Abstract
Purpose
Infantile esotropia is associated with maldevelopment of cortical visual motion processing, manifested as directional asymmetry of motion visual evoked potentials (mVEPs). The purpose of this study was to determine whether early surgery at or before age 11 months could promote the development of cortical visual motion processing in human infants, compared with standard surgery at age 11 to 18 months.
Methods
Sixteen children with a constant, infantile esotropia ≥30 prism diopters and onset before age 6 months were recruited prospectively. Eight of them underwent early surgery at ≤11 months of age, and eight underwent standard surgery at 11 to 18 months of age. Seven age-matched normal subjects served as the control. At 2 to 2.5 years of age, mVEPs were measured during monocular viewing of a grating that shifted between two positions with a lateral displacement of 90° at 10 Hz. Nasotemporal mVEP asymmetry was assessed by an amplitude asymmetry index and by the presence of a significant interocular phase difference.
Results
The mean asymmetry index and interocular phase difference in the early surgery group were comparable to that in age-matched control subjects, and they were significantly lower than those in the standard surgery group.
Conclusions
Early surgery for infantile esotropia promotes the development of cortical visual motion processing, whereas standard surgery is associated with abnormal mVEPs. The results provide additional evidence that early strabismus repair is beneficial for cortical development in human infants.
Infantile esotropia is a nasalward misalignment of the eyes that occurs within the first 6 months of life. Affected children exhibit several sensory and ocular motor deficits, including abnormal stereopsis1,2 and nasotemporal asymmetries of smooth pursuit,3,4 optokinetic responses,5–7 and visual motion processing (as measured by motion visual evoked potentials, or mVEPs).8–11 Nasotemporal asymmetries favoring nasalward movements are also present in these patients when they are asked to judge the speed of moving targets4,12 and during performance of global motion-perception tasks.13–16
Although there is a uniform agreement among pediatric ophthalmologists that infantile esotropia requires surgical correction, the most beneficial timing of surgery remains controversial.17,18 In North America, the typical age at surgery ranges from 11 to 18 months, and in many parts of Western Europe, surgery is delayed until 2 to 4 years of age.19 Despite successful surgical realignment of the eyes, subnormal stereopsis and ocular motor deficits often persist.1,2,6,10,20 Recently, surgery at or before age 11 months has been advocated, and it has been shown that early surgery restores binocular fusion and stereopsis,21–25 static eye alignment,25 and, rarely, symmetric mVEPs.8 The purpose of this prospective study was to investigate further the effects of early (≤11 months) versus standard surgery (11–18 months) for infantile esotropia on the development of cortical visual motion processing by using mVEPs.
Methods
Patients and Normal Subjects
Sixteen consecutive patients with onset of infantile esotropia before age 6 months were recruited from The Hospital for Sick Children in Toronto for this prospective, nonrandomized study. Eight of these infants presented at age 3 to 8 months, and they underwent surgery at or before age 11 months (early surgery). The other eight presented at age 9 to 16 months, and they underwent surgery at age 11 to 18 months (standard surgery). All infants had a constant esotropia ≥30 prism diopters (PD) at near (⅓ m) on two examinations separated by 2 to 4 weeks, and refractive error ≤ +3.00 D. Exclusion criteria were identical with those in the Congenital Esotropia Observational Study (CEOS)26: gestational age <34 weeks, birth weight ≤1500 g, ventilator treatment in the newborn period, history of meningitis or other major medical event, developmental delay, incomitant or paralytic strabismus, manifest nystagmus (other than latent nystagmus, which is typically seen in infantile strabismus syndrome) or head-bobbing, prior eye muscle surgery, prior treatment of amblyopia or spectacle correction for refractive errors, and presence of structural ocular anomalies.
The age at onset of a constant nasalward eye misalignment was estimated to the nearest month by the parent. Because infantile esotropia is rarely, if ever, present in the first 2 months of life,27,28 an age of onset of 2 months was assigned to infants whose parents reported the presence of esotropia at or within 1 month of birth.29 This is to ensure that our estimate of the age of onset is consistent with that in the literature and also to avoid potential bias in estimating the age of onset in older infants who were seen at a later age.29
A complete eye examination, including cycloplegic refraction, was performed on all patients. The amount of esotropia was measured by a certified orthoptist by one of two standard clinical methods: prism and alternate cover test (in cooperative patients) or the Krimsky method (in uncooperative patients). Another eye examination was repeated 2 to 4 weeks after the initial examination. Patients underwent bilateral medial rectus recession performed by three referring surgeons. An equal number of patients with early or standard surgery was recruited from each surgeon to account for potential differences in surgical techniques. After surgery, the patients were followed up by their surgeons at regular intervals, based on the surgeons’ usual clinical practice. Motion VEPs were performed when the children reached 2 years of age. During the same visit, a complete eye and orthoptic examination was performed to record visual acuity, ocular alignment, refractive error, any occlusion therapy or eyeglasses prescription, and the number and type of reoperation, if any. In addition, stereopsis was tested by the Lang stereo test; if negative, it was retested with patients wearing prisms that corrected for any residual deviations.
The characteristics of patients with early or standard surgery are summarized in Table 1. The mean age at onset (±SD) was 2.3 ± 0.5 months in the early surgery group and 3.0 ± 1.4 months in the standard surgery group (P = 0.19). The mean duration of esotropia was 5.6 ± 1.6 months in the early and 11.6 ± 3.0 months in the standard surgery group (P < 0.001). There was no significant difference in the angle of esotropia before surgery or in the magnitude of refractive error between the two groups.
Table 1.
Patient Characteristics
| Patient | Sex | Age at Onset (mo) | Age at Surgery (mo) | Duration of ET (mo) | Preop Angle (PD) | Adequate Alignment at 8 wk Postop* | Adequate Alignment at mVEP* | Age at mVEP (mo) | Amblyopia at mVEP† | Lang Stereo at mVEP (arc sec) | DVD at mVEP | Symmetric mVEP‡ (AI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early surgery (n = 8) | ||||||||||||
| E1 | F | 2 | 8 | 6 | 45 | Yes | Yes | 26 | No | 400 | No | Yes (0.43) |
| E2 | M | 2 | 8 | 6 | 30 | Yes | Yes | 25.5 | No | N§ | No | Yes (0.37) |
| E3 | M | 3 | 9.5 | 6.5 | 35 | Yes | Yes | 25.5 | No | 600 | No | Yes (0.43) |
| E4 | M | 2 | 8.5 | 6.5 | 40 | Yes | Yes | 26 | Yes | 200 | No | Yes (0.51) |
| E5 | F | 2 | 8.5 | 6.5 | 30 | Yes | Yes|| | 27 | No | N | Yes | Yes (0.25) |
| E6 | M | 2 | 9 | 7 | 40 | Yes | No | 25 | No | N | No | Yes (0.50) |
| E7 | F | 2 | 5 | 3 | 45 | Yes | Yes | 27 | Yes | N | No | Yes (0.52) |
| E8 | F | 3 | 6 | 3 | 45 | Yes | Yes | 24 | No | 400 | No | No (0.59) |
| Standard surgery (n = 8) | ||||||||||||
| S1 | M | 2 | 13 | 11 | 40 | Yes | No | 28 | Yes | 600¶ | No | Yes (0.49) |
| S2 | M | 3 | 14 | 11 | 45 | Yes | No | 26 | No | N | Yes | Yes (0.37) |
| S3 | M | 2 | 18 | 16 | 40 | Yes | Yes | 30 | No | 400 | No | No (0.78) |
| S4 | F | 4 | 16 | 12 | 45 | Yes | Yes | 25 | No | 400 | No | No (0.68) |
| S5 | F | 2 | 18 | 16 | 45 | Yes | Yes | 24 | No | N | No | No (0.59) |
| S6 | F | 2 | 11 | 9 | 30 | No | Yes|| | 25 | No | 600 | No | No (0.85) |
| S7 | M | 6 | 16 | 10 | 35 | Yes | No | 26 | Yes | N | No | Yes (0.53) |
| S8 | M | 3 | 11 | 8 | 50 | Yes | No | 24 | No | 600¶ | No | No (0.88) |
ET, esotropia; Preop, preoperative; PD, prism diopters; Postop, postoperative; DVD, dissociated vertical deviation.
Adequate alignment was defined as a microtropia or a small-angle esotropia ≤10 PD.
Amblyopia was defined as interocular acuity difference less than 0.3 logMAR.
Symmetric mVEP was defined as asymmetry index (AI) within 95% CI of normal group mean.
N denotes absent or not measurable Lang stereopsis due to poor cooperation.
After second surgery for residual ET.
After correction of residual esotropia with prisms.
Seven age-matched visually normal subjects without any history of prematurity or neurologic or ocular abnormality (except for refractive errors) were recruited through hospital advertisement. There were two females and five males. They all had monocular visual acuity of 0.3 logMAR or better, interocular acuity difference less than 0.3 logMAR, orthotropia or phoria ≤4 PD, fusion on Worth four-dot testing (both at 33 cm and 3 m), and normal stereopsis.
The research protocol complied with the tenets of the Declaration of Helsinki and was approved by the Research Ethics Board of The Hospital for Sick Children in Toronto.
Motion VEP Recordings
Motion VEPs were performed by placing electrodes according to the international 10–20 system for electrode placement.30–32 Three active gold cup electrodes were placed over the visual (occipital) cortex in positions Oz, O1, and O2, with CZ as the reference and PZ as the ground (they were placed at 50% and 30% of the inion–nasion distance at midline, respectively).
Power Diva Mac Software (ver. 1.6; developed by Anthony Norcia, Smith Kettlewell Eye Institute, San Francisco, CA)33 was used to test mVEPs. Motion VEPs were measured monocularly in response to a vertical sinusoidal grating displayed on a video monitor (18-inch Power Mac G4, with raster size of 1200 × 1600 pixels and refresh rate of 85 Hz; Apple Computer, Cupertino, CA), located 1 m away from the subject. Monocular viewing was achieved by occluding one eye with a translucent diffuser. The grating was presented with a space average luminance of 80 cd/m2 and Michaelson contrast of 80%. It was shifted synchronously with the video frame between two positions separated by 90° of spatial phase. A 3 cyc/deg grating alternating at a frequency of 10 Hz (50 ms per position, 20 changes of direction per second) was used.9,10 The grating was presented for 10 seconds, and multiple trials were recorded from each eye.
The analogue EEG signal was sampled and amplified (Grass Technologies, model 12 Data Acquisition System; AstroMed, Inc., West Warwick, RI) with a gain of 50,000 Hz. The EEG was digitized to 16-bit accuracy over a 1- to 100-Hz bandwidth at a sampling rate of 600 Hz. The analogue-to-digital signal conversion, data acquisition, stimulus presentation, and extrapolation of threshold estimates were controlled with the PowerDiva system.33
Data Analysis
The signals were subjected to Fourier analysis to extract the amplitude and phase of the evoked response at the first harmonic amplitude or F1 (10 Hz) and second harmonic amplitude or F2 (20 Hz). The analyses determined the asymmetry index and the presence or absence of a “bow tie” by a vector average of at least 10 trials in each eye.
Symmetric and asymmetric mVEPs yield distinct Fourier response spectra. A symmetric mVEP, which reflects equally strong cortical responses to rightward and leftward directions of motion, produces a response spectrum that is composed predominantly of even multiples of the stimulus frequency (e.g., F2, F4, F6). An asymmetric mVEP, which reflects cortical response in only one direction of motion, yields a response spectrum that is composed predominantly of odd harmonic multiples of the stimulus frequency (e.g., F1, F3, F5). Furthermore, the interocular phase of the asymmetric mVEP determines whether each eye responds to the same or different directions of motion. For instance, if both eyes respond to identical directions of motion, they will have the same phase. Conversely, if each eye produces a strong asymmetric mVEP to opposite directions of motion, the interocular phase difference of the F1 response will approximate 180°, and this will appear as a bow tie on a polar plot of amplitude and phase.
The symmetry of the mVEPs was quantified by calculating the amplitude asymmetry index, defined as F1/(F1 + F2). The asymmetry index can range from 1.0 (extremely asymmetric responses dominated by F1) to 0.0 (extremely symmetric responses dominated by F2). In all subjects, the asymmetry index and interocular phase difference in each eye did not differ between viewing with the right or left eye. We herein report the pooled data from either eye viewing. The data reported are amplitude and phase values of the channel with the highest signal-to-noise ratio and the most significant probability for the two eyes. The mean asymmetry index is the mean of the asymmetry indices measured in the left and right eyes.
Patient characteristics were compared using t-tests and Fisher exact tests for proportions. The mean asymmetry index and interocular phase difference were compared among the three groups (early surgery, standard surgery, and normal controls) by ANOVAs, with significance set at P < 0.05. Any significant difference was tested further by post hoc Tukey test. Mean asymmetry index and interocular phase difference of each patient was also compared to the 95% confidence interval of the control group mean.
Results
Patient Characteristics
All eight patients (100%) in early surgery and seven (88%) in standard surgery groups achieved adequate alignment (i.e., microtropia or small-angle esotropia ≤10 PD) at 8 weeks after surgery (P = 0.99). At the time of mVEP testing, one (13%) patient in each group (patients E5 and S6) had undergone a second surgery; the rest had only one surgery. Adequate alignment was achieved in seven (88%) patients in the early surgery group and in four (50%) in the standard surgery group at the time of mVEP testing (P = 0.28). Two (25%) patients in each group had significant amblyopia with interocular acuity difference ≥0.3 logMAR. Four (50%) patients in the early surgery and five (63%) in the standard surgery groups had Lang stereopsis of 600 seconds of arc or better (P = 0.99). Lang stereopsis was absent or was not measurable in the other patients because of poor cooperation. One (13%) patient in each group had dissociated vertical deviation.
Motion VEP Polar Plots
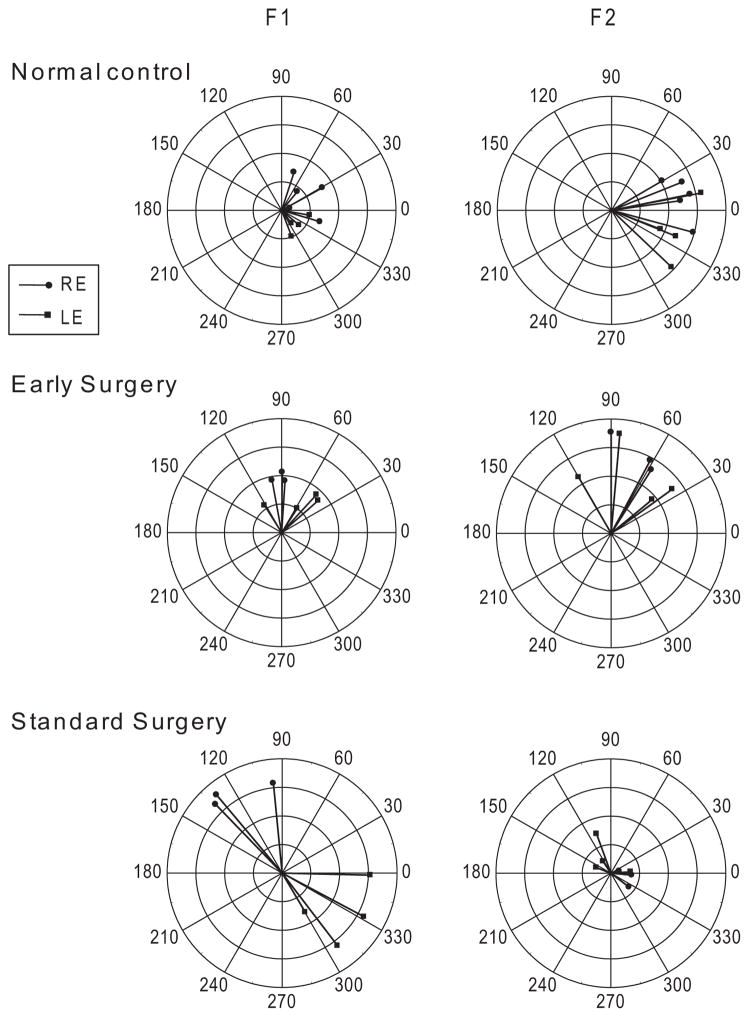
The mean age at mVEP testing was 27.4 ± 3.2 months (median, 30 mo: range, 24–30) in the control subjects, 25.8 ± 1.0 months (median, 26.0 mo; range, 24–27) in the early surgery group, and 26.0 ± 2.1 months (median, 25.5 mo; range, 24–30) in the standard surgery group (ANOVA, P = 0.35). Representative mVEP responses of individual subjects from each group are shown in the polar plots of Figure 1. Each vector is an individual response from a 10-second trial while viewing monocularly with the right or left eye. The length of the vector depicts amplitude (in microvolts) and the direction indicates phase (0–360°). The left graphs are plots of the first-harmonic (F1, asymmetric) responses and the right are of the second harmonic (F2, symmetric) responses. Note that the control subject (top) and patient with early surgery (middle) exhibited weak F1 but robust F2 amplitudes, with the phases of the responses congregating within similar angles for the right and left eyes. Similar findings were recorded in each of the other six control subjects and the other seven patients with early surgery (not shown).
Figure 1.
Polar plots of mVEP amplitude (4 μV, full scale) and phase in normal control subjects (top) and in patients who underwent early surgery (middle) or standard surgery (bottom). Each vector is an individual response from a 10-second trial while viewing monocularly with the right or left eye. The length of the vector depicts amplitude (in micro-volts) and the direction indicates phase (0–360°). F1, first harmonic response; F2, second harmonic response. Control subject and patient with early surgery had smaller F1 but larger F2 responses, with the phases of the responses for the right and left eyes congregated in roughly the same direction. Patient with standard surgery had larger F1 but smaller F2 responses, with the phases of the response for the right and left eyes in opposite directions (approximately 180° out of phase).
In contrast, patients with standard surgery showed a strikingly abnormal pattern of mVEP response (Fig. 1, bottom). They exhibited strong F1 but weaker F2 amplitudes, and the responses were approximately 180° out of phase between the right and left eyes. This interocular phase difference produced a bow-tie appearance in the F1 polar plots, and indicates asymmetrical responses in different directions of motion in each eye. Similar findings were recorded in other patients with standard surgery (not shown).
Asymmetry Index and Interocular Phase Difference
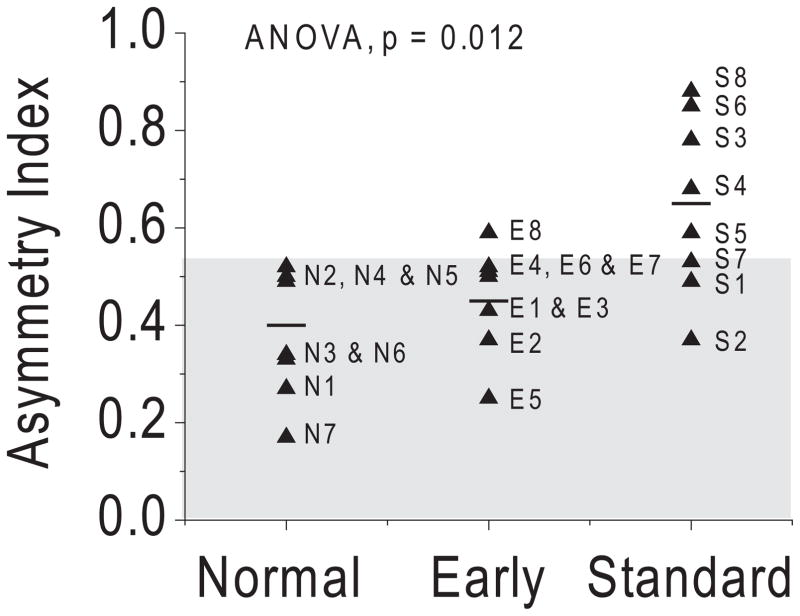
The mean asymmetry index and interocular phase difference for each patient in the two surgical groups (filled triangles), the patient group mean (short horizontal lines), and the 95% confidence interval in normal control subjects (shaded area) are shown in Figures 2 and 3. The mean asymmetry index differed across groups (ANOVA, P = 0.012; Fig. 2). Post hoc tests indicated that the mean asymmetry index in the early group (0.45 ± 0.11) was comparable to that in age-matched control subjects (0.40 ± 0.17; P = 0.505), but it was significantly lower than that in the standard group (0.65 ± 0.18; P = 0.018). Analysis of individual patient data showed one patient with early surgery (13%; patient E8) and five patients with standard surgery (63%; patients S3, S4, S5, S6, and S8) had abnormal individual asymmetry indices that fell outside the 95% confidence interval of the control group.
Figure 2.
Asymmetry indices in patients with early and standard surgery. (▲) Mean value of asymmetry index in each subject. Short horizontal lines: mean asymmetry index for each group. Shaded area: 95% CI in normal control subjects.
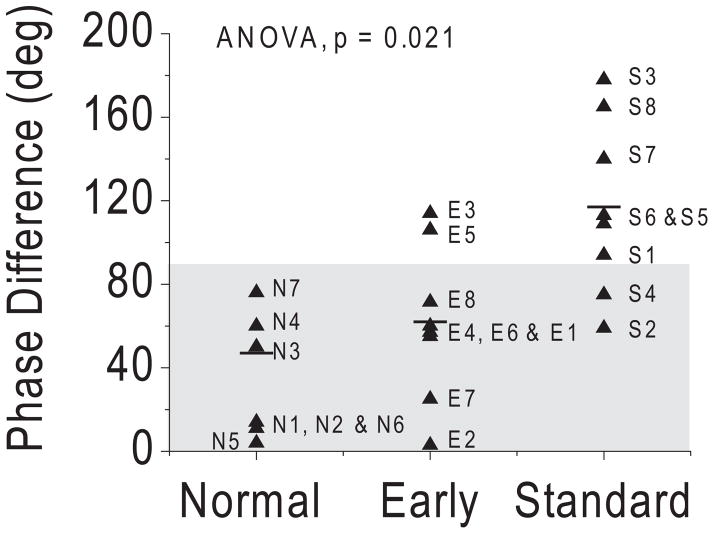
Figure 3.
Interocular phase differences in patients with early and standard surgery. (▲) Mean value of interocular phase difference in each subject. Short horizontal lines: mean phase difference for each group. Shaded area: 95% CI in normal control subjects.
Similarly, the mean interocular phase difference differed across groups (ANOVA, P = 0.021; Fig. 3). Post hoc tests indicated that the mean interocular phase difference in the early group (62 ± 40°) was comparable to that in the age-matched control subjects (47 ± 56°; P = 0.557), but it was significantly lower than that in the standard group (117 ± 45°; P = 0.022). Analysis of individual patient data showed two patients with early surgery (25%; patients E3 and E5) and six patients with standard surgery (75%; patients S1, S3, S5, S6, S7, and S8) had abnormal individual interocular phase differences that fell outside the 95% confidence interval of the control group.
Discussion
Motion VEPs are asymmetric in normal human infants at age 2 months, but become symmetric by approximately 5 to 6 months of age.34,35 The development of mVEPs closely parallels that of stereoscopic fusion.34,35 Motion VEPs remain asymmetric in human infants with infantile esotropia.8,9,11 The asymmetry is reduced by alternate occlusion of the eyes, which minimizes the interocular suppression caused by esotropia.36,37 Older children and adults who have persistent eye misalignment and interocular suppression, due either to delayed repair, unsuccessful repair, or nonrepair, show permanent mVEP asymmetries.9,38 The mVEP asymmetries are not caused by latent nystagmus; they remain robust under conditions that eliminate the oscillations of latent nystagmus.38 This fact suggests that mVEPs probably measure the activities of motion detection neurons in the visual cortex and are not just an epiphenomenon of interocular suppression. Birch et al.8 performed mVEPs on a prospective population of patients with infantile esotropia at 5 to 60 months. They found that most of them had asymmetric mVEPs regardless of the age at surgery and that only rare patients with surgery during the first 10 months of life achieved symmetric mVEPs. Unfortunately, they did not provide further details as to how many patients underwent early surgery and how many of these patients achieved symmetric mVEPs.
In this prospective study, we systematically investigated patients with early versus standard surgery and compared their mVEP results with the age-matched control. We found that the mean asymmetry index and interocular phase difference in the early group were similar to those in normal control subjects, and they were significantly better than in the standard surgery group. The decision to use a nonrandomized approach for this study is based on the experience from CEOS,26 which showed that recruitment would be too low to make randomization feasible. As patients were not randomized, the observed difference in mVEP outcomes cannot be definitively or exclusively attributed to the timing of surgery. However, the early and standard surgery groups had similar baseline characteristics. Our current finding in human infants is also consistent with the mVEP data we reported from a nonhuman primate model.39
We found that most patients with early surgery have symmetric mVEPs, whereas most patients with standard surgery do not. Of interest, two patients in the early group (E5 and E6) who had adequate alignment at 8 weeks after surgery but developed residual esotropia later, exhibited symmetric mVEP, whereas patient S6, who had standard surgery and displayed residual esotropia immediately and at 8 weeks after surgery but subsequently achieved adequate alignment after a second surgery at age 18 months, displayed asymmetric mVEPs. Taken together, these findings suggest that timely restoration of binocular image correlation during the early critical period is a major factor in promoting the development of cortical visual motion processing. As long as binocular alignment is achieved during the critical period of development for even a short duration after surgery, good mVEP outcomes can be achieved whether or not the child requires a second surgery for strabismus that develops at a later age. In addition, since the critical period of mVEP development occurs in the first 2 to 6 postnatal months, our finding that symmetric mVEPs can be achieved when surgery is performed by 10 months of age indicates that the critical period for functional recovery of mVEPs is slightly longer than that for the normal development of mVEPs. Although this observation applies to the majority of patients, the duration of the critical period for functional recovery of mVEPs may show individual variability. This notion is supported by the finding that one patient (E8) in the early group had asymmetric mVEPs, whereas three (S1, S2, and S7) in the standard group had symmetric mVEPs.
A possible explanation for the poorer mVEP results in the standard surgery group was that four of eight patients (S1, S2, S7, and S8) did not achieve adequate alignment at the time of mVEP testing (compared to only one patient in early surgery group). However, the individual mean asymmetry index of three of these four patients (S1, S2, and S7) fell within the 95% confidence interval of normal control subjects. In fact, even after excluding these four patients with residual esotropia from analysis, the mean group asymmetry index of the remaining four patients with adequate alignment (0.73 ± 0.11) was still significantly higher than those in the early and control groups (ANOVA, P = 0.004). Thus, the lack of adequate alignment at the time of mVEP testing could not explain the abnormal mVEP responses of the standard surgery group.
Early surgery in humans is believed to enhance sensory outcomes by re-establishing correlated binocular activity during an early critical period for the development of stereopsis.24,29,34 The stereopsis outcomes of infants who have early surgery are better than those in the standard surgery groups.21,23,29 Both age at alignment and duration of misalignment are linked to enhanced stereoacuity outcomes, but the duration of misalignment appears to be the more important factor.24,29 Because of the close correlation between age at alignment and duration of misalignment in this study, the relative contribution of each of these factors to the development of mVEP symmetry remains to be elucidated.
The potential for enhancing the development of normal binocular vision with early surgery must be balanced against the possibility of spontaneous resolution without surgery. The CEOS26 and the study by Birch et al.40 found that spontaneous resolution is <2% if the esotropia is constant and large (≥40 PD), with onset after 10 weeks of age. Recently, Fu et al.41 demonstrated that the constancy, rather than the angle, of strabismus, is the major determinant of spontaneous resolution. They found that infantile esotropia that is intermittent has a high likelihood of spontaneous resolution, whereas constant small-angle or variable-angle infantile esotropia seldom resolves.41 Surgery on patients in whom the esotropia may spontaneously resolve may lead to consecutive exotropia. Because all our patients had a constant deviation and because five had small-angle strabismus (30 – 40 PD; E2, E3, E5, S6, and S7), our findings that consecutive exotropia did not develop in any of them after surgery provides further support that the constancy of strabismus is an important factor in determining spontaneous resolution.
Other concerns for the timing of surgery include the potential risks or side effects associated with early surgery. Our data showed that there was no statistically significant differences between the early versus standard groups in achieving adequate alignment (i.e., microtropia or small-angle esotropia ≤10 PD) at 8 weeks after surgery or at 2 to 2.5 years of age. In addition, the rate of reoperation, the presence of amblyopia, and the presence of stereopsis (when measurable) were similar in both groups. Although the lack of differences in these clinical outcomes between surgical groups can be attributed to the relatively small sample size, our results are comparable to those reported previously,25 and provide further support that early surgery does not pose additional risk to young infants.
To the best of our knowledge, the present study provides the first prospective evidence that patients who undergo surgery at the standard age have worse mVEP outcomes than those with early surgery within 10 months of age. Our results indicate that adequate binocular alignment during the critical period of development for even a short duration is a major factor in achieving symmetric mVEPs, irrespective of whether additional surgery is required at a later time.
Acknowledgments
Supported by New Investigator Award MSH 55058; Grants MOP 67104 and MOP 57853 from the Canadian Institutes of Health Research; the University Health Network Ophthalmology Practice Plan; and the Department of Ophthalmology and Vision Science at The Hospital for Sick Children.
Footnotes
Disclosure: C. Gerth, None; G. Mirabella, None; X. Li, None; T. Wright, None; C. Westall, None; L. Colpa, None; A.M.F. Wong, None
References
- 1.Birch EE, Stager DR, Berry P, Everett ME. Prospective assessment of acuity and stereopsis in amblyopic infantile esotropes following early surgery. Invest Ophthalmol Vis Sci. 1990;31:758–765. [PubMed] [Google Scholar]
- 2.Birch EE, Stager DR, Everett ME. Random dot stereoacuity following surgical correction of infantile esotropia. J Pediatr Ophthalmol Strabismus. 1995;32:231–235. doi: 10.3928/0191-3913-19950701-07. [DOI] [PubMed] [Google Scholar]
- 3.Tychsen L, Hurtig RR, Scott WE. Pursuit is impaired but the vestibulo-ocular reflex is normal in infantile strabismus. Arch Ophthalmol. 1985;103:536–539. doi: 10.1001/archopht.1985.01050040078022. [DOI] [PubMed] [Google Scholar]
- 4.Tychsen L, Lisberger SG. Maldevelopment of visual motion processing in humans who had strabismus with onset in infancy. J Neurosci. 1986;6:2495–2508. doi: 10.1523/JNEUROSCI.06-09-02495.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schor CM, Levi DM. Disturbances of small-field horizontal and vertical optokinetic nystagmus in amblyopia. Invest Ophthalmol Vis Sci. 1980;19:668–683. [PubMed] [Google Scholar]
- 6.Westall CA, Eizenman M, Kraft SP, Panton CM, Chatterjee S, Sigesmund D. Cortical binocularity and monocular optokinetic asymmetry in early-onset esotropia. Invest Ophthalmol Vis Sci. 1998;39:1352–1360. [PubMed] [Google Scholar]
- 7.Westall CA, Shute RH. OKN asymmetries in orthoptic patients: contributing factors and effect of treatment. Behav Brain Res. 1992;49:77–84. doi: 10.1016/s0166-4328(05)80196-2. [DOI] [PubMed] [Google Scholar]
- 8.Birch EE, Fawcett S, Stager D. Co-development of VEP motion response and binocular vision in normal infants and infantile esotropes. Invest Ophthalmol Vis Sci. 2000;41:1719–1723. [PubMed] [Google Scholar]
- 9.Norcia AM, Garcia H, Humphry R, Holmes A, Hamer RD, Orel-Bixler D. Anomalous motion VEPs in infants and in infantile esotropia. Invest Ophthalmol Vis Sci. 1991;32:436–439. [PubMed] [Google Scholar]
- 10.Norcia AM, Hamer RD, Jampolsky A, Orel-Bixler D. Plasticity of human motion processing mechanisms following surgery for infantile esotropia. Vision Res. 1995;35:3279–3296. doi: 10.1016/0042-6989(95)00144-4. [DOI] [PubMed] [Google Scholar]
- 11.Tychsen L, Burkhalter A, Boothe RG. Neural mechanisms in infantile esotropia—what goes wrong? Am Orthoptic J. 1996;46:18–28. [PubMed] [Google Scholar]
- 12.Tychsen L, Rastelli A, Steinman S, Steinman B. Biases of motion perception revealed by reversing gratings in humans who had infantile-onset strabismus. Dev Med Child Neurol. 1996;38:408–422. doi: 10.1111/j.1469-8749.1996.tb15099.x. [DOI] [PubMed] [Google Scholar]
- 13.Shallo-Hoffmann J, Faldon M, Hague S, Riordan-Eva P, Fells P, Gresty M. Motion detection deficits in infantile esotropia without nystagmus. Invest Ophthalmol Vis Sci. 1997;38(1):219–226. [PubMed] [Google Scholar]
- 14.Bosworth RG, Birch EE. Motion detection in normal infants and young patients with infantile esotropia. Vision Res. 2005;45:1557–1567. doi: 10.1016/j.visres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Bosworth RG, Birch EE. Direction-of-motion detection and motion VEP asymmetries in normal children and children with infantile esotropia. Invest Ophthalmol Vis Sci. 2007;48:5523–5531. doi: 10.1167/iovs.07-0666. [DOI] [PubMed] [Google Scholar]
- 16.Fawcett S, Raymond JE, Astle WF, Skov CM. Anomalies of motion perception in infantile esotropia. Invest Ophthalmol Vis Sci. 1998;39(5):724–735. [PubMed] [Google Scholar]
- 17.Jampolsky A. When should one operate for congenital strabismus? In: Brockhurst RJ, Boruchoff SA, Hutchinson BT, Lessell S, editors. Controversy in Ophthalmology. Philadelphia: WB Saunders Co; 1977. pp. 416–422. [Google Scholar]
- 18.Parks MM. Operate early for congenital strabismus. In: Brockhurst RJ, Boruchoff SA, Hutchinson BT, Lessell S, editors. Controversy in Ophthalmology. Philadelphia: WB Saunders; 1977. pp. 423–430. [Google Scholar]
- 19.von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 5. St. Louis: CV Mosby; 1996. [Google Scholar]
- 20.Tychsen L. Improvements in smooth pursuit and fixational eye movements after strabismus surgery in infants. Ophthalmology. 1991;98(suppl):94. [Google Scholar]
- 21.Wright KW, Edelman PM, McVey JH, Terry AP, Lin M. High-grade stereo acuity after early surgery for congenital esotropia. Arch Ophthalmol. 1994;112:913–919. doi: 10.1001/archopht.1994.01090190061022. [DOI] [PubMed] [Google Scholar]
- 22.Ing MR. Early surgical alignment for congenital esotropia. Trans Am Ophthalmol Soc. 1981;79:625–663. [PMC free article] [PubMed] [Google Scholar]
- 23.Ing MR. Surgical alignment prior to six months of age for congenital esotropia. Trans Am Ophthalmol Soc. 1995;93:135–146. doi: 10.1016/s0002-9394(14)70552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ing MR, Okino LM. Outcome study of stereopsis in relation to duration of misalignment in congenital esotropia. J AAPOS. 2002;6:3–8. doi: 10.1067/mpa.2002.120172. [DOI] [PubMed] [Google Scholar]
- 25.Birch EE, Stager DR., Sr Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS. 2006;10:409–413. doi: 10.1016/j.jaapos.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Pediatric Eye Disease Investigator Group. Spontaneous resolution of early-onset esotropia: experience of the Congenital Esotropia Observational Study. Am J Ophthalmol. 2002;133:109–118. doi: 10.1016/s0002-9394(01)01316-2. [DOI] [PubMed] [Google Scholar]
- 27.Archer SM, Sondhi N, Helveston EM. Strabismus in infancy. Ophthalmology. 1989;96:133–137. doi: 10.1016/s0161-6420(89)32932-0. [DOI] [PubMed] [Google Scholar]
- 28.Nixon RB, Helveston EM, Miller K, Archer SM, Ellis FD. Incidence of strabismus in neonates. Am J Ophthalmol. 1985;100:798–801. doi: 10.1016/s0002-9394(14)73370-7. [DOI] [PubMed] [Google Scholar]
- 29.Birch EE, Fawcett S, Stager DR. Why does early surgical alignment improve stereopsis outcomes in infantile esotropia? J AAPOS. 2000;4:10–14. doi: 10.1016/s1091-8531(00)90005-3. [DOI] [PubMed] [Google Scholar]
- 30.Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr Clin Neurophysiol. 1985;10:370–375. [Google Scholar]
- 31.Harding GF, Odom JV, Spileers W, Spekreijse H. Standard for visual evoked potentials 1995. The International Society for Clinical Electrophysiology of Vision. Vision Res. 1996;36:3567–3572. doi: 10.1016/0042-6989(96)00125-3. [DOI] [PubMed] [Google Scholar]
- 32.Odom JV, Bach M, Barber C, et al. Visual evoked potentials standard. Doc Ophthalmol. 2004;108:115–123. doi: 10.1023/b:doop.0000036790.67234.22. [DOI] [PubMed] [Google Scholar]
- 33.Norcia A. PowerDiva Manual. San Francisco: Smith-Kettlewell Eye Research Institute; 1999. [Google Scholar]
- 34.Birch EE, Gwiazda J, Held R. Stereoacuity development for crossed and uncrossed disparities in human infants. Vision Res. 1982;22:507–513. doi: 10.1016/0042-6989(82)90108-0. [DOI] [PubMed] [Google Scholar]
- 35.Birch EE, Shimojo S, Held R. Preferential-looking assessment of fusion and stereopsis in infants aged 1–6 months. Invest Ophthalmol Vis Sci. 1985;26:366–370. [PubMed] [Google Scholar]
- 36.Norcia AM. Abnormal motion processing and binocularity: infantile esotropia as a model system for effects of early interruptions of binocularity. Eye. 1996;10:259–265. doi: 10.1038/eye.1996.55. [DOI] [PubMed] [Google Scholar]
- 37.Jampolsky A, Norcia A, Hamer R. Preoperative alternate occlusion decreases motion processing abnormalities in infantile esotropia. J Pediatr Ophthalmol Strabismus. 1994;31:6–17. doi: 10.3928/0191-3913-19940101-04. [DOI] [PubMed] [Google Scholar]
- 38.Anteby I, Zhai HF, Tychsen L. Asymmetric MVEPs in infantile strabismus are not an artifact of latent nystagmus. J AAPOS. 1998;2:153–158. doi: 10.1016/s1091-8531(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 39.Tychsen L, Wong AM, Foeller P, Bradley D. Early versus delayed repair of infantile strabismus in macaque monkeys: II. Effects on motion visually evoked potentials. Invest Ophthalmol Vis Sci. 2004;45:821–827. doi: 10.1167/iovs.03-0564. [DOI] [PubMed] [Google Scholar]
- 40.Birch E, Stager D, Wright K, Beck R. The natural history of infantile esotropia during the first six months of life. Pediatric Eye Disease Investigator Group. J AAPOS. 1998;2:325–328. doi: 10.1016/s1091-8531(98)90026-x. [DOI] [PubMed] [Google Scholar]
- 41.Fu VL, Stager DR, Birch EE. Progression of intermittent, small-angle, and variable esotropia in infancy. Invest Ophthalmol Vis Sci. 2007;48:661–664. doi: 10.1167/iovs.06-0717. [DOI] [PubMed] [Google Scholar]