Abstract
Prebiotics are nondigestible fermentable fibers that are reported to have health benefits for the host. Older as well as more recent studies show beneficial effects in experimental colitis and lately also in human inflammatory bowel diseases (IBD), such as Crohn’s disease, ulcerative colitis, and chronic pouchitis. In this review we give an overview of the benefits of prebiotics in rodent IBD models and in IBD patients and discuss their possible protective mechanisms. Commensal intestinal bacteria induce and perpetuate chronic intestinal inflammation, whereas others are protective. However, most of the current medications are directed against the exaggerated proinflammatory immune response of the host, some of them toxic and costly. Feeding prebiotics changes the composition of the intestinal microflora toward more protective intestinal bacteria and alters systemic and mucosal immune responses of the host. Therapy for IBD targeting intestinal bacteria and their function is just emerging. Prebiotics have the promise to be relatively safe, inexpensive, and easy to administer. Unraveling their protective mechanisms will help to develop rational applications of prebiotics. However, the initial promising results with dietary prebiotics in preclinical trials as well as small studies in human IBD will need to be confirmed in large randomized controlled clinical trials.
Keywords: ulcerative colitis, Crohn’s disease, prebiotics, bifidobacteria, lactobacilli
The incidence and prevalence of chronic inflammatory bowel diseases (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), are increasing in the developed northern hemisphere. Bernstein et al1 recently published data that showed that Canada has the highest incidence and prevalence of CD thus far reported. The incidence rate for CD ranged from 8.8–20.2 per 100,000 and the prevalence ranged from 161–319 per 100,000. The incidence and prevalence of UC was lower, ranging from 9.9–19.5 and 162–249 per 100,000, respectively. Approximately 0.5% of the Canadian population has IBD. Several studies concerning the pathogenesis of IBD indicate that a combination of factors such as genetic susceptibility, intestinal microflora, dietary factors, intestinal barrier dysfunction, and an abnormal immune response to intestinal bacteria lead to chronic intestinal inflammation.2–4
Current IBD Therapeutics
The current medical armamentarium to treat UC and CD consists of 5-aminosalicylic acid (ASA) compounds, corticosteroids, azathioprine/6-mercaptopurine, methotrexate, and cyclosporine. Recently several biologics have been added to this list.5 Most patients respond well to these therapies, but for some patients it is still inadequate and/or induces intolerable and serious side effects. In addition, the current therapies are mostly directed against the overly aggressive adaptive immune response of the host, but fail to correct potential environmental triggers such as the intestinal microflora that induces and perpetuates these disorders (Table 1). In addition, there is a dysbiosis between disease-inducing and protective intestinal bacteria in patients with IBD (Table 2).6
TABLE 1.
Research Findings that Suggest a Role for Intestinal Bacteria in the Pathogenesis of IBD
| Animals kept under specific germ free conditions do not develop inflammation until bacteria are introduced91,92 |
| The number of adherent mucosal bacteria is increased in Crohn’s disease patients93 |
| Inflammation and lesions generally occur in intestinal regions with the highest number of bacteria3 |
| Antibiotic treatment is a viable treatment option for some IBD patients94 |
| Luminal and mucosa-associated microflora of IBD patients differs from healthy controls95,96 |
| Diversion of the fecal stream induces clinical improvement in Crohn’s patients97 |
TABLE 2.
Dysbiosis Between Disease-inducing and Protective Intestinal Bacteria in Patients with IBD
| Protective Bacteria | Disease-inducing Bacteria |
|---|---|
| Bifidobacterium spp. | Selected Bacteroides spp. |
| Lactobacillus spp. | Enterococcus faecalis |
| Streptococcus salivarius | Enterobacter cloacae |
| Saccharomyces boulardii | Fusobacterium spp. |
| Clostridium butyricum | Intestinal Helicobacter spp. |
| E. coli Nissle 1917 | Entero-invasive E.coli |
| Ruminococci |
Eubacterium spp. Peptostreptococcus spp. |
This concept has led to research into alternative therapies for IBD such as probiotics and, lately, prebiotics, or their combination, also called “synbiotics.”
Probiotics
Probiotics have been the topic for research in IBD for quite some time. A probiotic has been defined as “a preparation of a product containing viable, defined microorganisms in sufficient numbers, which, when administered in adequate amounts, alters the microflora in a compartment of the host and by that exert beneficial effects in the host.”7
In order for microorganisms to be called probiotics they must 1) be able to withstand and survive the effect of gastric acid, biliary, and pancreatic secretions in order to reach the small and large intestines; 2) be nonpathogenic and nontoxic; 3) remain viable during transport and storage; 4) exert beneficial effects on the host; 5) stabilize the intestinal microflora; 6) adhere to the intestinal epithelial cell lining; and 7) produce antimicrobial substances toward pathogens.8,9 Probiotics exert several protective effects by altering the mucosal immune response resulting in less inflammation, prevention of colonization by intestinal pathogens, improving the intestinal epithelial barrier, stimulation of antiinflammatory cytokine production (such as IL-10 and TGF-β), and secretion of antibacterial substances.10
Probiotics may be useful in the treatment of several conditions, including diarrhea, atopic dermatitis, necrotizing enterocolitis, food intolerance, constipation, UC, and chronic pouchitis. So far, the current literature has not been conclusive on the effect of probiotics in the treatment of CD.11–13
Prebiotics
Prebiotics are nondigestible (oligo)saccharides, defined as “selectively fermented ingredients that allow specific changes, both in the composition and/or activity of the gastrointestinal microflora that confers benefits upon host well-being and health.”14 Substances are considered prebiotics when they meet the following criteria: 1) be neither hydrolyzed nor absorbed in the upper part of the gastrointestinal tract; 2) be selectively fermented by 1 or a limited number of potentially beneficial bacteria in the intestine; and 3) be able to alter the colonic microflora toward a healthier composition.14,15
The most commonly used prebiotics are beta-fructans oligosaccharides. Inulin and oligofructose are natural food ingredients or dietary fibers present in certain plants as storage carbohydrates. Wheat, chicory, bananas, onions, leek, artichoke, asparagus, and garlic contain prebiotics. Most commercially used prebiotics are synthesized from sucrose or are extracted from chicory roots. They are used in candies, confectioneries, bakery products, fermented products, fruit juices, desserts, spreads, fat replacers, etc.16
Inulins are composed of multiple fructose units with a terminal glucose (Fig. 1). The fructose units are joined by β-glycosidic links. Natural inulins typically consist of 2–140 beta-fructans units. Oligofructose is a degradation product of inulin and consists of fructose polymers with a lower degree of polymerization (DP) of ≤10. Higher DP inulin is less soluble and is suitable as a fat replacer. Lower DP oligofructose is more soluble and is used to replace sugar.
FIGURE 1.
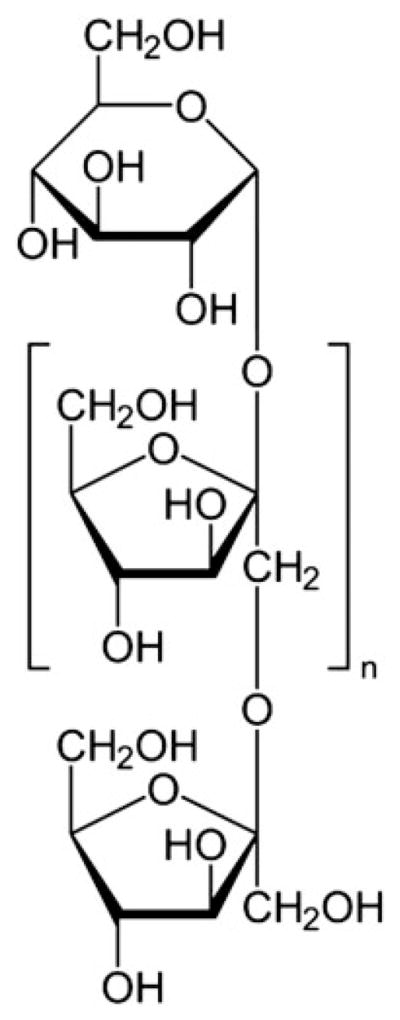
Molecular structure of inulin.
The daily intake of prebiotics is about 3–13 g/day, depending on the diet. Europeans consume on average 3–10 g/day, whereas Americans consume only 1–4 g/day.9,17 It is interesting that IBD develops in parts of the world with a relatively low daily intake of these prebiotics.
Prebiotics are not digested in the upper gastrointestinal tract and reach the colon intact, where they are selectively fermented by residential microbiota into short chain fatty acids (SCFAs) and lactate. Similar to oral administration of probiotics, only continuous intake of prebiotics will maintain their beneficial effects.18,19
Several studies show that factors such as lowering luminal pH, prebiotic dosage and concentration, duration of intake, fermentation site, and the initial composition of the intestinal microflora are important for the prebiotic effects.9,20–24
Several substances are claimed to be prebiotics, but so far only fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), lactulose, and inulin have met all 3 criteria listed above, as published by Roberfroid and Cherbut14,25 (Table 3). Other prebiotic candidates are promising, but only preliminary data exist for their health-inducing effects, whereas they do not meet all prebiotic criteria as stated above and therefore cannot be classified as prebiotics.14,25–27
TABLE 3.
Potential Candidate Prebiotic Substrates
| Fructo-oligosaccharides* | Lactosucrose |
| Galacto-oligosaccharides* | Mannan oligosaccharides |
| Inulin* | Melibiose oligosaccharides |
| Lactulose* | N-acetylchito-oligosaccharides |
| Gentio-oligosaccharides | Oligodextrans |
| Germinated Barley foodstuff | Pectic oligosaccharides |
| Gluco-oligosaccharides | Polydextrose |
| Gluconic acid | Resistant starch |
| Hemicellulose rich substrate | Soybean Oligosaccharides |
| Isomalto-oligosaccharides | Sugar alcohols |
| Lactoferrin derived peptide | Xylo-oligosaccharides |
Proven prebiotics.
(POTENTIAL) PROTECTIVE MECHANISMS OF PREBIOTICS (TABLE 4)
TABLE 4.
Proposed Benefits of Prebiotics to the Well-being and Health of the Animal Host
| Microflora changes | Selective stimulation of beneficial members of gut flora Inhibition of epithelial adherence and invasion of microbial pathogens Blocking of epithelial sites for microbial pathogens |
| Intestinal barrier function | Improving intestinal permeability Augmentation of mucus production |
| SCFA | Increased production of short-chain fatty acids, as fermentation product |
| Immune system | Increasing anti-inflammatory cytokines Decreasing pro-inflammatory cytokines |
Gastrointestinal protective mechanisms of prebiotics are still relatively unknown. Current hypotheses on protective mechanisms of prebiotics include changes in the intestinal microflora, improving intestinal barrier, regulating the mucosal and systemic immune response, and increasing the production of intestinal SCFAs.
Prebiotics and the Intestinal Microflora
Intestinal bacteria are thought to play an import role in the pathogenesis of IBD, especially in CD. This concept is supported by multiple publications (Table 1). Prebiotics alter the intestinal microbial composition by stimulating the growth of commensal protective bacteria and enhance resistance to colonization with disease-inducing bacteria, therefore contributing to colitis reduction.28,29 (Table 4)
Several studies showed that prebiotics shift the intestinal microflora toward a beneficial one in both animal models and human studies, as they increase the number of protective bacteria, such as bifidobacteria and lactobacilli, to the detriment of disease-inducing bacteria.19,30–34 Breast milk contains prebiotic milk-oligosaccharides. Several studies have shown that the intestinal microbiota of breast-fed infants is dominated by bifidobacteria and lactic acid bacteria, whereas the microflora of formula-fed infants contains lower numbers of these protective bacteria and more bacteroides, clostridia, and enterobacteriaceae.35–37
The current literature provides some explanations for the selective growth stimulation of protective bacteria by prebiotics. Some protective organisms have specific enzymes that can hydrolyze prebiotic oligosaccharides, which results in the proliferation of these protective bacteria. This mechanism has been reported in, e.g., Bifidobacterium infantis.38
In addition, fermentation of nondigestible carbohydrates in the proximal colon results in a lower luminal pH, resulting in growth inhibition of Bacteroides spp, which fails to grow at pH lower than 5.5.39
Prebiotic stimulation of intestinal microflora is not selective. Proliferation of intestinal bacteria other than the common “probiotics” is also stimulated by prebiotics, such as Eubacteria rectale, Clostridium coccoides, and Roseburia inulinivorans.19,40 This nonselective stimulation by prebiotics can also occur through cross-feeding mechanisms; Falony et al41 found that Bifidobacterium longum releases free fructose into the extracellular environment during prebiotic (oligofructose) degradation, which can then induce the proliferation of other bacteria such as Anaerostipus caccae, a bacterium that by itself is not capable of fermenting oligofructose. Despite the fact that “nonprobiotic” bacteria are able to ferment prebiotics, lactobacilli and bifidobacteria remain one of the most capable fermenters of prebiotics in a competitive intestinal environment.42
Prebiotics can also inhibit adherence of pathogenic bacteria to the gut epithelium and inhibit their colonization. The terminal sugars of prebiotic oligosaccharides can interfere with receptors on disease-inducing bacteria, thereby preventing their attachment to the intestinal epithelium. Hopkins and others showed that FOS, GOS, and inulin inhibit intestinal colonization of Clostridium difficile in vitro.43,44
Prebiotic oligosaccharides induce antimicrobial effects by selectively stimulating the growth of intestinal protective bacteria. Such intestinal organisms can then secrete antimicrobial compounds and compete with disease-inducing bacteria for intestinal epithelial receptors, reducing their ability to colonize and affect the host, as mentioned above.37,44
Effects on Intestinal Barrier Function
The intestinal barrier consists of a biofilm, a mucus layer, and the intestinal epithelium. Together with mucosal dendritic cells, Paneth cells, macrophages, and neutrophils, the intestinal barrier forms part of the innate immune response. The intestinal epithelium protects the host from invasion of disease-inducing intestinal bacteria. One factor for IBD pathogenesis involves a defective intestinal barrier.45 This intestinal epithelial barrier function is further impaired by inflammation; TNF-α and IFN-γ are proinflammatory cytokines released during inflammation that further increase epithelial permeability at tight junctions. This defect leads to translocation of endotoxins and bacterial antigens, resulting in a persistent activation of the adaptive immune system.46,47
Prebiotics are believed to improve the intestinal barrier by stimulating the growth of protective bacteria that upregulate epithelial defense mechanisms that protect against intestinal inflammation in animal models of colitis. Probiotics restore the intestinal epithelial integrity by enhancing tight junctions between intestinal epithelial cells and by increasing mucus production.35,48 Madsen49 showed that the probiotic cocktail VSL#3 improved intestinal barrier function in vivo in IL-10 KO mice.
The intestinal mucus layer prevents the attachment and translocation of bacteria across the epithelial barrier.50 Dietary fibers, such as guar gum and citrus fibers, increase mucin production, resulting in reduced bacterial translocation.51 An increase in sulphomucin production in inulin-fed rats has also been reported.52
Increased mucin production may result from fermentation and increased SCFA production by prebiotics. Barcelo et al53 showed that acetate and butyrate, resulting from fermentation of cellulose, pectin, and arabic gum, stimulated mucin production in an isolated perfused rat colon.
Effects on the Host Immune System
The balance between antiinflammatory and proinflammatory cytokines is disturbed in IBD. This is shown as a relative dominance of proinflammatory over antiinflammatory or regulatory cytokines. Analysis of the inflamed mucosa from patients with UC and CD revealed an increased expression of proinflammatory cytokines, such as IL-1, IL-6, IL-8, IL-12, IL-17, IL-23, IL-13, IFN-γ, and TNF-α and relatively less abundant immunoregulatory cytokines, such as IL-10 and TGF-β.4,5,46,54,55
Prebiotics can influence the production of pro- and antiinflammatory cytokines, resulting in less inflammation. Roller et al56 investigated the effect of pre- and probiotics on mesenteric lymph nodes and Peyer’s patches in rats. Feeding inulin-enriched oligofructose enhanced the production of IL-10 in Peyer’s patches and increased the secretion of IgA in the ileum. Secretory IgA prevents the attachment of intestinal pathogens and increases the phagocytic function of intraperitoneal macrophages. Feeding a combination of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 and prebiotics (inulin enriched with oligofructose) also enhanced IgA secretion in the ileum. In contrast, probiotics alone had only a slight immunomodulatory effect. Lactulose has also been associated with increased IgA secretion in gut-associated lymphoid tissue (GALT) in rats.57 In addition, feeding inulin-enriched oligofructose in colitis-susceptible HLA-B27 transgenic rats reduced colitis and increased intestinal TGF-β.58
Increase of Short Chain Fatty Acids
Prebiotics are fermented by anaerobic colonic microbiota into SCFAs such as acetate, butyrate, and propionate. The amount of SCFA produced in the colon depends on the composition of the intestinal microflora, their substrate, and the gut transit time. Most butyrate-producing microorganisms are related to Eubacterium rectale, Clostridium coccoidus, and Roseburia relatives.19,59 Acetate is metabolized in peripheral tissues; propionate is taken up by the liver, whereas butyrate is the major energy source for colonocytes. Butyrate has an essential role in the maturation of colonic epithelium, regeneration of mucosa in the case of atrophy, induction of cell differentiation and stimulation of apoptosis.60
Butyrate significantly inhibits proinflammatory cytokines IFN-γ and IL-2 production in rat mesenteric lymph nodes (MLNs). The ratio of the concentrations of IFN-γ to IL-10 measured in MLN was markedly decreased by butyrate.61
In addition, butyrate can reduce inflammation by inhibiting nuclear factor kappa B (NF-κB) activation and by increasing cytoplasmic inhibitor of nuclear factor kappa B levels, therefore inhibiting the production of proinflammatory cytokines.9,62,63
Administering short-chain fructo-oligosaccharides, which induce high amounts of butyrate in the colon, showed an increase in the mucosal crypt height and epithelial cell density in neonatal pigs.64 Prolonged administration of trans-galacto-oligosaccharides also changes the fermentative activity of colonic flora in humans, resulting in higher levels of SCFA.33
SCFAs can also alter the colonic physiology by decreasing the colonic pH. Lower luminal pH can inhibit growth and activity of pathogenic bacteria and can reduce several bacterial enzymatic activities detrimental for the intestinal epithelium.19,32
Reduced colonic SCFA, especially butyrate, could lead to chronic colonic injury. A decrease in SCFA concentration in the colonic lumen of UC patients with active disease compared to normal subjects has been reported.65,66 This suggested an association between decreased SCFA and UC. However, butyrate enemas have shown mixed results in the treatment of active UC.60,67
ADVERSE EFFECTS OF PREBIOTICS
Prebiotics have an excellent safety profile. However, they have been associated with symptoms of dose-dependent abdominal pain, flatulence, bloating, and diarrhea.16
Inulin and FOS increased colonization of Salmonella in cecal contents and enhanced translocation of Salmonella enterica serovar Enteritidis in rats despite proliferation of intestinal lactobacilli and bifidobacteria. This effect was counteracted by normalizing the calcium intake of the rats.68,69 In contrast, Osman et al70 showed a reduction of bacterial translocation to the liver in a dextran sulfate sodium (DSS)-induced colitis in rats fed oligofructose, whereas bacterial translocation was increased using a combination of oligofructose, inulin, and B. infantis DSM 15158. Mangell et al71 showed that Lactobacillus plantarum 299v reduced bacterial translocation to liver and mesenteric lymph nodes in rats, whereas prebiotics (oatmeal) did not prevent this. However, increased bacterial translocation was not found in synbiotic-treated patients (n = 72) compared to placebo-treated patients in elective surgery patients.72 Taken together, the effects of prebiotics on bacterial translocation are still unclear and further research is necessary.
PREBIOTICS IN EXPERIMENTAL CHRONIC INTESTINAL INFLAMMATION
The efficacies of prebiotics are most extensively studied in experimental colitis models (Table 5). In addition, these models have provided insight into possible mechanisms involved in the antiinflammatory effects of prebiotics, supporting its potential role for the treatment of human IBD. We highlight the following results.
TABLE 5.
Efficacy of Prebiotics or Synbiotics in the Prevention or Treatment in Rodent IBD Models
| Author | Animal Model | Treatment | Duration | Treatment Started Before or After Colitis Induction | Outcome |
|---|---|---|---|---|---|
| Madsen 1999 (n = 10)84 | IL-10 KO | Lactulose | 4 or 8 wks | Before + continued | Reduction of inflammation |
| Videla 2001 (n = 95)73 | DSS | Inulin | 5–14 days | After | Reduction of inflammation |
| Holma 2002 (n = 42)82 | TNBS | GOS | 13 days | Before + continued | No reduction of inflammation |
| Cherbut 2003 (n = 34)79 | TNBS | FOS | 2 wks | Before + continued | Reduction of inflammation No reduction of inflammation with FOS |
| Moreau 2003 (n = 96)74 | DSS | Starch or FOS | 7 or 14 days | After | Reduction of inflammation with starch |
| Rumi 2004 (n = 30)76 | DSS | Lactulose | 6 days | During | Reduction of inflammation |
| Lara-Villoslada 2005 (n = 20)75 | DSS | Goat milk oligosaccharides | 8 days | After | Reduction of inflammation |
| Camuesco 2005 (n = 30)77 | TNBS | Lactulose | 2 wks | Before + continued | Reduction of inflammation |
| Hoentjen 2005 (n = 16)58 | HLA-B27 | OFI | 7 wks | Before + continued | Reduction of inflammation |
| Daddaoua 2006 (n = 18)81 | TNBS | Goat milk oligosaccharides | 4 days | Before + continued | Reduction of inflammation |
| Schultz 2004 (n = 25)83 | HLA-B27 | Synbiotics | 8 wks | Before + continued | Reduction of inflammation |
| Osman 2006 (n = 36)70 | DSS | Synbiotics/OFI | 2 wks | Before + continued | Reduction of inflammation |
| Daddaoua 2007 (n = 30)80 | TNBS | AHCC | 8 days | Before + continued | Reduction of inflammation |
| Winkler 2007 (n = 10)78 | DSS | FOS | 19 days | Before and during or after | Reduction of inflammation |
DSS-induced Colitis
In a DSS-induced colonic injury model oral inulin reduced colonic injury,73 whereas rectal inulin or butyrate enemas had no effect. Resistant starch also reduced DSS-induced colitis, whereas fructo-oligosaccharides did not show improvement.74 A reduction of inflammation in the DSS model was also shown with goat milk oligosaccharides (GMO) as reported by Lara-Villoslada et al.75
Oligofructose and inulin (OFI) alone or 2 B. infantis strains (DSM 15158 and DSM 15159), isolated from infant feces, with and without OFI were fed to rats for 14 days in DSS-induced colitis. OFI alone or the B. infantis strains with and without OFI improved the DSS-induced acute colitis.70 Two studies using oral administration of lactulose in a DSS rat model (300–1000 mg/kg twice daily) and TNBS rat model showed reduction of colonic inflammation after 6 or 14 days, respectively. This effect was dose-dependent.76,77
Winkler et al78 reported a decrease in disease activity and colonic damage in a mouse model. Colitis in C57BL/6 mice was induced by DSS. FOS treatment was administered twice daily for 19 days. The administration of FOS reduced disease activity index, colonic crypt loss, and histological damage in all mice.
TNBS-induced Colitis
Oral administration of fructo-oligosaccharide was effective in a short-term study in hapten-induced colitis induced by trinitrobenzene sulfonic acid (TNBS).79 In the same model GMO showed reduction in inflammation after 4 days of oral administration. Active hexose-correlated compound, a product prepared from the mycelium of edible Basidiomycete fungi and which contains oligosaccharides, also showed disease reduction in TNBS-induced colitis.80,81 In contrast, treatment with galacto-oligosaccharides (GOS) had no effect, despite an increase of intestinal bifidobacteria,82 indicating that an increase of these bacteria is not always associated with protection.
Colitis in HLA-B27 Transgenic Rats
Prebiotics have also been administered in genetically induced chronic colitis. In HLA-B27 transgenic rats colonic inflammation was reduced after oral administration of the combination of inulin and oligofructose for 7 weeks.58 Synbiotic therapy with inulin plus a probiotic cocktail, containing lactobacilli and bifidobacteria, also reduced colitis in these rats.83
Colitis in IL-10 KO Mice
Treatment of IL-10 knockout (KO) mice with oral lactulose treatment (0.06%, wt/vol in drinking water) for 4 and 8 weeks significantly attenuated the development of colonic injury. Treatment was started before colitis develops.84
In summary, similar to probiotics, results from prebiotics in experimental colitis show that not all prebiotics are beneficial. Also, the protective effects depend on the colitis model used.
PREBIOTICS IN HUMAN IBD
Prebiotics
Results of prebiotics as therapeutics in human IBD are just emerging (Table 6). Lindsay et al48 performed a small open-label study in 10 patients with active ileocolonic CD using a combination of 15 g/day oligofructose and inulin (ratio 70:30%) for 3 weeks. This study showed a significant reduction in disease activity, concomitant with a significant increase in mucosal bifidobacteria, Interestingly, prebiotic treatment increased colonic dendritic cells expressing IL-10, Toll-like receptor (TLR)-2, and TLR-4, indicating that these prebiotics affected the innate mucosal immune response.
TABLE 6.
Clinical Studies Testing Prebiotics or Synbiotics in the Treatment of Human IBD
| Author | Patients | Treatment | Compared to | Duration | Outcome |
|---|---|---|---|---|---|
| Welters 2002 (n = 24)86 | Pouchitis | Inulin | Placebo (cross-over) | 3 wks | Reduction of inflammation |
| Furrie 2005 (n = 18)88 | UC | Synbiotics | Placebo (RCT) | 1 month | Reduction of inflammation |
| Lindsay 2006 (n = 10)48 | CD | FOS | None (open label) | 3 wks | Reduction of inflammation |
| Chermesh 2007 (n = 30)89 | Postop. CD | Synbiotics | Placebo (RCT) | 24 months | No prevention of relapse |
| Casellas 2007 (n = 19)85 | UC | Synergy | Placebo (pilot RCT) | 2 wks | Reduction of calprotectin level |
A small placebo-controlled study reported on the adjunct effect of 2 weeks oligofructose-enriched inulin (1:1) in 19 patients with mild to moderately active UC who also received 3 g/day of mesalamine. This study showed a significant reduction of the fecal inflammatory marker calprotectin in prebiotic-treated patients after 1 week compared to the placebo group, suggesting that these prebiotics were able to reduce chronic intestinal inflammation.85
Welters et al86 performed a crossover study in 24 patients with chronic pouchitis after colectomy for UC. The patients were given 24 g daily of inulin or placebo for 3 weeks. Inulin treatment resulted in decreased endoscopic and histological inflammation. This effect was associated with increased intestinal butyrate, lowered pH, and decreased numbers of Bacteroides fragilis.
Synbiotics
A special role is granted to synbiotics, the combination of pro- and prebiotics. Synbiotics immediately increase the amount of protective bacteria in the gut and have an indirect effect of stimulating endogenous protective intestinal bacteria by prebiotics.87 Furrie et al88 performed a double-blind randomized controlled study in 18 UC patients. Patients were fed a combination of Bifidobacterium longum, combined with oligofructose-enriched inulin (1:1) for 1 month. Rectal biopsies were collected before and at the end of treatment and mucosal immune markers were measured. After treatment there was a reduced endoscopic and microscopic colonic inflammation in synbiotic-treated patients versus controls, concomitant with a decrease of β-defensins mRNA, TNF-α, and IL-1α.
In contrast to these positive results, a randomized-controlled trial in 30 CD patients using a mixture of 4 lactic acid bacterial strains (1010 Pediococcus pentosaceus, 1010 L. raffinolactis, 1010 L. paracasei susp paracasei 19, 1010 L. plantarum 2362) and 4 different fermentable fibers (2.5 g β-glucans, 2.5 g inulin, 2.5 g pectin, and 2.5 g resistant starch) did not prevent endoscopic disease recurrence 24 months after ileocecal resection, compared to placebo,89 although this study was likely underpowered.
Beneficial Effects of Prebiotics in Other Medical Conditions
Prebiotics are also reported to have beneficial effects in other GI disorders, such as improvement of infant diarrhea, infectious colitis, reduced risk of experimentally induced colon cancer, improvement of lipid metabolism, increased calcium absorption, alleviation of constipation, and improvement of food allergy.16,90
CONCLUSION
Prebiotics and synbiotics are emerging as promising nutraceuticals in various medical conditions, including IBD. Since prebiotics are easy to administer, inexpensive, and lack significant toxic side effects they may become an attractive alternative or adjunct to standard therapeutics in IBD.
Further understanding of protective mechanisms of prebiotics and interactions between the gastrointestinal tract, host immune system, and intestinal microflora will help to identify which prebiotics or synbiotics may be most effective in IBD. Future randomized, placebo-controlled large clinical trials are needed before we can implement prebiotics in our standard medical armamentarium for IBD.
Acknowledgments
Dr. Dieleman is funded by an operating grant from the Canadian Institutes of Health and Research and an Aid in Research Grant from the Crohn’s and Colitis Foundation of Canada.
References
- 1.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–1568. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 2.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 3.Thompson-Chagoyan OC, Maldonado J, Gil A. Aetiology of inflammatory bowel disease (IBD): role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin Nutr. 2005;24:339–352. doi: 10.1016/j.clnu.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Bamias G, Nyce MR, De La Rue SA, et al. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 5.Korzenik JR, Podolsky DK. Evolving knowledge and therapy of inflammatory bowel disease. Nat Rev Drug Disc. 2006;5:197–209. doi: 10.1038/nrd1986. [DOI] [PubMed] [Google Scholar]
- 6.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9:497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 7.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am J Clin Nutr. 2001;73:361S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 8.Tomasik PJ, Tomasik P. Probiotics and prebiotics. Cereal Chem. 2003;80:113–117. [Google Scholar]
- 9.Delzenne NM. Oligosaccharides: state of the art. Proc Nutr Soc. 2003;62:177–182. doi: 10.1079/pns2002225. [DOI] [PubMed] [Google Scholar]
- 10.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2006;40:260–263. doi: 10.1097/00004836-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Galvez J, Rodriguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005;49:601–608. doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 14.Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 15.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota — introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 16.Swennen K, Courtin CM, Delcour JA. Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr. 2006;46:459–471. doi: 10.1080/10408390500215746. [DOI] [PubMed] [Google Scholar]
- 17.Niness KR. Inulin and oligofructose: What are they? J Nutr. 1999;129:1402S–1406S. doi: 10.1093/jn/129.7.1402S. [DOI] [PubMed] [Google Scholar]
- 18.Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004;80:1658–1664. doi: 10.1093/ajcn/80.6.1658. [DOI] [PubMed] [Google Scholar]
- 19.Kleessen B, Hartmann L, Blaut M. Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats associated with a human faecal flora. Br J Nutr. 2001;86:291–300. doi: 10.1079/bjn2001403. [DOI] [PubMed] [Google Scholar]
- 20.Palframan RJ, Gibson GR, Rastall RA. Effect of pH and dose on the growth of gut bacteria on prebiotic carbohydrates in vitro. Anaerobe. 2002;8:287–292. doi: 10.1006/anae.2002.0434. [DOI] [PubMed] [Google Scholar]
- 21.Rao VA. The prebiotic properties of oligofructose at low intake levels. Nutr Res. 2001:843–848. [Google Scholar]
- 22.Tuohy KM, Kolida S, Lustenberger AM, et al. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides—a human volunteer study. Br J Nutr. 2001;86:341–348. doi: 10.1079/bjn2001394. [DOI] [PubMed] [Google Scholar]
- 23.Tuohy KM, Finlay RK, Wynne AG, et al. A human volunteer study on the prebiotic effects of HP-inulin — faecal bacteria enumerated using fluorescent in situ hybridisation (FISH) Anaerobe. 2001;3:113–118. [Google Scholar]
- 24.Cavaglieri CR, Nishiyama A, Fernandes LC, et al. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. doi: 10.1016/s0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- 25.Cherbut C. Inulin and oligofructose in the dietary fibre concept. Br J Nutr. 2002;87:S159–S162. doi: 10.1079/BJNBJN2002532. [DOI] [PubMed] [Google Scholar]
- 26.Gibson GR, Probert HM, van Loo, et al. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 27.Quigley EM, Quera R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology. 2006;130:S78–S90. doi: 10.1053/j.gastro.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 28.Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr. 1999;129:1438S–1441S. doi: 10.1093/jn/129.7.1438S. [DOI] [PubMed] [Google Scholar]
- 29.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Santos A, San MM, Diaz DM. Prebiotics and their long-term influence on the microbial populations of the mouse bowel. Food Microbiol. 2006;23:498–503. doi: 10.1016/j.fm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Gibson GR, Beatty ER, Wang X, et al. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 32.Campbell JM, Fahey GC, Jr, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127:130–136. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- 33.Bouhnik Y, Flourie B, Gay-Abensour L, et al. Administration of trans-galacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J Nutr. 1997;127:444–448. doi: 10.1093/jn/127.3.444. [DOI] [PubMed] [Google Scholar]
- 34.Langlands SJ, Hopkins MJ, Coleman N, et al. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–1616. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruzzese E, Volpicelli M, Squaglia M, et al. Impact of prebiotics on human health. Dig Liver Dis. 2006;38(suppl 2):S283–S287. doi: 10.1016/S1590-8658(07)60011-5. [DOI] [PubMed] [Google Scholar]
- 36.Coppa GV, Zampini L, Galeazzi T, et al. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38(suppl 2):S291–S294. doi: 10.1016/S1590-8658(07)60013-9. [DOI] [PubMed] [Google Scholar]
- 37.Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93(suppl 1):S41–S48. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- 38.Perrin S, Warchol M, Grill JP, et al. Fermentations of fructo-oligosaccharides and their components by Bifidobacterium infantis ATCC 15697 on batch culture in semi-synthetic medium. J Appl Microbiol. 2001;90:859–865. doi: 10.1046/j.1365-2672.2001.01317.x. [DOI] [PubMed] [Google Scholar]
- 39.Walker AW, Duncan SH, William Leitch EC, et al. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duncan SH, Scott KP, Ramsay AG, et al. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl Environ Microbiol. 2003;69:1136–1142. doi: 10.1128/AEM.69.2.1136-1142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falony G, Vlachou A, Verbrugghe K, et al. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol. 2006;72:7835–7841. doi: 10.1128/AEM.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartemink R, Van Laere KM, Rombouts FM. Growth of enterobacteria on fructo-oligosaccharides. J Appl Microbiol. 1997;83:367–374. doi: 10.1046/j.1365-2672.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 43.Hopkins MJ, Macfarlane GT. Nondigestible oligosaccharides enhance bacterial colonization resistance against Clostridium difficile in vitro. Appl Environ Microbiol. 2003;69:1920–1927. doi: 10.1128/AEM.69.4.1920-1927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zopf D, Roth S. Oligosaccharide anti-infective agents. Lancet. 1996;347:1017–1021. doi: 10.1016/s0140-6736(96)90150-6. [DOI] [PubMed] [Google Scholar]
- 45.Meddings J. The significance of the gut barrier in disease. Gut. 2008;57:438–440. doi: 10.1136/gut.2007.143172. [DOI] [PubMed] [Google Scholar]
- 46.Kucharzik T, Maaser C, Lugering A, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 47.Berkes J, Viswanathan VK, Savkovic SD, et al. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsay JO, Whelan K, Stagg AJ, et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen KL. Inflammatory bowel disease: lessons from the IL-10 gene-deficient mouse. Clin Invest Med. 2001;24:250–257. [PubMed] [Google Scholar]
- 50.Katayama M, Xu D, Specian RD, et al. Role of bacterial adherence and the mucus barrier on bacterial translocation: effects of protein malnutrition and endotoxin in rats. Ann Surg. 1997;225:317–326. doi: 10.1097/00000658-199703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satchithanandam S, Vargofcak-Apker M, Calvert RJ, et al. Alteration of gastrointestinal mucin by fiber feeding in rats. J Nutr. 1990;120:1179–1184. doi: 10.1093/jn/120.10.1179. [DOI] [PubMed] [Google Scholar]
- 52.Fontaine N, Meslin JC, Lory S, et al. Intestinal mucin distribution in the germ-free rat and in the heteroxenic rat harbouring a human bacterial flora: effect of inulin in the diet. Br J Nutr. 1996;75:881–892. doi: 10.1079/bjn19960194. [DOI] [PubMed] [Google Scholar]
- 53.Barcelo A, Claustre J, Moro F, et al. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 55.Andoh A, Fujiyama Y. Therapeutic approaches targeting intestinal microflora in inflammatory bowel disease. World J Gastroenterol. 2006;12:4452–4460. doi: 10.3748/wjg.v12.i28.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roller M, Rechkemmer G, Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J Nutr. 2003;134:153–156. doi: 10.1093/jn/134.1.153. [DOI] [PubMed] [Google Scholar]
- 57.Schley PD, Field CJ. The immune-enhancing effects of dietary fibres and prebiotics. Br J Nutr. 2002;87(suppl 2):S221–S230. doi: 10.1079/BJNBJN/2002541. [DOI] [PubMed] [Google Scholar]
- 58.Hoentjen F, Welling GW, Harmsen HJ, et al. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11:977–985. doi: 10.1097/01.mib.0000183421.02316.d5. [DOI] [PubMed] [Google Scholar]
- 59.Pryde SE, Duncan SH, Hold GL, et al. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 60.Wong JM, de SR, Kendall CW, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Cavaglieri L, Orlando J, Etcheverry M. Rhizosphere microbial community structure at different maize plant growth stages and root locations. Microbiol Res. 2007 doi: 10.1016/j.micres.2007.03.006. in press. [DOI] [PubMed] [Google Scholar]
- 62.Tedelind S, Westberg F, Kjerrulf M, et al. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segain JP, Raingeard de la BD, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howard MD, Gordon DT, Pace LW, et al. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J Pediatr Gastroenterol Nutr. 1995;21:297–303. doi: 10.1097/00005176-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Den HE, Hiele M, Evenepoel P, et al. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology. 1998;115:584–590. doi: 10.1016/s0016-5085(98)70137-4. [DOI] [PubMed] [Google Scholar]
- 66.Chapman MA, Grahn MF, Hutton M, et al. Butyrate metabolism in the terminal ileal mucosa of patients with ulcerative colitis. Br J Surg. 1995;82:36–38. doi: 10.1002/bjs.1800820115. [DOI] [PubMed] [Google Scholar]
- 67.Steinhart AH, Hiruki T, Brzezinski A, et al. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther. 1996;10:729–736. doi: 10.1046/j.1365-2036.1996.d01-509.x. [DOI] [PubMed] [Google Scholar]
- 68.Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, et al. Dietary fructo-oligosaccharides and inulin decrease resistance of rats to salmonella: protective role of calcium. Gut. 2004;53:530–535. doi: 10.1136/gut.2003.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bovee-Oudenhoven IM, Ten Bruggencate SJ, Lettink-Wissink ML, et al. Dietary fructo-oligosaccharides and lactulose inhibit intestinal colonisation but stimulate translocation of salmonella in rats. Gut. 2003;52:1572–1578. doi: 10.1136/gut.52.11.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osman N, Adawi D, Molin G, et al. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. doi: 10.1186/1471-230X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mangell P, Lennernas P, Wang M, et al. Adhesive capability of Lacto-bacillus plantarum 299v is important for preventing bacterial translocation in endotoxemic rats. APMIS. 2006;114:611–618. doi: 10.1111/j.1600-0463.2006.apm_369.x. [DOI] [PubMed] [Google Scholar]
- 72.Anderson AD, McNaught CE, Jain PK, et al. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut. 2004;53:241–245. doi: 10.1136/gut.2003.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Videla S, Vilaseca J, Antolin M, et al. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96:1486–1493. doi: 10.1111/j.1572-0241.2001.03802.x. [DOI] [PubMed] [Google Scholar]
- 74.Moreau NM, Martin LJ, Toquet CS, et al. Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. Br J Nutr. 2003;90:75–85. doi: 10.1079/bjn2003867. [DOI] [PubMed] [Google Scholar]
- 75.Lara-Villoslada F, Debras E, Nieto A, et al. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin Nutr. 2006;25:477–488. doi: 10.1016/j.clnu.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Rumi G, Tsubouchi R, Okayama M, et al. Protective effect of lactulose on dextran sulfate sodium-induced colonic inflammation in rats. Dig Dis Sci. 2004;49:1466–1472. doi: 10.1023/b:ddas.0000042248.48819.ad. [DOI] [PubMed] [Google Scholar]
- 77.Camuesco D, Peran L, Comalada M, et al. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel Dis. 2005;11:265–271. doi: 10.1097/01.mib.0000160808.30988.d9. [DOI] [PubMed] [Google Scholar]
- 78.Winkler J, Butler R, Symonds E. Fructo-oligosaccharide reduces inflammation in a dextran sodium sulphate mouse model of colitis. Dig Dis Sci. 2007;52:52–58. doi: 10.1007/s10620-006-9224-z. [DOI] [PubMed] [Google Scholar]
- 79.Cherbut C, Michel C, Lecannu G. The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J Nutr. 2003;133:21–27. doi: 10.1093/jn/133.1.21. [DOI] [PubMed] [Google Scholar]
- 80.Daddaoua A, Martinez-Plata E, Lopez-Posadas R, et al. Active hexose correlated compound acts as a prebiotic and is antiinflammatory in rats with hapten-induced colitis. J Nutr. 2007;137:1222–1228. doi: 10.1093/jn/137.5.1222. [DOI] [PubMed] [Google Scholar]
- 81.Daddaoua A, Puerta V, Requena P, et al. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2006;136:672–676. doi: 10.1093/jn/136.3.672. [DOI] [PubMed] [Google Scholar]
- 82.Holma R, Juvonen P, Asmawi MZ, et al. Galacto-oligosaccharides stimulate the growth of bifidobacteria but fail to attenuate inflammation in experimental colitis in rats. Scand J Gastroenterol. 2002;37:1042–1047. doi: 10.1080/003655202320378239. [DOI] [PubMed] [Google Scholar]
- 83.Schultz M, Munro K, Tannock GW, et al. Effects of feeding a probiotic preparation (SIM) containing inulin on the severity of colitis and on the composition of the intestinal microflora in HLA-B27 transgenic rats. Clin Diagn Lab Immunol. 2004;11:581–587. doi: 10.1128/CDLI.11.3.581-587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 85.Casellas F, Borruel N, Torrejon A, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25:1061–1067. doi: 10.1111/j.1365-2036.2007.03288.x. [DOI] [PubMed] [Google Scholar]
- 86.Welters CF, Heineman E, Thunnissen FB, et al. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum. 2002;45:621–627. doi: 10.1007/s10350-004-6257-2. [DOI] [PubMed] [Google Scholar]
- 87.Yang SC, Chen JY, Shang HF, et al. Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J Gastroenterol. 2005;11:7413–7417. doi: 10.3748/wjg.v11.i47.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furrie E, Macfarlane S, Kennedy A, et al. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chermesh I, Tamir A, Reshef R, et al. Failure of synbiotic 2000 to prevent postoperative recurrence of Crohn’s disease. Dig Dis Sci. 2007;52:385–389. doi: 10.1007/s10620-006-9549-7. [DOI] [PubMed] [Google Scholar]
- 90.Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24:701–714. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- 91.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol. 2007;115:1–11. doi: 10.1016/j.ijfoodmicro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 94.Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223–228. doi: 10.1007/s11938-005-0014-z. [DOI] [PubMed] [Google Scholar]
- 95.Sokol H, Seksik P, Rigottier-Gois L, et al. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- 96.Kleessen B, Kroesen AJ, Buhr HJ, et al. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034–1041. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
- 97.Shanahan F. Host-flora interactions in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:S16–S24. doi: 10.1097/00054725-200402001-00004. [DOI] [PubMed] [Google Scholar]


