Abstract
Sweetened diets share commonalities with drugs of abuse, but studies comparing behavioral effects of different sweeteners are lacking. Common table sugar produces rewarding and withdrawal effects in planarians. We postulated that Splenda and Equal would produce similar responses and used a tetrad of behavioral assays to test this hypothesis. Acute exposure to a relatively high concentration (10%) of each sweetener produced stereotyped responses (C-shapes) and reduced motility, with Equal producing greater motor effects than sucrose or Splenda. In experiments testing for anxiogenic-like effects, planarians withdrawn from Splenda (1, 3%) or sucrose (1, 3%), but not Equal, and placed into a petri dish with dark and light compartments spent more time in the dark compared to water controls. In place conditioning experiments, both Splenda (0.01%) and sucrose (0.01%) produced an environmental preference shift. Maltodextrin (0.1%), a principal ingredient of Splenda and Equal, produced a significant preference shift. In contrast, sucralose, an indigestible polysaccharide contained in Splenda and Equal, was ineffective. Our data reveal that Splenda produces sucrose-like rewarding and withdrawal effects in planarians that may be dependent on maltodextrin and dextrose. The ineffectiveness of Equal may be due to the presence of aspartame, which is too water insoluble to test in our planarian assay, or to its bitter aftertaste that could mask any rewarding effects produce by maltodextrin or dextrose.
Keywords: planarians, invertebrate, sugar, artificial sweetener, Splenda, Equal, withdrawal, addiction, anxiety, place preference, reinforcing
1. Introduction
Sucrose produces behavioral and neurochemical effects in laboratory rats comparable to those produced by drugs of abuse, including conditioned place preference, locomotor sensitization, anxiogenic and depressant responses during forced abstinence, and enhancement of mesolimbic dopamine transmission [1–3]. Sucrose also produces a dopamine-dependent environmental preference shift and abstinence-induced withdrawal responses in planarians [16], which are the simplest living animals having bilateral symmetry and a CNS with cephalization [10]. Planarians utilize neurotransmitter systems, including glutamate, dopamine, serotonin, acetylcholine, and GABA [9], and display addictive-like behaviors including stereotypies, changes in motility, spontaneous withdrawal, behavioral sensitization, anxiogenic responses, and place conditioning, when exposed to drugs of abuse [6, 11–12]. Here, we compared behavioral effects of Splenda, Equal, and sucrose in planarians using a tetrad of behavioral assays, including anxiogenic and place conditioning assays that take advantage of the tendency of planarians to spend more time in dark environments [15]. We tested effects of the actual sweeteners, rather than the sweetening ingredients, to better model human exposure.
2. Experimental Procedures
2.1. Subjects and drugs
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA). Sucrose was purchased from Sigma-Aldrich (St. Louis MO, USA). Splenda and Equal packets were purchased from Acme Markets. Drugs were dissolved in spring water. Concentrations were based on prior work [15].
Stereotypy and motility experiments
An individual planarian was placed for 5 min into a petri dish (5.5 cm diameter) containing Splenda (0.1, 1, 10%) or spring water. The dish was placed over graphing paper with gridlines spaced 0.5 cm apart. Motility counts were quantified as the number of gridlines crossed or recrossed over the 5-min exposure. C-shaped movements, previously defined as stereotypical movements, were also quantified over the 5-min exposure interval [13–15]. Experiments were repeated with sucrose and Equal.
2.2. Light/dark experiments
An individual planarian was removed from its home jar and placed into a secondary jar (identical to their home jar) containing Splenda (0.1, 1, 3%) or spring water for 30 min. Each planarian was then removed and placed at the midline of a petri dish (5.5 cm diameter) containing spring water. A sleeve of black construction paper covered one half of the dish on the top, bottom and vertical sides to create a dark and ‘ambient’ light environment. Each planarian was given free access to roam the ‘ambient’ and dark sides of the dish, and time spent in each compartment was recorded over 10 min. Experiments were repeated with sucrose and Equal.
2.3. Environmental place conditioning (EPC) experiments
An individual planarian was placed at the midline of a petri dish split into light/dark compartments (as described above) containing spring water. Time spent in the least-preferred (or aversive) setting (light) was determined over a 5-min interval (pre-test). Planarians were then conditioned for 30 min with Splenda (0.01%) or spring water in the least-preferred environment followed by 30 min with spring water in the preferred environment (dark). Immediately following conditioning, planarians were placed at the midline of a petri dish containing just water and allowed free access to the light and dark sides of the dish for 10 min. Time spent in the drug-paired environment was again determined (post-test). The difference in time spent in the drug-paired environment was determined. Experiments were repeated with sucrose and Equal. Additional experiments were conducted with maltodextrin (0.1, 1, 3%) and sucralose (0.1, 1, 3%), which are two of the major individual ingredients of both Splenda and Equal.
2.4. Data analysis
Comparisons of group means (± S.E.M.) were evaluated by one- or two-way ANOVA followed by a Dunnett’s or Bonferroni test post-hoc test to identify group differences or a Student’s t-test. P < 0.05 was considered significant.
3. Results
3.1. Sweeteners produce motor effects
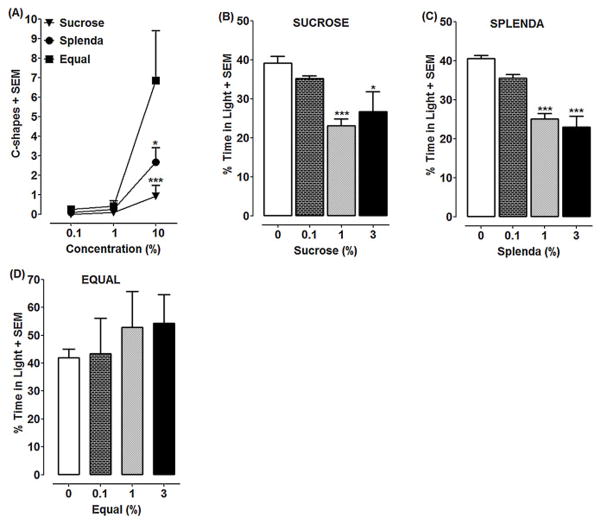
Two-way ANOVA revealed treatment [F(2, 101) = 3.501, P < 0.05] and concentration [F(2, 101) = 10.25, P < 0.001] effects and a significant interaction [F(4, 101) = 2.642, P < 0.05] (Fig. 1A). Equal produced more C-shapes (6.86 ± 2.54) than sucrose (0.92 ± 0.57) (P < 0.001) or Splenda (2.67 ± 0.74) (P < 0.05). Planarians tested in spring water did not display C-shapes. Decreased motility was detected only at a concentration of 10% for both Equal and Splenda, which coincided with enhanced C-shape activity [compound (percentage of water control: Equal (17 ±3.04%) (P < 0.5); Splenda (31 ± 4.41%) (P < 0.001)]. Sucrose did not reduce motility (0.1–10%) tested (P > 0.05). (data not shown).
Fig. 1. Splenda and sucrose produce withdrawal-induced anxiogenic effects.
(1A) Planarians were exposed to different concentrations (0.1, 1, 10%) of sucrose, Splenda or Equal for 5 min and the number of C-shapes was recorded. N=12–14 planarians/group. ***P < 0.001 or *P < 0.05 compared to 10% Equal. (1B–1D) Planarians were pretreated for 30 min with sucrose, Splenda or Equal and then tested in spring water for 10 min for time spent in the light or dark. N=11–12 planarians/group. ***P < 0.001 or *P < 0.05 compared to respective water control.
3.2. Splenda or sucrose withdrawal produces anxiogenic-type responses
One-way ANOVA revealed a main effect for sucrose [F(3, 44) = 7.076, P < 0.001] (Fig. 1B) and Splenda [F(3, 47) = 24.46, P < 0.0001] (Fig. 1C) but not Equal [F(3, 44) = 0.3889, P > 0.05] (Fig. 1D). Planarians pretreated with sucrose and tested in spring water spent more time in the dark relative to sucrose-naïve planarians (1%, P < 0.001 and 3%, P < 0.05). Similarly, Splenda-withdrawn planarians tested in spring water spent more time in the dark compared to Splenda-naïve planarians (1%, P < 0.001 and 3%, P < 0.001).
3.3. Splenda and sucrose produce environmental place conditioning (EPC)
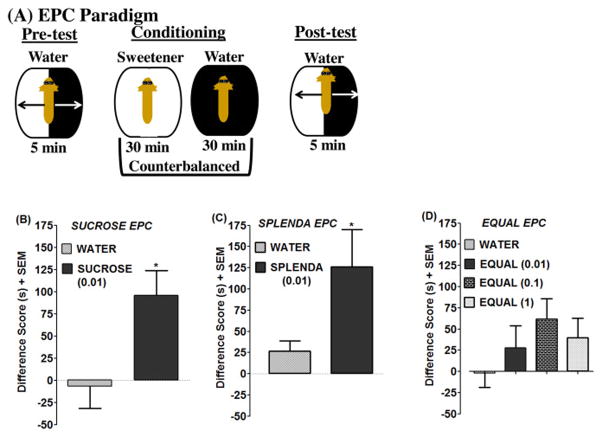
For the experimental set, 96% (72/75) of planarians spent more time in the dark versus light compartment during the pre-test. Planarians conditioned with Splenda (0.01%) displayed EPC (i.e., spent more time in ambient light after conditioning than before conditioning) compared to water controls (P < 0.05, Student’s t-test) (Fig. 2A). Equal (0.01–1%) did not produce place conditioning effects (P > 0.05) (Fig. 2C).
Fig. 2. Splenda and sucrose produce place conditioning effects.
(2A) Paradigm of EPC testing procedure. (2B–2D) Data are expressed as a difference score (post-test minus pre-test for time spent in light). N=12–14 planarians/group. *P < 0.05 compared to respective water control.
Sucrose (0.01%) conditioning also produced EPC compared to sucrose-naïve planarians (P < 0.5, Student’s t-test) (Fig. 2B). It should be noted that in our earlier study [16] 1% sucrose produced significant EPC relative to water controls, but a lower concentration of 0.1% did not reach statistical significance. In the present experiments, 0.01% did produce significant CPP relative to water controls. The difference between the two studies is most likely due to variability in the water control groups. A similar degree of variability is also observed in rat and mouse studies (i.e., the control group should theoretically be ‘zero’ but is normally going to present as a negative or positive shift). For the 0.01% concentration of sucrose tested here, the preference shift was 96 and the water control group was −6.5. In the earlier study, the 0.1% group (0.01% was not tested) showed about a 60 second preference shift and the water control was approximately 20. Therefore, concentrations of sucrose in the 0.01 to 1% range all produce a preference shift, but differences between the water control groups can ultimately affect the significance level.
3.4. Maltodextrin, but not sucralose, produces environmental place conditioning (EPC)
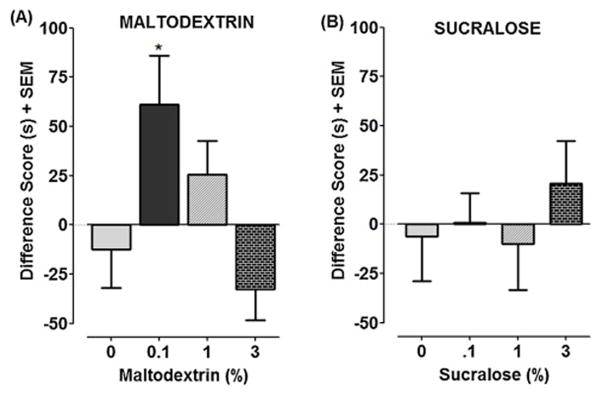
For the maltodextrin experiments, one-way ANOVA revealed a significant main effect [F(3, 44) = 4.52, P < 0.01] (Fig. 3A). Planarians conditioned with a concentration of 0.1% maltodextrin displayed a significant environmental shift compared to water controls (P < 0.05). Higher concentrations of maltodextrin (1, 3%) were ineffective. In contrast, sucralose did not produce significant EPC at any of the concentrations tested [F(3, 44) = 0.4263, P > 0.05] (Fig. 3B).
Fig. 3. Maltodextrin, but not sucralose, produces place conditioning effects.
Planarians were conditioned with either maltodextrin (3A) or sucralose (3B). Data are expressed as a difference score (post-test minus pre-test for time spent in light). N=12 planarians/group. *P < 0.05 compared to respective water control.
4. Discussion
Splenda produced place conditioning, suggest of a rewarding of drug-seeking effect, and an anxiogenic-like response following spontaneous withdrawal. The efficacy of Splenda, as well as its motor effects at a higher concentration, was similar to sucrose [16]. The main ingredient of Splenda is sucralose, which is approximately 1000-fold sweeter than sucrose [5]. Sucralose is made by replacing three hydrogen-oxygen groups on the sucrose molecule with three chlorine atoms, yielding an intense sweetener that activates the same taste buds as sucrose, which may explain its sucrose-like rewarding, drug-seeking and withdrawal effects in planarians.
Samples of Splenda, Equal and related artificial sweeteners do not list amounts or relative ratios of each of the individual components. However, Splenda and Equal do contain maltodextrin and dextrose, which are both easily digestible and readily absorbed in humans. For Splenda, which did produce significant place preference in our experiments, we conducted subsequent experiments identify which of the individual components were responsible for Splenda’s efficacy. We postulated that the place preference effects of Splenda must be due to one or more of the following ingredients – maltodextrin, dextrose or sucralose. In prior work, we showed that dextrose (glucose) produced significant place preference in planarians [16]. Here, our results indicated that maltodextrin, similar to dextrose, produced significant preference, with a concentration of 1% producing approximately a 6-fold shift in preference relative to water controls. In contrast, sucralose, tested across the same concentration range, was ineffective. The lack of efficacy of sucralose could be due to the fact that is indigestible, at least in humans. Interestingly, lactulose, another indigestible carbohydrate, also does not produce CPP in planarians [16].
Equal caused greater stereotypical activity and motor deficits than Splenda or sucrose but did not produce rewarding or withdrawal effects. A principal difference in constituency between Splenda and Equal is that Splenda contains sucralose whereas Equal contains aspartame and acesulfame potassium, both of which are approximately 200-fold sweeter than sucrose. It is possible that observed differences between Splenda and Equal relate to how their specific sweetening ingredients are catabolized rather than the relative sweetness of each ingredient. Sucralose is not considerably catabolized by humans, but aspartame is catabolized into phenylalananine, methanol and aspartate [8]. Aspartate, like glutamate, is an excitatory amino acid that produces C-shape behaviors and changes in motility in planarians [13, 14]. The breakdown of aspartame into aspartate may have contributed to Equal’s stereotypical and motor effects that, in turn, created an aversive state that counteracted, or masked, the rewarding effects of maltodextrin and dextrose. It should be noted that movement disorders in humans have also been reported following aspartame consumption [4]. We considered the idea of testing aspartame by itself, to determine if it did produce aversive effects in planarians, but aspartame is highly lipophilic and relatively water insoluble, physiochemical properties that limit experimentation in the ‘aquatic’ planarian assay.
Another principal difference between Splenda and Equal is that Equal, at least in humans, is associated with a bitter aftertaste. Although it is unknown whether or not planarians express sweet taste receptors, it has been suggested that they possess chemosensory neurons and a taste sensory organ [17]. Therefore, it is conceivable that planarians, like humans, detect a bitterness during Equal exposure that masks any potential rewarding effects of maltodextrin and dextrin. Since Splenda is not associated with a bitter taste, the maltodextrin and dextrose can combine to produce rewarding effects in planarians that present as a shift in place preference.
In conclusion, Splenda, but not Equal, produced sucrose-like behavioral effects in planarians suggestive of an addictive-like phenotype. Differences between Splenda and Equal may relate to catabolism and metabolites, although planarian pharmacokinetics is poorly defined. Future studies will investigate underlying dopaminergic and pharmacokinetic mechanisms, examine effects of the specific sweetening ingredients, and study additional sweeteners, including saccharin, which is reported to be more reinforcing than cocaine or heroin in rats [7].
Research Highlights.
Artificial sweeteners were compared with sucrose in planarians.
Splenda produced sucrose-like rewarding effects in a place conditioning assay.
Splenda produced sucrose-like anxiogenic effects following spontaneous withdrawal.
Splenda, Equal and sucrose produced motor effects at higher concentrations.
Maltodextrin, but not sucralose, produced rewarding effects.
Equal did not produce rewarding and withdrawal effects.
Acknowledgments
This study was supported by NIDA grants R25DA033270 and P30DA013429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avena NM, Bocarsly ME, Hoebel BG. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol. 2012;829:351–365. doi: 10.1007/978-1-61779-458-2_23. [DOI] [PubMed] [Google Scholar]
- 2.Avena NM, Gold MS. Food and addiction - sugars, fats and hedonic overeating. Addiction. 2011;106:1214–1215. doi: 10.1111/j.1360-0443.2011.03373.x. discussion 1219–1220. [DOI] [PubMed] [Google Scholar]
- 3.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerrard JW, Richardson JS, Donat J. Neuropharmacological evaluation of movement disorders that are adverse reactions to specific foods. Int J Neurosci. 1994;76:61–69. doi: 10.3109/00207459408985992. [DOI] [PubMed] [Google Scholar]
- 5.Kinghord AD, Compadre CM. Alternative sweetener. 3. Marcel Dekker; New York: Revised and expanded. [Google Scholar]
- 6.Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11:2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- 7.Madsen HB, Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. 2015;20:433–444. doi: 10.1111/adb.12134. [DOI] [PubMed] [Google Scholar]
- 8.Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, Pariza MW, Spencer PS, Waddell WJ, Walker R, Williams GM. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol. 2007;37:629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience. 2010;168:18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Pagan OR. The first Brain: The neuroscience of planarians. 1. Oxford University press; New York, NY: 2014. [Google Scholar]
- 11.Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur J Pharmacol. 2009;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- 13.Rawls SM, Patil T, Tallarida CS, Baron S, Kim M, Song K, Ward S, Raffa RB. Nicotine behavioral pharmacology: clues from planarians. Drug Alcohol Depend. 2011;118:274–279. doi: 10.1016/j.drugalcdep.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawls SM, Thomas T, Adeola M, Patil T, Raymondi N, Poles A, Loo M, Raffa RB. Topiramate antagonizes NMDA- and AMPA-induced seizure-like activity in planarians. Pharmacol Biochem Behav. 2009;93:363–367. doi: 10.1016/j.pbb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Tallarida CS, Bires K, Avershal J, Tallarida RJ, Seo S, Rawls SM. Ethanol and cocaine: environmental place conditioning, stereotypy, and synergism in planarians. Alcohol. 2014;48:579–586. doi: 10.1016/j.alcohol.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Tallarida CS, Raffa RB, Rawls SM. Sucrose produces withdrawal and dopamine-sensitive reinforcing effects in planarians. Physiol Behav. 2013;112–113:8–13. doi: 10.1016/j.physbeh.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto K, Takeuchi K, Agata K. Neural projections in planarian brain revealed by fluorescent dye tracing. Zoolog Sci. 2005;22:535–546. doi: 10.2108/zsj.22.535. [DOI] [PubMed] [Google Scholar]
- 18.Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]