA development, the data suggest several approaches to the development of new, potentially superior AA treatments. The current conventional therapy for human AA is based on nonselective immunoregulation achieved with corticosteroids or contact sensitizers. The emergence of more specific immune modulators in recent years has provided new candidate treatment options [174]. Targeting and modulation of the immune cells, their production and activity, which may include depleting pathogenic T cell populations and dampening the pathological immune response, may be effective in treating AA [175]. In principle, there are two basic approaches to the design of effective immunotherapies for human autoimmunity: non-antigen-specific immune checkpoint inhibitors which target MHC-TCR signaling, costimulation, and cytokine inputs [176], and antigen-specific therapies that induce tolerance to self-antigens [177]. As examples, immunotherapy development for other autoimmune diseases has focused on the induction of tolerance to beta cell antigens in Type I diabetes mellitus, anti-alpha 4 integrins and altered peptide ligand of myelin basic protein (MBP 83–99) in multiple sclerosis, anti-cytokine therapy (anti-TNFα and IL-1Rα) in rheumatoid arthritis, and anti-CD20 monoclonal antibody for in vivo B cells depletion and subsequent autologous peripheral stem cell transplants for systemic lupus erythematosus patients [178].
Targeting cytokine signaling
Activated CD8+ T cells can produce very high levels of TNFα and IFNγ, which may contribute directly and/or indirectly to target cell destruction in autoimmune mechanisms [179]. Blockade of TNFα has clear therapeutic benefit in some autoimmune diseases, particularly psoriasis [180]. TNFα is expressed in both lesions [181] and sera of patients with AA [182], but notably, TNFα inhibitors have been shown to have little effect on AA and may even be associated with AA development in some individuals [183,184]. The data suggest TNFα plays a rather more complicated role in AA pathogenesis than was previously thought and may even have some protective function [181]. In small scale studies, anti-IFNγ treatment showed significant hair restoration potential in patients with AA [185]. IFNγ deficient mice are resistant to the development of AA [70] and blockade of IFNγ prevented AA onset and reduced accumulation of CD8+NKG2D+ T cells in AA mouse skin [16,68], suggesting an approach to abrogate the IFNγ signaling in CD8+ T cells could be a therapy for AA. Targeting of other proinflammatory cytokines may also be effective.
Interference with antigen presentation and costimulation
Disruption of the cytotoxic T-lymphocyte-associated protein 4 (CTLA4) mediated CD28-CD80/CD86 costimulatory pathway can prevent onset of AA in the mouse model [186]. The absence of co-stimulation during antigen presentation can induce deletion and anergy of cognate T cells [187]. It implies that an AA treatment may interfere with co-stimulation or APC assisted activation of effector CD8+ T cells [188]. Cytotoxic T-lymphocyte–associated antigen 4–IgG (CTLA4-Ig), known as abatacept and belatacept, prevents binding of CTLA4 on T cells to CD80 and CD86 on APCs. These treatments have been approved for the treatment of rheumatoid arthritis in humans [189,190]. It may not be enough to target just one costimulatory pathway given the immune activity in AA is very strong. Importantly, new strategies are being devised to target several co-signaling molecules, or other components of the immune response, in combination therapies. These combination strategies include, but are not limited to, targeting additional surface molecules, targeting specific signaling molecules, cell-based vaccination strategies, or use in conjunction with more traditional drug treatments [190].
Skin localized immune checkpoint blockade
In AA, a treatment hindering the activation of tissue resident cells into effector CD8+ T cells may be effective. As skin TRM do not need to leave the epidermis and enter the draining lymph nodes to be primed, disruption of the interaction between TRM and APCs or co-stimulation in the skin could be an ideal target. Priming of CD8+ TRM within the tissue requires recruitment of APCs as well the help from specific CD4+ T cells [136,191]. A combination therapy aimed at targeting skin resident autoreactive CD4+, CD8+ T cells and APCs may be efficacious [179]. Once skin resident T cells are activated and the immune response is underway, neutralizing CTL action could be a potential treatment approach for AA. There are at least two CD8+ T-cell subsets (Tc1, Tc2) in human skin, both of which can express the Fas ligand and perforin cytotoxic pathways and display significant cytotoxic activity against keratinocytes [192]. Inactivation and degradation of perforin/granzyme B [193] and blockade of Fas-FasL signaling [194] may be novel therapeutic strategies for AA treatment. Early treatment with immune checkpoint inhibitors to delete active CD8+ T cells, or blockade CD8+ T cell recruitment, might hold promise when the antigen specificity is not known or the disease has already progressed to a stage where more specific therapy may be inefficient [179].
Promoting immune tolerance and Treg cell activity
It has recently been shown that Tregs residing in human skin have unique cell surface marker expression and cytokine production [120]. Interestingly, cutaneous Tregs preferentially localize to hair follicles and are more abundant in skin with high hair density [120], implying that modulating the balance of TRM and Tregs might be a potential AA treatment approach. Studies demonstrate the beneficial effects of Tregs in inducing tolerance in multiple autoimmune and inflammatory diseases such as cardiac fibrosis [195], bacteria induced sepsis [196], diabetes [197] and asthma [198]. Notably, in vitro expansion of Tregs followed by adoptive transfer has become an active area of research for autoimmune disease treatment [115]. However, such an approach requires knowledge of the specific target antigens to allow relevant epitope specific Tregs to be isolated. Non-specific Treg stimulation in situ may be an alternative. IL-2 at low dosages can non-specifically stimulate Treg cells. In a small pilot study, subcutaneous IL-2 injections increased Treg cell numbers and enabled partial hair regrowth in AA patients [199].
Vitamin D may hold promise for treatment and prevention of autoimmune disease [200]. The vitamin D receptor is expressed on immune cells (B cells, T cells and APCs), and vitamin D can suppress immune responses and promote a more tolerogenic status in part through supporting Treg cell function [201][202]. Notably, vitamin D affects the expression of the IFN signature in SLE [203] and immune cells from multiple autoimmune diseases appear to respond to the immunosuppressive effects of vitamin D [201]. Vitamin D deficiency may be a significant risk factor for AA occurrence [204]. Application of calcipotriol, a strong vitamin D analog, is associated with hair regrowth in AA patients [205]{Cerman, 2015 #674}.
Control of cell migration
Priming of CD8+ T cells requires recruitment of APCs as well as help from specific CD4+ T cells in secondary lymphoid tissues [136,191]. Lymphatic entry, which controls the recruitment of mature Ag-charged APCs from the periphery via afferent lymphatics, acts as a rate limiting step in the development of immunity and is a potential target for localized immunotherapy [206]. One of mechanisms by which contact sensitizers may work in AA is to impede skin-derived APC migration into draining lymph nodes, as shown in AA mice [21]. It has been proposed that therapeutic siRNA-mediated silencing of inflammatory monocyte migration is a potential strategy for the treatment of inflammatory diseases [207]. Alternatively and perhaps more practical, leukocyte traffic control into the skin has been proposed as a novel therapeutic strategy. Blockade of CD44v10 with mAbs impaired lymphocyte homing to the skin and prevented AA onset in a mouse model [208]. The expression of adhesion molecules on tissue microvasculature acts as a gateway, mediating the migration of activated leukocytes into tissue [209]. Natalizumab, a humanized monoclonal antibody against the cell adhesion molecule α4 integrin which inhibits leukocyte migration, is FDA-approved for the treatment of multiple sclerosis and Crohn’s disease [210]. These and other treatments to control cell migration may be effective for early stage AA.
Anergy promotion in pathogeneic T cells
Antigen-specific immunotherapy (ASIT) is based on the identification of pathogenic T cell subsets or the inciting autoantigen(s) that induce antigen-specific clonal responses [179,211]. ASIT provides a treatment approach focused on anergy and deletion of specific autoreactive T cells and/or promotion of regulatory cells. These therapies ideally promote the specific tolerance of self-reactive immune cells without altering host immunity to infectious insults [212]. ASIT strategies that are currently under investigation involve T-cell vaccination (TCV), T-cell receptor (TCR) peptide vaccination, DNA vaccination, and altered peptide ligand (APL) vaccination [213]. Encouraging results in animal models using vaccines have prompted the design of novel and selective immune-based therapies for human autoimmune disease [214]. Recent initiatives to optimize immune monitoring should facilitate rational design, monitoring and mechanistic understanding of ASIT for human autoimmune diseases [175].
Development of ASIT for human AA has not been launched since the primary autoantigens of AA remain undetermined [188]. Studies suggest enrichment of CD8+ T cell subsets that target hair follicle keratinocyte expressed trichohyalin and melanocyte expressed epitopes, though other antigens are also likely targeted [215]. In mice, some preliminary investigations suggest autoantigen peptides may be used to control immune responses. Vaccination of mice with keratin K71 or K31 peptides significantly retarded AA induction and prevented disease progression. Dendritic cell-presented K71 and K31 peptides induced long-lasting T cell anergy in vitro [216]. However it is not known whether these keratins are natural autoantigens targeted in AA.
Restoring hair follicle immune privilege
As an alternative to the specific targeting of pathogenic immune cells, a focus on rescue and reassertion of IP in and around the hair follicle unit may be effective. The two approaches to treatment development are not necessarily mutually exclusive and potentially, a single treatment could have an effect on both the immune system and hair follicle IP. Hair follicle IP is present during anagen, but is lost during the resting and regression phases of the hair cycle [217], indicating the state of IP during the anagen phase of the hair follicle might be taken into account when developing any treatment for AA. The mechanism of maintaining hair follicle IP could include downregulation of the MHC class I pathway and IFN regulatory factor-1 expression and/or upregulation of secreted immunosuppressive factors such as TGFβ1 and TGFβ2, ACTH, and αMSH among others [217]. Alternatively, hair follicle IP could be reinforced by addition of new IP defenses. Given its potent suppressive effects, bolstering PDL1 expression with the addition of PDL1 expressing cells may provide protective reinforcement [45,218]. Exposure of AA patients’ PBMCs ex vivo to cord blood-derived stem cells attenuates CD8+NKG2D+ T cells via PDL1 and B and T lymphocyte attenuator (BTLA)/herpesvirus entry mediator (HVEM) pathways. Reinfusion of the conditioned cells enables hair regrowth associated with increased TGFβ expression around hair follicles [219]. Whether this approach will prove to be practical is unknown, but a larger scale trial is underway. Provision of indoleamine 2,3-dioxygenase (IDO) expressing fibroblasts can significantly increase the number of CD25+FOXP3+ Treg cells and promote CD8+ T cell apoptosis [220]. Rodent model studies using injected IDO expressing cells to treat AA are ongoing (unpublished data).
Conclusions
Taken together, a large body of evidence has accumulated supporting a CD8+ T cell mediated attack on hair follicles with CD4+ T cell help as the underlying mechanism of AA. Though the primary targets of lymphocytes have not been confirmed, it seems likely that hair follicle specific autoantigens are the main focus for effector and memory T cells. Future treatments for AA could be immunosuppressive or immunomodulatory to shield selected tissue compartments from autoaggressive immune attack via proper adjuvants, delivery systems, and optimized administration schedules [39,160,221]. Topical or skin localized treatments are a key focus of interest since AA is a relatively organ-restricted autoimmune condition. In the near future, topical therapy with JAK inhibitors shows potential as a first line defense against skin immune cell activity [16].
Expert commentary
In the last 10 years significant progress has been made in understanding the pathogenesis of alopecia areata. Large scale genome wide association studies have revealed multiple loci associated with susceptibility towards disease onset. Several of the loci have been shown to be associated with other autoimmune diseases, suggesting alopecia areata exhibits some disease features common to other autoimmune conditions. Using rodent models, functional data has identified specific lymphocyte subsets that mediate the disease pathology. While the epitope targets in hair follicles have not yet been identified, almost all evidence confirms alopecia areata is primarily a CD8+ cytotoxic T cell driven autoimmune disease. With our much improved understanding of alopecia areata, there are opportunities to develop new treatments or to adapt treatments developed for other diseases.
Five-year view
Key issues in alopecia areata will likely be addressed with new research in the near term. Information is severely lacking on environmental inputs; particularly candidate triggers for the onset of alopecia areata. Investigations by several laboratories are already underway to identify the inciting (auto)-antigen epitopes. Further characterization of lymphocyte subsets and other cells types will occur with a specific focus on the nature of the mode of action utilized to disrupt hair follicles and prevent fiber growth. Though characterization of normal hair follicle immune privilege is progressing well, still relatively little is known about the changes that occur in alopecia areata and the true significance of these changes for alopecia areata onset. In the near term, new treatments targeting the JAK-STAT signaling pathway, as topical formulations, are likely to emerge as more effective therapies for alopecia areata.
Key issues.
Alopecia areata is a hair follicle – specific autoimmune disease involving both innate and adaptive immune cells
Activated lymphocytes, along with antigen presenting cells, synergize to initiate and promote alopecia areata
CD8+ cytotoxic T lymphocytes are fundamentally required for alopecia areata induction and perpetuation
The function of regulatory T cells may be defective in alopecia areata
Hair follicles normally exhibit immune privilege, but these properties may be deficient in alopecia areata
Blockade of antigen presentation and co-stimulation may prevent lymphocyte activation
Targeting immune cells and their cytokine production may be effective in alopecia areata treatment
Promoting regulatory T cells may restore immune system balance
Restoring or augmenting hair follicle immune privilege may prevent alopecia areata pathogenesis
Abbreviations
- APC
antigen presenting cell
- DS
dermal sheath
- IFN
interferon
- IL
interleukin
- IRS
inner root sheath
- MHC
major histocompatibility complex
- ORS
outer root sheath
- TNF
tumor necrosis factor
- TRM
tissue resident memory
Footnotes
Financial and competing interests disclosure
This work was supported by grants from the North American Hair Research Society (NAHRS) and the Canadian Dermatology Foundation (CDF). K McElwee is a recipient of the Canadian Institutes of Health Research (MSH-95328) and Michael Smith Foundation for Health Research [CI-SCH-00480(06-1)] investigator awards. K McElwee and J Shapiro are founders and shareholders of Replicel Life Sciences Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62(2):177–188. doi: 10.1016/j.jaad.2009.10.032. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 2.Gilhar A, Kalish RS. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev. 2006;5(1):64–69. doi: 10.1016/j.autrev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Thomas EA, Kadyan RS. Alopecia areata and autoimmunity: a clinical study. Indian J Dermatol. 2008;53(2):70–74. doi: 10.4103/0019-5154.41650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirzoyev SA, Schrum AG, Davis MD, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J Invest Dermatol. 2014;134(4):1141–1142. doi: 10.1038/jid.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kos L, Conlon J. An update on alopecia areata. Curr Opin Pediatr. 2009;21(4):475–480. doi: 10.1097/MOP.0b013e32832db986. [DOI] [PubMed] [Google Scholar]
- 6.McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2 Suppl):S49–51. doi: 10.1016/j.jaad.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 7*.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010;62(2):191–202. doi: 10.1016/j.jaad.2009.10.031. quiz 203–194. review of current alopecia areata treatments. [DOI] [PubMed] [Google Scholar]
- 8*.Wang E, McElwee KJ. Etiopathogenesis of alopecia areata: Why do our patients get it? Dermatologic therapy. 2011;24(3):337–347. doi: 10.1111/j.1529-8019.2011.01416.x. review of alopecia areata etiopathogenesis. [DOI] [PubMed] [Google Scholar]
- 9.Xiao FL, Yang S, Liu JB, et al. The epidemiology of childhood alopecia areata in China: a study of 226 patients. Pediatr Dermatol. 2006;23(1):13–18. doi: 10.1111/j.1525-1470.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 10.Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61(4):581–591. doi: 10.1016/j.jaad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Lomas M, Hanon E, Tanaka Y, Bangham CR, Gould KG. Presentation of a new H-2D(k)-restricted epitope in the Tax protein of human T-lymphotropic virus type I is enhanced by the proteasome inhibitor lactacystin. J Gen Virol. 2002;83(Pt 3):641–650. doi: 10.1099/0022-1317-83-3-641. [DOI] [PubMed] [Google Scholar]
- 12.Nanda A, Al-Fouzan AS, Al-Hasawi F. Alopecia areata in children: a clinical profile. Pediatr Dermatol. 2002;19(6):482–485. doi: 10.1046/j.1525-1470.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 13.Ma XJ, Cao SL, Lin L, et al. Hydrothermal pretreatment of bamboo and cellulose degradation. Bioresour Technol. 2013;148:408–413. doi: 10.1016/j.biortech.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Chu SY, Chen YJ, Tseng WC, et al. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. Journal of the American Academy of Dermatology. 2011;65(5):949–956. doi: 10.1016/j.jaad.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 15*.McElwee KJ, Gilhar A, Tobin DJ, et al. What causes alopecia areata? Experimental dermatology. 2013;22(9):609–626. doi: 10.1111/exd.12209. review of evidence in support of autoimmune mechanisms in alopecia areata pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. doi: 10.1038/nm.3645. demonstrates that CD8αβ+NKG2D+ T effector memory cells mediate alopecia areata in part through Janus kinase (JAK) signaling and that alopecia areata might be treated with JAK inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurashima Y, Amiya T, Fujisawa K, et al. The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity. 2014;40(4):530–541. doi: 10.1016/j.immuni.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 18*.Bertolini M, Zilio F, Rossi A, et al. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS One. 2014;9(5):e94260. doi: 10.1371/journal.pone.0094260. suggests the interaction of mast cells and CD8+T cells may initiate and promote alopecia areata. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou Rahal J, Kurban M, Kibbi AG, Abbas O. Plasmacytoid dendritic cells in alopecia areata: missing link? J Eur Acad Dermatol Venereol. 2014 doi: 10.1111/jdv.12932. [DOI] [PubMed] [Google Scholar]
- 20.Heffler LC, Kastman AL, Jacobsson Ekman G, Scheynius A, Fransson J. Langerhans cells that express matrix metalloproteinase 9 increase in human dermis during sensitization to diphenylcyclopropenone in patients with alopecia areata. The British journal of dermatology. 2002;147(2):222–229. doi: 10.1046/j.1365-2133.2002.04848.x. [DOI] [PubMed] [Google Scholar]
- 21.Gupta P, Freyschmidt-Paul P, Vitacolonna M, et al. A chronic contact eczema impedes migration of antigen-presenting cells in alopecia areata. J Invest Dermatol. 2006;126(7):1559–1573. doi: 10.1038/sj.jid.5700328. [DOI] [PubMed] [Google Scholar]
- 22*.Veldhoen M, Ferreira C. Influence of nutrient-derived metabolites on lymphocyte immunity. Nature medicine. 2015;21(7):709–718. doi: 10.1038/nm.3894. demonstrates contact sensitization treatment of alopecia areata creates a chemokine milieu severelyhampering dendritic cell migration from the skin towards the draining nodes. [DOI] [PubMed] [Google Scholar]
- 23*.Watanabe R, Gehad A, Yang C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7(279):279ra239. doi: 10.1126/scitranslmed.3010302. review of resident and recirculating T cells in human skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biran R, Zlotogorski A, Ramot Y. The genetics of alopecia areata: new approaches, new findings, new treatments. J Dermatol Sci. 2015;78(1):11–20. doi: 10.1016/j.jdermsci.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 25**.Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–117. doi: 10.1038/nature09114. Genomic study implies that alopecia areata is associated with both innate and adaptive immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64(1):26–30. [PubMed] [Google Scholar]
- 27.Wiesner-Menzel L, Happle R. Intrabulbar and peribulbar accumulation of dendritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res. 1984;276(5):333–334. doi: 10.1007/BF00404628. [DOI] [PubMed] [Google Scholar]
- 28.Ranki A, Kianto U, Kanerva L, Tolvanen E, Johansson E. Immunohistochemical and electron microscopic characterization of the cellular infiltrate in alopecia (areata, totalis, and universalis) J Invest Dermatol. 1984;83(1):7–11. doi: 10.1111/1523-1747.ep12261627. [DOI] [PubMed] [Google Scholar]
- 29*.McElwee KJ, Silva K, Boggess D, Bechtold L, King LE, Jr, Sundberg JP. Alopecia areata in C3H/HeJ mice involves leukocyte-mediated root sheath disruption in advance of overt hair loss. Vet Pathol. 2003;40(6):643–650. doi: 10.1354/vp.40-6-643. indicates focal follicular inflammation develops some time in advance of overt hair loss and focuses on the differentiating root sheaths in C3H/HeJ mice. [DOI] [PubMed] [Google Scholar]
- 30.Ono S, Otsuka A, Yamamoto Y, et al. Serum granulysin as a possible key marker of the activity of alopecia areata. J Dermatol Sci. 2014;73(1):74–79. doi: 10.1016/j.jdermsci.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 31*.Bodemer C, Peuchmaur M, Fraitaig S, Chatenoud L, Brousse N, De Prost Y. Role of cytotoxic T cells in chronic alopecia areata. J Invest Dermatol. 2000;114(1):112–116. doi: 10.1046/j.1523-1747.2000.00828.x. indicates apoptotic mechanisms involving granzyme B and Fas-Fas ligand pathways may play amajor role in the persistence of alopecia areata. [DOI] [PubMed] [Google Scholar]
- 32.Carroll JM, McElwee KJ, LEK, Byrne MC, Sundberg JP. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. The Journal of investigative dermatology. 2002;119(2):392–402. doi: 10.1046/j.1523-1747.2002.01811.x. [DOI] [PubMed] [Google Scholar]
- 33*.Freyschmidt-Paul P, McElwee KJ, Botchkarev V, et al. Fas-deficient C3.MRL-Tnfrsf6(lpr) mice and Fas ligand-deficient C3H/HeJ-Tnfsf6(gld) mice are relatively resistant to the induction of alopecia areata by grafting of alopecia areata-affected skin from C3H/HeJ mice. J Investig Dermatol Symp Proc. 2003;8(1):104–108. doi: 10.1046/j.1523-1747.2003.12182.x. indicates that the Fas/Fas ligand pathway plays an important pathogeneic role in an alopecia areatadisease model. [DOI] [PubMed] [Google Scholar]
- 34.Zoller M, McElwee KJ, Vitacolonna M, Hoffmann R. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13(7):435–444. doi: 10.1111/j.0906-6705.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 35.Zoller M, McElwee KJ, Vitacolonna M, Hoffmann R. Apoptosis resistance in peripheral blood lymphocytes of alopecia areata patients. J Autoimmun. 2004;23(3):241–256. doi: 10.1016/j.jaut.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Divito SJ, Kupper TS. Inhibiting Janus kinases to treat alopecia areata. Nat Med. 2014;20(9):989–990. doi: 10.1038/nm.3685. [DOI] [PubMed] [Google Scholar]
- 37.Geyfman M, Plikus MV, Treffeisen E, Andersen B, Paus R. Resting no more: re-defining telogen, the maintenance stage of the hair growth cycle. Biological reviews of the Cambridge Philosophical Society. 2014 doi: 10.1111/brv.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breitkopf T, Leung G, Yu M, Wang E, McElwee KJ. The basic science of hair biology: what are the causal mechanisms for the disordered hair follicle? Dermatol Clin. 2013;31(1):1–19. doi: 10.1016/j.det.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Paus R, Bertolini M. The role of hair follicle immune privilege collapse in alopecia areata: status and perspectives. J Investig Dermatol Symp Proc. 2013;16(1):S25–27. doi: 10.1038/jidsymp.2013.7. [DOI] [PubMed] [Google Scholar]
- 40.Westgate GE, Craggs RI, Gibson WT. Immune privilege in hair growth. J Invest Dermatol. 1991;97(3):417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- 41.Becker JC, Varki N, Brocker EB, Reisfeld RA. Lymphocyte-mediated alopecia in C57BL/6 mice following successful immunotherapy for melanoma. J Invest Dermatol. 1996;107(4):627–632. doi: 10.1111/1523-1747.ep12584237. [DOI] [PubMed] [Google Scholar]
- 42.McElwee KJ, Hoffmann R. Alopecia areata - animal models. Clin Exp Dermatol. 2002;27(5):410–417. doi: 10.1046/j.1365-2230.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 43.Boffa MJ, Wood P, Griffiths CE. Iron status of patients with alopecia areata. Br J Dermatol. 1995;132(4):662–664. doi: 10.1111/j.1365-2133.1995.tb08727.x. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson TA, Griffith TS. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev. 2006;213:228–238. doi: 10.1111/j.1600-065X.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Marr AK, Breitkopf T, et al. Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: a potential mechanism of immune privilege. J Invest Dermatol. 2014;134(3):736–745. doi: 10.1038/jid.2013.368. [DOI] [PubMed] [Google Scholar]
- 46.Guleria I, Khosroshahi A, Ansari MJ, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. The Journal of experimental medicine. 2005;202(2):231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habicht A, Dada S, Jurewicz M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179(8):5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 48.Ritprajak P, Hashiguchi M, Tsushima F, Chalermsarp N, Azuma M. Keratinocyte-associated B7-H1 directly regulates cutaneous effector CD8+ T cell responses. J Immunol. 2010;184(9):4918–4925. doi: 10.4049/jimmunol.0902478. [DOI] [PubMed] [Google Scholar]
- 49.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111(7):3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164(2):623–634. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breitkopf T, Lo BK, Leung G, et al. Somatostatin expression in human hair follicles and its potential role in immune privilege. J Invest Dermatol. 2013;133(7):1722–1730. doi: 10.1038/jid.2013.53. [DOI] [PubMed] [Google Scholar]
- 53.Mahi-Brown CA, Yule TD, Tung KS. Adoptive transfer of murine autoimmune orchitis to naive recipients with immune lymphocytes. Cellular immunology. 1987;106(2):408–419. doi: 10.1016/0008-8749(87)90183-3. [DOI] [PubMed] [Google Scholar]
- 54.Tung KS, Yule TD, Mahi-Brown CA, Listrom MB. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol. 1987;138(3):752–759. [PubMed] [Google Scholar]
- 55.Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. II. An analysis of F1 lymphocyte-induced immune deviation. J Immunol. 1978;120(3):689–693. [PubMed] [Google Scholar]
- 56.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270(5236):630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 57*.Kang H, Wu WY, Lo BK, et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J Invest Dermatol. 2011;130(11):2677–2680. doi: 10.1038/jid.2010.180. suggests the possibility of hair follicle immune privilege deficiency in AA pathogenesis in advance of overt hair loss. [DOI] [PubMed] [Google Scholar]
- 58.Saxena A, Martin-Blondel G, Mars LT, Liblau RS. Role of CD8 T cell subsets in the pathogenesis of multiple sclerosis. FEBS Lett. 2011;585(23):3758–3763. doi: 10.1016/j.febslet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 59*.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13(8):566–577. doi: 10.1038/nri3477. reviews the roles of dendritic cells in immunological tolerance, the effect of the gain or loss of dendritic cells on autoimmunity and dendritic cell-intrinsic molecular regulators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. The Journal of experimental medicine. 2003;198(10):1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McElwee KJ, Boggess D, Olivry T, et al. Comparison of alopecia areata in human and nonhuman mammalian species. Pathobiology. 1998;66(2):90–107. doi: 10.1159/000028002. [DOI] [PubMed] [Google Scholar]
- 62.Ito T, Ito N, Saatoff M, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128(5):1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 63.Gregoriou S, Papafragkaki D, Kontochristopoulos G, Rallis E, Kalogeromitros D, Rigopoulos D. Cytokines and other mediators in alopecia areata. Mediators Inflamm. 2010;2010:928030. doi: 10.1155/2010/928030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HS, Cho DH, Kim HJ, Lee JY, Cho BK, Park HJ. Immunoreactivity of corticotropin-releasing hormone, adrenocorticotropic hormone and alpha-melanocyte-stimulating hormone in alopecia areata. Exp Dermatol. 2006;15(7):515–522. doi: 10.1111/j.1600-0625.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 65.Megiorni F, Pizzuti A, Mora B, et al. Genetic association of HLA-DQB1 and HLA-DRB1 polymorphisms with alopecia areata in the Italian population. The British journal of dermatology. 2011;165(4):823–827. doi: 10.1111/j.1365-2133.2011.10466.x. [DOI] [PubMed] [Google Scholar]
- 66.Xiao FL, Zhou FS, Liu JB, et al. Association of HLA-DQA1 and DQB1 alleles with alolpecia areata in Chinese Hans. Archives of dermatological research. 2005;297(5):201–209. doi: 10.1007/s00403-005-0608-2. [DOI] [PubMed] [Google Scholar]
- 67.Akar A, Orkunoglu E, Sengul A, Ozata M, Gur AR. HLA class II alleles in patients with alopecia areata. European journal of dermatology : EJD. 2002;12(3):236–239. [PubMed] [Google Scholar]
- 68.Gilhar A, Kam Y, Assy B, Kalish RS. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: evidence for loss of immune privilege. J Invest Dermatol. 2005;124(1):288–289. doi: 10.1111/j.0022-202X.2004.23580.x. [DOI] [PubMed] [Google Scholar]
- 69.Sundberg JP, Silva KA, Edwards K, Black S, Bennett Jenson A, King LE. Failure to induce alopecia areata in C3H/HeJ mice with exogenous interferon gamma. Journal of Experimental Animal Science. 2007;43(4):265–270. [Google Scholar]
- 70.Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155(3):515–521. doi: 10.1111/j.1365-2133.2006.07377.x. [DOI] [PubMed] [Google Scholar]
- 71.Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Investig Dermatol Symp Proc. 2003;8(2):188–194. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 72.Kaka AS, Shaffer DR, Hartmaier R, et al. Genetic modification of T cells with IL-21 enhances antigen presentation and generation of central memory tumor-specific cytotoxic T-lymphocytes. J Immunother. 2009;32(7):726–736. doi: 10.1097/CJI.0b013e3181ad4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romagnani S. Immunological tolerance and autoimmunity. Intern Emerg Med. 2006;1(3):187–196. doi: 10.1007/BF02934736. [DOI] [PubMed] [Google Scholar]
- 74**.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126(1):32–41. doi: 10.1038/sj.jid.5700001. review of cytotoxic T cells in autoimmunity. [DOI] [PubMed] [Google Scholar]
- 75.Gravano DM, Hoyer KK. Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun. 2013;45:68–79. doi: 10.1016/j.jaut.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 76.McElwee KJ, Freyschmidt-Paul P, Zoller M, Hoffmann R. Alopecia areata susceptibility in rodent models. J Investig Dermatol Symp Proc. 2003;8(2):182–187. doi: 10.1046/j.1087-0024.2003.00806.x. [DOI] [PubMed] [Google Scholar]
- 77.Alli R, Nguyen P, Boyd K, Sundberg JP, Geiger TL. A mouse model of clonal CD8+ T lymphocyte-mediated alopecia areata progressing to alopecia universalis. J Immunol. 2012;188(1):477–486. doi: 10.4049/jimmunol.1100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paus R, Eichmuller S, Hofmann U, Czarnetzki BM, Robinson P. Expression of classical and non-classical MHC class I antigens in murine hair follicles. Br J Dermatol. 1994;131(2):177–183. doi: 10.1111/j.1365-2133.1994.tb08488.x. [DOI] [PubMed] [Google Scholar]
- 79.McElwee KJ, Hoffmann R, Freyschmidt-Paul P, et al. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4+ and CD8+ cells. J Invest Dermatol. 2002;119(6):1426–1433. doi: 10.1046/j.1523-1747.2002.19620.x. [DOI] [PubMed] [Google Scholar]
- 80.Zoller M, McElwee KJ, Engel P, Hoffmann R. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118(6):983–992. doi: 10.1046/j.1523-1747.2002.01745.x. [DOI] [PubMed] [Google Scholar]
- 81*.Zhang B, Zhao Y, Cai Z, et al. Early stage alopecia areata is associated with inflammation in the upper dermis and damage to the hair follicle infundibulum. Australas J Dermatol. 2013;54(3):184–191. doi: 10.1111/ajd.12065. characterises the pathology of very early stage alopecia areata in humans. [DOI] [PubMed] [Google Scholar]
- 82.McElwee KJ, Freyschmidt-Paul P, Sundberg JP, Hoffmann R. The pathogenesis of alopecia areata in rodent models. J Investig Dermatol Symp Proc. 2003;8(1):6–11. doi: 10.1046/j.1523-1747.2003.12164.x. [DOI] [PubMed] [Google Scholar]
- 83.McElwee KJ, Spiers EM, Oliver RF. In vivo depletion of CD8+ T cells restores hair growth in the DEBR model for alopecia areata. Br J Dermatol. 1996;135(2):211–217. [PubMed] [Google Scholar]
- 84.McElwee KJ, Spiers EM, Oliver RF. Partial restoration of hair growth in the DEBR model for Alopecia areata after in vivo depletion of CD4+ T cells. Br J Dermatol. 1999;140(3):432–437. doi: 10.1046/j.1365-2133.1999.02705.x. [DOI] [PubMed] [Google Scholar]
- 85.McElwee KJ, Tobin DJ, Bystryn JC, King LE, Jr, Sundberg JP. Alopecia areata: an autoimmune disease? Exp Dermatol. 1999;8(5):371–379. doi: 10.1111/j.1600-0625.1999.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 86*.McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124(5):947–957. doi: 10.1111/j.0022-202X.2005.23692.x. indicates CD8+T cells induce alopecia areata and Treg cells block disease onset in a rodent model. [DOI] [PubMed] [Google Scholar]
- 87.Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest. 1998;101(1):62–67. doi: 10.1172/JCI551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilhar A, Landau M, Assy B, Shalaginov R, Serafimovich S, Kalish RS. Mediation of alopecia areata by cooperation between CD4+ and CD8+ T lymphocytes: transfer to human scalp explants on Prkdc(scid) mice. Arch Dermatol. 2002;138(7):916–922. doi: 10.1001/archderm.138.7.916. [DOI] [PubMed] [Google Scholar]
- 89.Gebhardt T, Whitney PG, Zaid A, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 90.Yu M, Kissling S, Freyschmidt-Paul P, Hoffmann R, Shapiro J, McElwee KJ. Interleukin-6 cytokine family member oncostatin M is a hair-follicle-expressed factor with hair growth inhibitory properties. Exp Dermatol. 2008;17(1):12–19. doi: 10.1111/j.1600-0625.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 91.Tobin DJ. Morphological analysis of hair follicles in alopecia areata. Microsc Res Tech. 1997;38(4):443–451. doi: 10.1002/(SICI)1097-0029(19970815)38:4<443::AID-JEMT12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 92.Lu W, Shapiro J, Yu M, et al. Alopecia areata: pathogenesis and potential for therapy. Expert Rev Mol Med. 2006;8(14):1–19. doi: 10.1017/S146239940601101X. [DOI] [PubMed] [Google Scholar]
- 93*.Veldhoen M, Seddon B. Empowering T helper 17 cells in autoimmunity. Nature medicine. 2010;16(2):166–168. doi: 10.1038/nm0210-166. review of Th17 cells in autoimmunity. [DOI] [PubMed] [Google Scholar]
- 94.Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. Journal of autoimmunity. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 96.Tato CM, O’Shea JJ. Immunology: what does it mean to be just 17? Nature. 2006;441(7090):166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- 97.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature reviews Immunology. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lew BL, Cho HR, Haw S, Kim HJ, Chung JH, Sim WY. Association between IL17A/IL17RA Gene Polymorphisms and Susceptibility to Alopecia Areata in the Korean Population. Annals of dermatology. 2012;24(1):61–65. doi: 10.5021/ad.2012.24.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tembhre MK, Sharma VK. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. The British journal of dermatology. 2013;169(3):543–548. doi: 10.1111/bjd.12396. [DOI] [PubMed] [Google Scholar]
- 100.Tanemura A, Oiso N, Nakano M, Itoi S, Kawada A, Katayama I. Alopecia areata: infiltration of Th17 cells in the dermis, particularly around hair follicles. Dermatology. 2013;226(4):333–336. doi: 10.1159/000350933. [DOI] [PubMed] [Google Scholar]
- 101.Tojo G, Fujimura T, Kawano M, et al. Comparison of interleukin-17-producing cells in different clinical types of alopecia areata. Dermatology. 2013;227(1):78–82. doi: 10.1159/000353159. [DOI] [PubMed] [Google Scholar]
- 102.Avitabile S, Sordi D, Garcovich S, et al. Effective squaric acid dibutylester immunotherapy is associated with a reduction of skin infiltrating T-helper (Th)1 and Th17 cells in alopecia areata patients. The Journal of dermatology. 2015;42(1):98–99. doi: 10.1111/1346-8138.12740. [DOI] [PubMed] [Google Scholar]
- 103.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 104.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 105.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70(4):326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 107.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 108.Csencsits K, Wood SC, Lu G, Bishop DK. Transforming growth factor-beta1 gene transfer is associated with the development of regulatory cells. Am J Transplant. 2005;5(10):2378–2384. doi: 10.1111/j.1600-6143.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- 109.Fu S, Zhang N, Yopp AC, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 − precursors. Am J Transplant. 2004;4(10):1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 110.Andersson J, Tran DQ, Pesu M, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. The Journal of experimental medicine. 2008;205(9):1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daley SR, Ma J, Adams E, Cobbold SP, Waldmann H. A key role for TGF-beta signaling to T cells in the long-term acceptance of allografts. J Immunol. 2007;179(6):3648–3654. doi: 10.4049/jimmunol.179.6.3648. [DOI] [PubMed] [Google Scholar]
- 112.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 113.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 114*.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289–300. doi: 10.1111/j.1365-2567.2005.02317.x. indicates CD8+T cells induce alopecia areata and Treg cells block disease onset in a rodent model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nagahama K, Nishimura E, Sakaguchi S. Induction of tolerance by adoptive transfer of Treg cells. Methods Mol Biol. 2007;380:431–442. doi: 10.1007/978-1-59745-395-0_27. [DOI] [PubMed] [Google Scholar]
- 116.Bennett CL, Brunkow ME, Ramsdell F, et al. A rare polyadenylation signal mutation of the FOXP3 gene (AAUAAA-->AAUGAA) leads to the IPEX syndrome. Immunogenetics. 2001;53(6):435–439. doi: 10.1007/s002510100358. [DOI] [PubMed] [Google Scholar]
- 117.Mahic M, Henjum K, Yaqub S, et al. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. European journal of immunology. 2008;38(3):640–646. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 118.Shin BS, Furuhashi T, Nakamura M, Torii K, Morita A. Impaired inhibitory function of circulating CD4+CD25+ regulatory T cells in alopecia areata. J Dermatol Sci. 2013;70(2):141–143. doi: 10.1016/j.jdermsci.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 119.Conteduca G, Rossi A, Megiorni F, et al. Single nucleotide polymorphisms in the promoter regions of Foxp3 and ICOSLG genes are associated with Alopecia areata. Clinical and experimental medicine. 2014;14(1):91–97. doi: 10.1007/s10238-012-0224-3. [DOI] [PubMed] [Google Scholar]
- 120.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124(3):1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009;39(8):2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 122.Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol. 2013;13(4):257–269. doi: 10.1038/nri3403. [DOI] [PubMed] [Google Scholar]
- 123.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol. 2014;15(9):815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy. 2011;3(10):1223–1233. doi: 10.2217/imt.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 127*.McKinney EF, Lyons PA, Carr EJ, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16(5):586–591. 581–591. doi: 10.1038/nm.2130. indicates CD8+ T cell transcription signature predicts prognosis in autoimmune disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sundberg JP, Boggess D, Silva KA, et al. Major locus on mouse chromosome 17 and minor locus on chromosome 9 are linked with alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2003;120(5):771–775. doi: 10.1046/j.1523-1747.2003.12135.x. [DOI] [PubMed] [Google Scholar]
- 129.Tazi-Ahnini R, Cork MJ, Gawkrodger DJ, et al. Role of the autoimmune regulator (AIRE) gene in alopecia areata: strong association of a potentially functional AIRE polymorphism with alopecia universalis. Tissue Antigens. 2002;60(6):489–495. doi: 10.1034/j.1399-0039.2002.600604.x. [DOI] [PubMed] [Google Scholar]
- 130.McDonagh AJ, Tazi-Ahnini R. Epidemiology and genetics of alopecia areata. Clin Exp Dermatol. 2002;27(5):405–409. doi: 10.1046/j.1365-2230.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- 131.Laakso SM, Kekalainen E, Rossi LH, et al. IL-7 dysregulation and loss of CD8+ T cell homeostasis in the monogenic human disease autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2011;187(4):2023–2030. doi: 10.4049/jimmunol.1100212. [DOI] [PubMed] [Google Scholar]
- 132.Subramanya RD, Coda AB, Sinha AA. Transcriptional profiling in alopecia areata defines immune and cell cycle control related genes within disease-specific signatures. Genomics. 2010;96(3):146–153. doi: 10.1016/j.ygeno.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology. 2002;106(2):127–138. doi: 10.1046/j.1365-2567.2002.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4(9):835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 135.Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9(9):949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- 136.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev. 2013;255(1):165–181. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mueller SN, Zaid A, Carbone FR. Tissue-resident T cells: dynamic players in skin immunity. Front Immunol. 2014;5:332. doi: 10.3389/fimmu.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ariotti S, Beltman JB, Chodaczek G, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109(48):19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14(12):1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 140*.Sowell RT, Marzo AL. Resident-Memory CD8 T Cells and mTOR: Generation, Protection, and Clinical Importance. Front Immunol. 2015;6:38. doi: 10.3389/fimmu.2015.00038. review of tissue resident memory T cell manipulation for clinical benefit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389(6654):978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 142.Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176(7):4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 143.Tufail S, Badrealam KF, Sherwani A, Gupta UD, Owais M. Tissue specific heterogeneity in effector immune cell response. Front Immunol. 2013;4:254. doi: 10.3389/fimmu.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205(10):2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yano S, Nakamura K, Okochi H, Tamaki K. Analysis of the expression of cutaneous lymphocyte-associated antigen on the peripheral blood and cutaneous lymphocytes of alopecia areata patients. Acta Derm Venereol. 2002;82(2):82–85. doi: 10.1080/00015550252948077. [DOI] [PubMed] [Google Scholar]
- 146.Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harbor perspectives in biology. 2012;4(8):a007401. doi: 10.1101/cshperspect.a007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reizis B. Classical dendritic cells as a unique immune cell lineage. The Journal of experimental medicine. 2012;209(6):1053–1056. doi: 10.1084/jem.20121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nature immunology. 2004;5(12):1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 149*.Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. The Journal of investigative dermatology. 2009;129(2):302–308. doi: 10.1038/jid.2008.225. review of skin dendritic cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nature reviews Immunology. 2014;14(6):417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 151.Forster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends in immunology. 2012;33(6):271–280. doi: 10.1016/j.it.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 152*.Benoist C, Mathis D. Mast cells in autoimmune disease. Nature. 2002;420(6917):875–878. doi: 10.1038/nature01324. review of mast cells in autoimmune disease. [DOI] [PubMed] [Google Scholar]
- 153.Brown MA, Hatfield JK. Mast Cells are Important Modifiers of Autoimmune Disease: With so Much Evidence, Why is There Still Controversy? Frontiers in immunology. 2012;3:147. doi: 10.3389/fimmu.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Theoharides TC, Valent P, Akin C. Mast Cells, Mastocytosis, and Related Disorders. The New England journal of medicine. 2015;373(2):163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 155.Harvima IT, Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta dermato-venereologica. 2011;91(6):644–650. doi: 10.2340/00015555-1197. [DOI] [PubMed] [Google Scholar]
- 156.Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochimica et biophysica acta. 2012;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu Y, Chen G. Mast cell and autoimmune diseases. Mediators of inflammation. 2015;2015:246126. doi: 10.1155/2015/246126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Christy AL, Brown MA. The multitasking mast cell: positive and negative roles in the progression of autoimmunity. Journal of immunology. 2007;179(5):2673–2679. doi: 10.4049/jimmunol.179.5.2673. [DOI] [PubMed] [Google Scholar]
- 159.Sardesai VR, Prasad S, Agarwal TD. A study to evaluate the efficacy of various topical treatment modalities for alopecia areata. Int J Trichology. 2012;4(4):265–270. doi: 10.4103/0974-7753.111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Freyschmidt-Paul P, Happle R, McElwee KJ, Hoffmann R. Alopecia areata: treatment of today and tomorrow. J Investig Dermatol Symp Proc. 2003;8(1):12–17. doi: 10.1046/j.1523-1747.2003.12165.x. [DOI] [PubMed] [Google Scholar]
- 161.Freyschmidt-Paul P, Hoffmann R, Levine E, Sundberg JP, Happle R, McElwee KJ. Current and potential agents for the treatment of alopecia areata. Curr Pharm Des. 2001;7(3):213–230. doi: 10.2174/1381612013398266. [DOI] [PubMed] [Google Scholar]
- 162.Chu T, Al Jasser M, Alharbi A, Othman A, McElwee KJ, Shapiro J. Benefit of Different Concentrations of Intralesional Triamcinolone Acetonide in Alopecia Areata: An Intrasubject Pilot Study. J Am Acad Dermatol. 2015 doi: 10.1016/j.jaad.2015.04.049. In press. [DOI] [PubMed] [Google Scholar]
- 163.Happle R, Hausen BM, Wiesner-Menzel L. Diphencyprone in the treatment of alopecia areata. Acta Derm Venereol. 1983;63(1):49–52. [PubMed] [Google Scholar]
- 164.Happle R, Kalveram KJ, Buchner U, Echternacht-Happle K, Goggelmann W, Summer KH. Contact allergy as a therapeutic tool for alopecia areata: application of squaric acid dibutylester. Dermatologica. 1980;161(5):289–297. doi: 10.1159/000250380. [DOI] [PubMed] [Google Scholar]
- 165.Shapiro J. Topical immunotherapy in the treatment of chronic severe alopecia areata. Dermatol Clin. 1993;11(3):611–617. [PubMed] [Google Scholar]
- 166*.Sun J, Silva KA, McElwee KJ, King LE, Jr, Sundberg JP. The C3H/HeJ mouse and DEBR rat models for alopecia areata: review of preclinical drug screening approaches and results. Exp Dermatol. 2008;17(10):793–805. doi: 10.1111/j.1600-0625.2008.00773.x. reviews rodent model screening of novel or approved drugs for efficacy to treat human alopecia areata. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Herbst V, Zoller M, Kissling S, Wenzel E, Stutz N, Freyschmidt-Paul P. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16(5):537–542. [PubMed] [Google Scholar]
- 168.Zoller M, Freyschmidt-Paul P, Vitacolonna M, McElwee KJ, Hummel S, Hoffmann R. Chronic delayed-type hypersensitivity reaction as a means to treat alopecia areata. Clin Exp Immunol. 2004;135(3):398–408. doi: 10.1111/j.1365-2249.2003.02380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marhaba R, Vitacolonna M, Hildebrand D, Baniyash M, Freyschmidt-Paul P, Zoller M. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol. 2007;179(8):5071–5081. doi: 10.4049/jimmunol.179.8.5071. [DOI] [PubMed] [Google Scholar]
- 170.Freyschmidt-Paul P, Ziegler A, McElwee KJ, et al. Treatment of alopecia areata in C3H/HeJ mice with the topical immunosuppressant FK506 (Tacrolimus) Eur J Dermatol. 2001;11(5):405–409. [PubMed] [Google Scholar]
- 171.Price VH, Willey A, Chen BK. Topical tacrolimus in alopecia areata. J Am Acad Dermatol. 2005;52(1):138–139. doi: 10.1016/j.jaad.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 172.Ucak H, Kandi B, Cicek D, Halisdemir N, Dertlioglu SB. The comparison of treatment with clobetasol propionate 0.05% and topical pimecrolimus 1% treatment in the treatment of alopecia areata. J Dermatolog Treat. 2012;23(6):410–420. doi: 10.3109/09546634.2011.590788. [DOI] [PubMed] [Google Scholar]
- 173.Verma DD, Verma S, McElwee KJ, Freyschmidt-Paul P, Hoffman R, Fahr A. Treatment of alopecia areata in the DEBR model using Cyclosporin A lipid vesicles. Eur J Dermatol. 2004;14(5):332–338. [PubMed] [Google Scholar]
- 174.Masihi KN. Fighting infection using immunomodulatory agents. Expert Opin Biol Ther. 2001;1(4):641–653. doi: 10.1517/14712598.1.4.641. [DOI] [PubMed] [Google Scholar]
- 175.Anderson RP, Jabri B. Vaccine against autoimmune disease: antigen-specific immunotherapy. Curr Opin Immunol. 2013;25(3):410–417. doi: 10.1016/j.coi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marchingo JM, Kan A, Sutherland RM, et al. T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science. 2014;346(6213):1123–1127. doi: 10.1126/science.1260044. [DOI] [PubMed] [Google Scholar]
- 177**.Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435(7042):612–619. doi: 10.1038/nature03727. reviews the design of effective immunotherapies for human autoimmunity. [DOI] [PubMed] [Google Scholar]
- 178.Fong KY. Immunotherapy in autoimmune diseases. Ann Acad Med Singapore. 2002;31(6):702–706. [PubMed] [Google Scholar]
- 179.Liblau RS, Wong FS, Mars LT, Santamaria P. Autoreactive CD8 T cells in organ-specific autoimmunity: emerging targets for therapeutic intervention. Immunity. 2002;17(1):1–6. doi: 10.1016/s1074-7613(02)00338-2. [DOI] [PubMed] [Google Scholar]
- 180.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 181.Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996;288(3):153–156. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- 182.Kasumagic-Halilovic E, Prohic A, Cavaljuga S. Tumor necrosis factor-alpha in patients with alopecia areata. Indian J Dermatol. 2011;56(5):494–496. doi: 10.4103/0019-5154.87124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pan Y, Rao NA. Alopecia areata during etanercept therapy. Ocul Immunol Inflamm. 2009;17(2):127–129. doi: 10.1080/09273940802596559. [DOI] [PubMed] [Google Scholar]
- 184.Ettefagh L, Nedorost S, Mirmirani P. Alopecia areata in a patient using infliximab: new insights into the role of tumor necrosis factor on human hair follicles. Arch Dermatol. 2004;140(8):1012. doi: 10.1001/archderm.140.8.1012-a. [DOI] [PubMed] [Google Scholar]
- 185.Skurkovich S, Korotky NG, Sharova NM, Skurkovich B. Treatment of alopecia areata with anti-interferon-gamma antibodies. J Investig Dermatol Symp Proc. 2005;10(3):283–284. doi: 10.1111/j.0022-202X.2005.10130_6.x. [DOI] [PubMed] [Google Scholar]
- 186.Sundberg JP, McElwee KJ, Carroll JM, King LE., Jr Hypothesis testing: CTLA4 co-stimulatory pathways critical in the pathogenesis of human and mouse alopecia areata. J Invest Dermatol. 2011;131(11):2323–2324. doi: 10.1038/jid.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 2002;109(3):295–299. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leung MC, Sutton CW, Fenton DA, Tobin DJ. Trichohyalin is a potential major autoantigen in human alopecia areata. J Proteome Res. 2010;9(10):5153–5163. doi: 10.1021/pr100422u. [DOI] [PubMed] [Google Scholar]
- 189.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 190.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17(6):624–631. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 192.Traidl C, Sebastiani S, Albanesi C, et al. Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J Immunol. 2000;165(6):3058–3064. doi: 10.4049/jimmunol.165.6.3058. [DOI] [PubMed] [Google Scholar]
- 193.Chun-yan G, Bo H, Hong C, Hong-lei J, Xiu-zhen H. Anti-perforin neutralizing antibody reduces myocardial injury in viral myocarditis. Cardiol Young. 2009;19(6):601–607. doi: 10.1017/S104795110999182X. [DOI] [PubMed] [Google Scholar]
- 194.Ko GJ, Jang HR, Huang Y, et al. Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22(4):732–742. doi: 10.1681/ASN.2010010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cao Y, Xu W, Xiong S. Adoptive transfer of regulatory T cells protects against Coxsackievirus B3-induced cardiac fibrosis. PLoS One. 2013;8(9):e74955. doi: 10.1371/journal.pone.0074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Heuer JG, Zhang T, Zhao J, et al. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174(11):7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 197.Yi S, Ji M, Wu J, et al. Adoptive transfer with in vitro expanded human regulatory T cells protects against porcine islet xenograft rejection via interleukin-10 in humanized mice. Diabetes. 2012;61(5):1180–1191. doi: 10.2337/db11-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu W, Lan Q, Chen M, et al. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PLoS One. 2012;7(7):e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150(7):748–751. doi: 10.1001/jamadermatol.2014.504. [DOI] [PubMed] [Google Scholar]
- 200.Wong M. What has happened in the last 50 years in immunology. J Paediatr Child Health. 2015;51(2):135–139. doi: 10.1111/jpc.12834. [DOI] [PubMed] [Google Scholar]
- 201.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Peelen E, Knippenberg S, Muris AH, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmunity reviews. 2011;10(12):733–743. doi: 10.1016/j.autrev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 203.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170(6):1299–1304. doi: 10.1111/bjd.12980. [DOI] [PubMed] [Google Scholar]
- 205.Kim DH, Lee JW, Kim IS, et al. Successful treatment of alopecia areata with topical calcipotriol. Annals of dermatology. 2012;24(3):341–344. doi: 10.5021/ad.2012.24.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206*.Teoh D, Johnson LA, Hanke T, McMichael AJ, Jackson DG. Blocking development of a CD8+ T cell response by targeting lymphatic recruitment of APC. J Immunol. 2009;182(4):2425–2431. doi: 10.4049/jimmunol.0803661. indicates targeting lymphatic recruitment of antigen presenting cells ahead of other cell adhesion molecule-dependent events is effective. [DOI] [PubMed] [Google Scholar]
- 207.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Freyschmidt-Paul P, Seiter S, Zoller M, et al. Treatment with an anti-CD44v10-specific antibody inhibits the onset of alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2000;115(4):653–657. doi: 10.1046/j.1523-1747.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- 209.Fiorino G, Correale C, Fries W, Repici A, Malesci A, Danese S. Leukocyte traffic control: a novel therapeutic strategy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6(4):567–572. doi: 10.1586/eci.10.40. [DOI] [PubMed] [Google Scholar]
- 210.Pucci E, Giuliani G, Solari A, et al. Natalizumab for relapsing remitting multiple sclerosis. Cochrane Database Syst Rev. 2011;(10):CD007621. doi: 10.1002/14651858.CD007621.pub2. [DOI] [PubMed] [Google Scholar]
- 211.Peakman M, Dayan CM. Antigen-specific immunotherapy for autoimmune disease: fighting fire with fire? Immunology. 2001;104(4):361–366. doi: 10.1046/j.1365-2567.2001.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7(9):665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 213.Correale J, Farez M, Gilmore W. Vaccines for multiple sclerosis: progress to date. CNS Drugs. 2008;22(3):175–198. doi: 10.2165/00023210-200822030-00001. [DOI] [PubMed] [Google Scholar]
- 214.Fontoura P, Garren H, Steinman L. Antigen-specific therapies in multiple sclerosis: going beyond proteins and peptides. Int Rev Immunol. 2005;24(5–6):415–446. doi: 10.1080/08830180500379655. [DOI] [PubMed] [Google Scholar]
- 215.Wang EH, Breitkopf T, Akhoundsadegh N, Dutz JP, Shapiro J, McElwee KJ. Screening for autoantigen epitopes involved in the development of alopecia areata. J Invest Dermatol. 2014;134:S43. [Google Scholar]
- 216.Erb U, Freyschmidt-Paul P, Zoller M. Tolerance induction by hair-specific keratins in murine alopecia areata. J Leukoc Biol. 2013;94(4):845–857. doi: 10.1189/jlb.0413196. [DOI] [PubMed] [Google Scholar]
- 217.Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546. doi: 10.1155/2013/348546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang X, Hao J, Leung G, et al. Hair follicle dermal sheath derived cells improve islet allograft survival without systemic immunosuppression. J Immunol Res. 2015;2015:607328. doi: 10.1155/2015/607328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219*.Li Y, Yan B, Wang H, et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med. 2015;13:87. doi: 10.1186/s12916-015-0331-6. suggests patients with severe AA achieved improved hair regrowth after immune system eduction with Stem Cell Educator therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jalili RB, Forouzandeh F, Rezakhanlou AM, et al. Local expression of indoleamine 2,3 dioxygenase in syngeneic fibroblasts significantly prolongs survival of an engineered three-dimensional islet allograft. Diabetes. 2010;59(9):2219–2227. doi: 10.2337/db09-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ruckert R, Hofmann U, van der Veen C, Bulfone-Paus S, Paus R. MHC class I expression in murine skin: developmentally controlled and strikingly restricted intraepithelial expression during hair follicle morphogenesis and cycling, and response to cytokine treatment in vivo. J Invest Dermatol. 1998;111(1):25–30. doi: 10.1046/j.1523-1747.1998.00228.x. [DOI] [PubMed] [Google Scholar]