Abstract
The androgen receptor (AR) plays a key role in the development and progression of prostate cancer (CaP). Since the mid-1990s, reports in the literature pointed out higher incidences of CaP in some select groups, such as airline pilots and night shift workers in comparison to those working regular hours. The common finding in these “high-risk” groups was that they all experienced a deregulation of the body’s internal circadian rhythm. Here we discuss how the circadian rhythm affects androgen levels and modulates CaP development and progression. Circadian rhythmicity of androgen production is lost in CaP patients, with the clock genes Per1 and Per2 decreasing, and Bmal1 increasing, in these individuals. Periodic expression of the clock genes was restored upon administration of the neurohormone melatonin, thereby suppressing CaP progression. Activation of the melatonin receptors and the AR antagonized each other, and therefore the tumor suppressive effects of melatonin and the clock genes were most clearly observed in the absence of androgens, that is, in conjunction with androgen deprivation therapy (ADT). In addition, a large-scale study found that high-dose radiation was more effective in CaP patients when it was delivered before 5PM, compared to those delivered after 5PM, suggesting that the therapy was more effective when delivered in synchrony with the patient’s circadian clock. As CaP patients are shown to become easily resistant to new therapies, perhaps circadian delivery of these therapeutic agents or delivery in conjunction with melatonin and its novel analogs should be tested to see if they prevent this resistance.
Keywords: circadian clock, androgen receptor, melatonin, per1, bmal1
INTRODUCTION
The prostate is an androgen-dependent organ that relies on male hormones (androgens) for growth and maintenance. In disease states, androgens can stimulate oncogenes that promote cellular proliferation, thus increasing the chances of DNA copy errors. Rodent studies showed that excessive androgen production can induce prostate cancer (CaP) (Brown, et al. 1979) and this was later supported by human studies that explored long-term exposure to high levels of androgens (Gann, et al. 1996). The androgen receptor (AR) is a member of the nuclear steroid hormone receptor family that also includes the progesterone receptor (PR) and the estrogen receptor (ER). The AR is responsible for mediating the effects of androgens, which are synthesized primarily by the testes and the adrenal cortex, but also other organs such as skin and ovaries. Androgens play an important role in the development and maintenance of the male reproductive tissues. The AR, in its inactive state, is located in the cytoplasm complexed with other proteins such as the heat shock proteins (HSP). It is activated by binding to androgens which cause the AR to transmigrate to the nucleus, where it functions as a transcription factor. The main androgens in humans are testosterone and its more active metabolite, 5-alpha-dihydrotestosterone (DHT), although other androgens such as dehydroepiandrosterone (DHEA), androstenedione, androstenediol, and androsterone are also produced. Upon binding to androgens, the AR is freed from its protein complex and is allowed to move to the nucleus, binding target genes through their androgen response elements (ARE) located in the promoter or enhancer regions. Many of the genes targeted by the AR are involved in the growth and survival of prostate cells; therefore, dysregulation of AR activity is strongly implicated in CaP development and progression.
Primary CaP is mostly treated with either prostatectomy or with radiation therapy, although watchful waiting is a common option. Approximately 30–40% of those thus treated will experience a recurrence and will be administered androgen deprivation therapy (ADT), which consists of leutenizing hormone releasing hormone (LHRH) agonists or antagonists, or orchiectomy, to decrease the levels of circulating androgens. ADT is initially effective in >95% of patients, but many initial responders develop resistance to ADT and will eventually develop castration resistant CaP (CRPC). The majority of castration-resistant tumors still express AR, and androgen-regulated genes such as prostate specific antigen (PSA), indicating that the AR pathway is still active (Denmeade, et al. 2003). CRPC patients will be further treated with chemotherapy, immunotherapy, radiotherapy or the use of androgen signaling inhibitors (ASI): the AR inhibitor enzalutamide or the CYP17 inhibitor abiraterone acetate. However, the duration of efficacy of the inhibitors is short and eventually, most patients will develop resistance to these treatments as well.
A series of publications pointed to a pattern of CaP development in unique sets of conditions. A 1996 study in a cohort of 2,740 Air Canada pilots showed that standardized incidence ratio (SIR) for CaP was significantly increased among male pilots (SIR = 1.87, 90% confidence interval (CI) 1.38–2.49) compared to the general population, although there was an overall decrease in cancer-related mortality for all sites of cancer (Band, et al. 1996). Following this, in 2003, it was reported that a cohort of 10,051 male airline pilots from Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) experienced an increase in the relative risk of CaP with increasing number of flight hours in long-distance aircrafts (p = 0.01) (Pukkala, et al. 2003).
In addition, continuing with this pattern, a 2006 study from Japan reported that among 14,052 working men in Japan, compared with day workers, rotating-shift workers were significantly at risk for CaP (relative risk = 3.0, 95%CI: 1.2–7.7) (Kubo, et al. 2006). This was borne out by a meta-analysis of 2,459,845 individuals from eight published studies which showed that night-shift work was associated with a significantly increased risk of CaP (Rao, et al. 2015). The association between night shift work and CaP risk was particularly strong for tumors with worse prognosis (Papantoniou, et al. 2015a).
Based on the reports outlined in the last two paragraphs, a 2006 commentary hypothesized that “both altered-lighted environments and genetic variations in genes responsible for maintaining circadian rhythms may result in deregulation of clock-associated biological processes, such as androgen expression, and consequently influence an individual’s risk of prostate cancer” (Zhu, et al. 2006). In support of a role for altered light environments in CaP, a study linking a cohort of 17,557 persons with visual impairment demonstrated decreased incidence of CaP by degree of visual impairment (Pukkala, et al. 2006). These reports supported the validity of the chronodisruption hypothesis and indicated a role for the circadian rhythm in the development of CaP.
WHAT IS THE CIRCADIAN RHYTHM?
Circadian rhythms reflect daily oscillations in biological processes regulated by an internal timing system; for example, the sleep/wake cycles (Fu and Lee 2003). Circadian rhythm has a very important adaptive significance that greatly influences the behavior and physiology of all living organisms from prokaryotes to humans. Other functions in the human body that are influenced by the circadian rhythms include thermoregulation, arterial pressure in systemic circulation, endocrine functions, the digestive system and immune response (Reppert and Weaver 2002). Disruption of circadian rhythms has been linked to various human ailments including insomnia, jet lag, stomach ailments, coronary heart attacks, depression and cancer (Reppert and Weaver 2002).
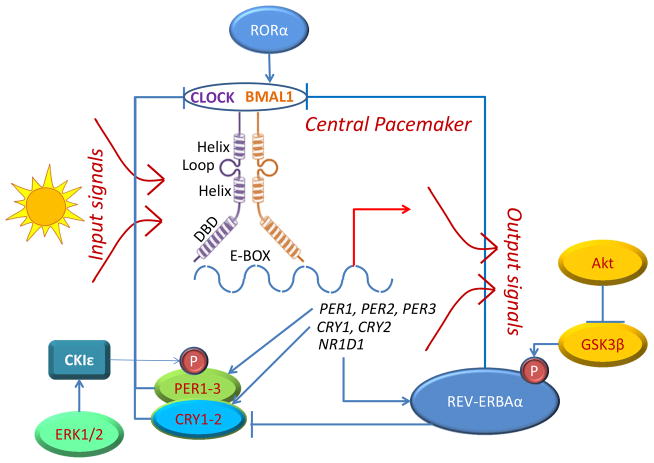
In mammals, the circadian system is made of three parts: the input pathways, the central pacemaker, and the output pathways (FIGURE 1). Environmental signals, such as external light, are transmitted via the input pathways to the central endogenous rhythm of the body, the central pacemaker, which transmits neural and hormonal signals to peripheral circadian systems throughout the body (output pathways) (Liu, et al. 1997). The central pacemaker resides in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus, which is made up of a number of single-cell circadian oscillators. In a normal individual, the single cell oscillators are synchronized and generate coordinated circadian outputs (Welsh, et al. 1995). However, in disease states, this cycle is disordered and leads to disruption of various functions of the related organs.
Figure 1. Relationship between the various clock genes.
Input signals such as sunlight activate the 8 known core clock genes: period (Per1-Per3), casein kinase Iε(CKIε), Clock/NPAS2, Bmal1/ARNTL, and cryptochrome (Cry1-2). Clock and Bmal1 are two basic helix-loop-helix (bHLH) transcription factors (each containing a bHLH region and a DNA binding domain – DBD), which bind the E-box sequence (CACGTG) in the promoter of target genes as a heterodimer. Target genes include Per, Cry, and NR1D1, which produces an orphan nuclear receptor protein REV-ERBAα. When REV-ERBAα is phosphorylated by GSK3β, a downstream target of Akt, it transcriptionally represses target genes such as Bmal1 and Cry. In this way, REV-ERBAα balances against the effects of another orphan nuclear receptor RORα, which transcriptionally upregulates Bmal1. Similarly, Per1 is regulated by phosphorylation by CKIε, which is itself regulated by ERK kinases. Activated Per dimerizes with Cry to form a complex that disrupt the Clock-Bmal1 complex and prevent transcription. P-denotes protein phosphorylation.
Circadian rhythms of the central pacemaker are generated by 8 known clock genes: period (Per1-Per3), casein kinase Iε(CKIε), Clock, Bmal1, and cryptochrome (Cry1-2) (Fu and Lee 2003). Proteins encoded by these genes form a molecular feedback loop that regulates the circadian rhythm (Ko and Takahashi 2006). Clock and Bmal1 are two basic helix-loop-helix (bHLH) transcription co-activators, which positively regulate the circadian cycle by forming a heterodimer that bind E-box sequences (typically CACGTG) in the promoter of target genes such as Per and Cry (FIGURE 1). At the protein level, intracellular expression of Clock remain more or less constant throughout the 24-hour period, whereas Bmal1 levels are upregulated at the beginning of the day and fall off as night approaches. At high levels of Bmal1, it forms a heterodimer with Clock which binds to E-box sequences in the promoters of the Cry, Per and NR1D1 genes to activate transcription at the beginning of a circadian day. NR1D1 encodes the protein Rev–ErbAα, which is an orphan nuclear receptor that acts as a transcriptional repressor and participates in the circadian regulation of organs like the brain, liver, skeletal muscle and adipose tissue. It is known to suppress transcription of Bmal1 and the Cry genes. Rev–ErbAα is phosphorylated on the amino terminus by glycogen synthase kinase (GSK 3β), which contributes to its protein stability. Bmal1 is coordinated by opposing effects of Rev–ErbAα and the RAR-related orphan receptor RORα, likely to fine-tune its effect on the circadian rhythm (FIGURE 1).
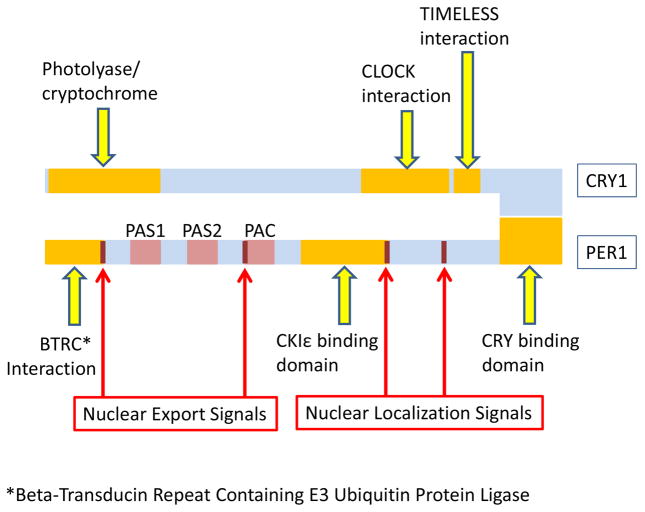
Per1 (rigui) is rhythmically transcribed in the SCN, with a period of approximately 24 hours. It is also expressed in other organs, including the prostate. This rhythm is sustained in constant darkness, and with changing light cycles, which likely explains why the circadian rhythm is maintained even in humans with partial or complete visual impairment (Pukkala et al. 2006). The three Per genes encode proteins that contain PER–ARNT–SIM (PAS)- and PAC-domains (Fu and Lee 2003) (FIGURE 2). All three contain a CKIε binding domain that allows interaction with this kinase and subsequent phosphorylation, and several nuclear localization and export sequences that allow the complex to enter and exit the nucleus as needed (Sun, et al. 1997; Tei, et al. 1997). Per2 has a similar function, but in addition, it was shown that Per2 contains glucocorticoid response elements (GREs) and can interact with PPARγ (So, et al. 2009). Cry1 and Cry2 belong to a class of flavoproteins that in plants and insects induce sensitivity to blue light (Hsu, et al. 1996). In mammals, they can also act in a light-independent manner as inhibitors of Clock-Bmal1 components of the circadian clock (Griffin, et al. 1999) (FIGURE 2).
Figure 2. General structure of core clock genes Per and Cry.
The three Per genes encode proteins that contain the PER–ARNT–SIM (PAS) and the related PAC motifs and form oligomers with the Cry proteins to form functional DNA binding complexes. Per1 contains a BTRC interaction domain at the N terminal end. In addition, Per proteins also contain a phosphorylation domain where Casein kinase 1 binds and phosphorylates these proteins. The very C terminus contains a specific domain that allows oligomerization with the Cry proteins. In addition, Per2 contains a PPAR gamma binding site between the CKIε and CRY binding sites that is not seen in Per1 or Per3. Per2 also has a stabilization domain upstream of the PAS motifs not seen in other Per proteins. All Per proteins, however, express nuclear localization and export sequences that allows them to enter and leave the nucleus as needed. The structure of the Cry proteins is much less complex. Both Cry1 and Cry2 contain a cryptochrome (flavoproteins that are sensitive to blue light) interaction domain, as well as a domain that allow them to interact with and inhibit the CLOCK/BMAL1 dimers. In addition, Cry1 also contain a domain that allows interaction with TIMELESS (which negatively regulates CLOCK/BMAL-1 interactions), but this domain is not found in Cry2. (Based on http://www.uniprot.org/).
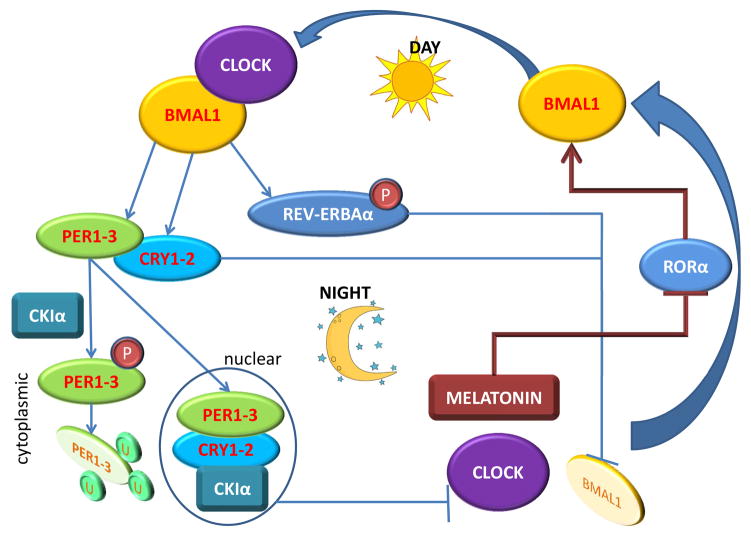
Per and Cry proteins form oligomers with each other in the cytoplasm, and the oligomeric structure likely stabilizes the complex. In particular, Per2, but not the other Per proteins, contain a stabilization sequence that may allow the stabilization of an oligomeric complex. As the day progresses, the oligomer complex translocate from the cytoplasm, where they are formed, to the nucleus. As night falls, the Per/Cry oligomer interfere with Clock/Bmal1 activity, thereby preventing further accumulation of the components of the oligomer. Any Per protein accumulated in the cytoplasm is phosphorylated by CKIε, an event that leads to a conformational change that masks the proteins stabilization sequence in Per2. This makes the protein complex unstable and it is degraded by ubiquitylation likely by recruitment of the BTRC E3 ubiquitin ligases to the N-terminal region of PER proteins. Interaction with TIMELESS leads to disruption of the Clock/Bmal1-associated transcriptional complex and results in the inhibition of Cry, Per and Rev–Erb α transcription, and de-repression of Bmal1 transcription (Reppert and Weaver 2001, 2002). The interacting positive and negative feedback loops of circadian genes ensure low levels of Per and Cry, and a high level of Bmal1 at the beginning of a new circadian day (FIGURE 3).
Figure 3. 24-hour cycle of the circadian clock.
The expression of Clock itself does not significantly vary during the 24-hour cycle, but it is only one of few genes in this group that does not. Major regulation of the circadian clock is mediated by the orphan nuclear receptors RORα and REV-ERBAα which determine the expression of Bmal1 (also known as ARNTL), the key binding partner of Clock. During the day, the Clock-Bmal1 dimer transcribes genes that result in the synthesis of Per and Cry, as well as REV-ERBAα. As the day progresses, Per and Cry dimerizes and forms a stable complex that translocates to the nucleus. Any free Per remaining in the cytoplasm is phosphorylated by CKIε, which causes it to be ubiquitinated and degraded. As night falls, the Per/Cry oligomer complex in the nucleus disrupts the formation of the Clock-Bmal1 heterodimer, which then stops producing Per and Cry. In addition, REV-ERBAα is phosphorylated by GSK3β which stabilizes the protein, and allow it to transcriptionally repress the expression of Bmal1. Bmal1 levels are also suppressed when melatonin levels increase in the circulation, which inhibits the expression of RORα that positively regulates Bmal1 expression. Decrease in Bmal1 further disrupts the Clock-Bmal1 complex and prevents the transcription of target genes, including Per and Cry. As REV-ERBAα levels in the cell, and melatonin levels in the circulation, decrease early in the morning, Bmal1 synthesis is resumed. Further, Per and Cry levels in the nucleus also fall off due to decreased synthesis, and Clock-Bmal1 complexes resume, which then allows the circadian clock to move forward. P-denotes protein phosphorylation, U- denotes protein ubiquitination.
REGULATION OF THE CIRCADIAN CLOCK BY MELATONIN
The pineal gland in the human brain (so called because it resembles a pine cone), is best known as a producer of the hormone melatonin, an indoleamine neurohormone derived from the neurotransmitter serotonin, which affects the modulation of sleep patterns in the circadian rhythm. Many of melatonin’s biological effects are produced by the interactions of melatonin with its two receptors MT1 and MT2. (Boutin, et al. 2005). Melatonin is a powerful antioxidant, and this function may be important in its interaction with the circadian rhythm. Serum melatonin levels are usually elevated nocturnally (80–120pg/ml) compared to its expression during the day (2–20pg/ml), MT1 and MT2 are G-protein coupled receptors (GPCR), which upon activation by melatonin binding, mediate the repression of RORα transcriptional activity, and hence blocks the expression of the clock gene BMAL1 (Hill, et al. 2009) (FIGURE 3). During the day, as melatonin levels are degraded, BMAL1 levels increase and resume binding to clock. Studies indicate improved sleep when melatonin is taken at the appropriate time for jet lag and shift work.
The U.S. Dietary Supplement Health and Education Act of 1994 allow synthetic melatonin to be sold as a dietary supplement; there have not been any reported cases of proven toxicity or overdose by melatonin; in fact, melatonin was found to prevent the cytotoxic effects of other drugs (Asghari, et al. 2016; Esteban-Zubero, et al. 2016), including chemotherapeutic drugs (Demir, et al. 2015). Clinical studies indicate a role for melatonin as an adjuvant therapy for sleep disorders of circadian etiology (jet lag, delayed sleep phase syndrome, sleep deterioration associated with aging, etc.) (Sanchez-Barcelo, et al. 2010). Melatonin seems to facilitate sleep in insomniac patients in some cases (Rohr and Herold 2002). However, the National Sleep Foundation warns that “the correct dosage, method and time of day it is taken must be appropriate to the sleep problem”.
RELATION BETWEEN THE CIRCADIAN CLOCK AND ANDROGENS
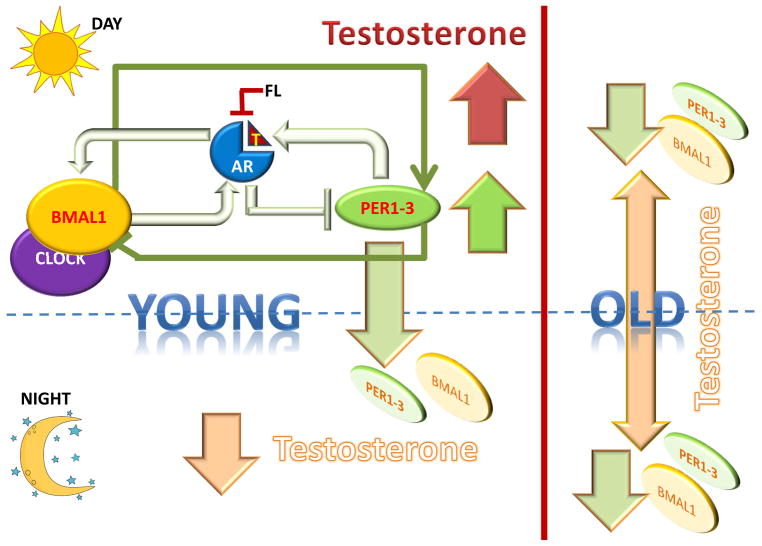
Circadian rhythms have been shown to play an important role in the expression of androgens, and studies show high levels of testosterone production in early mornings in healthy men that decline as the day progresses leading to lower levels by the evening (Cooke, et al. 1993). As a result, in the diagnosis of hypogonadism in males, it is recommended that testosterone levels be assessed in the morning before 10 AM. This decline was coincident with the initial rise in the concentration of cortisol (Cooke et al. 1993). Surprisingly, the early morning spike in testosterone levels characteristic of young men was blunted in old age (Bremner, et al. 1983). Additional studies indicated that an adrenal androgen-regulating system was impaired in the older subjects (Montanini, et al. 1988), especially over the age of 70 (Diver, et al. 2003). A comparison of young (3-month) and aged (18- and 24-month) rats showed that expression of several clock genes (Bmal1, Per1, Per2, Per3 and Rev-ErbAα) were reduced in the 24-month-old group compared to the younger groups (Baburski, et al. 2016) (FIGURE 4). These results seem to suggest that the reduced levels of the clock genes in aged males may be responsible for the loss of circadian rhythmicity observed in aging.
Figure 4. Circadian regulation of testosterone production in young and old men.
Healthy young men demonstrate high levels of testosterone production in early mornings that decline as the day progresses leading to lower levels by the evening. During the day, Per complexes promote testosterone production, as did Bmal1; the produced testosterone binds to and activates the androgen receptor (AR), but as night falls, activated AR suppresses Per levels, thereby decreasing testosterone production and deactivates the AR. This in turn suppressed Bmal1 levels, further preventing Per expression. As Per levels increase once more the next morning, along with Bmal1 levels, testosterone production is renewed. However, several clock genes (Bmal1, Per1, Per2, Per3 and Rev-ErbAα) are reduced in older males compared to younger ones, resulting in the loss of circadian rhythmicity in testosterone synthesis observed in aging males. T- testosterone.
In support of the above observations, delayed peak androgen production was identified in night shift workers compared with day workers (Papantoniou, et al. 2015b), and may have contributed in part to the increased risk for CaP observed in night shift workers (Kubo et al. 2006; Papantoniou et al. 2015a; Rao et al. 2015). Significantly, the rhythmicity of Per2 mRNA levels in the blood depended on the period of activity of the subjects, therefore, it was higher in the morning for day shift workers, similar to the general population, but higher in the evening for night shift workers (Fang, et al. 2015). This shift in Per2 levels was coincident with testosterone synthesis, suggesting strong association between androgen production and the expression of Per2. Additional reports showed that Bmal1 and Clock, but not Per2 and Nr1d1/Rev–ErbAα, are down-regulated by testosterone deficiency (Kawamura, et al. 2014). Thus, it is likely that Per2 that drives testosterone levels. This observation was borne out by another study in rat prostate mesenchymal cells, which showed that neither testosterone nor DHT caused any change in Per2 activity (Yoshida, et al. 2010). On the other hand, the same study showed that the anti-androgen flutamide, which is used for the treatment of advanced CaP, up-regulated the amplitude of circadian Per2 oscillations in a dose-dependent manner (Yoshida et al. 2010). Since flutamide binds to and inhibits the AR, it is possible that AR transcriptional activity, through activation of a downstream target of the AR, regulate Per2 activity and levels (FIGURE 4).
A significant relation exists between other clock genes and testosterone levels as well. Studies in rodent models revealed that the AR is concentrated in the SCN core in male mice (Iwahana, et al. 2008). Similar to Per2, Per1 also inhibited AR transactivation and diminished the expression of AR target genes following DHT stimulation; while Per1 itself is regulated by androgens in CaP cells (Cao, et al. 2009). Both male and female Bmal1 knockout (K/O) mice are infertile (Alvarez, et al. 2008). Male Bmal1 K/O mice had low testosterone and high luteinizing hormone serum concentrations, suggesting a role for Bmal1 in androgen production, while in turn, Bmal1 and Clock are down-regulated by testosterone deficiency (Kawamura et al. 2014). Importantly, Leydig cells, which produce testosterone, rhythmically express Bmal1 protein (Alvarez et al. 2008). Further evidence that the Bmal1-Clock heterodimer is involved in testosterone production and AR transactivation came from studies using mice homozygous for a dominant-negative allele of the Clock gene (Clock (Δ19/Δ19)), which have slightly but significantly decreased male fertility (Liang, et al. 2013). Taken together, these reports point to a process by which feedback loops may exist between the AR axis and the clock genes in males (FIGURE 4).
CLOCK GENES AND PROSTATE CANCER REGULATION
Clock genes are known to be directly involved in the regulation of tumorigenesis in the prostate and other organs. Per1 is down-regulated in human CaP samples compared with normal prostates (Cao et al. 2009). On the other hand, overexpression of Per1 in CaP cells resulted in significant growth inhibition and apoptosis (Cao et al. 2009). Other studies found that Clock and Per2 protein levels were downregulated whereas Bmal1 protein levels were upregulated in CaP cells, compared to normal prostate cells (Jung-Hynes, et al. 2010). Overexpression of Per2 reduced CaP cell growth and viability. Interestingly, melatonin treatment increased Per2 and Clock and reduced Bmal1, thereby causing resynchronization of oscillatory circadian rhythm genes in CaP cells (Jung-Hynes et al. 2010). It appears that resynchronization of the clock genes may have tumor suppressive effects in CaP cells.
Population-based genetic associations studies which genotyped single nucleotide polymorphisms (SNP) in known circadian-related genes showed that at least one SNP in each of the core circadian genes was significantly associated with susceptibility to CaP (Zhu, et al. 2009), and quite a few were associated with fatal CaP (Markt, et al. 2015) (TABLE 1). However, there are variations in the strength of association between fatal CaP and these SNPs between different cohorts showing variability in different patient cohorts (Markt et al. 2015). Taken together, these reports illustrate a possible role of multiple clock genes in the regulation of CaP progression and development.
TABLE 1.
Association of various single nucleotide polymorphisms (SNP) in known circadian-related genes with susceptibility to CaP (Zhu et al. 2009) or with fatal CaP (Markt et al. 2015).
| Clock Gene | SNP Associated with susceptibility to CaP | SNP associated with fatal CaP |
|---|---|---|
| PER1 | rs885747 and rs2289591 | rs2289591 |
| PER2 | rs7602358 | rs10462023 |
| PER3 | rs1012477 | |
| CRY1 | rs12315175 | rs7297614, rs1921126 and rs12315175 |
| CRY2 | rs2292912 | |
| CKIε | rs1534891 | |
| BMAL1 (ARNTL) | rs7950226 | rs969485 |
| CLOCK/NPAS2 | rs11133373, rs1369481, rs895521, and rs17024926 | rs3754674 and rs10206435 |
MELATONIN REGULATION OF PROSTATE CARCINOGENESIS
Recent studies showed that patients with high melatonin levels or a high melatonin/cortisol ratio were less likely to develop CaP or experience CaP progression (Tai, et al. 2016). Comparison of serum levels of melatonin in elderly men with benign prostatic hyperplasia (BPH), prostatic intraepithelial neoplasia (PIN), and localized CaP, as well as in normal adult men without CaP, demonstrated that melatonin induced significant circadian rhythms in normal men and patients with benign prostatic hyperplasia (BPH) (enlarged prostate) and prostatic intraepithelial neoplasia (PIN) (considered to be a precursor for prostate cancer) but not in patients with CaP (Bartsch, et al. 1985). In support of these reports, a rodent model affirmed that melatonin suppresses prostate tumorigenesis (Toma, et al. 1987).
However, further analysis found that, while afternoon melatonin administration induced tumor remission in castrated rodents, continuous melatonin administration did not have any significant effect on CaP tumors (Buzzell 1988), suggesting that melatonin is only effective when administered in synchronization with the patient’s natural circadian cycle,. Further, the study also emphasized that melatonin is more effective with low AR activity. In a study of 186 patients with previously untreated metastases from colorectal cancer, 93 patients were assigned chronotherapy with oxaliplatin, fluorouracil, and folinic acid and 93 were assigned constant-rate infusion via multichannel programmable ambulatory pumps (Levi, et al. 1997). An objective response was obtained in 47 (51%) of the chronotherapy group, and in 27 (29%) of the constant-rate group. Chronotherapy reduced five-fold the rate of severe mucosal toxicity and halved that of functional impairment from peripheral sensitive neuropathy (Levi et al. 1997). In vitro studies demonstrated higher growth inhibitory efficacy of melatonin not only in the absence of androgens (Siu, et al. 2002) but also in the presence of estrogens which are known to suppress androgen production (Lupowitz and Zisapel 1999). Thus, there is possible interaction between the melatonin-dependent pathway and the AR axis that likely antagonize each other.
The growth suppressive effects of melatonin seems to be dependent on the GPCRs MT1/Mel1A and MT2/Mel1B receptors (Moretti, et al. 2000; Xi, et al. 2001; Xi, et al. 2000); whereas in the absence of these receptors, melatonin does not appear to have a tumor suppressive role. The fact that melatonin’s effects on tumor suppression were only observed when melatonin was used in a circadian manner (afternoon administration), but not when it was continuous, suggest that continuous melatonin administration causes degradation of the melatonin receptors likely via a feedback mechanism, as is often seen in many GPCRs. Significantly, MT1 and MT2 are highly expressed in AR null cells (Gilad, et al. 1999; Marelli, et al. 2000; Sainz, et al. 2005), and in 22Rv1 cells expressing high levels of alternately spliced AR variants (and low levels of full length AR) (Tam, et al. 2007), but not in cells expressing high AR levels (Moretti et al. 2000). These results suggest that AR activity suppresses the expression of the melatonin receptors. Therefore, melatonin is perhaps more effective in CaP models of castration where AR activity is low, allowing MT1/2 expression, which can then bind melatonin (FIGURE 5).
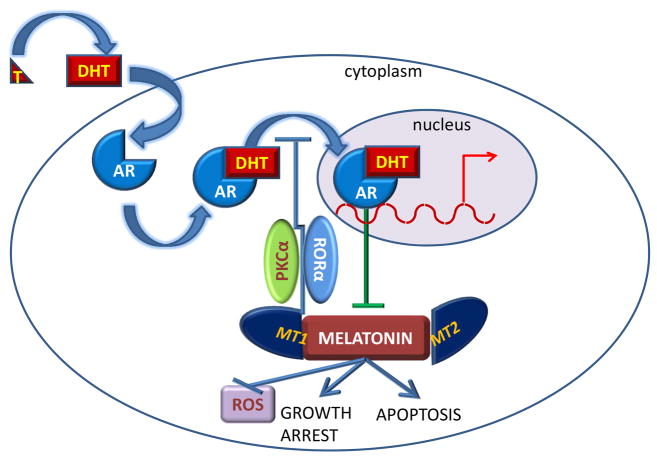
Figure 5. Negative feedback loop between melatonin and the androgen receptor.
Testosterone (T) is converted to dihydrotestosterone (DHT), which in the prostate, binds to the androgen receptor (AR) in the cytoplasm of prostate derived cells, including prostate cancer. DHT binding activates the AR which then translocates to the nucleus and binds to androgen response elements (ARE) in the target genes, thereby stimulating their transcription. AR transcriptional activity represses the expression of the melatonin receptors MT1 and MT2, thereby preventing their binding to melatonin. This prevents melatonin’s ability to induce growth arrest and apoptosis, and to prevent oxidative stress. In turn, melatonin and its receptors inhibit AR translocation to the nucleus, by preventing T conversion to DHT, but also by mechanisms involving PKCα or RORα.
In turn, it was reported that melatonin inhibits AR localization in the nucleus, where it is active, thereby suppressing AR activity (Lupowitz, et al. 2001; Rimler, et al. 2001; Rimler, et al. 2002a) and preventing AR binding to its target gene (Rimler, et al. 2002b). Other studies suggest that melatonin prevents testosterone conversion to DHT, a strong AR ligand (Philo and Berkowitz 1988). Melatonin’s effect on the AR has been suggested to be mediated by the inhibition of the RZR/ROR receptors (Karasek and Pawlikowski 1999), while inhibition of AR nuclear translocation by melatonin was likely via a PKCα-dependent mechanism (Sampson, et al. 2006). Thus, melatonin and the AR pathway inhibit each other (FIGURE 5), likely to gain control of the downstream targets leading to cell cycle progression and apoptosis. Whether the tumor progresses or not will depend on which pathway gains control in the end.
Melatonin’s effects on growth arrest are mediated by PKCα (Tam, et al. 2008), and by aldo-keto-reductase (AKR) and prostaglandin F-synthase (PGFS) dependent pathways (Byrns, et al. 2008), whereas its effects on cell death required elevation of p21 (Park, et al. 2009), p27Kip1 (Tam and Shiu 2011), activation of TNFα and NF-kB-dependent pathways (Rodriguez-Garcia, et al. 2013; Sainz, et al. 2008; Shiu, et al. 2013) and JNK and p38MAPK activation (Joo and Yoo 2009). Melatonin inhibition of HIF1α signaling and reactive oxygen species generation has also been established (Cho, et al. 2011; Park et al. 2009; Paroni, et al. 2014). Several microRNA (miRNA)s including miR3195 and miR374b were significantly up-regulated while other miRNAs were down-regulated in melatonin-treated cells under hypoxia compared to untreated control (Sohn, et al. 2015). In addition to hypoxia and androgen deprivation, melatonin also seems to sensitize CaP cell lines to cell death under hypoglycemic conditions (Gobbo, et al. 2015; Kabasakal, et al. 2011).
Since melatonin was effective in tumor suppression in castrated rodents, a Phase II clinical trial was initiated to test the efficacy of melatonin in conjunction with a LHRH agonist vs the LHRH agonist alone (Lissoni, et al. 1997). Men treated with the combination demonstrated a decrease in PSA serum levels greater than 50% in 57% patients, a normalization of platelet number in patients with persistent thrombocytopenia prior to study, and a survival longer than 1 year in 64% patients (Lissoni et al. 1997). Therefore, it appears that melatonin may actually prolong the effects of LHRH agonists in men with advanced CaP. In support of the involvement of the melatonin receptor in suppression of carcinogenesis by melatonin, a case report of a patient whose prostate tissue expressed the melatonin receptors demonstrated efficacy of melatonin in suppressing tumor progression (Shiu, et al. 2003).
EFFECT OF THE CIRCADIAN CLOCK IN HORMONE AND CHEMOTHERAPY
While melatonin is the direct effector of the circadian clock, our growing understanding of the circadian rhythm has produced various strategies to increase the effectiveness of cancer treatment. Hot flashes are a common side effect of ADT using LHRH agonists in men with advanced CaP. A recent study demonstrated that there were significant 24-h circadian rhythms of hot flashes following ADT administration (Hanisch and Gehrman 2011) where the peak of the rhythms occurred in early afternoon. Since altered circadian rhythms have been identified in untreated CaP patients, the authors conclude that the acrophases of hot flashes and elevated activity levels may represent a normalization of circadian rhythms following ADT (Hanisch and Gehrman 2011).
Recently, a retrospective study testing disease control and treatment-related toxicity in patients undergoing high-dose radiotherapy (HDRT) (median 78 Gy) for CaP evaluated those receiving daytime treatment (before 5 PM) (n = 267) vs evening treatment (after 5 PM) group (n = 142) (Hsu, et al. 2016). This study found that evening HDRT was significantly associated with worse freedom from ≥grade 2 late GI complications (hazard ratio = 2.96; p < 0.001), especially in in patients older than 70 years old, Moreover, biochemical failure-free survival (BFFS) was worse in the evening group than the daytime group (72% vs. 85%, hazard ratio = 1.95, p = 0.05) (Hsu et al. 2016). No known circadian based trials of hormone and chemotherapeutic agents that are effective in CRPC patients have been reported as yet. Hormone refractory metastatic CaP has been treated with circadian-timed fluorodeoxyuridine (FUDR) chemotherapy; however, without objective response (Rajagopalan, et al. 1998). The lack of objective response, of course, could result from the lack of efficacy of FUDR in CaP patients.
Since chronomodulation was not implemented in many CaP trials of chemotherapeutic agents, circadian-timed delivery of chemotherapeutic agents in other cancers will be examined. In 1985, 31 patients with advanced ovarian cancer received eight monthly courses of doxorubicin (adriamycin) that were followed 12 hours later by cisplatin, with doxorubicin randomly administered at either 6 a.m. or 6 p.m (Hrushesky 1985)., Those receiving doxorubicin in the evening and cisplatin in the morning required reductions in dosage and delays in treatment (indicative of greater toxicity), compared to those receiving doxorubicin in the morning and cisplatin in the evening (Hrushesky 1985). A similar study concluded that the combination (doxorubicin + cisplatin) is active against advanced ovarian cancer, but that its toxicities can be significantly decreased by dosing doxorubicin in the early morning and cisplatin in the late afternoon (Levi, et al. 1990).
Thereafter, this schedule (doxorubicin in the early morning and cisplatin in the late afternoon) has been used in studies on endometrial and bladder cancer patients, with considerable success (reviewed in (Kobayashi, et al. 2002)). Similar success was achieved in patients with metastatic renal cell carcinoma receiving fluorodeoxyuridine (FUDR) when delivered by a circadian-modified infusion schedule, in which 68% of the daily dose was administered between 15:00h and 21:00h, compared to continuous infusion (Hrushesky, et al. 1990) As mentioned earlier, a study of chronomodulated infusion (administered to coincide with relevant circadian rhythms) of oxaliplatin, fluorouracil, and folinic acid compared with a constant-rate infusion method demonstrated that chronotherapy was significantly less toxic and more effective than constant-rate infusion in patients with metastatic colorectal cancer (Levi et al. 1997). While no studies on the use of chronomodulation in hormone and chemotherapy in CaP patients have been reported, the above studies in ovarian, endometrial, bladder, renal and colorectal cancer patients suggest that the use of chronomodulation in CaP patients may yield considerable benefit as well.
CONCLUSION
This review concisely describes current knowledge of the mechanisms by which the circadian clock interacts with AR signaling pathway. While in normal individuals, the clock genes, especially Per1/per2 and Bmal1 were expressed in a circadian rhythmic manner, this rhythmicity was lost in CaP patients, while the neurohormone melatonin, which can regulate Bmal1 levels, restored circadian function. We provide evidence that melatonin reduced cytotoxicity in individuals receiving chemo- and hormonal therapy, and sensitized patients to LHRH agonists. A recent study suggests that chronomodulation of radiation therapy benefit CaP patients receiving radiotherapy in the morning compared to those receiving the treatment in the evening. Given the success of chronomodulation in the treatment of other cancers with chemotherapy, the studies references here hypothesize an improvement in outcome and a reduction in toxicity if CaP patients were to be tested for chronodependence in chemo- and hormonal therapy.
Acknowledgments
FUNDING:
This work was supported by a Biomedical Laboratory Research & Development (BLRD) Merit Award (I01BX000400, PMG) from the Department of Veterans Affairs, and by Award R01CA185509 (PMG) from the National Institutes of Health.
Footnotes
DECLARATION OF INTEREST
The authors declare no conflict of interest. The work reported here does not represent the views or opinions of the Department of Veteran Affairs or the United States Government.
References
- Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghari MH, Moloudizargari M, Bahadar H, Abdollahi M. A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opin Drug Metab Toxicol. 2016:1–10. doi: 10.1080/17425255.2016.1214712. [DOI] [PubMed] [Google Scholar]
- Baburski AZ, Sokanovic SJ, Bjelic MM, Radovic SM, Andric SA, Kostic TS. Circadian rhythm of the Leydig cells endocrine function is attenuated during aging. Exp Gerontol. 2016;73:5–13. doi: 10.1016/j.exger.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Band PR, Le ND, Fang R, Deschamps M, Coldman AJ, Gallagher RP, Moody J. Cohort study of Air Canada pilots: mortality, cancer incidence, and leukemia risk. Am J Epidemiol. 1996;143:137–143. doi: 10.1093/oxfordjournals.aje.a008722. [DOI] [PubMed] [Google Scholar]
- Bartsch C, Bartsch H, Fluchter SH, Attanasio A, Gupta D. Evidence for modulation of melatonin secretion in men with benign and malignant tumors of the prostate: relationship with the pituitary hormones. J Pineal Res. 1985;2:121–132. doi: 10.1111/j.1600-079x.1985.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–419. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Brown CE, Warren S, Chute RN, Ryan KJ, Todd RB. Hormonally induced tumors of the reproductive system of parabiosed male rats. Cancer Res. 1979;39:3971–3976. [PubMed] [Google Scholar]
- Buzzell GR. Studies on the effects of the pineal hormone melatonin on an androgen-insensitive rat prostatic adenocarcinoma, the Dunning R 3327 HIF tumor. J Neural Transm. 1988;72:131–140. doi: 10.1007/BF01250236. [DOI] [PubMed] [Google Scholar]
- Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol. 2008;75:484–493. doi: 10.1016/j.bcp.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Lee HJ, Jeong SJ, Lee HJ, Kim HS, Chen CY, Lee EO, Kim SH. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1alpha inactivation in hypoxic PC-3 prostate cancer cells. J Pineal Res. 2011;51:87–93. doi: 10.1111/j.1600-079X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf) 1993;39:163–171. doi: 10.1111/j.1365-2265.1993.tb01769.x. [DOI] [PubMed] [Google Scholar]
- Demir MG, Altintoprak N, Aydin S, Kosemihal E, Basak K. Effect of Transtympanic Injection of Melatonin on Cisplatin-Induced Ototoxicity. J Int Adv Otol. 2015;11:202–206. doi: 10.5152/iao.2015.1094. [DOI] [PubMed] [Google Scholar]
- Denmeade S, Sokoll L, Darlymple S, Rosen D, Gady A. Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific markers PSA, hK2 and PSMA in human prostate cancer models. Prostate. 2003;54:249–257. doi: 10.1002/pros.10199. [DOI] [PubMed] [Google Scholar]
- Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 2003;58:710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Esteban-Zubero E, Alatorre-Jimenez MA, Lopez-Pingarron L, Reyes-Gonzales MC, Almeida-Souza P, Cantin-Golet A, Ruiz-Ruiz FJ, Tan DX, Garcia JJ, Reiter RJ. Melatonin’s role in preventing toxin-related and sepsis-mediated hepatic damage: A review. Pharmacol Res. 2016;105:108–120. doi: 10.1016/j.phrs.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Fang MZ, Ohman-Strickland P, Kelly-McNeil K, Kipen H, Crabtree BF, Lew JP, Zarbl H. Sleep interruption associated with house staff work schedules alters circadian gene expression. Sleep Med. 2015;16:1388–1394. doi: 10.1016/j.sleep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- Gilad E, Laufer M, Matzkin H, Zisapel N. Melatonin receptors in PC3 human prostate tumor cells. J Pineal Res. 1999;26:211–220. doi: 10.1111/j.1600-079x.1999.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Gobbo MG, Dizeyi N, Abrahamsson PA, Bertilsson PA, Masiteli VS, Pytlowanciv EZ, Taboga SR, Goes RM. Influence of Melatonin on the Proliferative and Apoptotic Responses of the Prostate under Normal and Hyperglycemic Conditions. J Diabetes Res. 2015;2015:538529. doi: 10.1155/2015/538529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- Hanisch LJ, Gehrman PR. Circadian rhythm of hot flashes and activity levels among prostate cancer patients on androgen deprivation therapy. Aging Male. 2011;14:243–248. doi: 10.3109/13685538.2011.582528. [DOI] [PubMed] [Google Scholar]
- Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther. 2009;8:337–346. doi: 10.1177/1534735409353332. [DOI] [PubMed] [Google Scholar]
- Hrushesky WJ. Circadian timing of cancer chemotherapy. Science. 1985;228:73–75. doi: 10.1126/science.3883493. [DOI] [PubMed] [Google Scholar]
- Hrushesky WJ, von Roemeling R, Lanning RM, Rabatin JT. Circadian-shaped infusions of floxuridine for progressive metastatic renal cell carcinoma. J Clin Oncol. 1990;8:1504–1513. doi: 10.1200/JCO.1990.8.9.1504. [DOI] [PubMed] [Google Scholar]
- Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang RP, Todo T, Wei YF, Sancar A. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry. 1996;35:13871–13877. doi: 10.1021/bi962209o. [DOI] [PubMed] [Google Scholar]
- Hsu FM, Hou WH, Huang CY, Wang CC, Tsai CL, Tsai YC, Yu HJ, Pu YS, Cheng JC. Differences in toxicity and outcome associated with circadian variations between patients undergoing daytime and evening radiotherapy for prostate adenocarcinoma. Chronobiol Int. 2016;33:210–219. doi: 10.3109/07420528.2015.1130049. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53:422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SS, Yoo YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010;49:60–68. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabasakal L, Sener G, Balkan J, Dogru-Abbasoglu S, Keyer-Uysal M, Uysal M. Melatonin and beta-glucan alone or in combination inhibit the growth of dunning prostatic adenocarcinoma. Oncol Res. 2011;19:259–263. doi: 10.3727/096504011x13021877989748. [DOI] [PubMed] [Google Scholar]
- Karasek M, Pawlikowski M. Antiproliferative effects of melatonin and CGP 52608. Biol Signals Recept. 1999;8:75–78. doi: 10.1159/000014572. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Tasaki H, Misawa I, Chu G, Yamauchi N, Hattori MA. Contribution of testosterone to the clock system in rat prostate mesenchyme cells. Andrology. 2014;2:225–233. doi: 10.1111/j.2047-2927.2013.00161.x. [DOI] [PubMed] [Google Scholar]
- Ko C, Takahashi S. Molecular components of the mammalian circadian clock. Human molecular genetics. 2006;15:271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Wood PA, Hrushesky WJ. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int. 2002;19:237–251. doi: 10.1081/cbi-120002600. [DOI] [PubMed] [Google Scholar]
- Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- Levi F, Benavides M, Chevelle C, Le Saunier F, Bailleul F, Misset JL, Regensberg C, Vannetzel JM, Reinberg A, Mathe G. Chemotherapy of advanced ovarian cancer with 4′-O-tetrahydropyranyl doxorubicin and cisplatin: a randomized phase II trial with an evaluation of circadian timing and dose-intensity. J Clin Oncol. 1990;8:705–714. doi: 10.1200/JCO.1990.8.4.705. [DOI] [PubMed] [Google Scholar]
- Levi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997;350:681–686. doi: 10.1016/s0140-6736(97)03358-8. [DOI] [PubMed] [Google Scholar]
- Liang X, Cheng S, Jiang X, He X, Wang Y, Jiang Z, Hou W, Li S, Liu Y, Wang Z. The noncircadian function of the circadian Clock gene in the regulation of male fertility. J Biol Rhythms. 2013;28:208–217. doi: 10.1177/0748730413486873. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Cazzaniga M, Tancini G, Scardino E, Musci R, Barni S, Maffezzini M, Meroni T, Rocco F, Conti A, et al. Reversal of clinical resistance to LHRH analogue in metastatic prostate cancer by the pineal hormone melatonin: efficacy of LHRH analogue plus melatonin in patients progressing on LHRH analogue alone. Eur Urol. 1997;31:178–181. doi: 10.1159/000474446. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Lupowitz Z, Rimler A, Zisapel N. Evaluation of signal transduction pathways mediating the nuclear exclusion of the androgen receptor by melatonin. Cell Mol Life Sci. 2001;58:2129–2135. doi: 10.1007/PL00000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupowitz Z, Zisapel N. Hormonal interactions in human prostate tumor LNCaP cells. J Steroid Biochem Mol Biol. 1999;68:83–88. doi: 10.1016/s0960-0760(98)00164-2. [DOI] [PubMed] [Google Scholar]
- Marelli MM, Limonta P, Maggi R, Motta M, Moretti RM. Growth-inhibitory activity of melatonin on human androgen-independent DU 145 prostate cancer cells. Prostate. 2000;45:238–244. doi: 10.1002/1097-0045(20001101)45:3<238::aid-pros6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Markt SC, Valdimarsdottir UA, Shui IM, Sigurdardottir LG, Rider JR, Tamimi RM, Batista JL, Haneuse S, Flynn-Evans E, Lockley SW, et al. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control. 2015;26:25–33. doi: 10.1007/s10552-014-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanini V, Simoni M, Chiossi G, Baraghini GF, Velardo A, Baraldi E, Marrama P. Age-related changes in plasma dehydroepiandrosterone sulphate, cortisol, testosterone and free testosterone circadian rhythms in adult men. Horm Res. 1988;29:1–6. doi: 10.1159/000180956. [DOI] [PubMed] [Google Scholar]
- Moretti RM, Marelli MM, Maggi R, Dondi D, Motta M, Limonta P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep. 2000;7:347–351. [PubMed] [Google Scholar]
- Papantoniou K, Castano-Vinyals G, Espinosa A, Aragones N, Perez-Gomez B, Burgos J, Gomez-Acebo I, Llorca J, Peiro R, Jimenez-Moleon JJ, et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015a;137:1147–1157. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- Papantoniou K, Pozo OJ, Espinosa A, Marcos J, Castano-Vinyals G, Basagana X, Juanola Pages E, Mirabent J, Martin J, Such Faro P, et al. Increased and mistimed sex hormone production in night shift workers. Cancer Epidemiol Biomarkers Prev. 2015b;24:854–863. doi: 10.1158/1055-9965.EPI-14-1271. [DOI] [PubMed] [Google Scholar]
- Park JW, Hwang MS, Suh SI, Baek WK. Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J Pineal Res. 2009;46:415–421. doi: 10.1111/j.1600-079X.2009.00678.x. [DOI] [PubMed] [Google Scholar]
- Paroni R, Terraneo L, Bonomini F, Finati E, Virgili E, Bianciardi P, Favero G, Fraschini F, Reiter RJ, Rezzani R, et al. Antitumour activity of melatonin in a mouse model of human prostate cancer: relationship with hypoxia signalling. J Pineal Res. 2014;57:43–52. doi: 10.1111/jpi.12142. [DOI] [PubMed] [Google Scholar]
- Philo R, Berkowitz AS. Inhibition of Dunning tumor growth by melatonin. J Urol. 1988;139:1099–1102. doi: 10.1016/s0022-5347(17)42795-9. [DOI] [PubMed] [Google Scholar]
- Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hrafnkelsson J, Kyyronen P, Linnersjo A, et al. Cancer incidence among 10,211 airline pilots: a Nordic study. Aviat Space Environ Med. 2003;74:699–706. [PubMed] [Google Scholar]
- Pukkala E, Ojamo M, Rudanko SL, Stevens RG, Verkasalo PK. Does incidence of breast cancer and prostate cancer decrease with increasing degree of visual impairment. Cancer Causes Control. 2006;17:573–576. doi: 10.1007/s10552-005-9005-6. [DOI] [PubMed] [Google Scholar]
- Rajagopalan K, Peereboom D, Budd GT, Olencki T, Murthy S, Elson P, McLain D, Bukowski R. Phase II trial of circadian infusion floxuridine (FUDR) in hormone refractory metastatic prostate cancer. Invest New Drugs. 1998;16:255–258. doi: 10.1023/a:1006195815320. [DOI] [PubMed] [Google Scholar]
- Rao D, Yu H, Bai Y, Zheng X, Xie L. Does night-shift work increase the risk of prostate cancer? a systematic review and meta-analysis. Onco Targets Ther. 2015;8:2817–2826. doi: 10.2147/OTT.S89769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rimler A, Culig Z, Levy-Rimler G, Lupowitz Z, Klocker H, Matzkin H, Bartsch G, Zisapel N. Melatonin elicits nuclear exclusion of the human androgen receptor and attenuates its activity. Prostate. 2001;49:145–154. doi: 10.1002/pros.1129. [DOI] [PubMed] [Google Scholar]
- Rimler A, Culig Z, Lupowitz Z, Zisapel N. Nuclear exclusion of the androgen receptor by melatonin. J Steroid Biochem Mol Biol. 2002a;81:77–84. doi: 10.1016/s0960-0760(02)00050-x. [DOI] [PubMed] [Google Scholar]
- Rimler A, Lupowitz Z, Zisapel N. Differential regulation by melatonin of cell growth and androgen receptor binding to the androgen response element in prostate cancer cells. Neuro Endocrinol Lett. 2002b;23(Suppl 1):45–49. [PubMed] [Google Scholar]
- Rodriguez-Garcia A, Mayo JC, Hevia D, Quiros-Gonzalez I, Navarro M, Sainz RM. Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine-induced apoptosis. J Pineal Res. 2013;54:33–45. doi: 10.1111/j.1600-079X.2012.01017.x. [DOI] [PubMed] [Google Scholar]
- Rohr UD, Herold J. Melatonin deficiencies in women. Maturitas. 2002;41(Suppl 1):S85–104. doi: 10.1016/s0378-5122(02)00017-8. [DOI] [PubMed] [Google Scholar]
- Sainz RM, Mayo JC, Tan DX, Leon J, Manchester L, Reiter RJ. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate. 2005;63:29–43. doi: 10.1002/pros.20155. [DOI] [PubMed] [Google Scholar]
- Sainz RM, Reiter RJ, Tan DX, Roldan F, Natarajan M, Quiros I, Hevia D, Rodriguez C, Mayo JC. Critical role of glutathione in melatonin enhancement of tumor necrosis factor and ionizing radiation-induced apoptosis in prostate cancer cells in vitro. J Pineal Res. 2008;45:258–270. doi: 10.1111/j.1600-079X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- Sampson SR, Lupowitz Z, Braiman L, Zisapel N. Role of protein kinase Calpha in melatonin signal transduction. Mol Cell Endocrinol. 2006;252:82–87. doi: 10.1016/j.mce.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Sanchez-Barcelo EJ, Mediavilla MD, Tan DX, Reiter RJ. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- Shiu SY, Law IC, Lau KW, Tam PC, Yip AW, Ng WT. Melatonin slowed the early biochemical progression of hormone-refractory prostate cancer in a patient whose prostate tumor tissue expressed MT1 receptor subtype. J Pineal Res. 2003;35:177–182. doi: 10.1034/j.1600-079x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Shiu SY, Leung WY, Tam CW, Liu VW, Yao KM. Melatonin MT1 receptor-induced transcriptional up-regulation of p27(Kip1) in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-kappaB): potential implications on prostate cancer chemoprevention and therapy. J Pineal Res. 2013;54:69–79. doi: 10.1111/j.1600-079X.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- Siu SW, Lau KW, Tam PC, Shiu SY. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106–122. doi: 10.1002/pros.10098. [DOI] [PubMed] [Google Scholar]
- So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EJ, Won G, Lee J, Lee S, Kim SH. Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic Properties of Melatonin in Hypoxic PC-3 Prostate Cancer Cells. J Cancer. 2015;6:19–28. doi: 10.7150/jca.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- Tai SY, Huang SP, Bao BY, Wu MT. Urinary melatonin-sulfate/cortisol ratio and the presence of prostate cancer: A case-control study. Sci Rep. 2016;6:29606. doi: 10.1038/srep29606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CW, Chan KW, Liu VW, Pang B, Yao KM, Shiu SY. Melatonin as a negative mitogenic hormonal regulator of human prostate epithelial cell growth: potential mechanisms and clinical significance. J Pineal Res. 2008;45:403–412. doi: 10.1111/j.1600-079X.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- Tam CW, Mo CW, Yao KM, Shiu SY. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: implications for prostate cancer chemoprevention. J Pineal Res. 2007;42:191–202. doi: 10.1111/j.1600-079X.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Tam CW, Shiu SY. Functional interplay between melatonin receptor-mediated antiproliferative signaling and androgen receptor signaling in human prostate epithelial cells: potential implications for therapeutic strategies against prostate cancer. J Pineal Res. 2011;51:297–312. doi: 10.1111/j.1600-079X.2011.00890.x. [DOI] [PubMed] [Google Scholar]
- Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- Toma JG, Amerongen HM, Hennes SC, O’Brien MG, McBlain WA, Buzzell GR. Effects of olfactory bulbectomy, melatonin, and/or pinealectomy on three sublines of the Dunning R3327 rat prostatic adenocarcinoma. J Pineal Res. 1987;4:321–338. doi: 10.1111/j.1600-079x.1987.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Xi SC, Siu SW, Fong SW, Shiu SY. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate. 2001;46:52–61. doi: 10.1002/1097-0045(200101)46:1<52::aid-pros1008>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Xi SC, Tam PC, Brown GM, Pang SF, Shiu SY. Potential involvement of mt1 receptor and attenuated sex steroid-induced calcium influx in the direct anti-proliferative action of melatonin on androgen-responsive LNCaP human prostate cancer cells. J Pineal Res. 2000;29:172–183. doi: 10.1034/j.1600-079x.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K, He PJ, Yamauchi N, Hashimoto S, Hattori MA. Up-regulation of circadian clock gene Period 2 in the prostate mesenchymal cells during flutamide-induced apoptosis. Mol Cell Biochem. 2010;335:37–45. doi: 10.1007/s11010-009-0238-7. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Stevens RG, Hoffman AE, Fitzgerald LM, Kwon EM, Ostrander EA, Davis S, Zheng T, Stanford JL. Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res. 2009;69:9315–9322. doi: 10.1158/0008-5472.CAN-09-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zheng T, Stevens RG, Zhang Y, Boyle P. Does “clock” matter in prostate cancer? Cancer Epidemiol Biomarkers Prev. 2006;15:3–5. doi: 10.1158/1055-9965.EPI-05-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]