Abstract
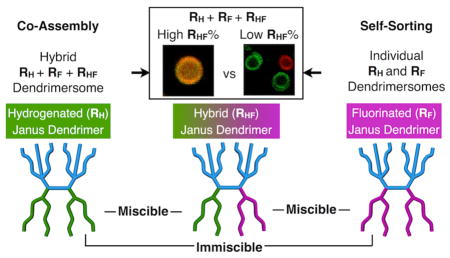
The modular synthesis of a library containing seven self-assembling amphiphilic Janus dendrimers is reported. Three of these molecules contain environmentally friendly chiral-racemic fluorinated dendrons in their hydrophobic part (RF), one contains achiral hydrogenated dendrons (RH), while one denoted hybrid Janus dendrimer, contains a combination of chiral-racemic fluorinated and achiral hydrogenated dendrons (RHF) in its hydrophobic part. Two Janus dendrimers contain either chiral-racemic fluorinated dendrons and a green fluorescent dye conjugated to its hydrophilic part (RF-NBD) or achiral hydrogenated and a red fluorescent dye in its hydrophilic part (RH-RhB). These RF, RH, and RHF Janus dendrimers self-assembled into unilamellar or onion-like soft vesicular dendrimersomes (DSs), with similar thicknesses to biological membranes by simple injection from ethanol solution into water or buffer. Since RF and RH dendrons are not miscible, RF-NBD and RH-RhB were employed to investigate by fluorescence microscopy the self-sorting and co-assembly of RF and RH as well as of phospholipids into hybrid DSs mediated by the hybrid hydrogenated-fluorinated RHF Janus dendrimer. The hybrid RHF Janus dendrimer co-assembled with both RF and RH. Three-component hybrid DSs containing RH, RF, and RHF were formed when the proportion of RHF was higher than 40%. With low concentration of RHF and in its absence, RH and RF self-sorted into individual RH or RF DSs. Phospholipids were also co-assembled with hybrid RHF Janus dendrimers. The simple synthesis and self-assembly of DSs and hybrid DSs, their similar thickness with biological membranes and their imaging by fluorescence and 19F-MRI make them important tools for synthetic biology.
Graphical Abstract
INTRODUCTION
Synthetic lipids1 such as phospholipids and glycolipids self-assemble into vesicles that mimic biological membranes. The amphiphilic structure of the lipids contains a hydrophilic head and hydrophobic tails. Lipophilic groups such as alkyl chains and fluorophilic groups such as fluorocarbon fragments have been successfully incorporated into lipids as their hydrophobic tails1b and therefore form hydrogenated and fluorinated vesicles with different stabilities and permeabilities.2 The immiscibility of hydrogenated and fluorinated components induced the phase separation of these domains.2h Fluorinated amphiphiles have been employed for biomedical applications including for drug and gene delivery.2d,e Fluorinated components including fluoropolymer nanoparticles also present potential functions such as 19F magnetic resonance imaging (MRI) agents.3 This requires stable and biocompatible fluorinated vesicles, which can be synthesized and self-assembled by simple methods. The miscibility of hybrid structures with both hydrogenated and fluorinated chains have been preliminarily investigated in phospholipids and glycolipids.4 However, very little is known about their assemblies.
Amphiphilc Janus dendrimers5 containing both hydrophobic (hydrogenated lipophilic chains) and hydrophilic dendrons self-assemble into nanoscale vesicular dendrimersomes (DSs) by simple injection of their solution in a water miscible organic solvent into water and buffer and into micrometer-scale giant DSs by film hydration. The sizes of DSs can be predicted from the thickness of their bilayer in the bulk state and from the concentration of the Janus dendrimer.5b It is expected that hydrogenated chains can be replaced with fluorinated chains leading to a new family of fluorinated vesicles named fluorinated DSs that will follow the same self-assembly principles as hydrogenated DSs.
Hybrid vesicles co-assembled from lipids and either block copolymers6 or Janus dendrimers5c provided a platform for the incorporation of biological cell membrane components into polymersomes and DSs.5c Polymersomes7 generate bilayers with a thickness of 8–50 nm and therefore are not compatible with biological membranes that have a thickness of ~4 nm,6c while DSs overcame this biocompatibility issue.5c Hence the design and synthesis of stable and biocompatible fluorinated DSs and of their hybrid structures with biological membranes including phospholipids and glycolipids became achievable.
Here we report the design and synthesis of a library containing hydrogenated, environmentally friendly chiral-racemic fluorinated and hybrid hydrogenated-fluorinated amphiphilic Janus dendrimers. Fluorinated and hydrogenated Janus dendrimers conjugated in their hydrophilic part with complementary fluorescent dyes were also elaborated. This library allowed for the first time to investigate the self-sorting and the co-assembly of fluorinated, hydrogenated and hybrid hydrogenated-fluorinated Janus dendrimers into fluorinated, hydrogenated and hybrid hydrogenated-fluorinated DSs as well as hybrid vesicles co-assembled from phospholipids and fluorinated Janus dendrimers.
RESULTS AND DISCUSSION
Design and Modular Synthesis of Amphiphilic Janus Dendrimers with Fluorinated, Hydrogenated, and Hybrid Fluorinated-Hydrogenated Chains
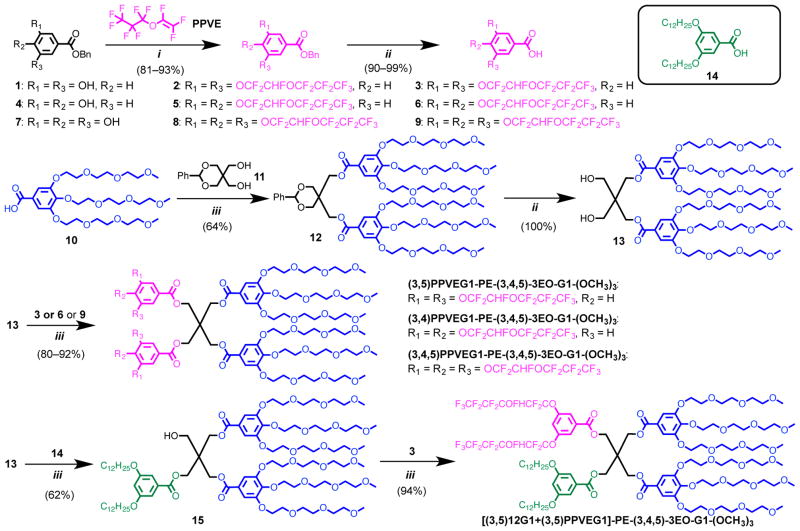
The chiral-racemic fluorinated dendrons employed in this study provide access to less ordered structures than those resulting from achiral hydrogenated or perfluorinated dendrons.8,9 Perfluoropropyl vinyl ether (PPVE) used in their synthesis is an environmentally tolerable fluorinated component that has been incorporated into chiral-racemic fluorinated dendrons in one step reaction performed under very mild reaction conditions (Scheme 1).9
Scheme 1. Synthesis of RF Janus Dendrimers and Hybrid RHF Janus Dendrimer with Both RF and RH Chains.a.
aReagents and conditions: (i) t-BuOK (cat.), DMF, 0 °C for 2 h, then 23 °C for 24 h; (ii) H2, Pd/C, DCM, methanol, 23 °C, 12 h; (iii) 4-(Dimethylamino)pyridinium 4-toluenesulfonate, DCC, DCM, 23 °C, 12 h.
With a catalytic amount of potassium tert-butoxide (t-BuOK) as base in anhydrous dimethylformamide, PPVE chains were introduced as side groups to benzyl (3,5)-, (3,4)-, and (3,4,5)-hydroxyl benzoates (Scheme 1). Previous studies have demonstrated that linear perfluorooctyl alkyl groups enhance the stability of vesicles2 and of other supramolecular assembles.8 However all linear perfluorooctyl based compounds degrade to the toxic and biopersistent perfluorooctanoic acid that was prohibited by the Environmental Protection Agency.9 The chiral-racemic fluorinated PPVE based dendrons employed in this study are prepared in one step, are soluble in ethanol rather than Freon-113 as the perfluorooctyl groups are, and degrade to environmental friendly natural hydroxybenzoic acids and perfluoropropyl chains that are nontoxic and nonbiopersistent.9
Subsequent hydrogenation with palladium on carbon in a solvent mixture of dichloromethane and methanol gave the respective fluorinated (RF) –COOH containing first generation dendrons (3,5)PPVE-G1 (3), (3,4)PPVE-G1 (6), and (3,4,5)PPVE-G1 (9) (Scheme S1). These RF dendrons were conjugated to form half of a Janus dendrimer via esterification with half protected pentaerythritol (PE) as the core and 3,4,5-tris(methyl triethylene glycol)benzoic acid [(3,4,5)-3EO-G1-(OCH3)3] (10) as the hydrophilic fragments, which has been previously developed for the modular synthesis of amphiphilic Janus dendrimers that formed stable but soft DSs with narrow size distribution (Scheme 1).5 A stepwise esterification strategy (Scheme 1) was employed for the synthesis of a hybrid Janus dendrimer containing both (3,5)PPVE-G1 (3) (RF) and 3,5-bis(dodecyloxy)benzoic acid [(3,5)12G1] (14) (RH) fragments. This hybrid compound was denoted as hybrid RHF Janus dendrimer.
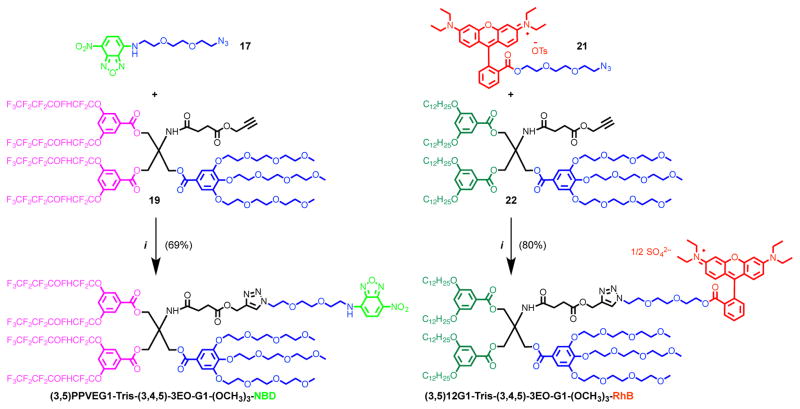
In order to elucidate the self-assembly and co-assembly/self-sorting of RF, RH, and hybrid RHF Janus dendrimers as individual and as a multi-component system and to investigate their miscibility/immiscibility, two Janus dendrimers with RF or RH chains, and with different dyes attached to their hydrophilic parts, were designed and synthesized (Scheme 2, and Scheme S4–5).
Scheme 2. Synthesis of NBD Conjugated Janus Dendrimer with RF Chains and Rhodamine B Labeled Janus Dendrimer with RH Chains.a.
aReagents and conditions: (i) CuSO4·5H2O, sodium ascorbate, THF, water, 23 °C, 24 h.
A Janus dendrimer with RH chains was labeled with a rhodamine B (RhB) red fluorescent dye, and a Janus dendrimer with RF chains was labeled with a 7-nitrobenzofurazan (NBD) green fluorescent dye. These azido triethylene glycol conjugated dyes were conjugated with the alkyne groups from the tris(hydroxymethyl)aminomethane (Tris) core via copper-catalyzed click chemistry10 which was previously developed for the synthesis of carbohydrates (glycan) conjugated Janus glycodendrimers.11
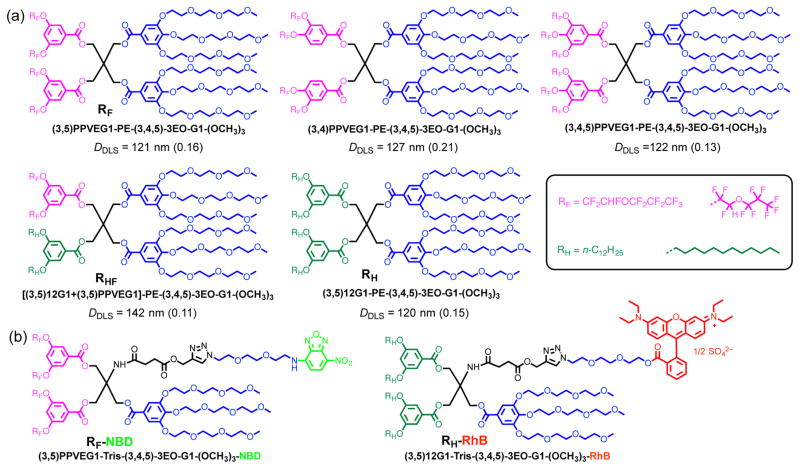
The molecular structures of three RF Janus dendrimers (3,5)PPVEG1-PE-(3,4,5)-3EO-G1-(OCH3)3, (3,4)PPVEG1-PE-(3,4,5)-3EO-G1-(OCH3)3, and (3,4,5)PPVEG1-PE-(3,4,5)-3EO-G1-(OCH3)3, one hybrid RHF Janus dendrimers [(3,5)12G1+(3,5)PPVEG1]-PE-(3,4,5)-3EO-G1-(OCH3)3 together with a RH Janus dendrimer (3,5)12G1-PE-(3,4,5)-3EO-G1-(OCH3)35a–5c are presented in Scheme 3a, in which magenta denotes RF chains, green denotes RH chains, and blue denotes hydrophilic parts. The fluorescent RF Janus dendrimer with NBD label (3,5)PPVEG1-Tris-(3,4,5)-3EO-G1-(OCH3)3-NBD and RH Janus dendrimer with RhB label (3,5)12G1-Tris-(3,4,5)-3EO-G1-(OCH3)3-RhB were presented in Scheme 3b, with abbreviations as RF-NBD and RH-RhB.
Scheme 3. The Library Containing (a) Three Janus dendrimers with RF, One Hybrid with RHF, and One with RH chains.a (b) Green Fluorescent Probe (NBD) Labeled Janus Dendrimer with RF Chains and Red Fluorescent Probe (RhB) Labeled Janus Dendrimer with RH Chains.
aDiameters (DDLS) and polydispersities (in between parentheses) indicated were obtained by DLS and refer to dendrimersomes obtained by injection of their ethanol solution into water (final concentration: 0.5 mg·mL−1).
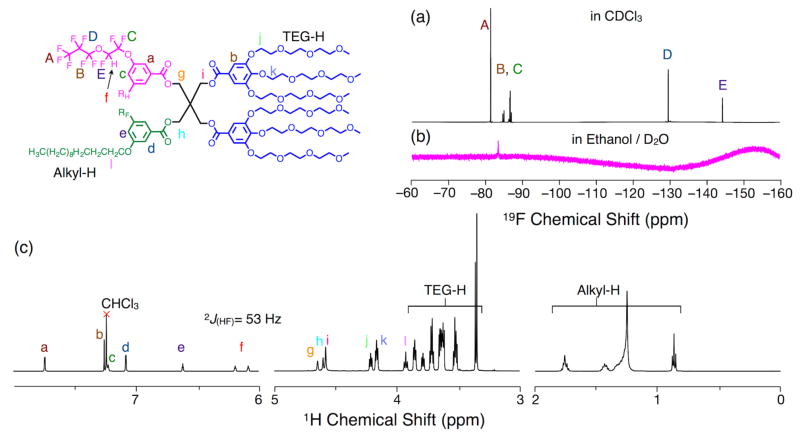
The structures of the newly synthesized RF and RHF Janus dendrimers were confirmed by NMR, in particular a 1H NMR chemical shift at 6.0–6.2 ppm, with a hydrogen–fluorine coupling 2J(HF) = 53 Hz (Figure 1c). The five different fluorine atoms in the PPVE chain were observed by 19F NMR as five groups of signals, from F-CF2 at the lowest field to F-C(O)H at the highest field (Figure 1a).
Figure 1.
Excerpt of (a–b) 19F NMR spectra (470 MHz) (a) RHF Janus dendrimer [(3,5)12G1+(3,5)PPVEG1]-PE-(3,4,5)-3EO-G1-(OCH3)3 in CDCl3, and (b) self-assembled dendrimersomes prepared by injection of an ethanol solution of RHF Janus dendrimer [(3,5)12G1+(3,5)PPVEG1]-PE-(3,4,5)-3EO-G1-(OCH3)3 into D2O. (c) Excerpt of 1H NMR spectrum (CDCl3, 500 MHz) of RHF Janus dendrimer [(3,5)12G1+(3,5)PPVEG1]-PE-(3,4,5)-3EO-G1-(OCH3)3.
Self-Assembly of Amphiphilic Janus Dendrimers with Fluorinated, Hydrogenated, and Hybrid Chains into Nanoscale Unilamellar or Onion-Like DSs
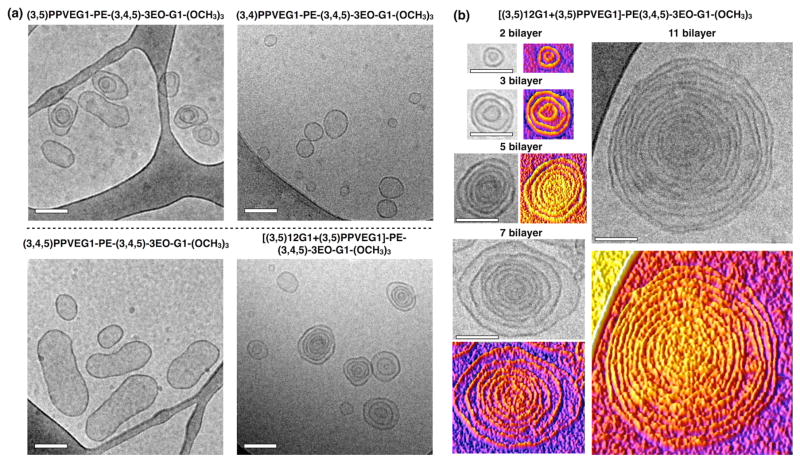
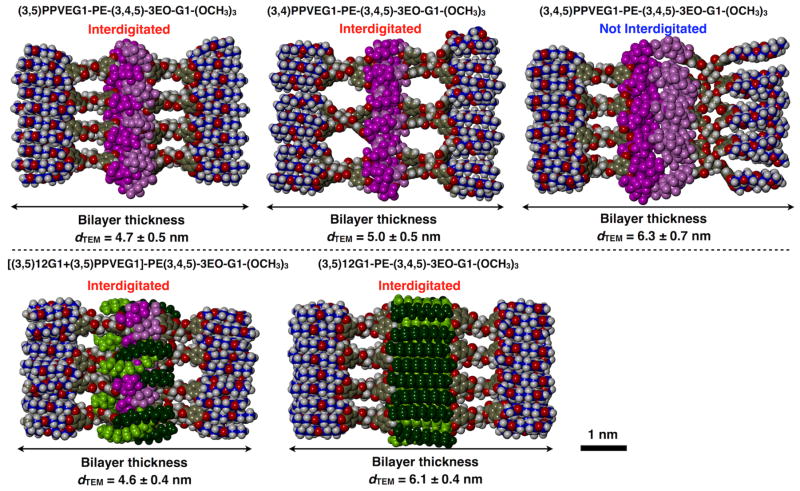
Nanoscale DSs of RF, hybrid RHF and of RH Janus dendrimers were prepared by injection of their ethanol solution into water. Monodisperse peaks with narrow polydispersity (PDI) obtained from dynamic light scattering (DLS) analysis (Scheme 3a, Figure S1) indicated the formation of nanoscale assemblies. RF, RHF and RH Janus dendrimers shared a similar trend of increasing the size of their assemblies with increasing concentration of the Janus dendrimer (Figure S1).5b The vesicular morphologies of the assemblies were characterized by cryogenic-transmission electron microscopy (Cryo-TEM) (Figure 2a). (3,4)PPVEG1-PE-(3,4,5)-3EO-G1-(OCH3)3 and (3,4,5)PPVEG1-PE-(3,4,5)-3EO-G1-(OCH3)3 self-assembled into unilamellar DSs with single bilayers, while (3,5)PPVEG1-PE-(3,4,5)-3EO-G1-(OCH3)3 and [(3,5)12G1+(3,5)PPVEG1]-PE-(3,4,5)-3EO-G1-(OCH3)3 formed multilamellar DSs.12 In samples of hybrid RHF Janus dendrimer [(3,5)12G1+(3,5)PPVEG1]-PE-(3,4,5)-3EO-G1-(OCH3)3, well-defined onion-like vesicles were observed, with numbers of bilayers from 2 to 11 (Figure 2b). The unilamellar vesicular assemblies from RH Janus dendrimers was reported before.7a The thickness of the bilayers of DSs was calculated from cryo-TEM data (Figure 3).8a,13 Cryo-TEM together with molecular modeling (Figure S2), demonstrated that RF Janus dendrimers with (3,5)- and (3,4)-substituted PPVE chains and the hybrid RHF Janus dendrimer showed interdigitation of their fluorinated fragments, with a membrane thickness in the range of 4.5 to 5 nm. These values are comparable to those those of the biological phospholipid-derived membranes (~4 nm) and thinner than that of the interdigitated RH Janus dendrimer (6.1 nm).5a This is due to the shorter PPVE chain compared to the dodecyl alkyl chain and/or the perfluorooctyl-based linear chains of the same length in the dendrons.8a In contrast, the (3,4,5)-substituted derivative showed a thicker bilayer (6.3 nm) indicative of no interdigitation. These results are consistent with the structures reported in a previous report on RH based Janus dendrimers.5b
Figure 2.
(a) Cryo-TEM images of DSs assembled by 0.5 mg·mL−1 (final concentration) of RF and RHF Janus dendrimers. (b) Selected cryo-TEM images of onion-like DSs self-assembled from 0.5 mg·mL−1 [(3,5)12G1+(3,5)PPVEG1]-PE-(3,4,5)-3EO-G1-(OCH3)3 with hybrid RHF chains and their 3D intensity-plotting images with different numbers of bilayers and diameters. Scale bar = 100 nm.
Figure 3.
Molecular models of the bilayer thickness with and without interdigitation of fluorinated or hydrogenated chains calculated from cryo-TEM images.
A representative 19F NMR spectrum of the DSs self-assembled from hybrid RHF Janus dendrimers exhibited a clear fluorine signal (Figure 1b) and suggests potential applications of these or related fluorinated DSs as 19F MRI imaging agents capable of being loaded with drugs, proteins, nucleic acids, or even with other imaging agents to generate hybrid imaging tools.5f
The non-crystallization nature of RF chains of these Janus dendrimers was also confirmed by a combination of differential scanning calorimetry (DSC) (Figure S3) and X-ray diffraction (XRD) (Figure S4) experiments. Below the phase transition temperature at −60 °C, all RF and hybrid RHF Janus dendrimers present a glassy phase. The RH Janus dendrimer exhibited a 2D hexagonal columnar phase as previously reported.5b These non-crystallizable RF chains help their self-assembly into soft vesicles.
Self-Sorting and Co-assembly of Janus Dendrimers with RF, RF, and Hybrid RHF chains
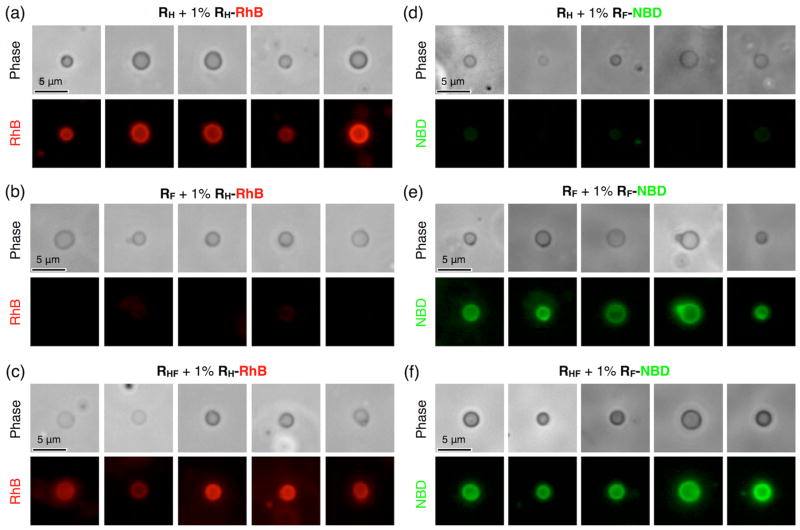
In order to be analyzable by fluorescence microscopy, giant DSs (diameter > 1 μm) in phosphate-buffered saline (PBS) from RF, RH, and hybrid RHF Janus dendrimers were prepared by film hydration on a Teflon sheet (Figure 4),5 via co-assembly with 1% of the dye-labeled Janus dendrimers RH-RhB with red fluorescence, and RF-NBD with green fluorescence. (3,5)PPVEG1-PE-(3,4,5)-3EO-G1-(OCH3)3 was selected as the fluorinated RF molecule based on its structural similarity with the RH and hybrid RHF Janus dendrimers (Scheme 3). Due to the expected immiscibility between RH and RF, the RH Janus dendrimer preferentially co-assemble with RH-RhB (Figure 4a), while the RF Janus dendrimer co-assemble with RF-NBD (Figure 4e), leading to bright red or green fluorescence along the boundary of the vesicles. By contrast, since RF and RH are not miscible and therefore attempts to co-assemble RF with RH-RhB (Figure 4b) and RH with RF-NBD (Figure 4d) produced vesicles that exhibit much weaker fluorescence. This miscibility problem was alleviated with the help of RHF which enabled both dye-labeled Janus dendrimers RH-RhB (Figure 4c) and RF-NBD (Figure 4f) to be incorporated into the giant DSs formed from RHF Janus dendrimers, resulting in intense red or green fluorescence (Figure 4c and 4f). A representative confocal scanning laser tomograph (Movie S1) of a giant DS formed from RF Janus dendrimer and a reconstructed 3D projection (Movie S2) demonstrate their self-assembly into vesicular structures.
Figure 4.
Representative microscopy images of giant unilamellar vesicles assembled from RH, RF and hybrid RHF Janus dendrimers with an additional 1% (wt/wt) RH-RhB, or RF-NBD. Exposure time for fluorescence = 5 ms. Phase-contrast images and fluorescence images under red fluorescence channel or green fluorescence channel were taken by successive exposures on the same vesicle. Images in the same fluorescence channel were normalized to have the same values for the darkest and brightest pixels.
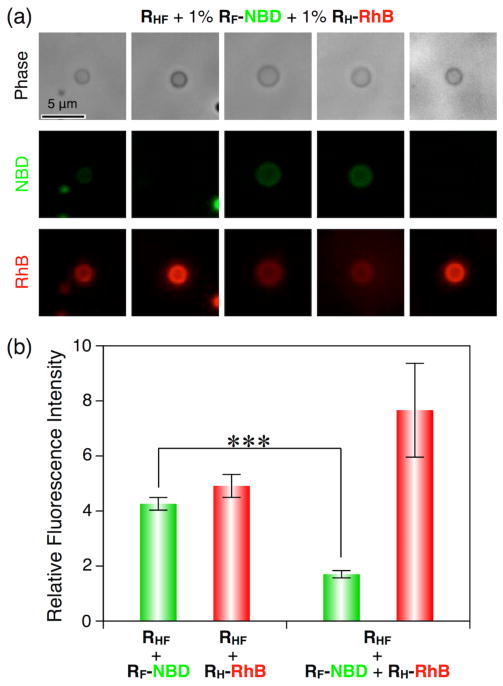
When both RH-RhB and RF-NBD were co-assembled with RHF Janus dendrimers, their giant DSs showed an expected decrease in green emission (Figure 5) (significant p-value < 0.001) due to intermolecular fluorescent resonance energy transfer (FRET)14 from the green dye to the red dye over a short distance (1–10 nm). This FRET experiment indicates that immiscible RH-RhB and RF-NBD Janus dendrimers mix well in giant DSs with the help of RHF Janus dendrimers without phase separation.
Figure 5.
(a) Representative microscopy images of giant unilamellar vesicles assembled from hybrid RHF Janus dendrimer with an additional 1% (wt/wt) RH-RhB, and 1% (wt/wt) RF-NBD. Exposure time for fluorescence = 5 ms. Phase-contrast images and fluorescence images under red fluorescence channel and green fluorescence channel were taken by successive exposures on the same vesicle. Images in the same fluorescence channel were normalized to have the same values for the darkest and brightest pixels. (b) Relative fluorescence intensity of giant vesicles in Figure 4c, 4f, and 5a, respectively. Green and red columns represent green and red fluorescence intensities of vesicles generated from hybrid RHF Janus dendrimer with additional RH-RhB or RF-NBD (left two columns), and of vesicles obtained from hybrid RHF Janus dendrimer with both RH-RhB and RF-NBD (right two columns). Relative intensity was calculated from the brightness histogram analysis by dividing the maximum value of each vesicle by the background. Error bars indicate standard error (SEM) of the mean based on data derived from five vesicles. *** denotes p-value < 0.001.
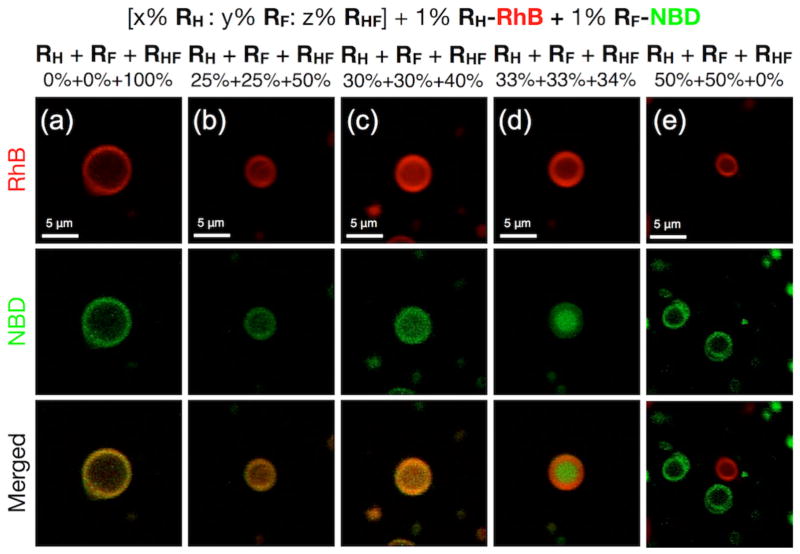
The phase diagram of the three-component assemblies generated from RH, RF, and hybrid RHF Janus dendrimers was investigated by confocal microscopy performed on giant DSs (Figure 6). The ratio of RH and RF was maintained 1:1. The proportion of RHF could be decreased to 50% and subsequently to 40% without phase isolation between the RH and RF components (Figure 6a–6c).
Figure 6.
Representative confocal fluorescent microscopy images of giant vesicles assembled from mixtures of RH, RF and hybrid RHF Janus dendrimers with proportions (in wt%) of (a) RH = 0%, RF = 0%, RHF = 100%; (b) RH = 25%, RF = 25%, RHF = 50%; (c) RH = 30%, RF = 30%, RHF = 40%; (d) RH = 33%, RF = 33%, RHF = 34%; (e) RH = 50%, RF = 50%, RHF = 0%. All mixtures contain an additional 1% (wt/wt) of RH-RhB and 1% (wt/wt) RF-NBD. Red and green fluorescent images were merged into one to demonstrated their co-assembly or self-sorting.
At 34% of the RHF component, the red and green colors from the dyes started to separate into a core-shell structure (Figure 6d). Without RHF in the mixture (0% of RHF), RF and RH self-assembled into individual green and red giant vesicles, indicating the self-sorting of RF and RH into incompatible perfluorinated and perhydrogenated incompatible DSs (Figure 6e). Self-sorting15 of immiscible fluorinated and hydrogenated components during their attempts to co-assemble was previously demonstrated with a hydrogenated-fluorinated peptide system.15a Here we demonstrate and visualize self-sorting of vesicular DSs self-assembled from individual RF and RH Janus dendrimers.
Co-assembly of Janus Dendrimers with Phospholipid
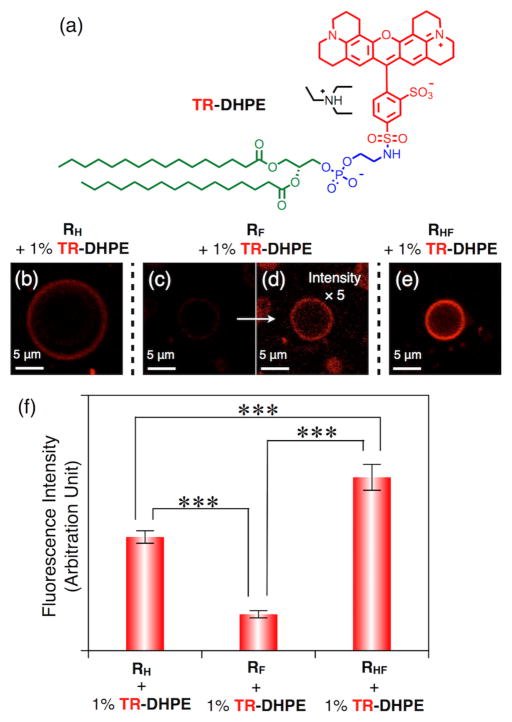
Finally, for potential biomedical applications of these RF and RHF DSs, the co-assembly of RH, RF and RHF Janus dendrimers with phospholipids was conducted by using a phospholipid labeled with the red dye, Texas Red (TR), denoted phospholipid TR-DHPE, where DHPE = 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (Figure 7a).
Figure 7.
(a) Chemical structure of Texas Red (TR)-labeled phospholipid TR-DHPE, DHPE = 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine. (b–e) Representative confocal fluorescent microscopy images of giant unilamellar vesicles assembled from (b) RH, (c,d) RF and (e) hybrid RHF Janus dendrimers with 1% (wt/wt) of Texas Red (TR)-labeled phospholipid TR-DHPE, DHPE = 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine. Images (c) and (d) show the same area, but the intensity has been multiplied by 5-fold in (d) to better display vesicle formation. (f) Fluorescence intensity of giant vesicles. Error bars indicate standard error (SEM) of the mean based on data derived from 25 vesicles of each sample. *** denotes p-value < 0.001.
Confocal images demonstrated that TR-DHPE could be incorporated into vesicles generated from RHF derived Janus dendrimers (Figure 7e, Movie S3) even more efficiently than in vesicles assembled from RH Janus dendrimers (Figure 7b, 7f) with higher intensity of fluorescence emission. As expected, giant DSs from RF Janus dendrimer showed quite weak fluorescence (Figure 7c, 7d, 7f). These co-assembly experiments are consistent with the previous co-assembly with dye-labeled Janus dendrimers and reveal that different fluorinated vesicles, miscible or immiscible with the biological membrane, can be used for different purposes. For example, DSs from RF Janus dendrimers can be used for remote lipophilic and hydrophilic drug loading due to low diffusion within biological membranes. In contrast, DSs from RHF Janus dendrimers may co-assemble with multiple components from biological membranes, including glycolipids, glycoproteins, and transmembrane proteins, to build biocompatible hybrids for immunology and targeted delivery. It is expected that these fluorinated components may co-assemble with bacterial and human cell membranes as demonstrated recently with RH Janus dendrimers.5c Therefore the building block reported here provide an important new toolbox for the area of synthetic biology.
CONCLUSIONS
A library containing achiral hydrogenated (RH), chiral-racemic fluorinated (RF), hybrid hydrogenated-fluorinated (RHF), hydrogenated conjugated in the hydrophilic part with a red fluorescent dye (RH-RhB) and a fluorinated conjugated in the hydrophilic part with a green fluorescent dye (RF-NBD) is reported. These RH, RF and RHF Janus dendrimers are soluble in ethanol and self-assemble into nanometer size unilamellar and multilamellar onion-like DSs by injection of their ethanol solution into water or buffer and by hydration into micrometer size DSs. The thickness of these narrow size distribution DSs is similar to that of biological membranes. Self-assembly and co-assembly of these Janus dendrimers were monitored by fluorescence microscopy to reveal self-sorting and self-assembly of the immiscible RH and RF Janus dendrimers into hydrogenated and fluorinated DSs. Co-assembly of RH with RF and RHF as a function of composition provided hybrid hydrogenated-fluorinated DSs when a high concentration (> 40%) of RHF was employed, and self-sorting into individual RH or RF DSs when low proportions of the RHF component were used. The simple synthesis and self-assembly, the similar thickness to biological membranes and the imaging capabilities via fluorescence and 19F MRI of these DSs make these building blocks and assemblies interesting tools for supramolecular science, medicine and synthetic biology,16 including hybrid hydrogenated-fluorinated cell-like assemblies.5c
Supplementary Material
Acknowledgments
Financial support from the National Science Foundation (grants DMR-1066116 and DMR-1120901), the P. Roy Vagelos Chair at the University of Pennsylvania, and the Humboldt Foundation (all to VP), National Science Foundation (grant DMR-1120901 to DAH, PAH, MG, and MLK) is gratefully acknowledged.
Footnotes
Notes: The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information: Synthetic procedures with complete structural and self-assembly analysis, sample preparation, experimental protocol, and movies. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Kunitake T, Okahata Y. J Am Chem Soc. 1977;99:3860. [Google Scholar]; (b) Ringsdorf H, Schlarb B, Venzmer J. Angew Chem Int Ed Engl. 1988;27:113–158. [Google Scholar]; (c) Thomas JL, Tirrell DA. Acc Chem Res. 1992;25:336–342. [Google Scholar]; (d) Kunitake T. Angew Chem Int Ed Engl. 1992;31:709–726. [Google Scholar]
- 2.(a) Ishikawa Y, Kuwahara H, Kunitake T. Chem Lett. 1989;10:1737–1740. [Google Scholar]; (b) Sakata K, Kunitake T. Thin Solid Films. 1992;210:26–28. [Google Scholar]; (c) Kuwahara H, Hamada M, Ishikawa Y, Kunitake T. J Am Chem Soc. 1993;115:3002. [Google Scholar]; (d) Krafft M. Adv Drug Deliv Rev. 2001;47:209–228. doi: 10.1016/s0169-409x(01)00107-7. [DOI] [PubMed] [Google Scholar]; (e) Vierling P, Santaella C, Greiner J. J Fluor Chem. 2001;107:337–354. [Google Scholar]; (f) Schutt EG, Klein DH, Mattrey RM, Riess JG. Angew Chem Int Ed. 2003;42:3218–3235. doi: 10.1002/anie.200200550. [DOI] [PubMed] [Google Scholar]; (g) Schmutz M, Michels B, Marie P, Krafft MP. Langmuir. 2003;19:4889–4894. [Google Scholar]; (h) Krafft MP, Riess JG. Chem Rev. 2009;109:1714–1792. doi: 10.1021/cr800260k. [DOI] [PubMed] [Google Scholar]; (i) Riess JG. Curr Opin Colloid Interface Sci. 2009;14:294–304. [Google Scholar]; (j) Homma T, Harano K, Isobe H, Nakamura E. Angew Chem Int Ed. 2010;49:1665–1668. doi: 10.1002/anie.200904659. [DOI] [PubMed] [Google Scholar]; (k) Homma T, Harano K, Isobe H, Nakamura E. J Am Chem Soc. 2011;133:6364–6370. doi: 10.1021/ja200498g. [DOI] [PubMed] [Google Scholar]; (l) Schwieger C, Achilles A, Scholz S, Rüger J, Bacia K, Saalwaechter K, Kressler J, Blume A. Soft Matter. 2014;10:6147–6160. doi: 10.1039/c4sm00830h. [DOI] [PubMed] [Google Scholar]; (m) Harano K, Takenaga S, Okada S, Niimi Y, Yoshikai N, Isobe H, Suenaga K, Kataura H, Koshino M, Nakamura E. J Am Chem Soc. 2014;136:466–473. doi: 10.1021/ja411235x. [DOI] [PubMed] [Google Scholar]; (n) Krafft MP. J Fluor Chem. 2015;177:19–28. [Google Scholar]; (o) Krafft MP, Riess JG. Curr Opin Colloid Interface Sci. 2015;20:192–212. [Google Scholar]
- 3.(a) Du W, Nystrom AM, Zhang L, Powell KT, Li Y, Cheng C, Wickline SA, Wooley KL. Biomacromolecules. 2008;9:2826–2833. doi: 10.1021/bm800595b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JWM. NMR Biomed. 2011;24:114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rolfe BE, Blakey I, Squires O, Peng H, Boase NRB, Alexander C, Parsons PG, Boyle GM, Whittaker AK, Thurecht KJ. J Am Chem Soc. 2014;136:2413–2419. doi: 10.1021/ja410351h. [DOI] [PubMed] [Google Scholar]; (d) Wang K, Peng H, Thurecht KJ, Puttick S, Whittaker AK. Polym Chem. 2014;5:1760–1771. [Google Scholar]; (e) Wang K, Peng H, Thurecht KJ, Puttick S, Whittaker AK. Biomacromolecules. 2015;16:2827–2839. doi: 10.1021/acs.biomac.5b00800. [DOI] [PubMed] [Google Scholar]
- 4.(a) Guillod F, Greiner J, Riess JG. Biochim Biophys Acta. 1996;1282:283–292. doi: 10.1016/0005-2736(96)00067-3. [DOI] [PubMed] [Google Scholar]; (b) Trabelsi S, Zhang S, Lee TR, Schwartz DK. Phys Rev Lett. 2008;100:037802. doi: 10.1103/PhysRevLett.100.037802. [DOI] [PubMed] [Google Scholar]
- 5.For selected examples of dendrimersomes, see: Percec V, Wilson DA, Leowanawat P, Wilson CJ, Hughes AD, Kaucher MS, Hammer DA, Levine DH, Kim AJ, Bates FS, Davis KP, Lodge TP, Klein ML, DeVane RH, Aqad E, Rosen BM, Argintaru AO, Sienkowska MJ, Rissanen K, Nummelin S, Ropponen J. Science. 2010;328:1009–1014. doi: 10.1126/science.1185547.Peterca M, Percec V, Leowanawat P, Bertin A. J Am Chem Soc. 2011;133:20507–20520. doi: 10.1021/ja208762u.Xiao Q, Yadavalli SS, Zhang S, Sherman SE, Fiorin E, da Silva L, Wilson DA, Hammer DA, André S, Gabius H-J, Klein ML, Goulian M, Percec V. Proc Natl Acad Sci USA. 2016;113:E1134–E1141. doi: 10.1073/pnas.1525589113.Filippi M, Martinelli J, Mulas G, Ferraretto M, Teirlinck E, Botta M, Tei L, Terreno E. Chem Commun. 2014;50:3453–3456. doi: 10.1039/c3cc49584a.Nazemi A, Gillies ER. Chem Commun. 2014;50:11122–11125. doi: 10.1039/c4cc05161k.Filippi M, Remotti D, Botta M, Terreno E, Tei L. Chem Commun. 2015;51:17455–17458. doi: 10.1039/c5cc06032j.Filippi M, Patrucco D, Martinelli J, Botta M, Castro-Hartmann P, Tei L, Terreno E. Nanoscale. 2015;7:12943–12954. doi: 10.1039/c5nr02695d.
- 6.(a) Ruysschaert T, Sonnen AFP, Haefele T, Meier W, Winterhalter M, Fournier D. J Am Chem Soc. 2005;127:6242–6247. doi: 10.1021/ja043600x. [DOI] [PubMed] [Google Scholar]; (b) Le Meins JF, Schatz C, Lecommandoux S, Sandre O. Mater Today. 2013;16:397–402. [Google Scholar]; (c) Schulz M, Binder WH. Macromol Rapid Commun. 2015;36:2031–2041. doi: 10.1002/marc.201500344. [DOI] [PubMed] [Google Scholar]
- 7.Discher BM, Won Y-Y, Ege DS, Lee JC-M, Bates FS, Discher DE, Hammer DA. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 8.For examples of semifluorinated linear alkyl compounds in self-assembly and their complex synthesis, see: Percec V, Schlueter D, Kwon YK, Blackwell J, Moeller M, Slangen PJ. Macromolecules. 1995;28:8807–8818.Johansson G, Percec V, Ungar G, Smith K. Chem Mater. 1997;9:164–175.Percec V, Johansson G, Ungar G, Zhou J. J Am Chem Soc. 1996;118:9855–9866.Percec V, Glodde M, Bera TK, Miura Y, Shiyanovskaya I, Singer KD, Balagurusamy VSK, Heiney PA, Schnell I, Rapp A, Spiess HW, Hudson SD, Duan H. Nature. 2002;419:384–387. doi: 10.1038/nature01072.
- 9.For environmentally friendly chiral-racemic semifluorinated compounds in self-assembly, see: Wu Y-C, Leowanawat P, Sun H-J, Partridge BE, Peterca M, Graf R, Spiess HW, Zeng X, Ungar G, Hsu C-S, Heiney PA, Percec V. J Am Chem Soc. 2015;137:807–819. doi: 10.1021/ja510643b.
- 10.(a) Kolb HC, Finn MG, Sharpless KB. Angew Chem, Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (b) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.(a) Percec V, Leowanawat P, Sun HJ, Kulikov O, Nusbaum CD, Tran TM, Bertin A, Wilson DA, Peterca M, Zhang S, Kamat NP, Vargo K, Moock D, Johnston ED, Hammer DA, Pochan DJ, Chen Y, Chabre YM, Shiao TC, Bergeron-Brlek M, André S, Roy R, Gabius HJ, Heiney PA. J Am Chem Soc. 2013;135:9055–9077. doi: 10.1021/ja403323y. [DOI] [PubMed] [Google Scholar]; (b) Zhang S, Xiao Q, Sherman SE, Muncan A, Ramos Vicente ADM, Wang Z, Hammer DA, Williams D, Chen Y, Pochan DJ, Vértesy S, André S, Klein ML, Gabius H-J, Percec V. J Am Chem Soc. 2015;137:13334–13344. doi: 10.1021/jacs.5b08844. [DOI] [PubMed] [Google Scholar]
- 12.(a) Zhang S, Sun HJ, Hughes AD, Moussodia RO, Bertin A, Chen Y, Pochan DJ, Heiney PA, Klein ML, Percec V. Proc Natl Acad Sci USA. 2014;111:9058–9063. doi: 10.1073/pnas.1402858111. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xiao Q, Zhang S, Wang Z, Sherman SE, Moussodia RO, Peterca M, Muncan A, Williams DR, Hammer DA, Vértesy S, André S, Gabius HJ, Klein ML, Percec V. Proc Natl Acad Sci USA. 2016;113:1162–1167. doi: 10.1073/pnas.1524976113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis KP, Lodge TP, Bates FS. Macromolecules. 2008;41:8289–8291. [Google Scholar]
- 14.Wu PG, Brand L. Anal Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 15.(a) Bilgiçer B, Xing X, Kumar K. J Am Chem Soc. 2001;123:11815–11816. doi: 10.1021/ja016767o. [DOI] [PubMed] [Google Scholar]; (b) Safont-Sempere MM, Fernández G, Würthner F. Chem Rev. 2011;111:5784–5814. doi: 10.1021/cr100357h. [DOI] [PubMed] [Google Scholar]
- 16.(a) Guo X, Szoka FC. Acc Chem Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]; (b) Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]; (c) Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]; (d) Menjoge AR, Kannan RM, Tomalia DA. Drug Discov Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]; (e) Deming TJ. WIREs Nanomed Nanobiotechnol. 2014;6:283–297. doi: 10.1002/wnan.1262. [DOI] [PubMed] [Google Scholar]; (f) Wei T, Chen C, Liu J, Liu C, Posocco P, Liu X, Cheng Q, Huo S, Liang Z, Fermeglia M, Pricl S, Liang XJ, Rocchi P, Peng L. Proc Natl Acad Sci USA. 2015;112:2978–2983. doi: 10.1073/pnas.1418494112. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Thota BNS, Urner LH, Haag R. Chem Rev. 2016;116:2079–2102. doi: 10.1021/acs.chemrev.5b00417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.