Abstract
Cdc37 is a relatively poorly conserved and yet essential molecular chaperone. It has long been thought to function primarily as an accessory factor for Hsp90, notably directing Hsp90 to kinases as substrates. More recent discoveries challenge this simplistic view. Cdc37 client proteins other than kinases have now been found, and Cdc37 displays a variety of Hsp90-independent activities both in vitro and in vivo. It can function as a molecular chaperone by itself, interact with other Hsp90 cochaperones in the absence of Hsp90, and even support yeast growth and protein folding without its Hsp90-binding domain. Thus, for many substrates, there may be many alternative chaperone pathways involving Cdc37, Hsp90, or both.
INTRODUCTION
The molecular chaperone Hsp90 requires a cohort of cochaperones to function correctly. Cochaperones bind Hsp90 directly and are thought to modulate its substrate recognition, specificity, stability, and binding (Picard 2002). The CDC37 gene was first identified in budding yeast as a temperature-sensitive cell-division cycle mutation that arrests cells in G1 at the nonpermissive temperature (Reed 1980). Cdc37 has proven to be essential for viability not only in yeast (Gerber et al 1995) but also in Drosophila (Cutforth and Rubin 1994) and Caenorhabditis elegans (Kamath et al 2003). Cdc37 was soon linked to Hsp90 because of its identification in Hsp90-dependent signaling pathways and its physical association with Hsp90 clients, such as Raf-1, Cdk4, and Src family kinases (Hunter and Poon 1997). In the literature, metazoan Cdc37 is sometimes referred to as p50Cdc37, but all orthologs will be referred to as Cdc37 in this article. A seminal review on Cdc37 described it as a substrate-specificity factor directing Hsp90 to kinases (Hunter and Poon 1997). We will critically appraise this notion by focusing on more recent discoveries, which demonstrate that Cdc37 has a wider range of clients and a more complex relationship with Hsp90 than was thought previously.
CDC37: AN HSP90 COCHAPERONE
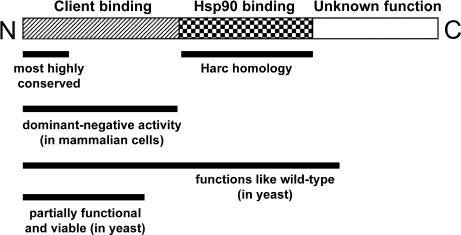
A variety of evidence demonstrates that Cdc37 acts, at least in part, as an Hsp90 cochaperone, although its precise role for Hsp90 function remains unclear. In vitro, Cdc37 binds Hsp90 directly and inhibits its adenosine triphosphatase (ATPase) activity (Siligardi et al 2002). Because client recruitment into the Hsp90 complex may require suppression of adenosine triphosphate turnover during the substrate-loading phase, Cdc37 may strengthen and prolong the interaction between Hsp90 and its client. Indeed, recombinant Cdc37 can enhance Hsp90 chaperone activity toward an artificial substrate in vitro (Kimura et al 1997). Further evidence comes from the observation that truncated Cdc37 mutants (Fig 1) that cannot interact with Hsp90 behave in a dominant-negative fashion by displacing full-length Cdc37 from the Hsp90-client complex and by reducing the affinity of the client for Hsp90 (Grammatikakis et al 1999; Rao et al 2001; Shao et al 2001). The intricate relationship between Cdc37 and Hsp90 is illustrated by the finding that their interaction is stabilized by the client protein (Hartson et al 2000). Like Hsp90, Cdc37 can specifically associate with nascent polypeptide chains for a prolonged period after synthesis. For example, Cdc37 is bound to the emerging heme-regulated inhibitor (HRI) before synthesis is complete and before the kinase is released from the ribosome (Shao et al 2001). Many clients are associated with both Cdc37 and Hsp90, and their folding, maturation, or stability (or all) presumably depend on the activity of both chaperones. Cdc37 mediates the formation of Hsp90–Raf-1 (Grammatikakis et al 1999) and Hsp90-Cdk4 complexes (Stepanova et al 1996). These interactions are necessary for protein stability and kinase function. Indeed, the transcription factor C/EBPα disrupts the Cdk4-Cdc37-Hsp90 complex and thereby triggers degradation of the client protein (Wang et al 2002a). Thus, by controlling kinase activity in a dose-dependent manner, Cdc37 becomes one of the rate-limiting components in certain signaling pathways (Grammatikakis et al 1999) and in cell transformation (Stepanova et al 2000a). In assisting Hsp90, Cdc37 may function primarily in the folding and stabilization of de novo–synthesized proteins rather than as a scavenger of denatured proteins, and its intrinsic chaperone activity may facilitate its association with these substrates (Kimura et al 1997).
Fig 1.
Schematic representation of Cdc37 domains. The exact boundaries of the domains and details of their functionality are described in more detail in the text and in Grammatikakis et al (1999), Scholz et al (2000, 2001), Rao et al (2001), and Lee et al (2002). The highest sequence conservation between species is found in the first 30 amino acids
HSP90-INDEPENDENT ACTIVITIES
Most studies have shown Cdc37 working in cooperation with Hsp90. However, Cdc37 also displays Hsp90-independent activities. In vitro, recombinant Cdc37 has intrinsic Hsp90-like molecular chaperone activity in the absence of Hsp90. It can stabilize inherently unstable kinases such as casein kinase II (CKII) and function as a “holding chaperone,” maintaining unfolded substrates in a refolding competent state for other chaperones (Kimura et al 1997). The combination of mutations in both HSP90 and CDC37 genes impairs viability in budding yeast and Drosophila, suggesting that Hsp90 and Cdc37 fulfill partially redundant functions. Increased levels of Cdc37 can partially compensate for a mutation in the Hsp90 gene to maintain the activity of the heterologously expressed kinase v-Src (Kimura et al 1997) and to increase androgen receptor (AR) protein levels (Rao et al 2001). Mutations in either CDC37 or the yeast Hsp90 genes HSP82/HSC82 lead to a decrease in v-Src activity and suppress its toxicity, but the alteration in phosphorylation activity of v-Src is different depending on which chaperone is defective (Kimura et al 1997). Similarly, for Mps1 the requirements for Cdc37 and Hsp90 can be separated. Mps1 is affected by mutations in Cdc37 but not by an alteration in Hsp90 activity (Schutz et al 1997).
Recently, Caplan and colleagues found that the Hsp90-binding domain of Cdc37 is dispensable for many Cdc37 functions (Lee et al 2002). High expression of this Cdc37 mutant is sufficient for viability under normal growth conditions, but it appears that some Hsp90-dependent functions of Cdc37 are required for viability at higher temperatures. This truncated Cdc37 still permits signal transduction through the pheromone-signaling pathway, which is mediated by the Cdc37 substrate Ste11 (Abbas-Terki et al 2000) but only at approximately 70% of the wild-type levels (Lee et al 2002). In an hsc82 mutant yeast strain, the truncated Cdc37 can partially suppress loss of v-Src activity. Consistent with a role for Hsp90-independent functions of Cdc37, not all of the endogenous Cdc37 is associated with Hsp90 in yeast (Chang and Lindquist 1994), and immunoprecipitation experiments revealed that only half the Cdc37-Cdk4 complexes in mammalian cells contain Hsp90 (Dai et al 1996). Thus, Cdc37 can target clients independently of Hsp90, and Hsp90 is not absolutely essential for Cdc37 function. Both yeast and mammalian Cdc37 are capable of some client recognition, chaperone activity, and stabilization of unfolded clients without Hsp90, but the reaction is optimal when both chaperones cooperate.
INTERACTION WITH CLIENT PROTEINS
Cdc37 has been demonstrated to interact with many kinases; consequently, it is involved in many cellular processes (often along with Hsp90) including deoxyribonucleic acid and protein synthesis, cell cycle regulation, signal transduction, and transcription. We have posted a comprehensive list of both genetic and biochemical interactions of Cdc37 at http://www.picard.ch/DP/DPhome.html.
It is not known how Cdc37 recognizes client proteins. In the case of kinases, Cdc37 could possibly recognize the structurally conserved catalytic domain because mutants lacking this domain fail to interact with Cdc37 (Nair et al 1996; Silverstein et al 1998). Moreover, in yeast 2-hybrid assays, Cdc37 interacts with the N-terminal portion of the catalytic domains of several protein kinases but not all cell cycle–dependent kinases (Lamphere et al 1997; Mort-Bontemps-Soret et al 2002). Interestingly, the direct interaction of Cdc37 with the kinase domain of the IκB kinase (IKK) is stabilized by Hsp90, which suggests a cooperative binding of Cdc37 and Hsp90 to IKK (Chen et al 2002). Binding of Cdc37 to the kinase domain of HRI is regulated by the nucleotide-mediated conformational switching of Hsp90 (Shao et al 2001), providing further evidence for the complex partnership between Cdc37 and Hsp90.
The precise requirements for Cdc37 for stability and activity may be as diverse as the kinases it interacts with. By and large, it remains unclear whether Cdc37 stabilizes clients by docking them to Hsp90, by enhancing the strength of the client-Hsp90 interaction, or by some direct chaperone action on the client. Studies in the mammalian system indicate that one of the key roles of Cdc37 for Raf-1 is to promote the assembly of a heterotrimeric complex with Hsp90 (Grammatikakis et al 1999). The cell cycle kinases Cdc28 and Cak1 are destabilized in a cdc37 yeast mutant and as a result fail to form complexes with cyclins (Farrell and Morgan 2000). Hck catalytic activity can also be significantly enhanced by overexpression of Cdc37 because Cdc37 can promote association with Hsp90 and folding into a catalytically active conformation (Scholz et al 2000). Cdc37 can bind directly to Akt (protein kinase B), and this interaction is not affected by Hsp90 inhibitors, even though they shorten the half-life of the kinase (Basso et al 2002).
Studies on the subcellular localization of Cdc37 provide further hints about its potential client proteins. Antibodies to Cdc37 stain the perinuclear region and give a punctate staining in the cytoplasm (Cutforth and Rubin 1994; Stepanova et al 1996; Perdew et al 1997). Some Cdc37 was also detected in the mitotic apparatus and colocalizes with the kinase Aurora B on the spindle microtubules and midbody during mitosis in Drosophila spermatocytes (Lange et al 2002). Abrogation of Cdc37 by ribonucleic acid interference in Drosophila results in cytokinesis failure and problems in chromosome segregation, a phenotype that may be due to the inactivation of Aurora B (Lange et al 2002). These findings suggest a role for Cdc37, which is involved in spindle pole body duplication (Schutz et al 1997), in meiosis and mitosis, consistent with the previously established genetic interaction between CDC37 and MPS1.
CDC37 EXTENDS ITS RANGE OF CLIENT PROTEINS
Cdc37 is clearly not entirely dedicated to kinases as substrates, although they dominate the currently known list of Cdc37 clients. In yeast, heterologously expressed AR requires Cdc37 function for full hormone-dependent transactivation (Fliss et al 1997). Different steroid receptors from vertebrates are differentially affected by cdc37 mutations (Fliss et al 1997) and have different binding affinities for Cdc37 (Rao et al 2001). Cdc37 binds the AR through its ligand-binding domain but not the closely related glucocorticoid receptor (Rao et al 2001). The interaction between Cdc37 and AR is partially dependent on Hsp90 and is disrupted by the Hsp90 inhibitor geldanamycin (Rao et al 2001). Cdc37 also interacts specifically with the hepadnavirus (hepatitis B virus) reverse transcriptase (RT) (Wang et al 2002b). Surprisingly, although RT is not a kinase, it nevertheless shares structural features with the kinase Raf-1. Cdc37 modulates the function of RT by mediating its interaction with Hsp90. It thus acts as a host cell cofactor for hepadnavirus replication.
Once the Cdc37 interactome is established, it will become clear to what extent Cdc37 is preferentially dedicated to kinases or whether a historical or experimental bias has precluded a more thorough evaluation of its role for other types of proteins.
CDC37 DOMAINS
In the budding yeast, Saccharomyces cerevisiae, Cdc37 is a protein of 506 amino acids (58.4 kDa) and comprises only about 0.01% of soluble protein (Kimura et al 1997). The human Cdc37 contains 378 residues and is only 19% identical to yeast Cdc37, with the highest conservation found through the first 30 amino acids (Fig 2). Even though mammalian and Drosophila Cdc37 sequences diverge considerably from yeast Cdc37, there is still some functional conservation between yeast and higher eukaryotes. For example, Drosophila Cdc37 can complement a yeast mutant strain (Cutforth and Rubin 1994), and as mentioned above, the yeast Hsp90-Cdc37 system supports proper folding of vertebrate v-Src (Dey et al 1996) and AR (Fliss et al 1997). Sequence analysis of Cdc37 does not reveal any homology with known domains or functional motifs in other proteins.
Fig 2.
Alignment of N-terminal Cdc37 sequences. Sequences corresponding to the 70–80 N-terminal amino acids were chosen from a range of representative species, aligned with ClustalW, and displayed with Boxshade. The alignment includes sequences from 6 different species (Gg, chicken; Hs, human; Dm, Drosophila melanogaster; Ce, Caenorhabditis elegans; Sp, Schizosaccharomyces pombe; and Sc, Saccharomyces cerevisiae), and the Cdc37 relative Harc from humans. Residues of identity and similarity are shown in black and gray, respectively
Several cdc37 yeast mutants that arrest growth at the nonpermissive temperature have been isolated. The cdc37-34 allele encodes a mutant with a substitution (S14L) in a conserved residue that corresponds to a CKII phosphorylation site (Dey et al 1996; Bandhakavi et al 2003). The mutation present in the cdc37-184 allele, which is synthetically lethal with a kin28 mutant (Mort-Bontemps-Soret et al 2002), results in a substitution in the putative Hsp90-binding site (A275D) (see below). A Cdc37 mutant that is C-terminally truncated at residue 388 behaves like a wild type (Lee et al 2002). In contrast, a strain with the cdc37-1 allele, which has a mutation at codon 360, resulting in a more extensive truncation, is thermosensitive and displays a decrease in client activity (Reed 1980; Gerber et al 1995).
Domain-mapping studies with mammalian Cdc37 first indicated that the C-terminal half is required for interaction with Hsp90 (Fig 1). A mutant lacking this portion fails to interact with Hsp90 (Grammatikakis et al 1999; Shao et al 2001), yet it is still capable of binding to client proteins (eg, Raf-1, HRI, and RT) (Grammatikakis et al 1999; Shao et al 2001; Wang et al 2002b). The first 8 amino acids at the N-terminus are essential for client-binding activity, but it is not known if they are required for direct binding to the client or for supporting the structure of the substrate-binding domain (Shao et al 2001). Conversely, an N-terminally truncated mutant that retains the Hsp90-binding domain is still able to suppress the ATPase activity of Hsp90 (Siligardi et al 2002).
A relative of Cdc37, termed Harc (Hsp90-associating relative of Cdc37), was identified in humans and other vertebrates, but no ortholog was found in other eukaryotes (Scholz et al 2001). Human Harc is 31% identical to human Cdc37. Interestingly, with 62% identity, the central region corresponding to the Hsp90-binding domain is particularly conserved (Scholz et al 2001). The Hsp90-binding domain of Cdc37 was thus narrowed down to a 120–amino acid midsection, and based on sequence similarity with human Cdc37, the putative Hsp90-binding site in yeast Cdc37 is proposed to lie between K243 and R343 (Fig 1) (Lee et al 2002). Remarkably, the C-terminal 118 amino acids are dispensable for function with respect to cell viability and v-Src folding, at least in yeast (Lee et al 2002). Therefore, Cdc37 can tentatively be divided into 3 domains: the N-terminal client-binding domain, the middle Hsp90-binding domain, and a C-terminal domain with unknown function (Fig 1).
COMPLEXES WITH OTHER HSP90 COCHAPERONES
The processing of substrates by the Hsp90 molecular chaperone system involves a complex choreography of interactions between Hsp90 and its cochaperones. A molecular understanding depends on establishing the precise nature and timing of these different complexes, including those with Cdc37. In this context, we know particularly little about Cdc37. Surprisingly, even its binding surface on Hsp90 is unknown (Picard 2002). In contrast, the binding domain of Hsp90 for cochaperones containing tetratricopeptide repeats (TPR) has been well characterized. When competition binding experiments between Cdc37 and TPR-containing proteins but not the TPR domain alone showed mutually exclusive binding to Hsp90, this was taken as an indication that the 2 binding sites overlap or are topologically adjacent (Silverstein et al 1998). However, we have recently discovered that Cdc37 can interact directly with the TPR-containing cochaperones Sti1 and Cpr7 in yeast (Abbas-Terki et al 2002), providing an alternative explanation for the aforementioned competition. An isolated TPR domain of Sti1 is apparently not sufficient to bind Cdc37 (our unpublished results), which correlates with the inability of the TPR domain to compete for binding to Hsp90. Cdc37 and the TPR-containing Hsp90 cochaperones Hop (Sti1 in yeast), FKBP52, cyclophilin-40, and protein phosphatase 5 also occur together with Hsp90 in native heterocomplexes in rabbit reticulocyte lysate, both in the presence and in the absence of a client kinase (Hartson et al 2000; Shao et al 2002). A small fraction of Cdc37 co-immunoadsorbs with Hsp90 complexes containing p23, their association being formed indirectly through Hsp90 (Hartson et al 2000).
A multitude of different interactions among cochaperones could contribute to building a variety of mixed Hsp90 heterocomplexes in vivo, which could differentially modulate Hsp90 substrate specificity and activity. The diversity of Cdc37 interactions with Hsp90 and its cochaperones is mirrored by a complex genetic interplay in yeast. Mutations of cdc37 and sti1 are synthetically lethal (Abbas-Terki et al 2002), and overexpression of Cdc37 suppresses part of the phenotype displayed by a Δsti1 strain (Lee et al 2002). Even a C-terminally truncated Cdc37, incapable of Hsp90 binding, can confer viability and partially stabilize v-Src independently of Sti1 (Lee et al 2002). It has been speculated that Cdc37 may be capable of sustaining a Sti1 bypass pathway by interacting directly with Hsp90 and the client. Similarly, increased levels of Cdc37 may be able to bypass the Hsp90 requirement of certain clients by reinforced collaboration with Sti1. It remains to be established to what extent Hsp90-dependent and -independent activities of Cdc37 involve the recruitment of Sti1 and other Hsp90 cochaperones.
OPEN QUESTIONS
We still do not know how and why Cdc37 recognizes clients, and what its full range of clients is, especially now that it has been shown to interact with proteins other than kinases (Rao et al 2001; Wang et al 2002b). For Cdc37 to determine the substrate specificity of Hsp90, one of the following modes of action would have to apply: (1) Cdc37 binds the substrate first and then recruits Hsp90 to it, (2) Cdc37 binds Hsp90 and modifies its substrate specificity, or (3) Cdc37 recognizes the substrate jointly with Hsp90. In all 3 cases, the recruitment of Hsp90 to substrate would be modified by the presence of Cdc37. At this point, however, there is no evidence either way, and it is equally conceivable that Cdc37 recognizes Hsp90 preferentially when the latter is bound to certain types of substrates. In this case the roles would be inverted, with Hsp90 serving to recruit Cdc37, and its primary role then might be to inhibit the ATPase activity of Hsp90 to allow it to hold on longer to a particular substrate. The rules for how client proteins that do not interact with Hsp90 are recognized by Cdc37 might yet be different, and what exactly Cdc37-Sti1 complexes do and whether they recognize yet other sets of substrates remain unknown as well.
Endogenous Cdc37 levels are normally low and certainly limiting for a variety of protein-folding pathways. Increased levels of Cdc37 may contribute to oncogenic transformation in the prostate because tumors contain much higher levels than normal prostate tissue (Stepanova et al 2000b). Targeting Cdc37 expression to mammary tissues, where it is not normally present, resulted in transgenic mice developing mammary gland tumors at the same rate as cyclin D1 transgenic mice, indicating that CDC37 can function as an oncogene in mice (Stepanova et al 2000a). Increased levels of Cdc37 can rescue destabilized kinase mutants (Scholz et al 2000); Cdc37 may therefore contribute to the capacitor function of Hsp90 (Rutherford and Lindquist 1998) in buffering against dramatic changes in evolution.
Very little is known about the transcriptional and posttranslational regulation of Cdc37. Both Cdc37 and Harc are serine phosphorylated (Scholz et al 2001), but the physiological significance and the regulation of this phosphorylation is only beginning to be elucidated. Serine 14 and 17 are the major sites of phosphorylation in yeast, possibly for CKII (Bandhakavi et al 2003). The cdc37-34 allele with a mutation at codon 14 confers a temperature-sensitive phenotype (see above), whereas the double mutant has a severe growth defect even at normal temperatures. This mutant has decreased CKII activity, demonstrating the existence of a positive feedback loop between Cdc37 and CKII, which is needed to maintain the activity of other Cdc37 clients (Bandhakavi et al 2003).
Significant sequence diversity occurs within metazoans and between metazoans and yeast (Fig 2). Even more strikingly, plants seem to lack Cdc37 altogether, or at least their genomes contain no recognizable homolog. Plants must have evolved other mechanisms to perform Cdc37 functions, perhaps with other Hsp90 cochaperones taking over some of them. Chaperoning protein folding being such a key process, considerable redundancy has been built into it. Different species may not need all systems, and a given species may not need all of them all the time.
Acknowledgments
We thank Peter Dudek for critical comments on the manuscript. M.M. is supported by a Federation of European Biochemical Societies postdoctoral fellowship. Chaperone projects in the D.P. laboratory are supported by the Canton de Genève, the Swiss National Science Foundation, and the Fondation Medic.
REFERENCES
- Abbas-Terki T, Briand P-A, Donzé O, Picard D. The Hsp90 co-chaperones Cdc37 and Sti1 interact physically and genetically. Biol Chem. 2002;383:1335–1342. doi: 10.1515/BC.2002.152. [DOI] [PubMed] [Google Scholar]
- Abbas-Terki T, Donzé O, Picard D. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 2000;467:111–116. doi: 10.1016/s0014-5793(00)01134-0. [DOI] [PubMed] [Google Scholar]
- Bandhakavi S, McCann RO, Hanna DE, Glover CV. A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J Biol Chem. 2003;278:2829–2836. doi: 10.1074/jbc.M206662200. [DOI] [PubMed] [Google Scholar]
- Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- Chang HCJ, Lindquist SL. Conservation of hsp90 macromolecular complexes in Saccharomyces cerevisiae. J Biol Chem. 1994;269:24983–24988. [PubMed] [Google Scholar]
- Chen G, Cao P, Goeddel D. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Rubin GM. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Dai K, Kobayashi R, Beach D. Physical interaction of mammalian CDC37 with CDK4. J Biol Chem. 1996;271:22030–22034. doi: 10.1074/jbc.271.36.22030. [DOI] [PubMed] [Google Scholar]
- Dey B, Lightbody JJ, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A, Morgan DO. Cdc37 promotes the stability of protein kinases Cdc28 and Cak1. Mol Cell Biol. 2000;20:749–754. doi: 10.1128/mcb.20.3.749-754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss AE, Fang Y, Boschelli F, Caplan AJ. Differential in vivo regulation of steroid hormone receptor activation by Cdc37p. Mol Biol Cell. 1997;8:2501–2509. doi: 10.1091/mbc.8.12.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber MR, Farrell A, Deshaies RJ, Herskowitz I, Morgan DO. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci U S A. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatikakis N, Lin JH, Grammatikakis A, Tsichlis PN, Cochran BH. p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartson SD, Irwin AD, Shao J, Scroggins BT, Volk L, Huang W, Matts RL. p50cdc37 is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules. Biochemistry. 2000;39:7631–7644. doi: 10.1021/bi000315r. [DOI] [PubMed] [Google Scholar]
- Hunter T, Poon RYC. Cdc37: a protein kinase chaperone. Trends Cell Biol. 1997;7:157–161. doi: 10.1016/S0962-8924(97)01027-1. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, and Dong Y. et al. 2003 Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 421:231–237. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Morimoto RI, Lindquist S. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997;11:1775–1785. doi: 10.1101/gad.11.14.1775. [DOI] [PubMed] [Google Scholar]
- Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta GF, Gyuris J. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- Lange BM, Rebollo E, Herold A, González C. Cdc37 is essential for chromosome segregation and cytokinesis in higher eukaryotes. EMBO J. 2002;21:5364–5374. doi: 10.1093/emboj/cdf531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Rao J, Fliss A, Yang E, Garrett S, Caplan AJ. The Cdc37 protein kinase-binding domain is sufficient for protein kinase activity and cell viability. J Cell Biol. 2002;159:1051–1059. doi: 10.1083/jcb.200210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort-Bontemps-Soret M, Facca C, Faye G. Physical interaction of Cdc28 with Cdc37 in Saccharomyces cerevisiae. Mol Genet Genomics. 2002;267:447–458. doi: 10.1007/s00438-002-0676-3. [DOI] [PubMed] [Google Scholar]
- Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgall TE, Smith DF. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor HSF1, and the arylhydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH, Wiegand H, Vanden Heuvel JP, Mitchell C, Singh SS. A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry. 1997;36:3600–3607. doi: 10.1021/bi9612529. [DOI] [PubMed] [Google Scholar]
- Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J, Lee P, Benzeno S, Cardozo C, Albertus J, Robins DM, Caplan AJ. Functional interaction of human Cdc37 with the androgen receptor but not with the glucocorticoid receptor. J Biol Chem. 2001;276:5814–5820. doi: 10.1074/jbc.M007385200. [DOI] [PubMed] [Google Scholar]
- Reed SI. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980;95:561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Scholz GM, Cartledge K, Hall NE. Identification and characterization of Harc: a novel Hsp90 associating relative of Cdc37. J Biol Chem. 2001;18:30971–30979. doi: 10.1074/jbc.M103889200. [DOI] [PubMed] [Google Scholar]
- Scholz G, Hartson SD, Cartledge K, Hall N, Shao J, Dunn AR, Matts RL. p50Cdc37 can buffer the temperature-sensitive properties of a mutant of Hck. Mol Cell Biol. 2000;20:6984–6995. doi: 10.1128/mcb.20.18.6984-6995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz AR, Giddings TH Jr, Steiner E, Winey M. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J Cell Biol. 1997;136:969–982. doi: 10.1083/jcb.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Grammatikakis N, Scroggins BT, Uma S, Huang W, Chen JJ, Hartson SD, Matts RL. Hsp90 regulates p50(Cdc37) function during the biogenesis of the active conformation of the heme-regulated eIF2a kinase. J Biol Chem. 2001;276:206–214. doi: 10.1074/jbc.M007583200. [DOI] [PubMed] [Google Scholar]
- Shao J, Hartson SD, Matts RL. Evidence that protein phosphatase 5 functions to negatively modulate the maturation of the Hsp90-dependent heme-regulated eIF2a kinase. Biochemistry. 2002;41:6770–6779. doi: 10.1021/bi025737a. [DOI] [PubMed] [Google Scholar]
- Siligardi G, Panaretou B, Meyer P, Singh S, Woolfson DN, Piper PW, Pearl LH, Prodromou C. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J Biol Chem. 2002;277:20151–20159. doi: 10.1074/jbc.M201287200. [DOI] [PubMed] [Google Scholar]
- Silverstein AM, Grammatikakis N, Cochran BH, Chinkers M, Pratt WB. p50(cdc37) binds directly to the catalytic domain of Raf as well as to a site on hsp90 that is topologically adjacent to the tetratricopeptide repeat binding site. J Biol Chem. 1998;273:20090–20095. doi: 10.1074/jbc.273.32.20090. [DOI] [PubMed] [Google Scholar]
- Stepanova L, Finegold M, DeMayo F, Schmidt EV, Harper JW. The oncoprotein kinase chaperone CDC37 functions as an oncogene in mice and collaborates with both c-myc and cyclin D1 in transformation of multiple tissues. Mol Cell Biol. 2000a;20:4462–4473. doi: 10.1128/mcb.20.12.4462-4473.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova L, Leng X, Parker SB, Harper JW. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1492–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- Stepanova L, Yang G, DeMayo F, Wheeler TM, Finegold M, Thompson TC, Harper JW. Induction of human Cdc37 in prostate cancer correlates with the ability of targeted Cdc37 expression to promote prostatic hyperplasia. Oncogene. 2000b;19:2186–2193. doi: 10.1038/sj.onc.1203561. [DOI] [PubMed] [Google Scholar]
- Wang H, Goode T, Iakova P, Albrecht JH, Timchenko NA. C/EBPa triggers proteasome-dependent degradation of cdk4 during growth arrest. EMBO J. 2002a;21:930–941. doi: 10.1093/emboj/21.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Grammatikakis N, Hu J. Role of p50/CDC37 in hepadnavirus assembly and replication. J Biol Chem. 2002b;277:24361–24367. doi: 10.1074/jbc.M202198200. [DOI] [PubMed] [Google Scholar]