Abstract
Inappropriate fear memory formation is symptomatic of many psychopathologies, and delineating the neurobiology of non-pathological fear learning may provide critical insight into treating these disorders. Fear memory formation is associated with decreased inhibitory signaling in the basolateral amygdala (BLA), and disrupted noradrenergic signaling may contribute to this decrease. BLA noradrenergic neurotransmission has been implicated in fear memory formation, and distinct adrenoreceptor (AR) subtypes modulate excitatory and inhibitory neurotransmission in this region. For example, α1-ARs promote GABA release from local inhibitory interneurons, while β3-ARs potentiate neurotransmission at lateral paracapsular (LPC) GABAergic synapses. Conversely, β1/2-ARs amplify excitatory signaling at glutamatergic synapses in the BLA. As increased BLA excitability promotes fear memory formation, we hypothesized that fear learning shifts the balanced regional effects of noradrenergic signaling toward excitation. To test this hypothesis, we used the fear-potentiated startle paradigm in combination with whole cell patch clamp electrophysiology to examine the effects of AR activation on BLA synaptic transmission following fear conditioning in male Long-Evans rats. We first demonstrated that inhibitory neurotransmission is decreased at both local and LPC synapses following fear conditioning. We next measured noradrenergic facilitation of BLA inhibitory signaling at local and LPC synapses using α1- and β3-AR agonists (1μM A61603 and 10μM BRL37344), and found that the ability of these agents to facilitate inhibitory neurotransmission is disrupted following fear conditioning. Conversely, we found that fear learning does not disrupt noradrenergic modulation of glutamatergic signaling via a β1/2-AR agonist (1μM isoproterenol). Taken together, these studies suggest that fear learning increases BLA excitability by selectively disrupting the inhibitory effects of noradrenaline.
Keywords: Fear-potentiated startle, GABA release, α1 Adrenoreceptor, β3 Adrenoreceptor, β1/2 Adrenoreceptor, norepinephrine
1. Introduction
Disruptions of fear and extinction learning are symptomatic of many psychopathologies, including anxiety disorders, post-traumatic stress disorder (PTSD), and substance-related and addictive disorders (Quirk and Gehlert, 2003; Maren et al., 2013; Torregrossa and Taylor, 2013; Dibbets et al., 2014; Waters et al., 2014). As such, elucidating the neural substrates of non-pathological fear memory formation may facilitate the identification of the maladaptive neurobiological alterations underlying these disorders. Many clinical and preclinical researchers use Pavlovian fear conditioning to study the neural correlates of fear learning (Fendt and Fanselow, 1999; LeDoux, 2000; Maren, 2001, 2005). This well-established behavioral paradigm pairs a noxious unconditioned stimulus (US), such as a footshock, with a neutral stimulus, such as a light, until an association is made between the two stimuli such that the formerly neutral light becomes a fear-eliciting conditioned stimulus (CS). Resultant fear memory formation can be assessed using the fear-potentiated startle paradigm, a highly translational assay that relies upon the mammalian acoustic startle reflex (Davis et al., 2010). Following fear conditioning, this reflexive startle response to a noise burst is potentiated when presented concurrently with the CS, and learning can be quantified by comparing the magnitude of the startle response to CS-paired and unpaired noise bursts.
Extant research has identified the lateral/basolateral amygdalar complex (BLA) as a crucial neural locus of fear memory formation. The BLA is well situated to modulate fear learning, as it receives direct projections from the thalamus (via the medial geniculate nucleus) and sensory association cortex (via the external capsule), and sends excitatory afferents to structures responsible for coordinating the behavioral and neuroendocrine responses to stress (van Vulpen and Verwer, 1989; McDonald and Mascagni, 1996; Shi and Cassell, 1997). Importantly, increased excitability of BLA pyramidal projection neurons has been associated with improved acquisition and expression of fear learning (Rogan et al., 1997; Maren, 1999; Gale et al., 2004; Phelps and LeDoux, 2005). As the excitability of BLA pyramidal neurons (and BLA output by extension) is known to be regulated by GABAergic interneurons (Quirk and Gehlert, 2003; Ehrlich et al., 2009), it follows that disinhibition might contribute to afferent excitability subsequent to fear memory formation. In fact, a growing body of evidence suggests that GABAergic signaling in this region is decreased during fear conditioning (Stork et al., 2002; Ehrlich et al., 2009; Rea et al., 2009; Lee et al., 2013), and disinhibition has been shown to promote LTP of BLA afferents and increase fear memory formation (Manzanares et al., 2005; Tully et al., 2007). Although the specific mechanisms contributing to this decreased GABAergic signaling after fear conditioning have yet to be fully elucidated, decreased noradrenergic facilitation of inhibitory neurotransmission may play a role.
Noradrenaline acts as a powerful neuromodulator within the BLA, and has both excitatory and inhibitory effects on synaptic transmission in this region (Braga et al., 2004; Buffalari and Grace, 2007; Miyajima et al., 2010; Silberman et al., 2010). For example, local inhibitory interneurons, which make perisomatic synapses on pyramidal cells and provide predominantly feed-back inhibition (Woodruff and Sah, 2007), express α1-ARs, and activation of these receptors increases GABA release onto pyramidal projection neurons (Braga et al., 2004). A second, lesser known population of interneurons, termed lateral paracapsular cells (LPCs), is found clustered along the external capsule border. These interneurons synapse on pyramidal cell distal dendrites and are activated by cortical afferents, thus providing feed-forward inhibition (Quirk and Gehlert, 2003; Marowsky et al., 2005). Our group has recently demonstrated that activation of post-synaptic β3-ARs selectively potentiates LPC inhibition onto BLA pyramidal neurons without affecting local GABAergic inhibition (Silberman et al., 2010). Noradrenergic signaling likewise modulates excitatory transmission in the BLA via activation of post-synaptic β1/2-adrenoreceptors (β1/2-ARs), which potentiate glutamatergic neurotransmission onto pyramidal projection neurons (Gean et al., 1992; Huang et al., 1996; Ferry et al., 1997; Liu et al., 2005; Buffalari and Grace, 2007).
Importantly, chronic stress uncouples noradrenaline/GABA interactions in the BLA (Braga et al., 2004; Buffalari and Grace, 2009), possibly shifting the balanced effects of noradrenaline toward excitability. If a similar uncoupling occurs following fear conditioning, the excitatory effects of BLA noradrenaline in response to subsequent US presentation might go unchecked. In support of this, pharmacological potentiation of noradrenergic signaling enhances BLA responses to fear signals in healthy humans (Onur et al., 2009). Interestingly, while in vivo electrophysiological recordings in anesthetized animals have revealed that noradrenaline has a predominately inhibitory effect on BLA pyramidal cell firing under basal conditions (Ferry et al., 1997; Buffalari and Grace, 2007), chronic stress exposure appears to increase the excitatory effects of noradrenaline in the BLA (Buffalari and Grace, 2009). Taken together, these findings suggest that exposure to stressors alters the balanced excitatory and inhibitory effects of noradrenaline release in the BLA, thereby promoting increased afferent activation in response to US presentation.
The studies described herein were designed to test the hypothesis that the equilibrated effects of noradrenergic signaling in the BLA are disrupted following Pavlovian fear conditioning. Specifically, we predicted that basal GABAergic signaling at local inhibitory synapses is decreased after fear conditioning, as has been reported in the lateral amygdala (Lin et al., 2009; Lin et al., 2011). Furthermore, as we have recently demonstrated that inhibitory signaling at LPC synapses contributes to fear learning (Skelly et al., 2016), we hypothesized that GABAergic signaling at these synapses is similarly disrupted following fear conditioning. We further hypothesized that the inhibitory effects α1- and β3-AR activation at local and LPC synapses are compromised by fear learning, while β1/2-AR facilitation of excitatory neurotransmission onto BLA projection neurons is preserved or possible sensitized. Such a shift in the balance of noradrenaline’s neuromodulatory effects could result in increased BLA output and subsequent hyperactivation of downstream structures. To directly assess these hypotheses, we combined Pavlovian fear conditioning and the fear-potentiated startle paradigm to induce and assess fear memory formation, and ex vivo whole cell patch clamp electrophysiology to compare noradrenergic facilitation of excitatory and inhibitory post-synaptic potentials in BLA pyramidal projection neurons in fear conditioned animals versus controls.
2. Methods
2.1 Subjects
Seventy-nine adult male Long-Evans rats (Harlan Laboratories, Indianapolis, IN, USA) were used in these studies. Animals arrived weighing approximately 250g and were allowed to acclimate to the housing environment for one week before experiments began. Rats were pair housed and maintained on a 12-h light/dark cycle (lights on 7:00 A.M. to 7:00 P.M.) with ad libitum access to food and water. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Wake Forest University School of Medicine Animal Care and Use Committee.
2.2 Pavlovian Fear Conditioning Procedure
During fear conditioning, animals were placed in individual plexiglass tubes (10″ (L) × 5″ (ID)) containing a grid floor equipped to convey footshocks and enclosed in sound-attenuated startle chambers (San Diego Instruments, San Diego, CA, USA). Following a five minute acclimation, a light conditioned stimulus (CS) was presented (duration 2 sec) with a 0.5 mA co-terminating foot shock (duration 0.5 sec). This combination was repeated 10 times, with each pairing separated by a two minute inter-stimulus interval. An unpaired control group received 10 presentations each of the same footshock and light stimuli used for fear conditioning; however, these stimuli were presented in an unpaired, pseudorandom order, with an average 1.5 min minute inter-trial interval (1–2 minute range). Following training, animals were returned to their home cages. Behaviorally naïve rats were pair housed and weighed once per week, but otherwise received no additional handling.
2.3 Fear-Potentiated Startle Paradigm
Both fear conditioned animals and unpaired controls were tested for fear learning 24 hours after training using the fear-potentiated startle paradigm. Animals were returned to the testing chambers described above. Following a five minute acclimation period, ten startle-inducing noise bursts (95 db) were presented alone to habituate the animals to the startle response. Following this, noise bursts were presented either alone (10 presentations) or paired with the light CS (10 presentations) in a pseudo-random order. Fear-potentiated startle was assessed by comparing the magnitude of accelerometer displacement in response to the noise alone versus the noise presented in the presence of the CS. Startle amplitude was defined as peak displacement within 200 ms after the onset of the startle stimulus. At the conclusion of testing, animals were returned to their home cages prior to being sacrificed for electrophysiological analysis.
2.4 Slice Preparation
One hour after the conclusion of the fear-potentiated startle test, fear conditioned and unpaired shock/light rats were anesthetized with halothane and rapidly decapitated; behaviorally naïve rats were simply removed from their homecage prior to anesthetization and decapitation. Brains were rapidly removed and isolated in ice cold artificial cerebral spinal fluid (aCSF) composed of (in mM): 124 NaCl, 3.3 KCl, 2.4 MgCl, 2.5 CaCl2, 1.2 KH2PO4, 10 d-glucose, and 25 NaHCO3, saturated with 95% O2 and 5% CO2. Transverse amygdala slices (400 μm) were prepared using a Leica VT1000S vibratome (Leica Microsystems Inc., Buffalo Grove, IL), and maintained at ambient temperature in oxygenated aCSF for at least one hour before recordings began.
2.5 Electrophysiological Recordings
Slices were transferred to a recording chamber and superfused with aerated aCSF at 2 ml/min using a calibrated flowmeter (Gilmont Instruments, Racine, WI). For whole cell patch clamp recordings of evoked GABAA inhibitory post-synaptic currents (eIPSCs) and AMPA excitatory post-synaptic currents (eEPSCs), we used an internal recording solution containing 130 mM K-gluconate, 10 mM KCl, 1 mM EGTA, 100 μM CaCl2, 2 mM Mg-ATP, 200 μM Tris-guanosine 5′-triphosphate, and 10 mM HEPES, pH adjusted with KOH, 275–280 mOsm. Miniature IPSCs (mIPSCs), spontaneous IPSCs (sIPSCs), and unitary IPSCs (uIPSCs) were recorded using a similar filling solution, exchanging equimolar CsCl for K-gluconate and KCl. In all experiments, 5 mM N-(2,6-dimethyl-phenylcarbamoylmethyl)-triethylammonium chloride (QX-314) was included in the internal solution to block voltage-gated sodium currents and GABAB IPSCs. Recording electrodes were prepared from filamented borosilicate glass capillary tubes (inner diameter, 0.86 mm) using a horizontal micropipette puller (P-97; Sutter Instruments, Novato, CA). Whole cell patch clamp recordings were conducted from BLA pyramidal neurons voltage-clamped at −30 to −40 mV for eIPSCs and at −60 to −70 mV for eEPSCs, sIPSCs, mIPSCs, and uIPSCs (not corrected for junction potential). One to two cells were recorded from each animal, and all cells included in these analyses maintained a stable access resistance of 5–20 MΩ. Pyramidal neurons were readily distinguished from other cell types (GABAergic interneurons, glial cells) by their characteristic membrane properties (ie, capacitance, resistance, tau). Whole cell currents were acquired using an Axoclamp 2B amplifier, digitized (Digidata1200, Axon Instruments, Union City, CA), and analyzed online and offline using an IBM-compatible personal computer and pClamp 9.0 software (Axon Instruments).
2.6 Pharmacological Isolation of Synaptic Currents
Drugs were prepared as 100-fold concentrates and bath applied to the slices via calibrated syringe pumps (Razel Scientific Instruments, Stamford, CT). GABAA IPSCs were pharmacologically isolated using a mixture of 50 μM APV and 20 μM DNQX to block NMDA and AMPA/kainate receptors, and AMPA EPSCs were pharmacologically isolated using a mixture of 50 μM APV and 20 μM bicuculline to block NMDA and GABAA receptors. Synaptic currents were evoked every 20 s by electrical stimulation (0.2 ms duration) using a concentric bipolar stimulating electrode (FHC, Bowdoinham, ME), placed along the external capsule to target LPC interneurons (Silberman et al, 2008, 2009) or the medial input to generate EPSCs. uIPSCs were evoked by electrical stimulation (0.2 ms duration) with a glass stimulating electrode (septum theta tubing: 1.5 mm outer diameter, 1.2 mm inner diameter, 0.2 mm thick septum; World Precision Instruments, Sarasota, FL) placed along the external capsule to target LPCs. Minimal stimulation was applied at 0.1 Hz and adjusted to threshold levels to produce both synaptic responses and response failures. Failures were identified by visual inspection methods similar to those reported by our lab and others (Braga et al., 2003; Silberman et al., 2010). Increasing the stimulation intensity by ∼50% above the threshold required to generate a post-synaptic response did not change uIPSC amplitude, indicating stimulation of a single presynaptic site. No stimulation was used to record sIPSCs or mIPSCs; these responses were digitized at 5–10 kHz in continuous 3 min epochs. mIPSCs were recorded in the presence of 500 nm tetrodotoxin, to suppress action potential-dependent synaptic activity. The β3-AR agonist BRL37344, the α1-AR agonist A61603, and the β1/2-AR agonist isoproterenol were acquired from Tocris (Ellisville, MO); all other drugs used were purchased from Sigma (St Louis, MO).
2.7 Statistics
Paired t-tests were used to assess fear-potentiated startle magnitude within groups (light alone vs light + tone responses), and unpaired t-tests were used to compare the percent change in startle amplitude across conditions (fear conditioned vs unpaired shock and light). Unpaired t-tests were also used to analyze differences in the frequency and amplitude of mIPSCs at local GABAergic synapses and uIPSCs at LPC synapses between fear conditioned and naïve subjects. The percent change in sIPSC frequency and amplitude following A61603 application in each treatment condition was assessed using a one-way ANOVA and Newman-Keuls post-hoc tests. Follow up paired t-tests were used to determine whether A61603 increased sIPSC frequency and amplitude relative to baseline within each condition. Differences in the inter-event interval of spontaneous events at baseline (prior to A61603 application) were analyzed using separate Kolmogorov–Smirnov tests (K–S tests; naïve vs unpaired and naïve vs fear conditioned). Differences in the percent change in eIPSC amplitude across treatment conditions (fear conditioned, unpaired, and naïve) following application of BRL37344 were analyzed using a one-way ANOVA and Newman-Keuls post-hoc analyses, and paired t-tests were used to assess the ability of this agonist to increase eIPSC amplitude in each condition. Finally, isoproterenol-induced facilitation of eIPSCs was analyzed using an unpaired t-test to compare the average percent change in response to drug application in slices obtained from naïve and fear conditioned animals.
3. Results
3.1 Fear conditioning decreases GABAergic signaling at local and LPC synapses
We first aimed to replicate earlier findings that GABAergic inhibition mediated by local interneurons is decreased following fear conditioning (Lin et al., 2009; Lin et al., 2011). As anatomical and pharmacological evidence suggests that LPCs synapse on the distal dendrites of BLA pyramidal neurons (Marowsky et al., 2005; Silberman et al., 2008; Silberman et al., 2010; Diaz et al., 2011), spontaneous fusion events occurring at these synapses are not readily detectible at the soma; as such, synaptic strength must be approximated using uIPSCs. In contrast, miniature and spontaneous IPSCs can be used to measure the activity of local inhibitory interneurons, which make perisomatic synapses onto pyramidal cells (Muller et al., 2006). As expected, fear conditioned animals (n = 8), on average, exhibited a potentiated startle response to a CS-paired noise burst (Figure 1a; t(7) = 5.615, p < 0.001). We found that the frequency of action potential-independent miniature inhibitory post-synaptic currents (mIPSCs) is significantly reduced at local interneuron synapses after fear conditioning (Figure 1c; n = 10 cells/8 rats), as compared to behaviorally naïve controls (Figure 1c; n = 11 cells/10 rats; t(19) = 5.959, p < 0.001). In contrast to earlier reports (Lin et al., 2009; Lin et al., 2011), we did not see a difference in mIPSC amplitude between groups (Figure 1d; t(19) = 0.210, p = 0.836).
Figure 1.
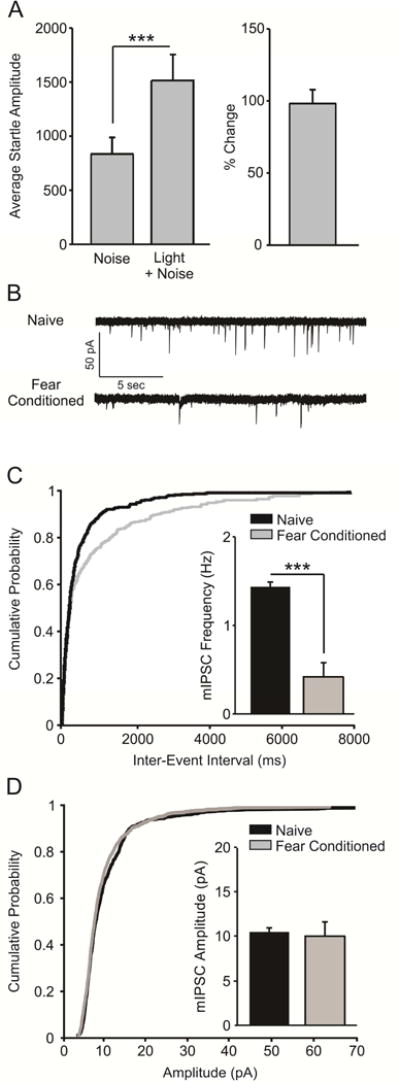
Fear conditioning decreases the frequency of miniature inhibitory post-synaptic currents (mIPSCs) at local GABAergic synapses. A) Fear-conditioned animals (n = 8) exhibited a potentiated startle response to a CS-paired startle-inducing noise burst, indicating acquisition of fear learning (p < 0.001). B) Representative traces of mIPSCs recorded in BLA slices from naïve and fear-conditioned animals. C) Cumulative probability plot demonstrating that mIPSC frequency is lower in slices obtained from fear conditioned animals (bar graph inset, p < 0.001). D) Cumulative probability plot demonstrating that mIPSC amplitude does not differ in slices obtained from naïve and fear conditioned animals (bar graph inset, p > 0.05).
We next used uIPSCs to examine the strength of inhibitory signaling at LPC synapses after fear conditioning. These animals acquired fear learning, as evidenced by exhibiting a more robust startle response to a CS-paired noise burst versus the noise burst alone (Figure 2a; n = 5; t(4) = 4.201, p = 0.014). Subsequent electrophysiological analysis revealed that uIPSC amplitude is decreased in slices obtained from fear conditioned animals (Figure 2b; n = 5 cells/5 rats) as compared to naïve conspecifics (n = 5 cells/5 rats; t(8) = 3.339, p = 0.049). Taken together, these results indicate that fear conditioning is associated with a significant decrease in both local and LPC GABAergic inhibition of BLA pyramidal neurons.
Figure 2.
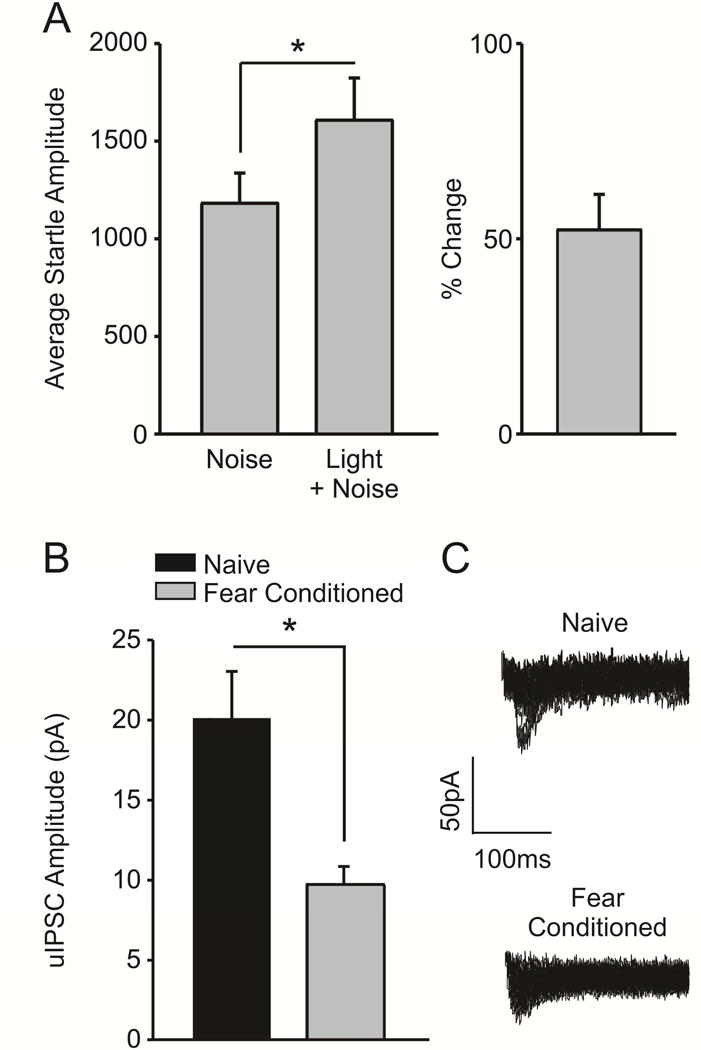
Fear conditioning decreases the amplitude of unitary inhibitory post-synaptic currents (uIPSCs) at LPC synapses. A) Fear-conditioned animals (n = 5) exhibited a potentiated startle response to a CS-paired startle-inducing noise burst, indicating acquisition of fear learning (p < 0.05). B) The average amplitude of LPC uIPSCs is significantly decreased in slices obtained from fear conditioned animals (n = 5 cells/5 rats), as opposed to naïve controls (n = 5 cells/5 rats, p < 0.05). C) Representative traces illustrating that the amplitude of uIPSCs is decreased in fear conditioned animals, as opposed to naïve controls.
3.2 Fear conditioning promotes fear memory formation
Slices from fear conditioned- and unpaired control animals were used to examine the effects of both A61603 and BRL37344 on local and LPC-mediated facilitation of IPSCs; as such, fear-potentiated startle in both groups were combined and analyzed together. Fear conditioned animals (n = 12) exhibited a potentiated startle response to the CS-paired tone (Figure 3a; FC: t(11) = 6.069, p < 0.001) while animals that received footshocks and light presentations in a pseudorandom, unpaired manner did not acquire fear learning (Figure 3a; UP: n = 11; t(10) = 0.604, p = 0.560). Accordingly, the percent increase in startle responding in the presence of the light CS was greater among fear conditioned animals than unpaired controls (Figure 3b; t(21) = 2.887, p = 0.009).
Figure 3.
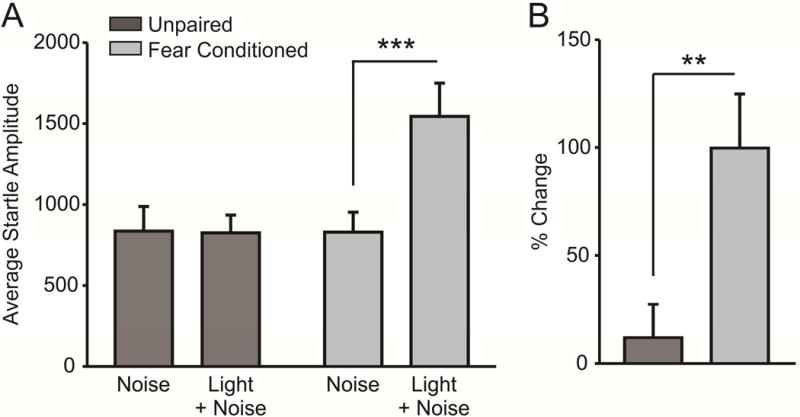
Fear conditioning, but not unpaired shock/light presentation, induces a fear-potentiated startle response. A) Animals exposed to 10 unpaired shock and light presentations do not exhibit a potentiated startle response to a light-paired noise burst (n = 8, p > 0.05), while animals exposed to paired shock/light presentations do exhibit fear-potentiated startle (n = 8, p < 0.001). B) The percent change in average startle amplitude exhibited in response to a light-paired noise is significantly increased in fear conditioned animals as compared to unpaired shock/light controls (p < 0.01).
3.3 Fear conditioning decreases α1-AR facilitation of sIPSCs
We next examined the ability of an α1-AR agonist, A61603 (1μM), to facilitate presynaptic GABA release from local GABAergic synapses in BLA slices taken from FC animals, as compared to naïve animals and UP controls. We found that application of this agonist significantly increased the frequency of sIPSCs in naïve animals (Figure 4a,c; n = 8 cells/7 animals; t(7) = 3.946, p = 0.006) and UP controls (Figure 4a,c; n = 8 cells/8 animals; t(7) = 4.459, p = 0.003), but this potentiation was not observed in BLA slices taken from FC animals (Figure 4a,c; n = 8 cells/8 animals; t(7) = 1.274, p = 0.243). A one-way ANOVA comparing the percent change in the frequency (Hz) of sIPSCs in response to application of A61603 was significant (Figure 4f; F(2, 21) = 3.618, p = 0.045), and post-hoc analyses revealed that the agonist-induced increase in sIPSC frequency was significantly greater in naïve animals as compared to FC animals (Figure 4f; q = 3.777, p = 0.037). No difference was detected when comparing the percent increase in sIPSC frequency between naïve vs UP (Figure 4f; q = 1.492, p = 0.303) or FC vs UP (Figure 4f; q = 2.284, p = 0.121) conditions. There was no change in the amplitude of sIPSCs upon application of the agonist in any condition (Figure 4b,c; naïve, t(7) = 1.490, p = 0.180; FC, t(7) = 1.252, p = 0.251; UP, t(7) = 1.490, p = 0.180), and a one-way ANOVA comparing the percent change in amplitude across groups did not prove significant (Figure 4i; F(2, 21) = 0.218, p = 0.806).
Figure 4.
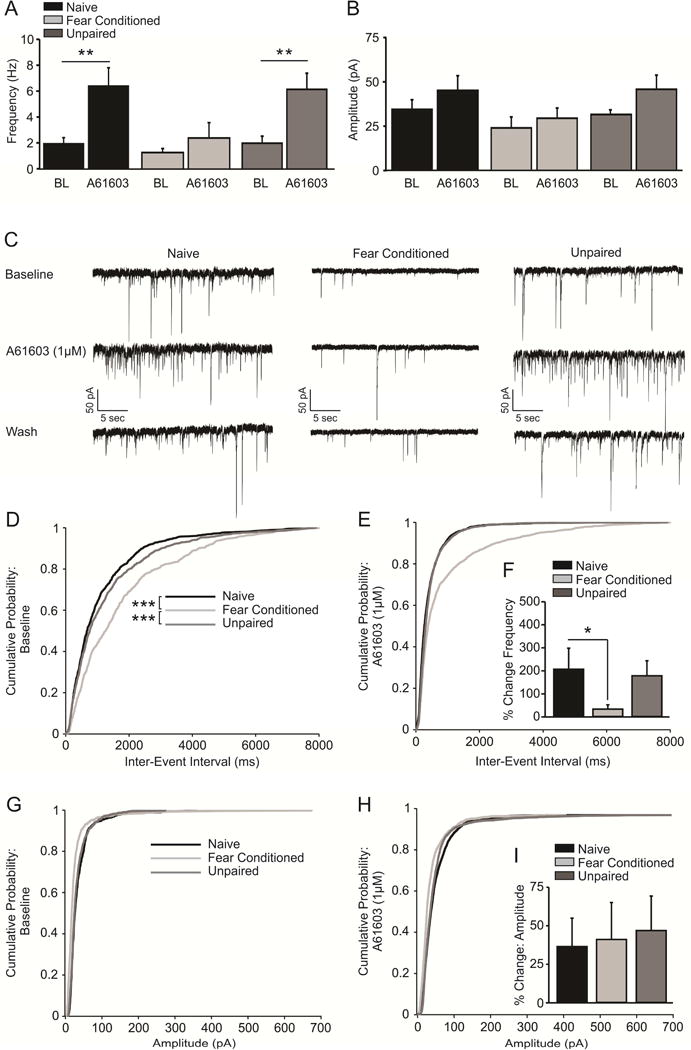
Fear conditioning decreases α1-AR facilitation of spontaneous inhibitory post-synaptic currents (sIPSCs) at local GABAergic synapses. A) The α1-AR agonist, A61603 (1μM), facilitates presynaptic GABA release from local GABAergic synapses in BLA slices taken from naïve (n = 8 cells/7 animals, p < 0.01) and unpaired (n = 8 cells/8 animals, p < 0.01), but not fear conditioned animals (n = 8 cells/8 animals, p > 0.05). B) Activation of α1-ARs has no effect on sIPSC amplitude across any condition (p > 0.05). C) Representative traces demonstrating the increased frequency of sIPSCs in response to A61603 application in slices taken from naïve animals and unpaired shock/light controls, but not fear conditioned animals. D) Cumulative probability plot demonstrating that sIPSC frequency is significantly decreased in slices obtained from fear conditioned animals, as compared to naïve and unpaired controls (p > 0.001). E) Cumulative probability plot demonstrating that activation of α1-ARs via application of the agonist A61603 results in potentiation of the frequency of sIPSCs in slices obtained from naïve animals and unpaired controls, but not in slices taken from fear conditioned animals. F) The percent increase in sIPSC frequency in response to A61603 application is significantly increased in slices from naïve animals, as compared to animals that underwent fear conditioning (p > 0.05). G) Cumulative probability plots indicating that sIPSC amplitude is equivalent in slices obtained from fear conditioned animals and both naïve and unpaired controls. H) Cumulative probability plot demonstrating that activation of α1-ARs has no effect on sIPSC amplitude in any condition. I) The percent change in the amplitude of sIPSCs in response to activation of α1-ARs via application of A61603 does not differ across conditions (p > 0.05).
As we had previously detected a decrease in the frequency of mIPSCs from fear conditioned (FC) animals versus naïve controls (Figure 1c), we also assessed whether there was a similar difference in the frequency of sIPSCs between animals that had undergone fear conditioning versus naïve animals and UP. Using separate Kolmogorov–Smirnov tests, we found that the cumulative probability distributions of inter-event intervals, a measure of event frequency, were significantly different between naïve and FC animals (Figure 4d; D = 0.219, p < 0.0001). Similarly, there was a significant difference between the cumulative probability distributions of events between FC animals and UP controls (Figure 4d; D = 0.169, p < 0.0001). In contrast, the inter-event interval population distributions did not differ between naïve animals and UP controls (Figure 4d; D = 0.064, p = 0.060). Taken together, these findings are consistent with our mIPSC result (Figure 1c) and indicate that the frequency of both miniature and spontaneous IPSCs is selectively decreased by fear conditioning, but not by acute non-contingent footshock administration.
3.4 Fear conditioning decreases β3-AR facilitation of eIPSCs
Similarly, we examined the ability of a β3-AR agonist (BRL37344, 10μM) to facilitate inhibitory signaling at LPC synapses. We found that application of BRL37344 significantly potentiated evoked IPSCs (eISPCs) in slices from naive (Figure 5a,b; n = 6 cells/animals, t(5) = 4.098, p = 0.009) and UP (Figure 5a,b; n = 6 cells/animals, t(5) = 2.653, p = 0.045) animals, and that this facilitation was attenuated after fear conditioning (Figure 5a,b; n = 6 cells/animals, t(5) = 1.417, p = 0.216). A one-way ANOVA comparing the percent change in eIPSC amplitude in the presence of BRL37344 vs baseline across conditions was significant (Figure 5c; F(2,15) = 4.895, p = 0.023), and post-hoc analysis revealed that the agonist-induced potentiation was significantly greater in naïve slices when compared to FC (Figure 5c; q = 3.671, p = 0.020). Similarly, eIPSC amplitude was significantly potentiated in UP slices as compared to those taken from FC animals (Figure 5c; q = 3.975, p = 0.033). There was no significant difference in UP and naïve conditions (Figure 5c; q = 0.304, p = 0.833).
Figure 5.
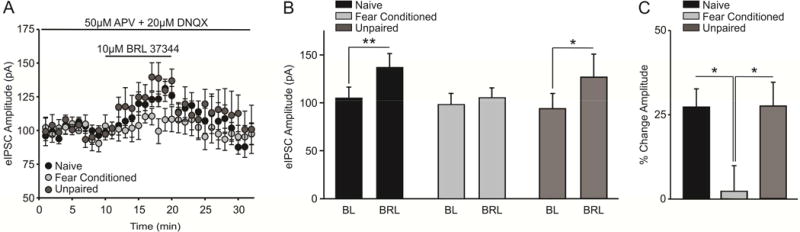
Fear conditioning decreases β3-AR facilitation of eIPSCs. A) Timecourse graph demonstrating that application of the β3-AR agonist, BRL37344 (10μM), potentiates evoked inhibitory post-synaptic current (eIPSC) amplitude in slices obtained from naïve animals and UP controls. B) Application of the β3-AR agonist BRL37344 facilitates LPC eIPSC amplitude in slices obtained from naïve (n = 6 cells/animals, p < 0.01) and UP animals (n = 6 cells/animals, p < 0.05), but this facilitation was abolished in slices taken from fear conditioned animals (n = 6 cells/animals, p > 0.05). C) The percent change in eIPSC amplitude in the presence of BRL37344 was greater in naïve and UP slices, as compared to slices taken from FC animals (p < 0.05).
3.5 Fear conditioning does not alter β1/2-AR potentiation of eEPSCs
Finally, to assess whether the excitatory effects of β1/2-AR activation are disrupted by fear conditioning, we measured isoproterenol-induced (1 μM) potentiation of evoked excitatory post-synaptic current (eEPSC) amplitude. We first confirmed that fear conditioning produced an increased startle response to a noise-paired light CS, as opposed to a noise burst presented alone (Figure 6a; n = 7; t(6) = 2.563, p = 0.043). We found that application of isoproterenol potentiated eEPSCs in slices from behaviorally naïve animals (Figure 6b; n = 12 cells/7 animals; t(11) = 3.517, p = 0.005) as well as FC animals (Figure 6b; n = 9 cells/7 animals; t(8) = 3.744, p = 0.006). A t-test comparing the percent change in eEPSC amplitude following isoproterenol application was non-significant (Figure 6c; t(19) = 0.119, p = 0.907).
Figure. 6.
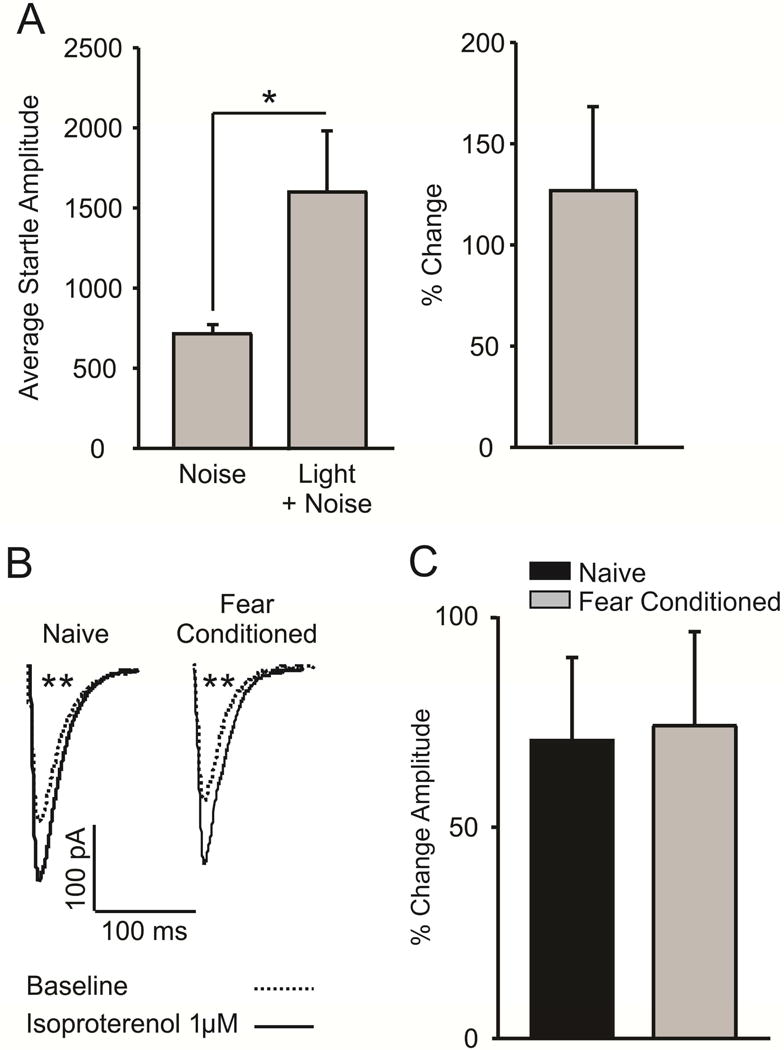
Fear conditioning does not alter β1/2-AR facilitation of evoked excitatory post-synaptic currents (eEPSCs). A) Fear conditioning resulted in a significant potentiation of the acoustic startle response (n = 7, p < 0.05). B) Representative traces demonstrating that application of the β1/2-AR agonist isoproterenol (1μM) potentiates the amplitude of evoked excitatory post-synaptic currents in fear conditioned animals (n = 9 cells/7 animals, p > 0.01) and naïve controls (n = 12 cells/7 animals, p > 0.01). C) Isoproterenol-induced potentiation of eIPSC amplitude does not differ between naïve and fear conditioned animals (p > 0.05).
4. Discussion
The main findings of these studies are as follows: (i) BLA inhibitory signaling is reduced at local and LPC GABAergic synapses after fear conditioning; (ii) fear conditioning decreases α1-AR mediated potentiation of GABA release from local inhibitory interneurons; (iii) β3-AR facilitation of inhibitory signaling at post-synaptic GABAA receptors is likewise attenuated after fear conditioning; (iv) facilitation of BLA excitatory neurotransmission by β1/2-ARs is not significantly altered after fear memory formation. Taken together, the results of these experiments support our hypothesis that Pavlovian fear conditioning selectively alters noradrenergic facilitation of GABAergic inhibition in the BLA. These novel findings may have important implications for normal and pathological fear memory formation.
Consistent with previous work in the lateral amygdala (Lin et al., 2009; Lin et al., 2011), we found that the frequency of action potential-independent mIPSCs is decreased in BLA slices obtained from animals that underwent fear conditioning. As the frequency with which mIPSCs occur is generally thought to represent presynaptic GABA release probability, our results suggest that fear memory formation significantly reduces GABA release from local inhibitory interneurons onto post-synaptic pyramidal projection cells. Similarly, we report that the amplitude of LPC uIPSCs is decreased after fear conditioning. This result suggests that post-synaptic modulation of inhibitory transmission at LPC synapses is likewise diminished following fear learning. Although we did not perform any experiments to directly assess the mechanism underlying these observed disruptions of basal inhibitory signaling at local and LPC synapses, previous investigations have demonstrated that fear conditioning results in endocytosis of BLA GABAA receptors (Lin et al., 2009), and that activation of adrenergic receptors may promote GABAA receptor endocytosis and fear memory formation (Lin et al., 2011).
Interestingly, both local and LPC interneuron synapses are known to be potentiated by the activation of adrenergic receptors (Braga et al., 2004; Silberman et al., 2010), and our results suggest that the ability of noradrenaline to increase inhibitory signaling at these synapses may be reduced following fear conditioning. Specifically, we have shown that both α1-AR mediated facilitation of GABA release at local interneuron synapses onto BLA pyramidal neurons and β3-AR mediated facilitation of IPSCs at LPC synapses onto pyramidal neurons are decreased in recordings from fear conditioned animals, relative to controls. Importantly, these disruptions are not observed in BLA slices obtained from animals exposed to multiple unpaired presentations of the same shock and light stimuli used to train fear conditioning, suggesting that it is fear memory formation specifically, and not acute stress exposure in general, that disrupts the inhibitory effects of noradrenaline in the BLA.
These data are important, as they are the first to demonstrate that noradrenergic facilitation of inhibitory neurotransmission in the BLA is decreased following Pavlovian fear conditioning. Furthermore, these data provide strong evidence that inhibitory synaptic transmission at LPC synapses is directly influenced by fear learning. We recently demonstrated that in vivo activation of LPC synapses via intra-BLA microinjection of β3-AR agonists disrupts fear memory formation, and that ex vivo activation of these receptors blocks the induction of synaptic plasticity at LPC synapses (Skelly et al., 2016). These results, combined with the evidence presented herein, demonstrate a potentially important role for these synapses in modulating BLA output following fear memory formation.
Our finding that acute footshock does not significantly disrupt the inhibitory effects of BLA noradrenaline is consistent with previous work demonstrating that acute stress has no detectable effect on GABAergic transmission in this region (Lin et al., 2009; Lin et al., 2011). In contrast, protracted stress has been shown to disrupt BLA inhibitory signaling; for example, Braga and colleagues (2004) elegantly established that α1-AR mediated potentiation of local interneuron synapses is uncoupled by chronic restraint stress. Similarly, Buffalari and Grace (2009) used in vivo recordings to demonstrate that chronic cold stress shifts the balanced effects of BLA noradrenaline toward increased excitation. These results are compelling, as they suggest that chronic stress-induced alterations in noradrenergic modulation of BLA inhibitory transmission mimic the changes induced by fear conditioning. Prior stress has been shown to promote fear learning and disrupt acquisition of extinction memories (Cordero et al., 2003; Manzanares et al., 2005; Miracle et al., 2006; Skelly et al., 2015; Perusini et al., 2016), and our results suggest that stress-induced alterations to BLA noradrenergic receptors may partly explain these outcomes.
We further demonstrate that β1/2-AR mediated facilitation of EPSCs is not affected by fear conditioning. This result is consistent with our hypothesis that fear-related noradrenaline release promotes BLA output by selectively enhancing signaling at excitatory synapses. In support of this, activation of BLA β1/2-ARs has been shown to enhance fear learning (Ferry and McGaugh, 1999; Ferry et al., 1999) and excitatory synaptic plasticity (Wang et al., 1999; Huang et al., 2000), while blockade of these receptors blocks fear memory consolidation (Qu et al., 2008). Taken together, our findings support the hypothesis that fear conditioning promotes BLA excitability by attenuating the inhibitory effects of noradrenaline acting at α1- and β3-ARs in this region, without affecting excitatory signaling at β1/2-ARs.
A limitation of this study is that we have not identified the mechanisms whereby noradrenergic facilitation of inhibitory synaptic transmission is decreased by fear conditioning. As mentioned previously, extant research has demonstrated that fear conditioning promotes GABAA receptor endocytosis in BLA pyramidal neurons (Lin et al., 2009), and that β1/2-AR activation may facilitate this process (Lin et al., 2011). Although it is possible that GABAA receptor endocytosis resulting from β3-AR activation underlies the decrease in uIPSC amplitude observed in slices obtained from fear conditioned animals, this seems unlikely as previous studies in our lab have not shown any effect of β-AR activation on the amplitude of IPSCs evoked by LPC stimulation in the absence of fear conditioning (Silberman et al., 2010; Silberman et al., 2012). Similarly, although Lazzaro and colleagues (2010) found that blockade of α1-ARs decreases the amplitude of eIPSCs at local interneuron synapses onto BLA PNs, these receptors are thought to increase inhibitory signaling at local interneuron synapses via a presynaptic mechanism (Braga et al., 2004). It seems unlikely, therefore, that their activation results in alterations in post-synaptic GABAA receptor expression. Although the decrease in eIPSC amplitude reported by these authors following application of an α1-AR antagonist suggests that fear conditioning-induced reductions in noradrenergic tone might explain the decreased frequency of mIPSCs observed herein, this explanation seems somewhat unlikely as application of an α1-AR agonist failed to restore these miniature events in slices obtained from fear conditioned animals. Similarly, our prior studies have found no evidence of noradrenergic tone at either the β1 or β3-ARs that enhance LPC synapses (Silberman et al., 2010; Silberman et al., 2012), making it unlikely that fear conditioning-related changes in noradrenaline release contribute to the observed decreases in LPC inhibition. Future studies are needed to directly assess the mechanisms whereby fear conditioning decreases basal inhibitory signaling at local and LPC synapses and whether adrenoreceptor activation plays a role in these processes.
Although these studies provide strong evidence that noradrenergic facilitation of inhibitory signaling at BLA local and LPC interneuron synapses is disrupted following fear memory formation, by no means do these experiments represent a complete investigation of the net effect of adrenoreceptor activation on BLA excitability following fear conditioning. There are other subtypes of adrenergic receptors expressed in this region, including α2-ARs (Ferry and McGaugh, 2008), and the subtypes examined here are also expressed at other synapses. For example, our lab has demonstrated that β1-ARs at LPC synapses may play a role in facilitating inhibitory signaling (Silberman et al., 2012). However, application of a β1/2-AR agonist has been shown to potentiate BLA field potentials (Abraham et al., 2008), suggesting that the predominate effect of activating these receptors is increased excitation. As such, we chose to focus our attention on alterations in the ability of these receptors to facilitate excitatory neurotransmission in this region. Similarly, while postsynaptic α1-ARs have also been shown to contribute to excitatory signaling in the BLA (Domyancic and Morilak, 1997; Ferry et al., 1999), we chose to investigate the effect of fear conditioning on α1-AR modulation of BLA GABAergic transmission, as activation of these receptors has been demonstrated to have a net inhibitory effect on BLA output (Braga et al., 2004). Additionally, both α1- and β3-AR mediated facilitation of BLA inhibitory transmission have recently been shown to influence fear learning and BLA synaptic plasticity (Lazzaro et al., 2010; Skelly et al., 2016). This is important, as reduced inhibitory signaling in this region promotes the induction of long-term synaptic plasticity, thought to be the physiological correlate of fear learning (Tully et al., 2007). Future studies are needed to examine the impact of fear conditioning on the global effects of noradrenaline on pyramidal neuron excitability and the induction of synaptic plasticity in this region.
Acute stress exposure has been shown to increase phosphorylation of adrenoreceptors in various brain regions, inducing receptor desensitization (Glavin, 1985). Future studies should also address whether the observed impairments in noradrenergic modulation of inhibitory signaling in the BLA result from receptor downregulation, internalization, or desensitization, or by disruption of intracellular signaling cascades. While other groups have observed a down-regulation of post-synaptic GABAA receptors at local interneurons after fear conditioning (Lin et al., 2009; Lin et al., 2011), we did not replicate these findings (specifically, the amplitude of mIPSCs and sIPSCs was unaltered after fear learning in our studies). This divergence may be explained by the specific subnuclei being targeted; Lin and colleagues recorded primarily from neurons in the lateral amygdala, while we aimed to record from neurons in the basal nucleus. While these nuclei are often studied in combination, the distribution of GABAA receptor subtypes actually varies significantly between these two regions (Marowsky et al., 2012; Marowsky and Vogt, 2014), and these differences may explain our contradictory results. Future studies should directly assess GABAA receptor expression and function in the BLA after fear conditioning.
Central noradrenergic signaling has been implicated in both healthy and pathological fear and anxiety (Southwick et al., 1999; Southwick et al., 2002; McGaugh, 2004). While increased noradrenergic signaling in the basolateral amygdala promotes the encoding of memories related to trauma, which is an appropriate and adaptive response, stress-induced sensitization of noradrenaline release is thought to contribute to the development of PTSD following trauma exposure (Geracioti et al., 2001; Strawn and Geracioti, 2008). As such, the results of these studies, which are among the first to directly assess adaptive changes in BLA adrenoceptor signaling following non-pathological fear memory formation, may further the identification and treatment of the deleterious adaptations to this system that underlie pathological conditions. Specifically, although pharmacotherapeutic interventions presumed to decrease the excitatory effects of central noradrenaline have been somewhat efficacious in reducing the symptoms of stress-related anxiety disorders, substance abuse disorders, and PTSD (Raskind et al., 2003; Simpson et al., 2009; Fox et al., 2012), identifying the specific noradrenergic receptor subtypes and locations that are impacted in individuals suffering from these conditions could lead to the development of improved pharmacological interventions. Additionally, these studies indicate that restoring the balanced effects of BLA noradrenaline by reversing the uncoupled neuromodulatory actions of noradrenaline at local and LPC GABAergic synapses might be a promising therapeutic objective. Thus, future studies should be conducted to investigate the role of BLA noradrenergic signaling in animal models of human psychopathology.
Highlights.
Basolateral amygdala (BLA) GABAergic signaling is reduced after fear conditioning
Fear conditioning reduces noradrenergic facilitation of BLA GABAergic signaling
Noradrenergic facilitation of excitatory signaling is not altered by fear learning
Fear conditioning disrupts the excitatory/inhibitory balance of BLA noradrenaline
Acknowledgments
These studies were supported by R37 AA017531 (J.L.W.), P01 AA21099 (J.L.W.), R37 AA010422 (J.L.W.), and F31 AA022275 (M.J.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
M.J.S. and J.L.W. designed the research; M.J.S. and O.J.A. performed experiments; M.J.S. analyzed data and drafted the paper, and J.L.W. provided critical feedback which significantly shaped the manuscript.
References
- Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH, Li H. beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res. 2008;1209:65–73. doi: 10.1016/j.brainres.2008.02.082. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23:442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychoph. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Hormones and behavior. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbets P, van den Broek A, Evers EA. Fear conditioning and extinction in anxiety- and depression-prone persons. Memory. 2014 doi: 10.1080/09658211.2014.886704. [DOI] [PubMed] [Google Scholar]
- Domyancic AV, Morilak DA. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol. 1997;386:358–378. doi: 10.1002/(sici)1096-9861(19970929)386:3<358::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and biobehavioral reviews. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of learning and memory. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Involvement of basolateral amygdala alpha2-adrenoceptors in modulating consolidation of inhibitory avoidance memory. Learn Mem. 2008;15:238–243. doi: 10.1101/lm.760908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Magistretti PJ, Pralong E. Noradrenaline modulates glutamate-mediated neurotransmission in the rat basolateral amygdala in vitro. European Journal of Neuroscience. 1997;9:1356–1364. doi: 10.1111/j.1460-9568.1997.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism, clinical and experimental research. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. Journal of Neuroscience. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gean PW, Huang CC, Lin JH, Tsai JJ. Sustained enhancement of NMDA receptor-mediated synaptic potential by isoproterenol in rat amygdalar slices. Brain research. 1992;594:331–334. doi: 10.1016/0006-8993(92)91146-6. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. The American journal of psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Glavin GB. Stress and brain noradrenaline: a review. Neuroscience and biobehavioral reviews. 1985;9:233–243. doi: 10.1016/0149-7634(85)90048-x. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS, Gean PW. Isoproterenol potentiates synaptic transmission primarily by enhancing presynaptic calcium influx via P- and/or Q-type calcium channels in the rat amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:1026–1033. doi: 10.1523/JNEUROSCI.16-03-01026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro SC, Hou M, Cunha C, LeDoux JE, Cain CK. Antagonism of lateral amygdala alpha1-adrenergic receptors facilitates fear conditioning and long-term potentiation. Learn Mem. 2010;17:489–493. doi: 10.1101/lm.1918210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim SJ, Kwon OB, Lee JH, Kim JH. Inhibitory networks of the amygdala for emotional memory. Front Neural Circuit. 2013;7 doi: 10.3389/fncir.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Block of gamma-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol Psychiatry. 2009;66:665–673. doi: 10.1016/j.biopsych.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lin HC, Tseng YC, Mao SC, Chen PS, Gean PW. GABAA receptor endocytosis in the basolateral amygdala is critical to the reinstatement of fear memory measured by fear-potentiated startle. J Neurosci. 2011;31:8851–8861. doi: 10.1523/JNEUROSCI.0979-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XQ, Cao XH, Li BM. beta-Adrenergic activation enhances NMDA-induced current in pyramidal cells of the basolateral nucleus of amygdala. Chinese Sci Bull. 2005;50:1966–1968. [Google Scholar]
- Manzanares PAR, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. Journal of Neuroscience. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual review of neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Vogt KE. Delta-subunit-containing GABAA-receptors mediate tonic inhibition in paracapsular cells of the mouse amygdala. Front Neural Circuits. 2014;8:27. doi: 10.3389/fncir.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy JM, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for alpha3 subunit-containing GABAA receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Cortico-cortical and cortico-amygdaloid projections of the rat occipital cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1996;71:37–54. doi: 10.1016/0306-4522(95)00416-5. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual review of neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of learning and memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Ozaki M, Wada K, Sekiguchi M. Noradrenaline-induced spontaneous inhibitory postsynaptic currents in mouse basolateral nucleus of amygdala pyramidal neurons: comparison with dopamine-induced currents. Neuroscience letters. 2010;480:167–172. doi: 10.1016/j.neulet.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onur OA, Walter H, Schlaepfer TE, Rehme AK, Schmidt C, Keysers C, Maier W, Hurlemann R. Noradrenergic enhancement of amygdala responses to fear. Soc Cogn Affect Neur. 2009;4:119–126. doi: 10.1093/scan/nsn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, Maksymetz J, Spigelman I, Fanselow MS. Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress. Neuropsychopharmacology. 2016;41:45–57. doi: 10.1038/npp.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Qu LL, Guo NN, Li BM. Beta1- and beta2-adrenoceptors in basolateral nucleus of amygdala and their roles in consolidation of fear memory in rats. Hippocampus. 2008;18:1131–1139. doi: 10.1002/hipo.20478. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. The American journal of psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Rea K, Lang Y, Finn DP. Alterations in extracellular levels of gamma-aminobutyric acid in the rat basolateral amygdala and periaqueductal gray during conditioned fear, persistent pain and fear-conditioned analgesia. The journal of pain: official journal of the American Pain Society. 2009;10:1088–1098. doi: 10.1016/j.jpain.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. The Journal of comparative neurology. 1997;382:153–175. [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. beta1-adrenoceptor activation is required for ethanol enhancement of lateral paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2012;343:451–459. doi: 10.1124/jpet.112.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology. 2010;35:1886–1896. doi: 10.1038/npp.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcoholism, clinical and experimental research. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, Weiner JL. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology. 2015;97:149–159. doi: 10.1016/j.neuropharm.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AM, Ariwodola OJ, Weiner JL. Behavioral and neurophysiological evidence that lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the acquisition and extinction of fear learning. Neurobiol Learn Mem. 2016;127:10–16. doi: 10.1016/j.nlm.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Davis M, Horner B, Cahill L, Morgan CA, Gold PE, Bremner JD, Charney DC. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am J Psychiat. 2002;159:1420–1422. doi: 10.1176/appi.ajp.159.8.1420. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neuroscience letters. 2002;327:138–142. doi: 10.1016/s0304-3940(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depression and anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 2013;226:659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci U S A. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vulpen EH, Verwer RW. Organization of projections from the mediodorsal nucleus of the thalamus to the basolateral complex of the amygdala in the rat. Brain research. 1989;500:389–394. doi: 10.1016/0006-8993(89)90337-5. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Cheng LL, Gean PW. Cross-modulation of synaptic plasticity by beta-adrenergic and 5-HT1A receptors in the rat basolateral amygdala. Journal of Neuroscience. 1999;19:570–577. doi: 10.1523/JNEUROSCI.19-02-00570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Peters RM, Forrest KE, Zimmer-Gembeck M. Fear acquisition and extinction in offspring of mothers with anxiety and depressive disorders. Developmental cognitive neuroscience. 2014;7:30–42. doi: 10.1016/j.dcn.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


