Abstract
Sonic hedgehog (Shh) is a secreted protein that controls the patterning of neural progenitor cells, and their neuronal and glial progeny, during development. Emerging findings suggest that Shh also plays important roles in the formation and plasticity of neuronal circuits in the hippocampus, a brain region of fundamental importance in learning and memory. Shh mediates activity-dependent and injury-induced hippocampal neurogenesis. Activation of Shh receptors in the dendrites of hippocampal neurons engages a trans-neuronal signaling pathway that accelerates axon outgrowth and enhances glutamate release from presynaptic terminals. Impaired Shh signaling may contribute to the pathogenesis of several developmental and adult-onset neurological disorders that affect the hippocampus, suggesting a potential for therapeutic interventions that target Shh pathways.
Hippocampal Plasticity and Vulnerability
The hippocampus is of critical importance for learning and memory because inputs conveyed from multiple sensory association cortices converge on neurons in hippocampal circuits resulting in the potentiation of activated synapses [1]. The neuronal circuits of the hippocampus exhibit remarkable adaptive structural and functional responses to environmental demands; new synapses form, existing synapses can be pruned, and new neurons are generated from neural progenitor cells (NPCs) in the subgranular region of the dentate gyrus [2]. When rats or mice are challenged to perform cognitive tasks, such as maze learning or living in an ‘enriched’ environment, the number of synapses on hippocampal pyramidal and dentate granule neurons is increased [3]. Cognitive and bioenergetic challenges (exercise and food restriction) can also enhance the proliferation of NPCs, their differentiation into neurons, and/or their survival [4].
Hippocampal neurons, particularly CA1 and CA3 pyramidal neurons, are prone to dysfunction and atrophy in three major neurological disorders, Alzheimer’s disease (AD), depression and temporal lobe epilepsy. Their degeneration involves excessive activation of glutamate receptors, bioenergetic/mitochondrial deficits, and compromised cellular stress resistance and repair mechanisms [3]. One general approach to protecting the hippocampus from injury and disease is to activate signaling pathways that promote neuronal plasticity and cell survival during development [3, 6]. Many of the cellular signaling pathways that regulate the formation of hippocampal neuronal circuits during development also mediate the structural and functional plasticity of the adult hippocampus, including pathways activated by the neurotransmitter glutamate, and brain-derived neurotrophic factor (BDNF) [3]. Here we focus on emerging evidence supporting important roles for sonic hedgehog (Shh), a protein that controls early cell patterning and axon growth during embryonic development [7, 8], in hippocampal neuroplasticity and vulnerability.
Shh signal transduction
The core sequence of molecular events that mediate Shh signal transduction include (Figure 1) [7, 8]: 1) binding of Shh to a plasma membrane receptor called Patched (Ptch); 2) activation of a membrane GTP-binding protein coupled receptor (GPCR)-like protein called Smoothened (Smo) which, in the absence of Shh is tonically inhibited by Ptch; 3) recruitment/activation of a downstream protein complex that remains to be fully understood, but likely includes the kinesin-like protein Costal2, a kinase called Fused (FU), and a suppressor of FU (SUFU); 4) activation of one or more of three transcription factors, Gli1, Gli2 and Gli3; and 5) induction (Gli1 and Gli2) or repression (Gli3) of target genes that effect cellular responses to Shh (Figure 1).
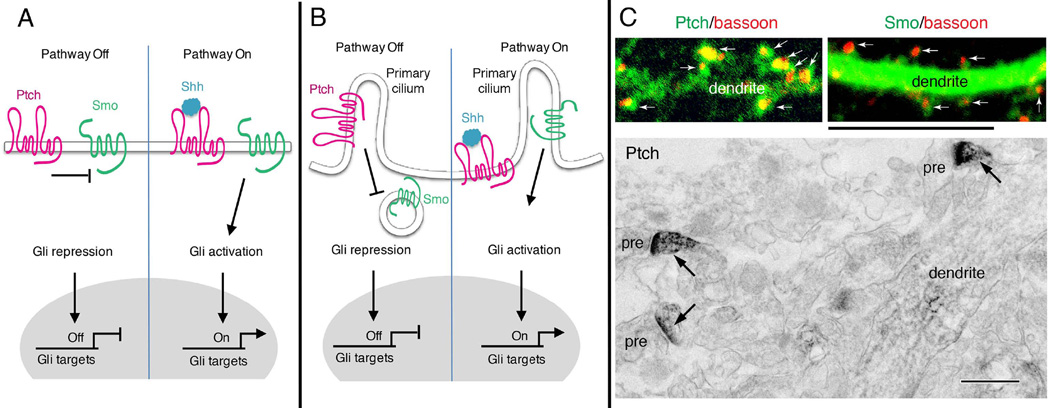
Figure 1. Shh signal transduction pathways.
(A) In the absence of Shh, the transmembrane receptor Patched (Ptch) keeps the transmembrane transducer Smoothened (Smo) in an inactive state. Transcription of Gli target genes is repressed, so the pathway is off. Binding of Shh inactivates Ptch and releases its suppression of Smo. Smo subsequently activates Gli, allowing transcription of Gli target genes. (B) In many cell types, Shh signaling may occur in the primary cilium, a small cellular protrusion. Without Shh, Ptch resides on the membrane of the primary cilium whereas Smo is located on intracellular vesicles that are closely apposed to the cilium membrane. Upon binding Shh, inactivated Ptch leaves the cilium and relieves its suppression of Smo. Smo moves to the cilium, where a sequence of events occur that lead to the activation Gli transcription factors. (C) Distribution of Ptch and Smo in dendrites and synaptic spines of neurons resembles that found in ciliated cells in the absence of Shh binding (left in B), that is, with Ptch on the cilium and Smo in vesicles in the cell cytoplasm. Confocal images show green immunofluorescence labeling for Ptch and Smo in cultured rat hippocampal neurons. Ptch is concentrated mainly in the spines (arrows; red labeling is bassoon, used to indicate presynaptic terminals) with much less in the dendrite. Smo, on the other hand, is concentrated in the dendrite, with relatively little in most spines. The electron micrograph from the CA1 region of the hippocampus from an adult rat shows that immunoperoxidase labeling for Ptch is concentrated in spines (arrows) with much less seen in the dendrite shaft (pre, presynaptic terminal). Smo shows the opposite pattern with high labeling in the dendrite shaft and relatively less in spines (not shown; see [49]). Scale bar is 5 µm for immunofluorescence and 500 nm for immunoperoxidase. The immunofluorescence Ptch image is previously unpublished, and the immunofluorescence Smo image and electron micrograph are modified from Petralia et al. [29].
The precise molecular mechanism by which Ptch inhibits Smo has not been established, with data suggesting a range of possibilities from direct physical interactions of the two proteins, to formation of a membrane channel by Ptch via its sterol-binding domain that is required for inhibition of Smo. The structure of Smo suggests that it is a GPCR, and evidence from studies of cultured cell lines suggest that Smo can functionally couple to a Gi protein resulting in a reduction in levels of cyclic AMP in the cells. There is evidence that basal levels of PKA (cyclic AMP-dependent protein kinase) activity inhibit Smo signaling tonically, suggesting that activation of Gi by Smo upon Shh binding to Ptch relieves this inhibition.
Smo can be directly activated by the small molecular agonist SAG, and Smo is inhibited selectively by cyclopamine; SAG and cyclopamine have therefore proved to be valuable tools for elucidating Shh signaling downstream of Smo. Upon activation, the Costal2 – FU –SUFU complex is recruited to Smo where Costal2 interacts with the C-terminal cytoplasmic tail of Smo. Data suggest that, in the absence of Shh, SUFU interacts with Gli transcription factors and PKA thereby preventing activation of the Gli proteins. Smo activation may result in phosphorylation of SUFU and release of Gli proteins, which then translocate into the nucleus. Gli proteins are also targeted for proteasomal degradation by the ubiquitin ligase beta-transducin repeat-containing protein (βTrCP), thereby providing another level of control of Shh signaling [9].
The genes encoding Ptch and Gli1are targets of Gli-mediated Shh signaling; depending upon the amounts of Shh and Smo relative to Ptch, Shh-induced expression of Ptch and Gli1 genes can either enhance or reduce cellular responsiveness to Shh [10]. Shh signaling via Gli activity controls the proliferation, patterning/migration and survival of NPCs, in part by regulating genes encoding G1 cell cycle proteins (cyclin D and N-Myc) and the anti-apoptotic protein Bcl-2 [11]. Numerous other potential Shh signaling pathway targets are suggested from gene expression analyses of neural cells; some of the genes up-regulated in response to Shh encode proteins involved in hippocampal NPC proliferation, and neuronal differentiation and plasticity [12].
Shh and hippocampal neurogenesis
The vigorous neurogenesis that generates all neurons of the cerebral cortex during embryonic development is influenced greatly by Shh signaling. Thus, selective knockout of Shh and Smo in the embryonic telencephalon results in reduced proliferation of NPCs, reduced neurogenesis and abnormal positioning of neurons in the neocortex [13]. NPCs generated in the ventral hippocampus during late embryonic development migrate to the dorsal hippocampus postnatally, a process that may be regulated by Shh produced by distinct subpopulations of neurons within the hippocampus and medial entorhinal cortex neurons that project to the dentate gyrus [14]. When the transcription factor Sox2 is deleted from NPCs the brain appears relatively normal at birth, but the late-developing dentate gyrus exhibits stem cell loss and severe hypoplasia [15]. It turns out that the Shh gene is a Sox2 target, and treatment with a Shh agonist partially rescues hippocampal hypoplasia in Sox2-deficient mice, demonstrating a critical role for Shh downstream of Sox2 in hippocampal development.
In at least some cell types, including hippocampal NPCs, the primary cilium is where the Shh signal is transduced [16]. Cells in the developing cerebral cortex of homozygous mutant Stumpy mice lack a cilium and exhibit a hypoplastic hippocampus as the result of a deficiency of NPCs [17]. The Stumpy-deficient NPCs do not respond to Shh, consistent with a key role for the cilium in Shh signaling. In the adult hippocampus, NPCs fail to develop after embryonic ablation of genes necessary for cilium formation resulting in a hypotrophic dentate gyrus [18]. Conversely, overexpression of a constitutively active Smo results in an increased size of the dentate gyrus [18]. Because NPCs have only one cilium, it will be of considerable interest to determine whether Shh signaling emanating from the cilium determines whether cell divisions are symmetric (giving rise to two self-renewing daughter NPCs) or asymmetric (giving rise to one neuron and one new NPC) [19].
Emerging findings suggest that Shh signaling also regulates the proliferation and differentiation of adult hippocampal NPCs. Hippocampal NPCs express Ptch, and when NPCs are isolated from the hippocampus and maintained in cell culture, Shh stimulates their proliferation [20]. Moreover, adeno-associated virus-mediated overexpression of Shh in the hippocampus stimulates NPC proliferation in vivo, whereas cyclopamine inhibits their proliferation. The adult hippocampus harbors NPCs in the subgranular region of the dentate gyrus. These NPCs can generate neurons that integrate into the dentate gyrus and receive synaptic inputs from other hippocampal neurons and from neurons in the entorhinal cortex, septum and thalamus [21]. Hippocampal neurogenesis plays an important role in spatial pattern separation, a type of learning and memory of fundamental importance for the generation of ‘cognitive maps’ which are neuronal network-encoded patterns (particularly images and sequences of sounds) of one’s experiences [22]. Shh signaling is required for basal hippocampal neurogenesis in the adult mouse because conditional deletion of either Shh or Smo in nestin-expressing NPCs results in reduced neurogenesis [23].
Regulation of axon elongation and synaptic plasticity
While studies of commissural axons demonstrated that Shh can act directly on axons to regulate their growth [24–26], recent findings suggest that the outgrowth of axons of embryonic rat hippocampal neurons is not affected directly by Shh. Instead, hippocampal neuron axon elongation is stimulated by activation of Shh signaling in the dendrites [27]. Ptch and Smo are present in the somatodendritic compartment of growing hippocampal neurons, where local activation of Smo results in the activation of Gli1 which induces the expression of the axonal actin-binding protein profilin. Mutations in profilin abolish the axon outgrowth-promoting effect of Shh [27]. Interestingly, and in apparent contrast to Shh signaling in NPCs, the axon elongation-promoting effect of Shh can occur even in embryonic hippocampal neurons that lack a primary cilium [27]. Collectively, the data from developing hippocampal neurons suggest a mechanism for regulation of neuronal connectivity in which activation of Shh signaling in dendrites enhances the outgrowth of the axon on the same neuron, thereby hastening its interaction and synaptic connectivity with dendrites of target neurons (Figure 2).
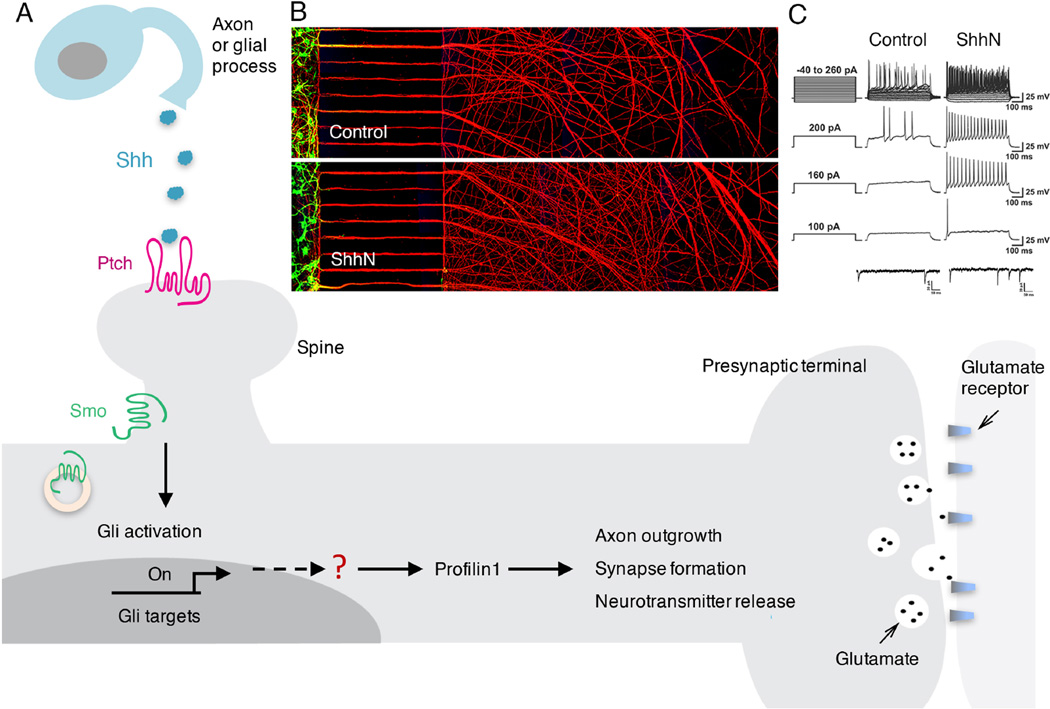
Figure 2. Mechanisms by which Shh may regulate axon outgrowth and synaptic plasticity.
(A) Shh molecules, released from neurons or glial cells, bind to the receptor Ptch located on the dendrites, in particular the dendritic spines, of receiving neurons. The binding of Shh to Ptch allows activation of Smo, which subsequently triggers transcription of target genes through the Gli family of transcription factors. Proteins that are not direct targets of Gli, such as profilin1, can also be upregulated by Shh through not-yet-known mechanisms. Upregulated prolilin1, and likely additional proteins, then promote and regulate axon outgrowth, presynaptic terminal formation, and synaptic plasticity. (B) Example of an experiment with embryonic hippocampal neurons in a compartmentalized culture system shows that Shh stimulates axon outgrowth indirectly and trans-neuronally via Shh receptors in the somatodendritic compartment. Neurons were plated in the compartment at the left and their axons (red; immunostained with an antibody against the axonal marker Smi312) grew through parallel microgrooves and into the compartment at the right. In the compartment at the left are cell bodies and dendrites (green; immunostained with an antibody against the somatodendritic marker MAP2). Shh was applied to the somatodendritic compartment, but not the axonal compartment. Notice higher density of axons in Shh-treated neurons. Scale bar is 450 µm. The images are related to experiments reported in ref. 27. (C) The upper four rows of traces show membrane voltage recordings in control and Shh-treated cultured hippocampal neurons at the indicated current steps. Neurons exposed to Shh exhibit increased axon potential frequency. The lower two traces show recordings of miniature excitatory postsynaptic currents in hippocampal neurons that were exposed to vehicle or Shh. Shh increased the mEPSC frequency indicating increased glutamate release from presynaptic terminals (modified from ref. 31).
Immuno-electron microscope analyses have shown that Ptch and Smo are present in the growth cones of immature neurons in the developing cerebellum, and are located in the postsynaptic dendrites in the adult cerebellum [28]. Similarly, Ptch and Smo are clustered in dendritic growth cones of immature embryonic hippocampal neurons and in dendrites and dendritic spines in the adult hippocampus [29]. The presence of Shh, Ptch and Smo at synapses suggests potential roles for Shh signaling in synaptic plasticity. Indeed, it was reported that Shh increases the size of presynaptic terminals at hippocampal neuron synapses, and this is associated with an increase in the frequency of miniature excitatory postsynaptic currents [30]. The source of Shh that is presumed to regulate axon outgrowth and synaptic plasticity in the hippocampus has not been established. However, data suggest that Shh is present in both pre- and post-synaptic terminals where it may be associated with synaptic vesicles and endosomes [31]. Considerable further research will be required to fully understand where, when and how Shh influences synaptic plasticity and associated functions such as learning and memory.
Neurotrophic proteins, including Shh, are generally believed to be released from cells in a soluble form and act locally or move through the extracellular milieu and act on distant target cells. However, it is increasingly appreciated that growth factors and morphogens can be released from cells in or on the surface of extracellular vesicles (EVs)/exosomes. For example, FGF2 and IGFs are present in EVs released from myocytes, which can stimulate myogenesis from stem cells [32], and EVs released from astrocytes contain glial cell line-derived neurotrophic factor [33]. Primary chick notochord cells release two populations of Shh-containing EVs, with only one population containing integrins that exhibit Shh bioactivity [34]. EVs released from cells overexpressing Shh can activate the Shh signaling pathway in a reporter cell line, and cultured embryonic hippocampal neurons release EVs that contain Shh [35]. Shh is present in EVs located at the surface of neurites, but the roles of EV-associated Shh in hippocampal neuroplasticity remain to be determined.
Shh interactions with Notch and BDNF signaling pathways
Shh can affect the responsiveness of NPCs and neurons to other signals in their cellular niches such that a cell will respond qualitatively or quantitatively differently to a local cue, depending upon the location of that cell within a Shh concentration gradient. Prominent among the signals that regulate hippocampal neurogenesis and synaptic plasticity are Notch ligands and BDNF [36, 37]. Notch is a transmembrane protein that transduces signals from cell surface-associated ligands such as Jagged. Several studies have described interactions between Shh and Notch signaling in regulating neural cell plasticity. By increasing the concentration of Smo in primary cilia, Notch signaling enhances responsiveness of proliferating cells to Shh [38]. Activation of Notch can cause the subcellular relocalization of Ptch and Smo such that Ptch is excluded from the primary cilium, while Smo moves into the primary cilium where it engages downstream signaling to Gli proteins [39]. Notch overexpression induces hippocampal NPC proliferation, whereas Notch inhibition triggers NPC cell cycle exit and dendrite outgrowth in newly generated neurons [40]. Thus, Shh and Notch signaling pathways may cooperate to control the fates of NPCs in the hippocampus.
In the postnatal mouse hippocampus Notch and its ligand Jagged 1 are located at synapses and, in response to synaptic activity, Notch signaling is increased greatly by a mechanism involving activation of the immediate early gene Arc [41]. LTP and LTD at CA1 synapses, and hippocampus-dependent learning and memory are impaired in mice in which Notch is conditionally deleted from hippocampal neurons [41–43]. The effects of Notch on hippocampal synaptic plasticity are mediated, at least in part, by the downstream transcription-regulating protein RBP-J [44]. The enhancement of synaptic plasticity by Notch signaling is mediated by postsynaptic mechanisms that remain to be established. Interestingly, Shh signaling can enhance release of glutamate from presynaptic terminals of hippocampal axons [31], suggesting that combined activation of presynaptic Shh signaling and postsynaptic Notch may be particularly effective in enhancing LTP and associated learning and memory. It will be important to better understand the molecular mechanisms by which Shh and Notch signaling influence activity-dependent hippocampal synaptic plasticity.
BDNF plays major roles in hippocampal neurogenesis and synaptic plasticity [37]. BDNF expression is induced in hippocampal neurons by excitatory synaptic activity, and by physical exercise and fasting [37]. BDNF promotes the differentiation of hippocampal NPCs into neurons, and the growth and integration of newly generated dentate granule neurons into functional neuronal circuits. The formation, maintenance, growth, and plasticity of hippocampal synapses is enhanced by BDNF and, accordingly, BDNF plays very important roles in learning and memory [37]. Emerging findings suggest that Shh and BDNF interact to regulate neuroplasticity. Shh increases the levels of BDNF in regenerating axons, and the ability of Shh to enhance axon outgrowth is compromised when BDNF signaling is inhibited [45]. Interestingly, Shh can increase the production of BDNF by suppressing a micro-RNA (miR-206) that otherwise inhibits translation of the Bdnf mRNA [46].
Shh signaling and neurological disorders
Genetic defects in Shh signaling and teratogens such as cyclopamine that selectively inhibit Shh signaling can cause severe developmental abnormalities in the nervous systems of animals and humans, including holoprosencephaly [47]. Alterations of Shh signaling may also contribute to other neurodevelopmental disorders. Down syndrome (DS) is a developmental caused by triplication of chromosome 21. DS patients exhibit impaired development of cognitive abilities and motor coordination skills, and also develop neuropathological features of Alzheimer’s disease (AD), including amyloid plaques and neurofibrillary tangles in the hippocampus [48]. Shh signaling is reduced and Ptch expression is increased in neural cells in mouse models of DS [49, 50]. Many of the genes on chromosome 21 in humans are located on chromosome 16 in mice; and mice with some or all of chromosome 16 triplicated are models relevant to DS. Studies of a mouse DS model suggest that the mechanism by which trisomy 16 causes increased Ptch expression may involve increased γ-secretase-mediated cleavage of the amyloid precursor protein (APP) to generate an intracellular domain (AICD) that acts as a transcriptional repressor [50]. Treatment of newborn Ts65Dn mice, a mouse model of DS, with the Shh pathway agonist SAG ameliorates cerebellar development defects, effectively restoring the granule cell precursor pool [49]. Hippocampal neurogenesis is impaired in Ts65Dn mice, a model of Down syndrome, and neurogenesis is normalized when the mice are treated with a γ-secretase inhibitor [51]. Shh signaling is required for restoration of neurogenesis because inhibition of γ-secretase does not restore neurogenesis when mice are treated with cyclopamine. Treatment of newborn Ts65Dn mice with SAG also rescues hippocampal phenotypes including cognitive deficits [52]. However, a deficit in a cerebellum-dependent motor learning task (phase reversal adaptation and consolidation of the vestibulo-ocular reflex) is not ameliorated by postnatal SAG treatment in Ts65Dn mice, demonstrating brain region/neuronal circuit-dependent effects of Shh signaling in this DS model [53]. Consistent with complex roles for Shh in DS are data showing that crossing of Ts65Dn mice with mice with reduced Ptch expression (which increases Shh signaling) normalizes some, but not all, of the brain structural and behavioral phenotypes in the DS mice [54].
While abnormal Shh signaling may contribute to DS neural phenotypes, it is not known whether Shh signaling is involved in the pathogenesis of other common developmental neurological disorders. Two findings suggest a potential role for impaired Shh signaling in autism. First, mutations in the cholesterol biosynthetic enzyme 7-dehydrocholesterol reductase cause an autosomal recessive disorder with autistic features called Smith-Lemli-Optiz syndrome, and evidence suggests that reduced cholesterol levels impair Shh signaling in this disorder [55]. Second, an X-linked inherited neurodevelopmental disorder caused by deletion of the gene encoding a Ptch homolog is characterized by mental retardation and autism-like behaviors [56]. Phosphatidylinositol 4-phosphate (PI4P) is found in high levels in the cilia of NPCs and inactivation of inositol polyphosphate 5-phosphatase E depletes PI4P from the cilium, reduces Shh signaling and impairs hippocampal neurogenesis [57]. Inositol polyphosphate 5-phosphatase E mutations cause Joubert syndrome a developmental disorder characterized by abnormal brain development and intellectual disability. Finally, a possible role for Shh signaling in schizophrenia is suggested by data showing that the expression of Disc 1 (disrupted in schizophrenia 1) is reduced in Smo mutants and elevated in Ptch mutants. Interestingly Disc 1 is expressed in high amounts in oligodendrocyte progenitors, and these progenitors are absent in Smo mutants [58].
Shh may play roles in adult brain injury responses and age-related neurodegenerative disorders (Figure 3). Intrathecal administration of Shh, or topical application of Shh to the brain surface above the cerebral infarct region, results in improved functional outcome, reduced neuronal degeneration and increased neurogenesis in a rat model of focal ischemic stroke [59, 60]. Similarly, treatment of mice with the Smo agonist purmorphamine beginning shortly after experimental focal cerebral ischemia improves functional outcome and lessens brain damage and neuroinflammation in a stroke model [61]. Conversely, cyclopamine treatment impairs the ability of mesenchymal stem cells (MSCs) to promote neurite outgrowth, synaptogenesis, remyelination and recovery of function following focal ischemic stroke in mice [62]. The latter study included evidence that MSCs provide a signal(s) that stimulates the production of Shh and tissue plasminogen activator in neurons and astrocytes which, in turn, promote neurite outgrowth, synaptogenesis and myelination. The proliferation of hippocampal NPCs is increased in response to cerebral ischemic stroke in animal models, and Shh signaling plays a key role in this presumptive adaptive response to neuronal injury [63]. Shh may act downstream of melanocortin in the neurogenic response to ischemia because melanocortin 4 receptor agonist-induced neurogenesis is prevented when Shh signaling is selectively blocked [64]. Astrocytes may be a major source of Shh generated in response to brain injury. As evidence, Shh expression is up-regulated in reactive astrocytes in the hippocampus of mice in response to experimental excitotoxic seizures, and the Shh produced by the astrocytes may stimulate proliferation of both astrocytes and microglia [65]. In addition to potential direct effects on NPCs and neurons, Shh signaling may stimulate cerebral angiogenesis [66] which could contribute to improved functional outcomes in stroke and chronic neurodegenerative disorders such as AD that manifest reduced blood flow to affected brain regions, including the hippocampus.
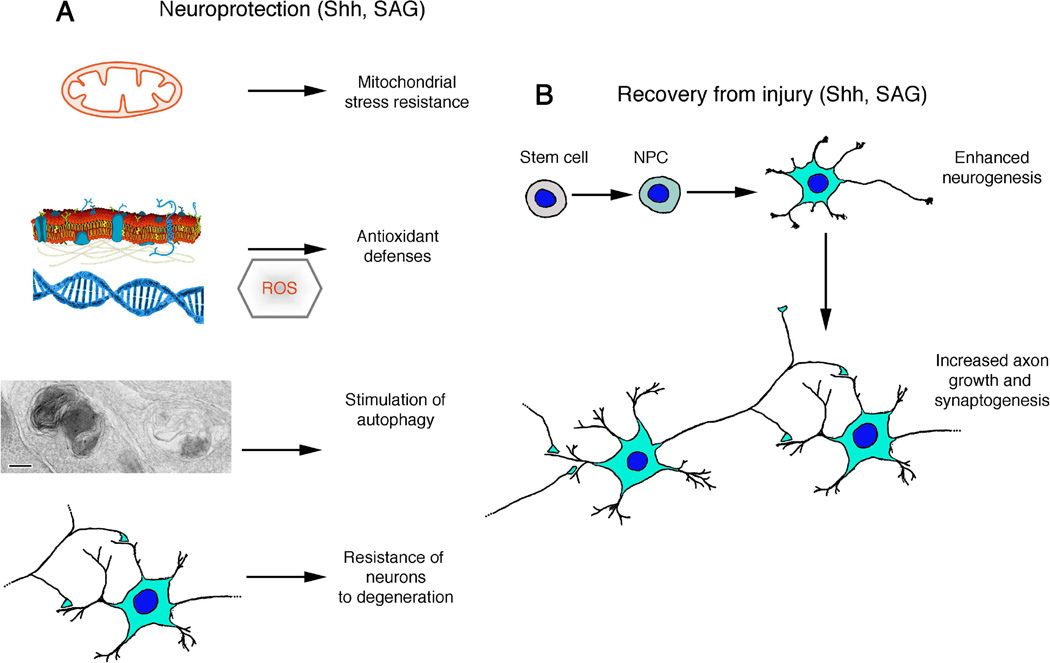
Figure 3. Potential applications of Shh pathway agonists in the treatment of nervous system injury and neurological disorders.
(A) Shh signaling may protect neurons against dysfunction and degeneration by engaging multiple pathways that bolster the resistance of neurons to excitotoxic, metabolic and proteotoxic stress. Among the neuroprotective mechanisms of action of Shh are enhancement of mitochondrial stress resistance and antioxidant defenses and stimulation of autophagy. Two different autophagosomes are shown in a presynaptic terminal from a hippocampal neuron culture treated with Shh (scale bar is 100 nm; modified from Fig. 3E in [75]). Cell membrane and DNA drawings are public domain images. (B) Shh signaling can enhance recovery of function following a traumatic or ischemic injury to the nervous system by stimulating neurogenesis, axon growth and synaptogenesis. NPC, neural progenitor cell; ROS, reactive oxygen species.
The possible involvement of perturbed Shh signaling in the pathogenesis of AD, a disorder in which the hippocampal synapses and neurons degenerate, has begun to be investigated. It was reported that levels of Ptch and Gli1 are reduced in brain tissue samples from AD patients and APP mutant transgenic mice [67]. Self-aggregation of the amyloid β-peptide (Aβ) is a prominent feature in the hippocampus of AD patients, and studies of experimental cell culture and animal models of AD have shown that Aβ can impair hippocampal neurogenesis and synaptic plasticity, and can trigger excitotoxic neuronal calcium overload [68, 69]. It is unknown whether Shh or Shh agonists can protect brain cells in experimental models of AD. However, it is reasonable to consider that Shh can protect hippocampal neurons against Aβ toxicity by inducing BDNF production [37], because BDNF has been shown to be neuroprotective in experimental models of AD [70–72]. Another mechanism by which Shh might bolster neuronal resistance in degeneration is by enhancing autophagy. Impaired autophagy is implicated in the accumulation of cytotoxic proteins (Aβ and p-Tau) and dysfunctional mitochondria in AD [73], and enhancement of autophagy by bolstering mitochondrial bioenergetics can ameliorate AD-like Aβ and p-Tau pathologies in a mouse model of AD [74]. Shh signaling can stimulate autophagy in neurons [75], but it is not known whether Shh signaling can protect neurons against dysfunction and degeneration in AD or neurodegenerative disorders. Future studies of patients, and of animal models of AD and PD in which Shh signaling is manipulated using pharmacological or molecular genetic technologies, will advance an understanding of these diseases and may lead to novel approaches for their prevention and treatment.
The hippocampus is adversely affected in depression, a psychiatric disorder that is a major cause of morbidity and mortality [76]. Studies of animal models suggest that cognitive deficits in depression result from a deficit in BDNF signaling, synapse loss and impaired neurogenesis [76]. Emerging findings suggest that Shh may have an anti-depressant action. Electroconvulsive shock (ECS) therapy is often very effective in patients with severe depression. ECS induces proliferation of hippocampal NPCs in rats, and blockade of Shh signaling with cyclopamine completely prevents ECS-induced NPC proliferation [77]. Interestingly, relief of the inhibition of BDNF translation by miR-206 may mediate the production of BDNF in the hippocampus in response to ketamine, a drug with potent and rapid antidepressant actions [78]. It is not known whether Shh signaling is impaired in depression nor whether Shh agonists such as SAG exhibit antidepressant actions. The ability of Shh to induce BDNF expression provides a rationale for preclinical studies of Shh signaling modulators in models of depression.
Conclusions and Future Directions
The emerging findings described in this article suggest that Shh signaling plays roles in the proliferation and differentiation of hippocampal NPCs into neurons. By regulating axon outgrowth, synaptogenesis and synaptic plasticity, Shh may regulate the formation and adaptive plasticity of hippocampal neuronal circuits throughout life. An unexplored question is if and how Shh signaling is involved in the mechanisms by which exercise, dietary energy restriction and engagement in intellectually challenging endeavors enhance hippocampal neuroplasticity [79]. It is also not known whether impaired Shh signaling contributes to the adverse effects of sedentary overindulgent lifestyles on hippocampal neuroplasticity [80]. Because aberrant Shh signaling can cause developmental defects, and may also contribute to the pathogenesis of a range of adult onset neurological disorders, there is a potential for the development of interventions that target the Shh pathway. Preclinical findings suggest that the Shh agonist SAG can reverse deficits in hippocampal synaptic plasticity and spatial learning and memory in a mouse model of relevance to Down syndrome and AD [52]. Further studies of the potential therapeutic benefits of agents that target Shh signaling in animal models of disorders that affect the hippocampus including AD, depression, epilepsy and cerebral ischemia may provide a rationale for future clinical trials of Shh-modifying interventions in human patients.
Trends Box.
Sonic hedgehog (Shh) regulates hippocampal stem cell proliferation, neuronal differentiation, axon outgrowth and synaptic plasticity.
Cell responses to Shh are mediated by a membrane receptor Patched, a transducer protein Smoothened, and Gli transcription factors.
Regulation of axon elongation is a function of Shh in the developing and adult hippocampus.
Emerging findings suggest roles for Shh signaling in hippocampal synaptic plasticity.
Results of studies of animal models suggest potential therapeutic applications of Shh receptor agonists in several neurological disorders.
Outstanding Questions Box.
What are the signaling pathways that regulate the production and release of Shh from neurons and glial cells?
Does Shh convey a trans-neuronal signal(s) that mediates activity-dependent formation of neuronal circuits (synaptogenesis)?
Does Shh regulate synaptic plasticity independently of its effects on axon outgrowth and, if so, what are the underlying mechanisms?
What are the major Shh signaling gene targets that regulate each of the different Shh-responsive cell types?
Is Shh signaling altered in various neurological disorders and, if so, what is the contribution of abnormal Shh signaling in disease pathogenesis.
Will small molecule Shh activators such as SAG prove effective in mitigating hippocampal dysfunction and neurodegeneration and improving functional outcome in developmental and neurodegenerative disorders?
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institutes of Health/National Institute on Aging and the National Institutes of Health/National Institute on Deafness and Other Communication Disorders. The code for the Advanced Imaging Core is ZIC DC000081-03.
Glossary Terms
- Axon
a long process emanating from the cell body of a neuron and forming a presynaptic terminal with a postsynaptic neuron.
- BDNF
brain-derived neurotrophic factor, a protein involved in neurogenesis, synaptic plasticity, and neuronal stress resistance
- Cyclopamine
a chemical that inhibits sonic hedgehog signaling.
- Dentate gyrus
a neuronal cell layer in the hippocampus where neurogenesis occurs, and which plays a key role in spatial learning and memory.
- Down syndrome
a developmental disorder caused by triplication of chromosome 21 in humans.
- Gli proteins
a family of transcription factors that mediate many biological actions of sonic hedgehog.
- Hippocampus
a brain region that plays a fundamental role in short-term memory and which is adversely affected in Alzheimer’s disease and depression.
- Neurogenesis
a process in which neural stem cells differentiate into neurons.
- Notch
a cell surface receptor that plays important roles in hippocampal neurogenesis and synaptic plasticity.
- Patched
a sonic hedgehog receptor that tonically inhibits the downstream sonic hedgehog transducer protein smoothened; upon binding sonic hedgehog, patched no longer inhibits smoothened.
- Presynaptic terminal
the enlarged end of an axon where neurotransmitter vesicles accumulate and release their contents into the synaptic cleft in response to membrane depolarization.
- Primary cilium
a microtubule-based antenna-like projection emanating from the surface of a cell.
- Smoothened
a G protein-coupled membrane receptor that transduces sonic hedgehog signalling to downstream transcription factors.
- Sonic hedgehog
a protein encoded by the Shh gene which is secreted from cells and acts on other cells to regulate their motility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McClelland JL. In: Perception, Memory, and Emotion : Frontier in Neuroscience. Ono T, McNaughton BL, Molotchnikoff S, Rolls E, Nichijo H, editors. Oxford: Elsevier Science, Ltd.; 1996. pp. 601–613. [Google Scholar]
- 2.Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci. 2015;19:151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Bartsch T, Wulff P. The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience. 2015;309:1–16. doi: 10.1016/j.neuroscience.2015.07.084. [DOI] [PubMed] [Google Scholar]
- 6.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 7.Briscoe J, Small S. Morphogen rules: design principles of gradient-mediated embryo patterning. Development. 2015;142:3996–4009. doi: 10.1242/dev.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matise MP, Wang H. Sonic hedgehog signaling in the developing CNS where it has been and where it is going. Curr Top Dev Biol. 2011;97:75–117. doi: 10.1016/B978-0-12-385975-4.00010-3. [DOI] [PubMed] [Google Scholar]
- 9.Huntzicker EG, et al. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006;20:276–281. doi: 10.1101/gad.1380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shikata Y, et al. Ptch1-mediated dosage-dependent action of Shh signaling regulates neural progenitor development at late gestational stages. Dev Biol. 2011;349:147–159. doi: 10.1016/j.ydbio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Cayuso J, et al. The sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development. 2006;133:517–528. doi: 10.1242/dev.02228. [DOI] [PubMed] [Google Scholar]
- 12.Wu SM, et al. Enhanced production of neuroprogenitors, dopaminergic neurons, and identification of target genes by overexpression of sonic hedgehog in human embryonic stem cells. Stem Cells Dev. 2012;21:729–741. doi: 10.1089/scd.2011.0134. [DOI] [PubMed] [Google Scholar]
- 13.Komada M, et al. Hedgehog signaling is involved in development of the neocortex. Development. 2008;135:2717–2727. doi: 10.1242/dev.015891. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Fang L, Fernández G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78:658–672. doi: 10.1016/j.neuron.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favaro R, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 16.Métin C, Pedraza M. Cilia: traffic directors along the road of cortical development. Neuroscientist. 2014;20:468–482. doi: 10.1177/1073858414543151. [DOI] [PubMed] [Google Scholar]
- 17.Breunig JJ, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han YG, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 19.Crowther AJ, Song J. Activity-dependent signaling mechanisms regulating adult hippocampal neural stem cells and their progeny. Neurosci Bull. 2014;30:542–556. doi: 10.1007/s12264-014-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai K, et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 21.Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattson MP. Superior pattern processing is the essence of the evolved human brain. Front Neurosci. 2014;8:265. doi: 10.3389/fnins.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 24.Charron F, et al. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 25.Yam PT, et al. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Sloan TF, Qasaimeh MA, Juncker D, Yam PT, Charron F. Integration of shallow gradients of Shh and Netrin-1 guides commissural axons. PLoS Biol. 2015;13(3):e1002119. doi: 10.1371/journal.pbio.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao PJ, et al. Dendrosomatic sonic hedgehog signaling in hippocampal neurons regulates axon elongation. J Neurosci. 2015;35:16126–16141. doi: 10.1523/JNEUROSCI.1360-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petralia RS, et al. Subcellular distribution of patched and smoothened in the cerebellar neurons. Cerebellum. 2012;11:972–981. doi: 10.1007/s12311-012-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petralia RS, et al. Subcellular localization of Patched and Smoothened, the receptors for Sonic hedgehog signaling, in the hippocampal neuron. J Comp Neurol. 2011;519:3684–3699. doi: 10.1002/cne.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell N, et al. Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. J Cell Sci. 2012;125:4207–4213. doi: 10.1242/jcs.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petralia RS, et al. Sonic hedgehog distribution within mature hippocampal neurons. Commun Integr Biol. 2011;4:775–777. doi: 10.4161/cib.17832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JS, Yoon HI, Lee KS, Choi YC, Yang SH, Kim IS, Cho YW. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J Control Release. 2016;222:107–115. doi: 10.1016/j.jconrel.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. FASEB J. 2016 Aug 10; doi: 10.1096/fj.201600756R. 2016 pii: fj.201600756R. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vyas N, Walvekar A, Tate D, Lakshmanan V, Bansal D, Lo Cicero A, Raposo G, Palakodeti D, Dhawan J. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep. 2014;4:7357. doi: 10.1038/srep07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eitan E, Petralia RS, Wang YX, Indig FE, Mattson MP, Yao PJ. Probing extracellular Sonic hedgehog in neurons. Biol Open. 2016;5:1086–1092. doi: 10.1242/bio.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lathia JD, Mattson MP, Cheng A. Notch: from neural development to neurological disorders. J Neurochem. 2008;107:1471–1481. doi: 10.1111/j.1471-4159.2008.05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stasiulewicz M, et al. A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development. 2015;142:2291–2303. doi: 10.1242/dev.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong JH, et al. Notch activity modulates the responsiveness of neural progenitors to sonic hedgehog signaling. Dev Cell. 2015;33:373–387. doi: 10.1016/j.devcel.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alberi L, Liu S, Wang Y, Badie R, Smith-Hicks C, Wu J, Pierfelice TJ, Abazyan B, Mattson MP, Kuhl D, Pletnikov M, Worley PF, Gaiano N. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron. 2011;69:437–444. doi: 10.1016/j.neuron.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–1354. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Wang Y, Worley PF, Mattson MP, Gaiano N. The canonical Notch pathway effector RBP-J regulates neuronal plasticity and expression of GABA transporters in hippocampal networks. Hippocampus. 2015;25:670–678. doi: 10.1002/hipo.22402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bond CW, Angeloni N, Harrington D, Stupp S, Podlasek CA. Sonic Hedgehog regulates brain-derived neurotrophic factor in normal and regenerating cavernous nerves. J Sex Med. 2013;10:730–737. doi: 10.1111/jsm.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci. 2011;31:15407–15415. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schachter KA, Krauss RS. Murine models of holoprosencephaly. Curr Top Dev Biol. 2008;84:139–170. doi: 10.1016/S0070-2153(08)00603-0. [DOI] [PubMed] [Google Scholar]
- 48.Wiseman FK, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16:564–574. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roper RJ, et al. Defective cerebellar response to mitogenic Hedgehog signaling in Down [corrected] syndrome mice. Proc Natl Acad Sci U S A. 2006;103:1452–1456. doi: 10.1073/pnas.0510750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trazzi S, et al. APP-dependent up-regulation of Ptch1 underlies proliferation impairment of neural precursors in Down syndrome. Hum Mol Genet. 2011;20:1560–1573. doi: 10.1093/hmg/ddr033. [DOI] [PubMed] [Google Scholar]
- 51.Giacomini A, Stagni F, Trazzi S, Guidi S, Emili M, Brigham E, Ciani E, Bartesaghi R. Inhibition of APP gamma-secretase restores Sonic Hedgehog signaling and neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis. 2015;82:385–396. doi: 10.1016/j.nbd.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das I, et al. Hedgehog agonist therapy corrects structural and cognitive deficits in a Down syndrome mouse model. Sci Transl Med. 2013;5(201):201ra120. doi: 10.1126/scitranslmed.3005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutierrez-Castellanos N, et al. Size does not always matter; Ts65Dn Down syndrome mice show cerebellum-dependent motor learning deficits that cannot be rescued by postnatal SAG treatment. J Neurosci. 2013;33:15408–15413. doi: 10.1523/JNEUROSCI.2198-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutka T, et al. Chronic up-regulation of the SHH pathway normalizes some developmental effects of trisomy Ts65Dn mice. Mech Dev. 2015;135:68–80. doi: 10.1016/j.mod.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blassberg R, et al. Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum Mol Genet. 2016;25:693–705. doi: 10.1093/hmg/ddv507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filges I. Deletion in Xp22.11: PTCHD1 is a candidate gene for X-linked intellectual disability with or without autism. Clin Genet. 2011;79:79–85. doi: 10.1111/j.1399-0004.2010.01590.x. [DOI] [PubMed] [Google Scholar]
- 57.Chávez M, Ena S, Van Sande J, de Kerchove d'Exaerde A, Schurmans S, Schiffmann SN. Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev Cell. 2015;34:338–350. doi: 10.1016/j.devcel.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Boyd PJ, et al. Sonic hedgehog functions upstream of disrupted-in-schizophrenia 1 (disc1): implications for mental illness. Biol Open. 2015;4:1336–1343. doi: 10.1242/bio.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bambakidis NC, et al. Improvement of neurological recovery and stimulation of neural progenitor cell proliferation by intrathecal administration of Sonic hedgehog. J Neurosurg. 2012;116:1114–1120. doi: 10.3171/2012.1.JNS111285. [DOI] [PubMed] [Google Scholar]
- 60.Huang SS, et al. Anti-oxidative, anti-apoptotic, and pro-angiogenic effects mediate functional improvement by sonic hedgehog against focal cerebral ischemia in rats. Exp Neurol. 2013;247:680–688. doi: 10.1016/j.expneurol.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Chechneva OV, et al. A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis. 2014;5:e1481. doi: 10.1038/cddis.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding X, et al. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab. 2013;33:1015–1024. doi: 10.1038/jcbfm.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, Moskowitz MA. Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke. 2009;40:3618–3626. doi: 10.1161/STROKEAHA.109.561951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spaccapelo L, Galantucci M, Neri L, Contri M, Pizzala R, D'Amico R, Ottani A, Sandrini M, Zaffe D, Giuliani D, Guarini S. Up-regulation of the canonical Wnt-3A and Sonic hedgehog signaling underlies melanocortin-induced neurogenesis after cerebral ischemia. Eur J Pharmacol. 2013;707:78–86. doi: 10.1016/j.ejphar.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 65.Pitter KL, Tamagno I, Feng X, Ghosal K, Amankulor N, Holland EC, Hambardzumyan D. The SHH/Gli pathway is reactivated in reactive glia and drives proliferation in response to neurodegeneration-induced lesions. Glia. 2014;62:1595–1607. doi: 10.1002/glia.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teng H, et al. Tissue plasminogen activator and plasminogen activator inhibitor 1 contribute to sonic hedgehog-induced in vitro cerebral angiogenesis. PLoS One. 2012;7(3):e33444. doi: 10.1371/journal.pone.0033444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He P, et al. Deficiency of patched 1-induced Gli1 signal transduction results in astrogenesis in Swedish mutated APP transgenic mice. Hum Mol Genet. 2014;23:6512–6527. doi: 10.1093/hmg/ddu370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 70.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwasaki Y, Negishi T, Inoue M, Tashiro T, Tabira T, Kimura N. Sendai virus vector-mediated brain-derived neurotrophic factor expression ameliorates memory deficits and synaptic degeneration in a transgenic mouse model of Alzheimer's disease. J Neurosci Res. 2012;90:981–989. doi: 10.1002/jnr.22830. [DOI] [PubMed] [Google Scholar]
- 72.Criscuolo C, Fabiani C, Bonadonna C, Origlia N, Domenici L. BDNF prevents amyloid-dependent impairment of LTP in the entorhinal cortex by attenuating p38 MAPK phosphorylation. Neurobiol Aging. 2015;36:1303–1309. doi: 10.1016/j.neurobiolaging.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 73.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 74.Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, Kawamoto EM, Mattson MP. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging. 2013;34:1564–1580. doi: 10.1016/j.neurobiolaging.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petralia RS, et al. Sonic hedgehog promotes autophagy in hippocampal neurons. Biol Open. 2013;2:499–504. doi: 10.1242/bio.20134275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ota KT, Duman RS. Environmental and pharmacological modulations of cellular plasticity: role in the pathophysiology and treatment of depression. Neurobiol Dis. 2013;57:28–37. doi: 10.1016/j.nbd.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banerjee SB, Rajendran R, Dias BG, Ladiwala U, Tole S, Vaidya VA. Recruitment of the Sonic hedgehog signalling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur J Neurosci. 2005;22:1570–1580. doi: 10.1111/j.1460-9568.2005.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang X, Yang Q, Wang X, Luo C, Wan Y, Li J, Liu K, Zhou M, Zhang C. MicroRNA expression profile and functional analysis reveal that miR-206 is a critical novel gene for the expression of BDNF induced by ketamine. Neuromolecular Med. 2014;16:594–605. doi: 10.1007/s12017-014-8312-z. [DOI] [PubMed] [Google Scholar]
- 79.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]