Abstract
Aims
To determine (1) whether prescription opioid poisoning (PO) hospital discharges spread across space over time, (2) the locations of ‘hot-spots’ of PO-related hospital discharges, (3) how features of the local environment contribute to the growth in PO-related hospital discharges and (4) where each environmental feature makes the strongest contribution.
Design
Hierarchical Bayesian Poisson space–time analysis to relate annual discharges from community hospitals to postal code characteristics over 10 years.
Setting
California, USA.
Participants
Residents of 18 517 postal codes in California, 2001–11.
Measurements
Annual postal code-level counts of hospital discharges due to PO poisoning were related to postal code pharmacy density, measures of medical need for POs (i.e. rates of cancer and arthritis-related hospital discharges), economic stressors (i.e. median household income, percentage of families in poverty and the unemployment rate) and concentration of manual labor industries.
Findings
PO-related hospital discharges spread from rural and suburban/exurban ‘hot-spots’ to urban areas. They increased more in postal codes with greater pharmacy density [rate ratio (RR) = 1.03; 95% credible interval (CI) = 1.01, 1.05], more arthritis-related hospital discharges (RR = 1.08; 95% CI = 1.06, 1.11), lower income (RR = 0.85; 95% CI = 0.83, 0.87) and more manual labor industries (RR = 1.15; 95% CI = 1.10, 1.19 for construction; RR = 1.12; 95% CI = 1.04, 1.20 for manufacturing industries). Changes in pharmacy density primarily affected PO-related discharges in urban areas, while changes in income and manual labor industries especially affected PO-related discharges in suburban/exurban and rural areas.
Conclusions
Hospital discharge rates for prescription opioid (PO) poisoning spread from rural and suburban/exurban hot-spots to urban areas, suggesting spatial contagion. The distribution of age-related and work-place-related sources of medical need for POs in rural areas and, to a lesser extent, the availability of POs through pharmacies in urban areas, partly explain the growth of PO poisoning across California, USA.
Keywords: Bayesian space-time models, drug overdose, geography, hospital discharges, prescription opioids, rural and urban
INTRODUCTION
Opioid abuse is an important global problem with far-reaching implications for the health, social and economic wellbeing of all populations—32.4 million people abuse opioids across the world [1]. The health consequences of this abuse are severe and on the rise. For example, in the United States, prescription opioid (PO) poisoning deaths have increased more than 400% between 1999 and 2014, and account for more deaths than heroin, cocaine and stimulant poisoning combined [2]. Opioid overuse-related hospital stays more than doubled between 1993 and 2012, and continue to grow at a rate of 5% per year [3].
Rates of PO poisoning are concentrated in ‘hot-spots’, usually in rural areas, as well as in small towns and suburban areas [4]. The ways in which such hot-spots develop and spread across space over time, as well as the factors that explain why PO poisoning increases in rural and suburban areas, are not well understood [5,6].
Three factors may drive PO poisoning in local areas: (1) PO availability; (2) medical need for POs; and (3) economic stressors (4). First, growth in the rate of PO poisoning is correlated strongly with an increase in PO supply at national [7] and local levels [8]. Pharmacies could thus provide a local source of access to POs, as they constitute the main dispensation site for the drugs. Secondly, medical need for POs in the local community, resulting from a high density of patients affected by leading causes of pain such as arthritis and cancer pain [9,10], or from a high concentration of residents with work-place-related physical injuries [4], may increase the community supply of POs and the risk of poisoning. Thirdly, communities with more economic stressors such as unemployment, low median income and poverty may be particularly vulnerable to PO abuse, among other reasons, as a way to manage chronic stress and resulting anxious and mood disorders [11,12].
While prior studies point to systematic spatial patterns in PO poisoning, no study has yet examined whether PO poisoning is subject to a process of spatial contagion, as has been shown for other types of drugs. Further, we do not understand what factors may explain the concentration of PO poisoning in certain hot-spots, or understand whether different types of community-level factors matter in rural and suburban versus urban areas. To address these gaps, we focused upon PO poisoning in California—the most populous and ethnically diverse US state. We asked the following four questions: (1) do hospital discharges due to PO poisoning spread across space over time; (2) in what types of geographic regions can we find hot-spots of PO-related hospital discharges; (3) what contribution do three ecological factors (i.e. formal access to POs through higher density of pharmacies, medical need for POs due to chronic disease or work-place injury and economic stressors) make to the growth in PO-related hospital discharges; and (4) in what types of geographic areas is the contribution of each ecological factor the strongest?
METHODS
The study related PO poisoning hospitalizations in California postal (zip) codes from 2001 to 2011 (n = 18 517 space × time units) to population and environmental characteristics.
Data sources and variables
Outcome measure
We obtained annual zip code-level counts of hospital discharges related to PO poisoning from the California Office of Statewide Health Planning and Development. These records identify all hospital discharges from community hospitals that result in at least one overnight stay [13].
PO-related hospital discharge rates were measured by the annual counts of hospital discharges with Principal or Additional E-codes indicating poisoning by methadone or other opiates and narcotics (E850.1, E850.2, E935.1 and E935.2), located by the patient residence zip code. This excluded poisoning by heroin and non-narcotic analgesics. Reporting of E-codes is mandatory in California.
Ecologic measures
Availability of POs: pharmacy density
Densities of pharmacies and overall retail per square mile were derived from Zip Code Business Patterns [14] data by using North American Industry Classification System (NAICS) codes to aggregate industry counts to the zip code. Overall retail clutter including ‘retail trade’ (sectors 44, 45) and ‘accommodations and food service’ (sector 72) was included to isolate the pharmacy density effect from that of general retail concentration.
Medical need for POs: manual labor industries
The concentration of manual labor industries in the zip code was used as a proxy for medical need for POs due to work-place-related physical injury, as areas with a higher proportion of individuals involved in manual labor have higher rates of self-reported injury and chronic pain [15]. Zip Code Business Patterns data provided counts of business locations in six industries that involve manual labor: agriculture, forestry, fishing and hunting (sector 11); mining, quarrying, and oil and gas extraction (sector 21); construction (sector 23); manufacturing (sectors 31–33); wholesale trade (sector 42); and transportation, warehousing and utilities (sectors 22, 48–49) [16]. Densities for these six variables were calculated as number of locations per 1000 people.
Age-related medical need for POs: rates of cancer and arthritis
Rates of cancer and arthritis-related hospital discharges were used as proxies for an age-related source of medical need for POs, as cancer and arthritis are leading age-related sources of chronic pain [10]. Opioids are the mainstay of treatment for cancer patients [17], and the three-step World Health Organization (WHO) analgesic ladder (non-opioids, weak opioids, strong opioids), is also now used for the treatment of arthritis [18]. Rates of cancer and arthritis were obtained from the hospital discharge data. Cancer cases were identified as all discharges classified with ICD-9 diagnostic codes for malignant neoplasms (140–165, 170–176, 179–196, 199–208 and supplementary classification V10). Arthritis cases were identified as all discharges classified as osteoarthrosis and allied disorders (E715). Counts of cases were aggregated by residential zip code and rates were calculated as a percentage of hospital discharges.
Economic stressors
Three indicators of local economic stressors were measured: median household income, percentage of families in poverty and the unemployment rate. These block group-level estimates were based on between-census projections supplied by GeoLytics [19]. Economic variables were aggregated from the census block group level up to the zip code boundaries specific to each year. Because block groups are not nested within zip codes, economic variables had to be allocated for block groups that cross zip code boundaries. The block group demographic variables were converted to the zip code-level by assigning block group economic values to all Census blocks nested within each zip code, and then aggregating these to zip codes using appropriate block weights.
Control variables
Estimated annual zip code-level demographic data included racial distribution (percentage of non-Hispanic white, black and Hispanic); age distribution (percentage age 0–19, 20–24, 25–44 and 45–64 years); population density; and percentage male. The overall hospitalization rate, calculated as the number of discharges per capita, was also included as a covariate to control for differences in access to in-patient care.
Zip code definitions
Zip codes were chosen as the unit of analysis because they were the most resolved geographic unit at which California hospital discharges were identified. An important drawback of using zip codes for statistical analysis is that they are defined for efficiency of mail delivery and were thus altered periodically at the discretion of postal authorities. Spatial polygons encompassing these routes were available across all years [20]. To ensure complete geographical coverage of the state, uninhabited zip codes were assigned state-wide average values for rate variables (e.g. proportion Hispanic) and were assigned a population value of 1 to allow for non-zero population risks in all areas.
Unit misalignment
The statistical models outlined below allow for unexplained spatial variation in underlying PO-related risk even in the presence of geographic units that change over time. An independent variable measuring the geographic instability of each zip code was computed to test whether alteration of its boundaries was directly related to PO risks. This was calculated as the percentage of year-2000 Census block populations within a given year’s zip code definition that would not have fallen within the boundaries of the best-matched zip code in the prior year (range: 0–59%). This instability measure tested the assumption that zip code boundary shifts did not bias other effects estimates substantively.
Data analysis
We used a hierarchical Bayesian hierarchical space–time misalignment Poisson model [21] to analyze statistically counts of hospital discharges related to prescription opioids. The model assumes that counts are log-linear functions of exogenous measures and correlated across adjacent zip code areas (i.e. conditionally autoregressive). Observed counts are not statistically independent, nor are they globally correlated, but rather they are correlated with counts observed among nearest neighbors (here defined as zip codes with shared boundaries); covariances among units have local spatial structure. Neglect of this particular feature of spatial data leads to Type I errors in analysis, misinterpretation of nominally significant or well-supported effects and miscalculation of relative rates of disease outcomes in disease mapping models [22,23] Observed changes in disease rates may be a function of time and the creation or rearrangement of zip code units over time; thus the analyses require characterizing spatial relationships between units at each time step and measures of changes in population coverage induced by the addition or rearrangement of zip code areas (misalignment). While maximum likelihood solutions are available for statistical analyses of Poisson distributed data, no such general approach exists for spatial Poisson models and, as there are no conjugate priors for Bayesian Poisson models, empirical Bayesian analyses are required [24]; low-precision uninformative prior estimates of expected effects and their error variances are input to iterative computational procedures that converge on best posterior estimates. Added benefits of the approach are: (1) it provides an evaluation of the spatial gradient of risk across California, allowing some areas of the state to have higher or lower problem rates across years despite zip code misalignments; (2) it addresses small area problems by allowing poorly estimated risks in sparsely populated areas to ‘borrow strength’ from rates observed rates in nearby communities [25]; and (3) it allows for over-dispersion about as effectively as do zero-infiated models [26] (technical details regarding the Bayesian Misalignment Poisson model are described in Supporting information, Appendix S1).
Two main models were estimated: model 1 included basic zip code population demographics (age distribution, racial distribution and percentage male), as well as zip code characteristics (retail density, population density, hospitalization rate and rates of cancer and arthritis-related discharges). Model 2 incorporated covariates to test our hypotheses that increased rates of PO-related hospital discharges will be found in areas that are poor (proportion of families in poverty, median household income and unemployment rate); have large numbers of manual labor industries (density of six industry classes); and have high pharmacy density (pharmacies per square mile).
Posteriors estimates (model-based predicted values) were joined to annual zip code units and mapped using the Aeronautical Reconnaissance Coverage Geographic Information System (ArcGIS). This allowed us to examine the changing distributions of PO-related hospital discharges over space and time due to the effects of individual covariates as well as of groupings of covariates. We were particularly interested in examining the changing distribution of PO-related hospital discharges across rural areas [small town and rural zip codes (i.e. up to 64 housing units per square mile and a low degree of commuting to a metropolitan core area)], suburban and exurban areas (i.e. zip codes with 16–64 housing units per square mile with a high degree of commuting; 65–640 housing units per square mile; or 641–1600 housing units per square mile) and urban areas (i.e. zip codes with more than 1600 housing units per square mile) [27].
RESULTS
Descriptive results
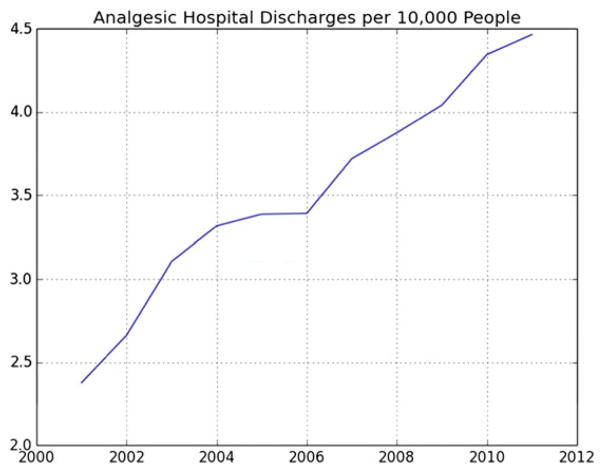
The rates of PO-related hospital discharges per capita increased during the study period from 2.4 cases per 10 000 people in 2001 to 4.5 cases per 10 000 people in 2011 (Fig. 1). There was an average of 7.6 PO-related hospital discharges per zip code during this time-period (0.3% of all hospital discharges), ranging from 0 to 101 discharges across zip codes (Table 1).
Figure 1.
Prescription opioid hospital discharges per 10 000 people in California zip codes, 2001–11
Table 1.
Zip code level characteristics, California, 2001–2011.a
| Variable description | Mean | SD |
|---|---|---|
| Population | 21573.1 | 21846.3 |
| Zip code area (square miles) | 93.5 | 248.2 |
| Count of prescription opioid hospital discharges | 7.6 | 8.9 |
| Pharmacy density (per square mile) | 0.5 | 1.6 |
| Age-related medical need for prescription opioids | ||
| Proportion of hospital discharges with arthritis diagnosis | 0.1 | 0.04 |
| Proportion of hospital discharges with cancer diagnosis | 0.1 | 0.1 |
| Work-related medical need for prescription opioids | ||
| Agriculture and forestry industry density (per 1000 people) | 0.8 | 19.9 |
| Construction industry density (per 1000 people) | 7.02 | 94.0 |
| Manufacturing industry density (per 1000 people) | 3.2 | 46.3 |
| Mining industry density (per 1000 people) | 0.3 | 7.5 |
| Transportation, warehousing and utility industry density (per 1000 people) | 4.3 | 86.1 |
| Wholesale trade industry density (per 1000 people) | 8.9 | 197.8 |
| Median household real income (US$100 000 2009) | 0.5 | 0.2 |
| Proportion of families in poverty | 0.1 | 0.1 |
| Unemployment rate | 0.1 | 0.1 |
| Overall hospitalization rate (discharges per capita) | 0.2 | 2.4 |
| Proportion age 0–19 | 0.3 | 0.1 |
| Proportion age 20–24 | 0.1 | 0.03 |
| Proportion age 25–44 | 0.3 | 0.1 |
| Proportion age 45–64 | 0.3 | 0.1 |
| Proportion white non-Hispanic | 0.6 | 0.3 |
| Proportion black | 0.05 | 0.1 |
| Proportion Hispanic | 0.3 | 0.2 |
| Proportion male | 0.5 | 0.05 |
| Population density (100 000 per square mile)b | 0.03 | 0.1 |
| Retail density (hundreds of establishments per square mile) | 0.2 | 1.0 |
| Zip code instability | 0.01 | 0.03 |
n = 18 517 zip codes over years 2001–11;
the average population density is 0.033 × 100 000 people, or 3300 per square mile.
SD= standard deviation.
Associations between zip code-level characteristics and the rate of PO poisoning discharges
Table 2 presents posterior estimates of the association of a 1-unit shift in each fixed-effect variable with the rate of PO-related hospital discharges. To allow readers to compare the magnitude of the relationship between each exposure and PO-related hospital discharges in a common metric, in the text below we present results associated with a standard deviation shift in zip code-level characteristics. A greater rate of PO-related hospital discharges was found in zip codes with a higher concentration of arthritis-related hospital discharges, with an 8% increase in the rate of PO-related discharges predicted by a standard deviation increase in the arthritis discharge rate [rate ratio (RR) = 1.08; 95% confidence interval (CI) = 1.06, 1.11]. Zip codes with greater pharmacy density had higher rates of PO-related hospital discharges with a 3% increase in discharges predicted from a standard deviation increase in pharmacy density (RR = 1.03; 95% CI = 1.01, 1.05). Zip codes with more manual labor industries also had higher rates of PO-related hospital discharges: in particular, construction (RR = 1.15; 95% CI = 1.10, 1.19) and manufacturing (RR = 1.12; 95% CI = 1.04, 1.20) each predicted a 15 and 12% increase, respectively, in PO-related hospital discharge rates from a standard deviation increase in industry density. Higher median income was associated with lower PO-related hospital discharge rates, with a 15% decrease in PO-related hospital discharge rates predicted from a standard deviation increase in income (RR = 0.85; 95% CI = 0.83, 0.87).
Table 2.
Relative rates of prescription opioid hospital discharges associated with zip code characteristics, California, 2001–11 (n = 18 517 zip codes).
| Variable description |
Model 1
|
Model 2
|
||
|---|---|---|---|---|
| Median | (95% CI) | Median | (95% CI) | |
| Time trend | 1.06 | (1.05, 1.07) | 1.05 | (1.05, 1.06) |
| Constant | 5.16 | (4.01, 6.59) | 4.42 | (3.42, 5.38) |
| Pharmacy density (per square mile) | 1.02 | (1.01, 1.03) | ||
| Age-related medical need for prescription opioids | ||||
| Proportion of hospital discharges with arthritis diagnosis | 11.05 | (5.87, 22.72) | 6.99 | (3.99, 11.86) |
| Proportion of hospital discharges with cancer diagnosis | 0.71 | (0.44, 1.17) | 1.56 | (1.07, 2.35) |
| Work-related medical need for prescription opioids | ||||
| Agriculture and forestry industry density | 1.000 | (0.988, 1.005) | ||
| Construction industry density (per 1000 people) | 1.001 | (1.001, 1.002) | ||
| Manufacturing industry density (per 1000 people) | 1.002 | (1.001, 1.004) | ||
| Mining industry density (per 1000 people) | 1.007 | (0.997, 1.014) | ||
| Transportation, warehousing, utility industry density (per 1000 people) | 1.001 | (1.000, 1.001) | ||
| Wholesale trade industry density (per 1000 people) | 0.999 | (0.998, 0.999) | ||
| Median household real income (US$100 000 2009) | 0.52 | (0.48, 0.57) | ||
| Proportion of families in poverty | 1.04 | (0.82, 1.33) | ||
| Unemployment rate | 0.62 | (0.51, 0.75) | ||
| Random effects | ||||
| County-level time trend | 0.03 | (0.02, 0.04) | 0.03 | (0.02, 0.04) |
| County-level random effect | 0.21 | (0.18, 0.26) | 0.23 | (0.29, 0.18) |
| CAR spatial random effect | 0.29 | (0.28, 0.32) | 0.28 | (0.26, 0.30) |
| Non-spatial random effect | 0.16 | (0.14, 0.18) | 0.15 | (0.13, 0.17) |
| Spatial proportion of zip code variance | 0.77 | (0.72, 0.81) | 0.77 | (0.71, 0.84) |
Both models controlled for zip code hospitalization rate, age, sex and racial composition, population density, retail density and zip code instability. CAR = conditional autoregressive model; CI = confidence interval.
Growth of PO-related hospital discharges across space and time
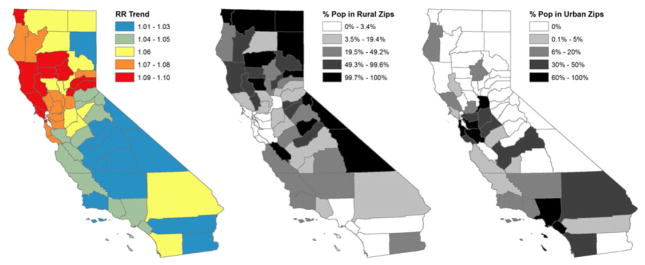
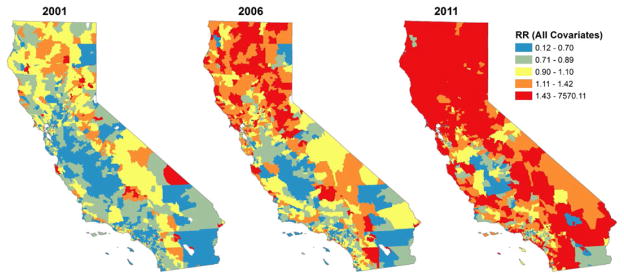
The time trend effect suggests that, even after accounting for the covariates considered above, state-wide PO-related hospital discharge rates grew by an average of 5.6% per year (95% CI = 4.95, 6.51) (see model 1). Growth was not consistent across the state. Figure 2 combines this state-wide time trend, with the county-level random time effect to map spatial variation in risk of growth beyond that explained by the demographic characteristics considered in model 1, and presents the distribution of the state population in urban and rural zip codes. Figure 3 presents predicted relative incidence rates of PO-related hospital discharges estimated from model 1 for 3 years at the beginning, middle and end of the study period: 2001, 2006 and 2011. Growth in use appears most substantial in the central and northern parts of the state, including urban and suburban/exurban areas in the San Francisco peninsula and rural areas in the central valley and north of the state.
Figure 2.
Estimated growth in relative rate per year by county and the distribution of the population in rural and urban zip codes, California, 2001–11. Note that the growth in relative rate is estimated after controlling for all of the variables in our model (model 1)
Figure 3.
Posterior estimated growth of relative incidence rates of prescription opioid poisoning hospital discharges by zip code for selected years, California (model 1). Relative rate values are symbolized by quantiles across all 11 years
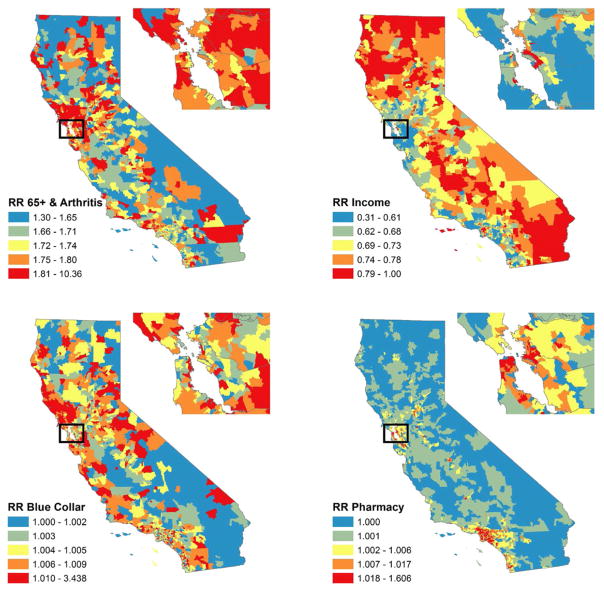
We were interested in investigating the specific contribution that our leading predictors identified in model 2 made to the incidence rate of PO-related hospital discharges in different types of geographic areas. Figure 4 represents the zip code-specific relative rate of PO-related hospital discharges associated with four types of characteristics: (a) age-related medical need for POs, including the concentration of residents aged 65+ and the concentration of hospital discharges due to arthritis in the zip code; (b) median income; (c) work-related medical need (i.e. concentration of construction and manufacturing industries in the zip code); and (d) pharmacy density in each zip code. The figure also presents a magnified picture of zip code-specific rate ratios for each type of characteristic in the San Francisco Bay area. A qualitative review of the figure suggests that these demographic and economic characteristics were associated with different patterns of risk across the state. The risk of PO-related hospital discharges associated with work-place-related medical need was strongest in suburban/exurban and rural areas along the coast and in the Sierra foothills. The contribution of household income to PO-related hospital discharges was strongest outside the coastal urban areas. In contrast, the relative rate of PO-related hospital discharges predicted by pharmacy density was highest in urban areas.
Figure 4.
Posterior estimated relative incidence rates of prescription opioid poisoning hospital discharges in California in 2011 contributed by four combinations of model covariates: % 65+ and arthritis rate; median household income; construction industries per capita and manufacturing industries per capita (blue collar); and pharmacies per square mile (model 2)
DISCUSSION
This study has three major findings. First, akin to other types of drugs, the spatially correlated growth in PO-related hospital discharge rates across a large, geographically diverse US state suggests spatial contagion, where poisoning spreads from rural and suburban/exurban hot-spots to urban areas. These patterns are consistent with findings in other regions of the United States, Canada and Australia, which showed a concentration of PO poisoning in rural areas [28–33]. Secondly, pharmacy density, and community-level concentrations of age- and work-related sources of medical need for POs were associated with higher rates of PO-related hospital discharges across the state. Thirdly, the effects of these community-level factors were heterogeneous across urban and rural areas of the state—a finding that was revealed only by focusing on a state that exhibits the entire continuum across the rural–urban divide.
Sources of PO dispensation were associated modestly with PO-related hospital discharge rates: in particular, zip codes with greater pharmacy density had higher rates of PO-related hospital discharges. This finding is consistent with a prior study of county-level variation in PO abuse, which found that the rate of PO abuse was associated with the rate of PO dispensations and the density of pharmacies at the local level [8]. Prior studies have also found that use of multiple prescribers and multiple pharmacies in California were associated with the number of licensed physicians and surgeons in a county [34]. The modest average association between pharmacy density and PO-related hospital discharges across the state conceals great heterogeneity in the effect: while we saw no effect of pharmacy density on PO-related hospital discharges in rural areas, urban areas exhibited up to a 65% increase in PO-related hospital discharges from a standard deviation increase in pharmacy density. This finding suggests that interventions regulating formal sources of PO supply such as pharmacies may achieve the largest effect in urban contexts.
Local sources of medical need for POs were also an important driver of hospitalizations: as hypothesized, zip codes with more hospital discharges with an arthritis diagnosis and areas with more businesses that might lead to work-place injury (i.e. the construction and manufacturing industries) had higher rates of PO-related hospital discharges. Chronic pain and injury can drive greater medical PO use and thus increase the local supply of POs [15,35]. A greater availability of POs can create opportunities for illegal markets to arise, as family/friends are a primary distribution source of non-medical POs [36]. ‘Pill brokers’, organized dealers who often source from pharmacies or physicians, can also divert a large proportion of POs for non-medical use [36]. The contribution of work-place-related sources of medical need for POs was concentrated in rural and suburban/exurban areas: higher levels of chronic pain associated with the predominance of manual labor industries may, partly, explain the higher rates of non-medical PO use in rural and suburban/exurban compared to urban areas [4]. The effect of manual labor industries on PO poisoning also points to an opportunity for PO abuse prevention interventions in work-place settings.
PO poisoning discharges were concentrated in areas with lower household income, but also lower rates of unemployment. The negative relationship between median household income and PO-related hospital discharges is consistent with findings reported in prior studies of drug poisoning, and in particular with prior studies on PO poisoning [37]. At the same time, the negative association between unemployment rates and PO-related hospital discharges highlights a key distinction between PO abuse and other types of drug abuse. While areas with lower income have higher rates of PO-related hospital discharges, this type of substance abuse is not concentrated in the most economically disadvantaged areas that have high rates of unemployment. Loss of health insurance associated with unemployment may reduce access to POs through formal channels, while the higher street price of POs compared to heroin may limit abuse of POs in the most disadvantaged areas. This finding is consistent with a prior study which found that while PO poisoning fatalities were concentrated in lower-income neighborhoods than non-poisoning unintentional fatalities, they were concentrated in higher-income neighborhoods than heroin poisoning fatalities [37]. The spatial pattern of the association between income and PO-related hospital discharges also suggests that lower levels of income may contribute particularly to PO poisoning risk in exurban and rural areas.
The study findings should be considered with the following limitations. First, population-level analyses present aggregate patterns of PO-related hospital discharges—hence, we suggest inference regarding the relationships between characteristics of small areas and rates of PO-related hospital discharges. However, inferences cannot be made about the impact of local features of the environment on the risk of individual-level non-medical PO use. Secondly, it was not possible to identify the mechanisms that connect ecological features to PO-related hospital discharges rates. Thirdly, this study used measures of hospital discharges related to PO poisoning. Hence, we could not make inferences about the spread of less severe types of PO use, PO poisoning cases that were not hospitalized or PO poisoning fatalities over space and time. However, with these data we could evaluate reliably the geographic distribution of serious, non-fatal manifestations of PO use. Hospitalization data are highly accurate, have been tested rigorously and are used widely to estimate diagnoses [38].
Public health implications
While this study focused upon one US state, it also produces new generalizable findings about the spatial spread of PO poisoning and about the types of community-level factors that shape poisoning in rural versus urban areas. In particular, the concentration of work-place-related sources of medical need for opioids and lower income may contribute to the proliferation of PO-related hospital discharges in rural and suburban/exurban hot-spots of risk. These findings point to the importance of making policies to prevent non-medical use of POs among populations with a medical need for POs a priority in the public health response to the PO epidemic. This is consistent with the public health response to the prescription opioid epidemic, which has focused efforts particularly upon users who access opioids through the health-care system. Current responses, including regulatory and enforcement efforts to reduce over-prescribing, mandated prescriber education programs, the release of evidence-based opioid prescribing guidelines by the Centers for Disease Control, expansion of prescription drug monitoring programs, reformulation of prescription opioids and expansion of naloxone access to treat overdoses and have contributed to a decline in over-prescribing and non-medical prescription opioid use [39–47] Our findings also suggest that policies to address work-place-related needs for pain management and investment in screening and treatment programs for PO abuse in communities with a high prevalence of manual labor occupations might also be a promising approach to reduce PO poisoning [48–57]. Further, increased access to screening and treatment programs by low-income populations may reduce geographic socio-economic disparities in PO poisoning. The impact that such strategies could have on PO poisoning deserves investigation. At the same time, an important fraction of the spatial spread of poisoning rates remains unexplained, suggesting that important drivers of this problem are still to be discovered.
Footnotes
Declaration of interests
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Appendix S1 Technical appendix.
References
- 1.United Nations Office on Drugs and Crime (UNODC) World Drug Report. Vienna: UNODC; 2015. [Google Scholar]
- 2.Compton WM, Jones C, Baldwin G. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–63. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens P, AHRQ, Barrett M, ML Barrett, Inc, Weiss A, Truven Health Analytics, Washington R, AHRQ, Kronick R., AHRQ . Hospital Inpatient Utilization Related to Opioid Overuse Among Adults, 1993–2012. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 4.Keyes KM, Cerda M, Brady JE, Havens JR, Galea S. Understanding the rural–urban differences in nonmedical prescription opioid use and abuse in the United States. Am J Public Health. 2014;104:e52–9. doi: 10.2105/AJPH.2013.301709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havens JR, Talbert JC, Walker R, Leedham C, Leukefeld CG. Trends in controlled-release oxycodone (OxyContin) prescribing among Medicaid recipients in Kentucky, 1998–2002. J Rural Health. 2006;22:276–8. doi: 10.1111/j.1748-0361.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 6.Brownstein JS, Green TC, Cassidy TA, Butler SF. Geographic information systems and pharmacoepidemiology: using spatial cluster detection to monitor local patterns of prescription opioid abuse. Pharmacoepidemiol Drug Saf. 2010;19:627–37. doi: 10.1002/pds.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulozzi LJ, Kilbourne EM, Shah NG, Nolte KB, Desai HA, Landen MG, et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95. doi: 10.1111/j.1526-4637.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 8.Wright ER, Kooreman HE, Greene MS, Chambers RA, Banerjee A, Wilson J. The iatrogenic epidemic of prescription drug abuse: county-level determinants of opioid availability and abuse. Drug Alcohol Depend. 2014;138:209–15. doi: 10.1016/j.drugalcdep.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Kuehn BM. Opioid prescriptions soar. JAMA. 2007;297:249–53. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- 10.Zech DFJ, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63:65–76. doi: 10.1016/0304-3959(95)00017-M. [DOI] [PubMed] [Google Scholar]
- 11.King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am J Public Health. 2014;104:e32–42. doi: 10.2105/AJPH.2014.301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boardman JD, Finch BK, Ellison CG, Williams DR, Jackson JS. Neighborhood disadvantage, stress, and drug use among adults. J Health Soc Behav. 2001;42:151–65. [PubMed] [Google Scholar]
- 13.Puckett C. The Educational Annotation of ICD-9_CM. Reno, NV: Channel Publishing, Ltd; 1991. [Google Scholar]
- 14.US Census Bureau. Zip Code Business Patterns. Washington, DC: Department of Commerce; 2013. [Google Scholar]
- 15.Leff M, Stallones L, Keefe TJ, Rosenblatt R, Reeds M. Comparison of urban and rural non-fatal injury: the results of a statewide survey. Inj Prev. 2003;9:332–7. doi: 10.1136/ip.9.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Census Bureau. [accessed 5 September 2014];North American Industry Classification System. Available at: http://www.census.gov/cgi-bin/sssd/naics/naicsrch?chart=2007.
- 17.Pergolizzi J, Boger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 18.Bergman S. Management of musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:153–66. doi: 10.1016/j.berh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Geolytics Estimates Premium. East Brunswick, NJ: Geolytics, Inc; 2011. [Google Scholar]
- 20.Environmental Systems Research Institute (ESRI) ESRI Data and Maps (DVD) Redlands, CA: ESRI; 2012. [Google Scholar]
- 21.Zhu L, Waller LA, Ma J. Spatial–temporal disease mapping of illicit drug abuse or dependence in the presence of misaligned ZIP codes. Geo Journal. 2013;78:463–74. doi: 10.1007/s10708-011-9429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Best N, Richardson S, Thomson A. A comparison of Bayesian spatial models for disease mapping. Stat Methods Med Res. 2005;14:35–59. doi: 10.1191/0962280205sm388oa. [DOI] [PubMed] [Google Scholar]
- 23.Morrison C, Cerda M, Gorman DM, Gruenewald PJ, Mair CF, Naimi TS, et al. Commentary on Gmel et al. (2016): Are alcohol outlet densities strongly associated with alcohol-related outcomes? A critical review of recent evidence. Drug Alcohol Rev. 2016:35. doi: 10.1111/dar.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]; Special Section: Alcohol policy. :55–57. [Google Scholar]
- 24.Jackman S. Bayesian Analysis for the Social Sciences. New York: Wiley; 2009. [Google Scholar]
- 25.Waller L, Gotway C. Applied Spatial Statistics for Public Health Data. Hoboken, NJ: Wiley Publishers; 2004. [Google Scholar]
- 26.Lord D, Washington SP, Ivan JN. Poisson, Poisson-gamma and zero-inflated regression models of motor vehicle crashes: balancing statistical fit and theory. Accid Anal Prev. 2005;37:35–46. doi: 10.1016/j.aap.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Housing Assistance Council. Taking Stock: Rural People, Poverty, and Housing in the 21st Century. Washington, DC: Housing Assistance Council; 2012. [Google Scholar]
- 28.Havens JR, Oser CB, Leukefeld CG, Webster JM, Martin SS, O’Connell DJ, et al. Differences in prevalence of prescription opiate misuse among rural and urban probationers. Am J Drug Alcohol Abuse. 2007;33:309–17. doi: 10.1080/00952990601175078. [DOI] [PubMed] [Google Scholar]
- 29.Sadowski CA, Carrie AG, Grymonpre RE, Metge CJ, St John P. Access and intensity of use of prescription analgesics among older Manitobans. Can J Clin Pharmacol. 2009;16:e322–30. [PubMed] [Google Scholar]
- 30.Pulver A, Davison C, Pickett W. Recreational use of prescription medications among Canadian young people: identifying disparities. Can J Public Health. 2014;105:E121–6. doi: 10.17269/cjph.105.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban–rural status and by drug type. Pharmacoepidemiol Drug Saf. 2008;17:997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
- 32.Fischer B, Ialomiteanu A, Boak A, Adlaf E, Rehm J, Mann RE. Prevalence and key covariates of non-medical prescription opioid use among the general secondary student and adult populations in Ontario. Canada Drug Alcohol Rev. 2013;32:276–87. doi: 10.1111/dar.12025. [DOI] [PubMed] [Google Scholar]
- 33.Rintoul AC, Dobbin MD, Drummer OH, Ozanne-Smith J. Increasing deaths involving oxycodone, Victoria, Australia, 2000–09. Inj Prev. 2011;17:254–9. doi: 10.1136/ip.2010.029611. [DOI] [PubMed] [Google Scholar]
- 34.Han H, Kass PH, Wilsey BL, Li CS. Individual and county-level factors associated with use of multiple prescribers and multiple pharmacies to obtain opioid prescriptions in California. PLOS ONE. 2012;7:e46246. doi: 10.1371/journal.pone.0046246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inciardi JA, Surratt HL, Cicero TJ, Kurtz SP, Martin SS, Parrino MW. The ‘black box’ of prescription drug diversion. J Addict Dis. 2009;28:332–47. doi: 10.1080/10550880903182986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerda M, Ransome Y, Keyes KM, Koenen KC, Tardiff K, Vlahov D, et al. Revisiting the role of the urban environment in substance use: the case of analgesic overdose fatalities. Am J Public Health. 2013;103:2252–60. doi: 10.2105/AJPH.2013.301347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa K, Brown DF, Tsugawa Y, Camargo CA., Jr Epidemiology of emergency department visits for opioid overdose: a population-based study. Mayo Clin Proc. 2014;89:462–71. doi: 10.1016/j.mayocp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Reifler LM, Droz D, Bailey JE, Schnoll SH, Fant R, Dart RC, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13:434–42. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 40.Delcher C, Wagenaar A, Goldberger B, Cook R, Maldonado-Molina M. Abrupt decline in oxycodone-caused mortality after implementation of Florida’s Prescription Drug Monitoring Program. Drug Alcohol Depend. 2015;150:63–8. doi: 10.1016/j.drugalcdep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Simeone R, Holland L. An Evaluation of Prescription Drug Monitoring Programs. Washington, DC: Department of Justice, Office of Justice Programs; 2006. [Google Scholar]
- 42.Bao YH, Pan YJ, Taylor A, Radakrishnan S, Luo FJ, Pincus HA, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Affair. 2016;35:1045–51. doi: 10.1377/hlthaff.2015.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–45. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martins SS, Sampson L, Cerda M, Galea S. Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature. Am J Public Health. 2015;105:e29–49. doi: 10.2105/AJPH.2015.302843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sessler NE, Downing JM, Kale H, Chilcoat HD, Baumgartner TF, Coplan PM. Reductions in reported deaths following the introduction of extended-release oxycodone (OxyContin) with an abuse-deterrent formulation. Pharmacoepidemiol Drug Saf. 2014;23:1238–46. doi: 10.1002/pds.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O’Malley AJ, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375:44–53. doi: 10.1056/NEJMsa1514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boothby LA, Doering PL. Buprenorphine for the treatment of opioid dependence. Am J Health Syst Pharm. 2007;64:266–72. doi: 10.2146/ajhp060403. [DOI] [PubMed] [Google Scholar]
- 49.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008;9:360–72. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cochran G, Rubinstein J, Bacci JL, Ylioja T, Tarter R. Screening community pharmacy patients for risk of prescription opioid misuse. J Addict Med. 2015;9:411–6. doi: 10.1097/ADM.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeFulio A, Everly JJ, Leoutsakos JM, Umbricht A, Fingerhood M, Bigelow GE, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48–54. doi: 10.1016/j.drugalcdep.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunn K, DeFulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, et al. Employment-based reinforcement of adherence to oral naltrexone in unemployed injection drug users: 12-month outcomes. Psychol Addict Behav. 2015;29:270–6. doi: 10.1037/adb0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirchmayer U, Davoli M, Verster AD, Amato L, Ferri A, Perucci CA. A systematic review on the efficacy of naltrex-one maintenance treatment in opioid dependence. Addiction. 2002;97:1241–9. doi: 10.1046/j.1360-0443.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 54.Reisfield GM, Shults T, Demery J, Dupont R. A protocol to evaluate drug-related workplace impairment. J Pain Palliat Care Pharmacother. 2013;27:43–8. doi: 10.3109/15360288.2012.753975. [DOI] [PubMed] [Google Scholar]
- 55.Weiner SG, Horton LC, Green TC, Butler SF. A comparison of an opioid abuse screening tool and prescription drug monitoring data in the emergency department. Drug Alcohol Depend. 2016;159:152–7. doi: 10.1016/j.drugalcdep.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garg RK, Fulton-Kehoe D, Turner JA, Bauer AM, Wickizer T, Sullivan MD, et al. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J Pain. 2013;14:1620–8. doi: 10.1016/j.jpain.2013.08.001. [DOI] [PubMed] [Google Scholar]