Abstract
A complementary deoxyribonucleic acid (cDNA) and the corresponding gene segment encoding a member of the 70-kDa heat shock protein (Hsp70) family have been cloned and sequenced from Locusta migratoria, the African migratory locust. These animals are noted for their thermotolerance, which can exceed temperatures of 50°C. Conceptually translated, the sequence shows a 654-residue protein with theoretical molecular weight of 71.4 kDa, which more closely resembles the mammalian Hsp70 (84–85% similarity) than Hsp70 from other insects, with ∼75% similarity to the sequence from the fruit fly. Comparisons of cDNA and genomic sequences show that the gene contains 2 introns, a 245-bp intron located in the 5′ untranslated region and a 91-bp intron in the coding region. Transcript abundance, as estimated by Northern blot analysis and reverse transcription–polymerase chain reaction, shows that heat shock treatment (45°C for 3 hours) does not elevate hsp70 messenger ribonucleic acid levels in fat bodies or in neural tissues. Immunological assays of Hsp70 show that the protein is constitutively expressed, with a modest, ∼2-fold induction after a 3-hour heat shock in fat body preparations. Although this sequence could be an hsc70 rather than an hsp70, it was the only cDNA isolated from heat-shocked tissue. Whatever the formal designation, such modest induction and constitutive expression may be ideally suited as an adaptation to the locust's chronic exposure to heat shock temperatures and the consequent demand for chaperone proteins.
INTRODUCTION
Heat stress as well as cold stress, anoxia, heavy metals, and other stresses typically enhance the production of heat shock proteins (Hsps) of ∼20 kDa to ∼90 kDa, grouped into several families based on size. One family with molecular weights close to 70 kDa contains the best-known Hsp, Hsp70, and its corresponding complementary deoxyribonucleic acid (cDNA) has been isolated from a variety of organisms. These sequences have been traditionally divided into 2 groups based on their expression pattern: those with low expression under nonstress conditions, but which can be quickly induced under stress conditions (Hsp70), and a second group consisting of sequences that are constitutively expressed under nonstress conditions, but with little or no induced expression after heat shock (Hsc70) (Denlinger et al 2001). Both Hsp70 and Hsc70 act as molecular chaperones helping to stabilize proteins during folding, and both participate in reactions to remove abnormal cellular proteins (Terlecky et al 1992). Because heat shock is associated with the misfolding of proteins, the presence of these proteins, especially Hsps, is correlated with the acquisition of thermotolerance (Loomis and Wheeler 1980; Li and Werb 1982).
African migratory locusts are among the most thermotolerant of metazoans and are routinely exposed to temperatures up to 50°C in their native habitat. In addition to their intrinsic thermotolerance, they have a modest, rapid heat-hardening response, also known as inducible thermotolerance, as evidenced by enhanced survival and the protection of flight rhythms at elevated temperatures after heat shock treatment (Whyard et al 1986; Robertson et al 1996; Wu et al 2001). Although it might be assumed that Hsp70 levels, which are thought to contribute to thermotolerance (Feder and Krebs 1997), might be dramatically induced by heat stress in this species, recent evidence brings this supposition into question. Certain strains of Drosophila species, either selected for heat resistance or originating from subtropical Africa, have decreased messenger ribonucleic acid (mRNA) and protein expression of hsp70 after heat shock compared with less thermotolerant strains (Bettencourt et al 1999; Zatsepina et al 2001) and similar, if not lower, levels of constitutively expressed forms (Zatsepina et al 2001). The intent of this study was to facilitate the understanding of the high intrinsic thermotolerance of locusts, long adapted to the rigors of the equatorial continental climate, by cloning Hsp70 cDNA and its corresponding gene to monitor transcript and protein expression levels after heat shock stress.
MATERIALS AND METHODS
Animals
Adult Locusta migratoria L. were obtained from a crowded colony maintained at 25–30°C on a 16:8 hour light-dark cycle (Chinzei et al 1982). The animals were fed daily with wheat seedlings, carrots, and dry food (a mixture of bran and milk powder).
Cloning a cDNA of the hsp70 family
Adult female locusts were heat shocked for 3 hours at 45°C and allowed to recover for 1 hour at 25°C. Total RNA was isolated from 100 mg of dissected fat bodies from a pool of several locusts by using Trizol reagent (GIBCO BRL, Rockville, MD, USA). First-strand cDNA was made using oligo dT17-CSX (5′-GACTCGAGTCGACATCGAT17-3′) according to the cDNA synthesis kit (Stratagene Corp, LaJolla, CA, USA).
Degenerate oligonucleotide primers were designed by alignment of the hsp70 nucleotide (nt) sequences derived from humans, flies, rats, and yeast (Homo sapiens GenBank accession no. U56725; D auraria, AJ001365; Rattus norvegicus, M11942; and Saccharomyces cerevisiae, M25395). PCR was performed using the designed forward primer 5′-GGGCACCACITACTCCTG (Fig 1) and the oligo dT17-CSX race anchor primer. Thirty-five cycles of amplification were performed: 1 minute at 94°C, 1 minute at 52°C, and 3 minutes at 72°C, followed by a 5-minute extension at 72°C, using Taq DNA polymerase under standard reaction conditions. A sample of the amplified product (1 μL) was used in a second, nested PCR reaction, using the same thermocycle parameters and the same forward primer, but with another nested internal reverse primer that was degenerate 5′-TCRAAYGTIACYTCIATYTGIGG (where I = inosine, R = A + G, and Y = C + T). The resulting amplified fragment was cloned in pBluescript SK+ and sequenced in both directions.
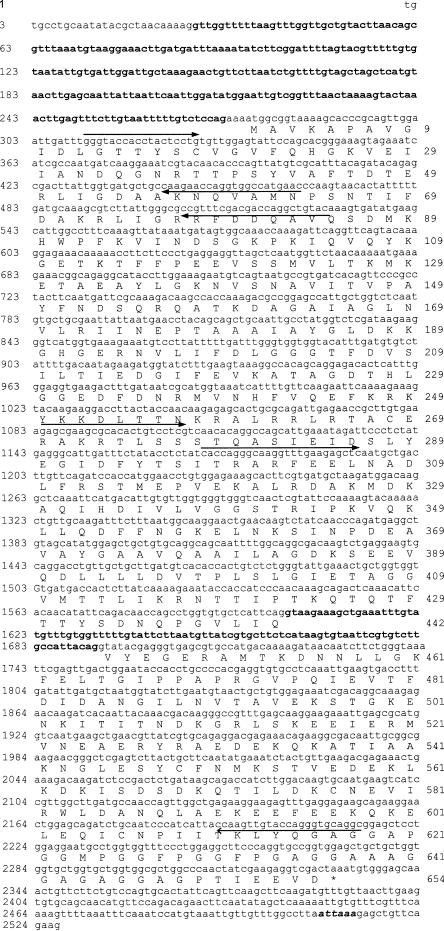
Fig 1.
Nucleotide sequence and deduced amino acid sequence of the Lmhsp70 family member (accession no. AY178988). The 245-bp intron in the 5′ untranslated region and the 91-bp intron in the open reading frame are shown in bold, lower-case letters. The polyadenylation signal sequence, ATTAAA, is shown in bold, italic, lower-case letters, and the asterisk denotes the translational stop codon. Closed arrows indicate the location and direction of gene-specific primers used for cloning the complementary deoxyribonucleic acid or gene, including the degenerate forward primer at position 311 bp, LEPrimerA at 464 bp, LERev at 528 bp, 5′Lmhsp70p1 at 1084 bp, and 5′Lmhsp70p5 at 1169 bp (see Materials and Methods), with the open arrow indicating the sequence presumably recognized by the multiply degenerate, second-nested reverse primer at position 2214 bp
The 5′-end of the cDNA sequence was extended by PCR, using a λgt10 cDNA library template with the vector-specific primer 5′-AGCAAGTTCAGCCTGGTTAG and a primer based on the cDNA fragment LERev: 5′-GTACAGCCTGGTCGTCGAAACG (Fig 1). After 5 minutes of initial denaturation at 94°C, 30 cycles were done at 94°C for 60 seconds, 53°C for 60 seconds, 72°C for 90 seconds, and with 7 minutes of further extension at 72°C. The amplified product was used as a template with the same vector-specific primer but with a second hsp70-specific primer, LEPrimerA: 5′-GTTCATGGCCACCTGGTTCTTG, using identical amplification conditions. After separating the amplification products on a 1.2% agarose gel, the DNA in the gel slice was isolated using the QIAquick gel extraction kit (Qiagen GmbH, Hilden, Germany) and cloned into the PCR4-TOPO vector (Invitrogen, Carlsbad, California, USA). The insert was verified by sequencing both strands of the DNA and designated as a member of the hsp70 gene family (occasionally referred to as putative hsp70 for simplicity).
The 3′-end of the cDNA sequence was cloned by 3′-RACE (rapid amplification of cDNA ends; Kawasaki et al 1989). First-strand cDNA was synthesized from total RNA using the SMART® RACE cDNA amplification kit (Clontech Laboratories Inc, Palo Alto, CA, USA). PCR was performed using this first-strand cDNA and the universal primer mix from the kit and a gene-specific primer 5′LMhsp70P1: 5′-GAGCGAAGCGCACACTGTCCTCG (Fig 1). A second amplification was performed with 0.2 μL of the amplified product as template by using the nested universal kit primer and a second gene-specific primer, 5′LMhsp70P5: 5′-CACCAGGGCAAGGTTTGAAGAGCTC, at 94°C for 5 minutes and 30 cycles of 94°C for 50 seconds, 57°C for 50 seconds, 72°C for 80 seconds, followed by 72°C for 20 minutes. The nested PCR product was directly ligated into the PCR4-TOPO vector and sequenced as described for the 5′-end fragment.
Amplification of genomic DNA
Adult locust genomic DNA was isolated using the DNeasy Tissue Kit (Qiagen) and used as a template for PCR amplifications with Taq DNA polymerase (MBI Fermentas, Hanover, MD, USA) under standard conditions and with primers derived from the cDNA sequence. Three sets of primer pairs (2350LP2: 5′-TGTGCCTGCAATATACGCTAAC with 2350RP3: 5′-ATGGCCACCTGGTTCTTGGC; LG41/42intron: 5′-GTAGCTAGCTCATGTAACTTGAG with 2350RP5: 5′-GCCTTGTCCATCTTAGCATCACG; and InvRP2: 5′-GATAAGAAGGGTCATGGTGAAAGAAATG with HSP70RE: 5′-CTGAACAGCTCTTTAATTAAGGC) were used to amplify the putative hsp70 genomic sequence. Amplified fragments were cloned into PCR4-TOPO and sequenced on both strands.
All sequence translations and analyses were performed using the ExPasy translation tool (Swiss Institute of Bioinformatics, Basel, Switzerland) and aligned using the ClustalW and Align programs on that Web site. Sequences in GenBank were searched using the BLAST sequence similarity algorithm on the National Center for Biotechnology Information (NCBI) Web site.
Transcript accumulation
For Northern blots, total fat body RNA was isolated from locusts that were either not heat shocked or heat shocked for 3 hours at 45°C, with 1 hour of recovery at 25°C. RNA was isolated using Trizol reagent and then electrophoresed (12 μg in each lane) on a 1.4% formaldehyde–agarose gel and subsequently capillary transferred to Hybond N+ nylon membrane (Amersham-Pharmacia, Quebec, Canada). The blot was hybridized for 16 hours at 42°C in 50% formamide, 5× standard saline citrate (SSC), 5× Denhardt, 0.5% sodium dodecyl sulfate (SDS) solution, and 200 μg of sheared salmon sperm DNA (Sambrook et al 1989). The locust 1681-bp hsp70 cDNA fragment was labeled with α-32P deoxycytidine triphosphate (dCTP) to 5 × 108 cpm/mL using DNA-labeling beads (Amersham-Pharmacia). Two high-stringency washes were done in 0.1× SSC or 0.5% SDS at 65°C for 20 minutes each. Detection of the signal was done using X-Omat film for 4 days at −80°C with an intensifying screen. The blot was reprobed with Drosophila alpha-tubulin (Mischke and Pardue 1982) to assess loading in each lane.
Reverse transcription–PCR (RT-PCR) analysis was also used to assess putative hsp70 mRNA levels. Mature (≥3 weeks old) adult female locusts were heat shocked at 45°C for 3 hours and allowed to recover for 1 hour (HS-1hR), and control females were kept at 25°C (No HS). Fat bodies and ganglia were dissected in Ringers, and total RNA from these tissues was isolated as described previously. The RNA (200 ng, 100 ng/μL) was used as an RT-PCR template with an hsp70 gene–specific primer set 5′LMhsp70P1 and 3′LMhsp70P2: 5′-GAGTTGTCATCACACCACCAGCAG. A control sequence from the locust insulin–related peptide gene (LIRP; GenBank accession no. X17024), which encodes a transcript that does not vary in abundance either spatially or temporally (Kromer-Metzger et al 1994; Zhou et al 2002), was amplified using a 1-step RT-PCR kit (Clontech) and the primers described by Zhou et al (2002). The amplified products were electrophoresed on 1.2% agarose gels and stained with ethidium bromide, and band intensity was estimated by the GeneGenius Gel Documentation and Analysis System (Syngene, Cambridge, UK).
Immunological and protein analyses
Mature adult female locusts (≥3 weeks old) were transferred to 45°C for 3 hours (HS-NoR), allowed to recover for 1 hour at 25°C (HS-1hR), or kept at 25°C as controls (No HS) before dissection. Fat bodies from at least 2 locusts were pooled and homogenized in the extraction reagent, with protease inhibitors provided by the Hsp70 EIA kit (Stressgen Biotechnologies Corp, Victoria, BC, Canada catalogue number SPA-812), and centrifuged at 21 000 × g for 10 minutes at 4°C. After collection of the supernatant, proteins were quantified by dye binding (BCA kit, Pierce, Rockford, IL, USA), and 250 μg of protein was electrophoresed for 1 hour on polyacrylamide gels (with 5% stacking and 8% resolving gels). After transfer onto a nylon membrane, bands were detected using a chemiluminescent substrate, as described by the manufacturer (POD, Boehringer Mannheim, Ingelheim, Germany), and anti-human Hsp70 antibody (Stressgen Biotechnologies Corp.).
Fat body proteins, as described above, were also used for enzyme-linked immunosorbent assay (ELISA), according to the Hsp70 EIA kit protocol. The abundance of Hsp70 cross-reacting protein from each sample was determined from a set of standard curves.
Newly synthesized proteins were identified by the culture of dissected fat body and thoracic ganglia at 30°C and 45°C in 35S-methionine, as described previously (Whyard et al 1986), except that the leucine concentration was increased to 5.0 mM and the methionine concentration was decreased to 0.03 mM in the incorporation medium. Fat body cultures were labeled for ∼1 hour, whereas the ganglia were labeled for 5 hours because of the low synthetic rate of the latter tissue; then, gel electrophoresis and analysis were performed as described.
RESULTS
Cloning and sequencing of the hsp70 family member cDNA and genomic DNA
Previous attempts to clone hsp70 cDNA using primers designed solely on the basis of fruit fly and mosquito sequences, as well as hybridization of the D melanogaster hsp70 cDNA sequence with genomic and cDNA libraries, were unsuccessful (not shown), suggesting that the locust hsp70 sequence did not have sufficient identity with these previously isolated insect cDNAs. With “near-universal” degenerate primers and a first-strand cDNA template derived from heat-shocked tissues, however, a 1681-bp cDNA fragment was amplified, subcloned, and sequenced. Conceptual translation showed that this fragment had 75–79% identity with mammalian Hsp70 sequences (eg, M11717) and 80% identity with the brine shrimp sequence (AAL27404) but only 68% identity with D melanogaster hsp70 (NM080187), explaining the initial difficulty in the cDNA cloning.
The sequence of the cDNA fragment was used to obtain 5′ and 3′ flanking sequences using a cDNA library and RACE, respectively. After assembly of the 3 sequences, a 2350-bp cDNA sequence was obtained (Fig 1). It had an open reading frame (ORF) of 1962 bp, encoding a 654–amino acid protein with a theoretical molecular weight of 71.4 and a pI of 5.4. Comparisons with other locust cDNAs (not shown) indicated that there were no unusual amino acid codon preferences in the putative hsp70 cDNA from locusts.
A genomic fragment, corresponding to the fully assembled cDNA, was amplified using primers derived from the 5′ and 3′ terminal sequences of the cDNA, subcloned, sequenced, and designated as a gene fragment from an Lmhsp70 family member. Much of the gene, with the exception of the promoter region, has been isolated since a portion of the 5′ untranslated region (UTR), as well as the putative poly(A) signal sequence, ATTAAA, at positions 2508–2513, was identified. Comparison of the cDNA and genomic sequence revealed 2 introns. A small phase 0 intron of 91 bp interrupts the ORF between residues 442 and 443, and a larger intron of 245 bp is located in the 5′-UTR, just 3 nts upstream of the translational start site (Fig 1). Analysis of the intron sequences using Web-based algorithms indicated that they conformed to consensus intron identification rules. When the locust amino acid sequence was aligned with intron-containing hsp70 sequences from other organisms, the location of the intron interrupting the ORF appeared to be novel (Fig 2).
Fig 2.
Intron positions of Locusta migratoria Hsp70 family member aligned with Hsp70s from organisms known to have intervening sequences interrupting the coding region of the corresponding gene. Intron sites are indicated by shading over the amino acid where the site interrupts the codon or over 2 amino acids where the site falls between 2 codons. Conserved residues are indicated with asterisks, whereas similar residues are indicated by dots beneath the sequence. Gaps are inserted to optimize alignments and are shown by dashes. The sequences are available from GenBank under the accession numbers shown in brackets. The fungus Neurospora crassa (Nc) has 4 introns in hsp70's coding region (U10443), the pathogenic fungus Trichophyton rubrum (Tr) has 2 (AF052391), and the aquatic fungus Blastocladiella emersonii (Be) has 1 intron (L22497). The worm Caenorhabditis elegans (Ce) has a number of hsp70s with introns, only 2 of which are shown here; there are 3 introns in hsp-1 (formerly hsp70A; M18540) and 5 introns in hsp70-4 (AF000264)
Transcript accumulation after heat shock
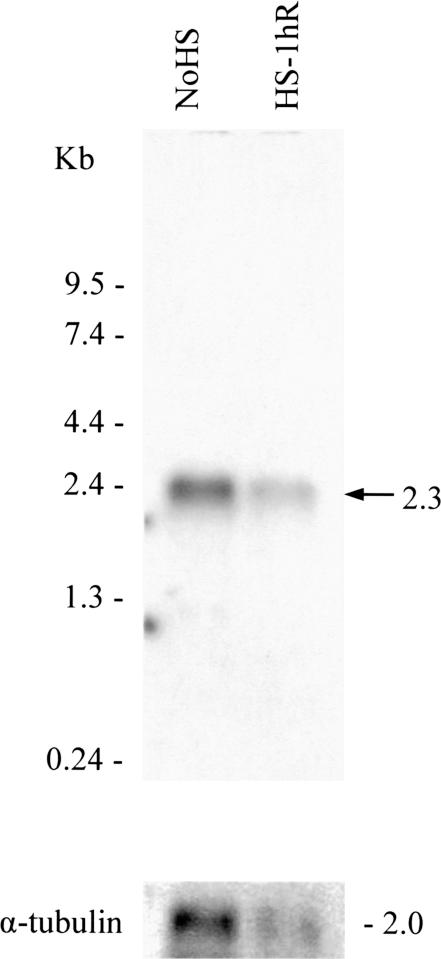
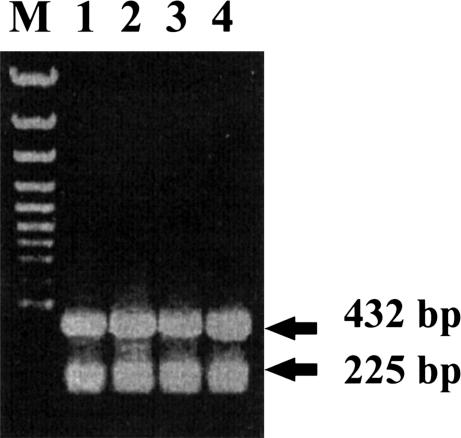
To examine the mRNA accumulation of the hsp70 family member after heat shock, Northern blots of fat body RNA that had been isolated from control or heat shock–treated locusts were hybridized with the 1681-bp locust cDNA fragment. A single 2.3-kb band was seen, and after correcting for loading differences in several blots, it appeared that this band was of similar intensity in each heat shock or non–heat shock treatment sample (Fig 3). To partially quantify the accumulation of the putative hsp70 mRNA, RT-PCR analysis, using first-strand cDNA prepared from mRNA isolated from heat-shocked fat bodies and ganglia, was done. Amplified products of an expected size, 432 bp, were obtained, and the abundance of these was compared with the amplified 225-bp LIRP fragments, used as an internal control (Fig 4). Levels of the putative hsp70 mRNA were approximately the same in both the tissues analyzed. After heat shock, the relative abundance of mRNA, normalized to non–heat shock controls did not significantly increase (with mean ratios of 1:1.09 ± 0.05 in fat bodies and 1:1.01 ± 0.01 in ganglia).
Fig 3.
Transcript accumulation of the Lmhsp70 family member. Total ribonucleic acid (RNA) was isolated from fat bodies obtained from control animals, non–heat-shocked animals, and those that had been heat shocked at 45°C for 3 hours, with 1 hour of recovery. RNAs (approximately12 μg) were loaded in each lane, and after electrophoresis the blot was hybridized with a 1681-bp putative locust hsp70 complementary ribonucleic acid fragment, labeled with [α-32P]deoxycytidine triphosphate. The northern blot was reprobed with α-tubulin. A representative gel of the 3 that were run is shown
Fig 4.
A typical 1.2% agarose gel showing amplified products from template ribonucleic acids (RNAs) prepared from adult female locusts. RNAs were isolated from fat bodies with no heat shock (1, No-HS) or fat bodies with heat shock for 3 hours at 45°C, with 1 hour of recovery (2, HS-1hR), as well as ganglia with no heat shock (3, No-HS) or that had been heat shocked for 3 hours at 45°C, with 1 hour of recovery (4, HS-1hR). M indicates the size marker lane. The 432-bp putative hsp70–amplified products and the 225-bp control LIRP products are shown
Immunological analysis
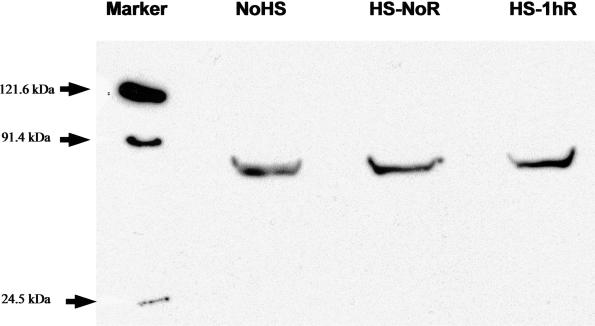
To assess the levels of Hsp70 in selected locust tissues, total proteins were isolated as described. Initially, Western blots containing proteins from several locust tissues were performed using antibodies prepared against D melanogaster Hsp70 (kindly provided by Dr R. Tanguay, Université de Laval, Quebec, Canada), but only faint cross-reactivity was observed (results not shown). Subsequently, anti-human Hsp70 antibody was used on similar blots containing locust fat body samples (100 mg total protein). All 3 samples, control (no HS) and heat shock with or without recovery at 25°C (HS-NoR and HS-1hR, respectively) showed a clear protein band at ∼70 kDa (Fig 5), indicating that the locust protein and human Hsp70 share epitopes and suggesting that the antiserum would be useful to quantify levels of Hsp70 cross-reacting material in locusts. Indeed, the region of the peptide fragment of the human Hsp70 used in the generation of the commercial antiserum shows 93% identity with the translated sequence of our locust cDNA.
Fig 5.
Western blot analysis of Hsp70 cross-reacting proteins from fat bodies. Proteins were isolated from fat bodies with no heat shock (No-HS), with 3 hours of heat shock at 45°C but with no recovery (HS-NoR), and with 3 hours of heat shock at 45°C followed by 1 hour of recovery at 25°C (HS-1hR). The first lane contains the size markers, with arrows indicating the position on the gel. The putative Hsp70 band from locusts is shown in all 3 lanes
ELISA assays with the anti-human Hsp70 antibody showed that levels of the locust protein were modestly increased in fat bodies and ganglia. In fat bodies, for example, there was a 1.6-fold (range: 1.3- to 2-fold) increase after 3 hours of heat shock and a 2.3-fold (range: 2.2- to 2.3-fold) increase after the same heat shock treatment followed by a 1-hour recovery period, relative to non–heat shock controls. When fat body or thoracic ganglia are cultured in radiolabled methionine at 30°C or 45°C, followed by the separation of the newly synthesized proteins on polyacrylamide gels, there was a substantial increase in the labeled band migrating at ∼70 kDa in both tissues after heat shock treatment, compared with the 30°C controls (Whyard et al 1986; results not shown). Also observed was the continued synthesis of a wide range of proteins, whose presence appeared to be unaffected by the 45°C treatment, as has been previously reported (Whyard et al 1986; Baldaia et al 1987).
DISCUSSION
Hsp70 is a stress protein that plays a critical role in normal cellular function and in recovery and survival after heat shock (Feder and Krebs 1997; Mayer and Bukau 1998). As a molecular chaperone, Hsp70 prevents protein aggregation and refolds damaged protein, thus presumably facilitating the survival of organisms under stressful conditions. In accordance with this crucial role, Hsp70 has been found in organisms as diverse as bacteria, fungi, humans, and insects, and sequences encoding these proteins have been isolated from several dozen different species.
It was anticipated that the locust hsp70 cDNA sequence could be obtained by using redundant PCR primers derived from the previously sequenced cDNAs from insects or, alternatively, by using reduced stringency hybridization to the D melanogaster sequence, but neither of these approaches was successful. Rather, the putative locust hsp70 cDNA was cloned using cDNA templates from heat-shocked locust tissues and near-universal PCR primers with subsequent 5′ and 3′ extensions to obtain full-length sequences. Comparison of the sequence to known hsp70 cDNAs confirmed its identity as a member of the hsp70 family but also revealed, perhaps surprisingly, that, overall, the encoded residues were more similar to hsp70 from mammals and brine shrimp (eg, 84% similarity to both human, M11717, and shrimp, AAL27404, hsp70s) than to hsps from flies (75–79% similarity). Comparisons with the cognates are more difficult to assess because they vary widely; even hsc70s from a single organism, D melanogaster, have only 61–87% similarity among themselves.
In another unusual feature of the sequence, the coding region of our gene contains 2 introns, a 91-bp intervening sequence in the amino acid coding–region and a 245-bp sequence in the 5′-UTR, just 3 nts 5′ to the translational start. Although it appears that some hsp70 gene homologues have introns in the 5′-UTR, including hsp70 from the lung pathogen Pneumocystis carinii (Stedman et al 1998), hsp70-3 from the rat (Friedrich et al 1998), and hspA1L from human testicular cells, the latter with an intron located 13 bp upstream of the ATG (Ito et al 1998), introns in the coding region are more remarkable. In certain worm and fungal species, however, hsp70 genes are known to have introns that interrupt the coding region. The fungus Neurospora crassa has 4 introns in the coding region (Kapoor et al 1995), one of which is in a position similar to that of one of the 2 introns found in hsp70 from the pathogenic fungus Trichophyton rubrum (Rezaie et al 2000). The nematode Caenorhabditis elegans has 3 introns in hsp-1 (formerly hsp-70A; Snutch et al 1988) and 5 in hsp70-4 (AF000264), with 2 of these in positions similar to that in fungal species. The gene from the aquatic fungus Blastocladiella emersonii contains 1 intron (Stefani et al 1995). The position of the phase 0 intron in the locust ORF, however, appears unique and could therefore have been derived from a relatively recent insertion event, rather than reflect the retention of an ancient intron (Tyshenko and Walker 1997).
L migratoria is native to the semiarid regions of equatorial Africa, where the average air temperature is 32°C and the exposed sand temperature can be as high as 55°C (Uvarov 1977). Locusts have a broad heat shock temperature of 39–50°C, with an optimum induction of new Hsp70 synthesis in fat body at 43–45°C (Whyard et al 1986). The neural tissue also shows a clear heat shock–hardening response (Wu et al 2001), and new Hsp70 synthesis is seen after culture of thoracic ganglia at 45°C. Interestingly, although newly synthesized Hsp70 appears after heat shock in cultured locust fat bodies, epidermis, and ganglia (Whyard et al 1986; Baldaia et al 1987; data not shown), this emerges against a background of constitutive mRNA and protein synthesis (Figs 3–5), so that the overall increase in the levels of Hsp70 cross-reacting material after heat shock is only ∼2-fold. This compares with increases that vary considerably in different organisms, such as the 1- to 4-fold (isoform dependent) induction of Hsp70 in the parasite Trypanosoma cruzi (Requena et al 1992), the “slight” to 10-fold increase of Hsp70, depending on the tissue type, in humans (Oehler et al 2001), and the spectacular 1000-fold increase in hsp70 transcripts reported in a laboratory stock of D melanogaster (Lindquist 1986). Curiously, it appears that transcript induction also can vary depending on the particular assay; the modest 2- to 6-fold induction of C elegans hsp-1 (Snutch et al 1988) appears as a “significant induction” after the transfection of expression constructs consisting of the hsp-1 promoter region ligated to a reporter sequence, as in the case of hsp70-4 (J. Tyson and D. Ballie, personal communication). In Drosophila species selected for thermotolerance or collected from subtropical regions, there is a reduced expression of hsp70 mRNA and protein after heat shock compared with nonselected or temperate strains (Bettencourt et al 1999; Zatsepina et al 2001), prompting Zatsepina et al (2001) to suggest that high levels of Hsp70, if chronically expressed, are detrimental to the organism and would be negatively selected.
The increase in Hsp70 cross-reacting material, albeit modest, combined with the knowledge that this cDNA fragment corresponds to the most abundant mRNA hsp70–related sequence in heat-shocked animals (the original fragment was isolated more than once, not shown) argues that the Lmhsp70 family member encodes Hsp70. The presence of 2 introns in the gene, however, could suggest that the sequence is an hsc. Splicing of intervening sequences is inhibited at heat shock temperatures in certain organisms (Yost and Linquist 1988), but this, apparently, is not universally applicable because the splicesome complex is significantly thermotolerant in B emeronii, a fungus with an intron-containing hsp70 (Stefani and Gomes 1995). Other intron-containing Hsp genes, such as Drosophila hsp83 (Holmgren et al 1979), and the vertebrate hsp90 and hsp105 genes (Ali et al 1996; Shen et al 1997; Yasuda et al 1999), as well as the list of hsp70s in Fig 2, also bring this reason for the general lack of introns in hsp70 into question. Rather, it is possible that the need to respond quickly to a stress-generated requirement for chaperones in most organisms would have resulted in the evolution of more streamlined genes, which, if they contained introns, would have been reduced in size and number by selection. Locusts have a genome that is twice the size of the human genome, and large introns (∼0.5–9 kb) have been found in every gene described, with the exception of the currently isolated Lmhsp70 family member. At only 91 bp, one of the 2 small introns described here is the smallest reported in Locusta. Unlike D melanogaster, and more similar to mammals, there appears to be no translational control of non-hsp proteins in the locust heat shock response at 45°C; other proteins, even those encoded by genes containing huge introns, continue to be synthesized and secreted at heat shock temperatures (Whyard et al 1986; Baldaia et al 1987; results not shown). It is reasonable that intrinsic thermotolerance must be accompanied by the retention of splicesome function, and, in addition, according to Zatsepina et al (2001), there may be a disadvantage to the chronic production of high levels of Hsp70. Thus, the locust's adaptation to the African climate, where temperatures frequently exceed 40°C and migratory flight can further increase thoracic temperatures by 6–10°C (Weis-Fogh 1956), may be facilitated by the constitutive production of Hsp70 and by increasing new synthesis at 45°C, with a resulting modest increase in Hsp70 (or alternatively, Hsc70) abundance above its constitutive level. Presumably, such production satisfies the near-constant demand for chaperone proteins while continuing the synthesis and secretion of other proteins.
Acknowledgments
We thank Shutang Zhou and Ping Yang for their excellent care of the locust colony, Dr R. M. Tanguay for the Drosophila antibodies, Mr G. Molaei and Dr A. Lange for the cDNA library template used to extend the cDNA, and Drs D. Ballie and J. Tyson for sharing their unpublished data on C elegans. A special thanks goes to Dr G.R. Wyatt and an anonymous referee for their comments on the manuscript. This work was supported by a Natural Sciences and Engineering Research Council of Canada/Canadian Institutes of Health Research (NSERC/CIHR) grant to R.M.R. and an NSERC grant to V.K.W.
REFERENCES
- Ali A, Krone PH, Pearson DS, Heikkila JJ. Evaluation of stress-inducible hsp90 gene expression as a potential molecular biomarker in Xenopus laevis. Cell Stress Chaperones. 1996;1:62–69. doi: 10.1379/1466-1268(1996)001<0062:eosihg>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldaia L, Maisonhaute C, Porcheron P, Best-Belpomme M. Effect of heat shock on protein synthesis in Locusta migratoria epidermis. Arch Insect Biochem Physiol. 1987;4:225–231. [Google Scholar]
- Bettencourt BR, Feder ME, Cavicchi S. Experimental evolution of Hsp70 expression and thermotolerance in Drosophila melanogaster. Evolution. 1999;53:484–492. doi: 10.1111/j.1558-5646.1999.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Chinzei Y, White BN, Wyatt GR. Vitellogenin mRNA in locust fat body: identification, isolation and quantitative changes induced by juvenile hormone. Can J Biochem. 1982;60:243–251. doi: 10.1139/o82-029. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Rinehart JP, and Yocum GD 2001 Stress proteins: A role in insect diapause. In: Insect Timing: Circadian Rhythmicity to Seasonality, ed Denlinger DL, Giebultowicz J, Saunders DS. Elsevier Sciences B. V., Amsterdam, 155–171. [Google Scholar]
- Feder ME, Krebs RA. Ecological and evolutionary physiology of heat shock proteins and the stress response in Drosophila: complementary insights from genetic engineering and natural variation. EXS. 1997;187:155–173. doi: 10.1007/978-3-0348-8882-0_9. [DOI] [PubMed] [Google Scholar]
- Holmgren R, Livak K, Morimoto R, Freund R, Meselson M. Studies of cloned sequences from four Drosophila heat shock loci. Cell. 1979;18:1359–1370. doi: 10.1016/0092-8674(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Curle CA, Runham C. The hsp70 gene family of Neurospora crassa: cloning, sequence analysis, expression, and genetic mapping of the major stress-inducible member. J Bacteriol. 1995;177(1):212–221. doi: 10.1128/jb.177.1.212-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer-Metzger E, Lagueux M. Expression of the gene encoding an insulin-related peptide in Locusta (Insecta, Orthoptera) Eur J Biochem. 1994;221:427–434. doi: 10.1111/j.1432-1033.1994.tb18755.x. [DOI] [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Loomis WF, Wheeler SA. Heat shock responses in Dictyostelium. Dev Biol. 1980;79:399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperone systems: diversity of cellular functions and mechanisms of action. J Biol Chem. 1998;379:261–268. [PubMed] [Google Scholar]
- Mischke D, Pardue ML. Organization and expression of alpha-tubulin genes in Drosophila melanogaster. One member of the alpha-tubulin multigene family is transcribed in both oogenesis and later embryonic develpoment. J Molec Biol. 1982;156:449–466. doi: 10.1016/0022-2836(82)90260-1. [DOI] [PubMed] [Google Scholar]
- Oehler R, Pusch E, and Zellner M. et al. 2001 . Cell Stress Chaperones. 6:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena JM, Jimenez-Ruiz A, and Soto M. et al. 1992 Regulation of hsp70 expression in Trypanosoma cruzi by temperature and growth phase. Mol Biochem Parasitol. 53:201–211. [DOI] [PubMed] [Google Scholar]
- Rezaie S, Ban J, Mildner M, Poitschek C, Brna C, Tschachler E. Characterization of a cDNA clone, encoding a 70 kDa heat shock protein from the dermatophyte pathogen Richophyton rubrum. Gene. 2000;241(1):27–33. doi: 10.1016/s0378-1119(99)00475-8. [DOI] [PubMed] [Google Scholar]
- Robertson RM, Xu H, Shoemaker KL, Dawson-Scully K. Exposure to heat shock affects thermosensitivity of the locust flight system. J Neurobiol. 1996;29:367–383. doi: 10.1002/(SICI)1097-4695(199603)29:3<367::AID-NEU8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fitsch EF, and Maniatis T 1989 Molecular cloning: A laboratory manual, 2nd edition. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- Shen Y, Liu J, Wang X, Cheng X, Wang Y, Wu N. Essential role of the first intron in the transcription of hsp90 beta gene. FEBS Lett. 1997;413:92–98. doi: 10.1016/s0014-5793(97)00883-1. [DOI] [PubMed] [Google Scholar]
- Snutch TP, Heschl MF, Baillie DL. The Caenorhabditis elegans hsp70 gene family: a molecular genetic characterization. Gene. 1988;64:241–255. doi: 10.1016/0378-1119(88)90339-3. [DOI] [PubMed] [Google Scholar]
- Stefani RM, Gomes SL. A unique intron-containing hsp70 gene induced by heat shock and during sporulation in the aquatic fungus Blastocladiella emersonii. Gene. 1995;152(1):19–26. doi: 10.1016/0378-1119(95)00645-m. [DOI] [PubMed] [Google Scholar]
- Terlecky SR, Chiang HL, Olson TS, Dice JF. Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock protein. J Biol Chem. 1992;267:9202–9209. [PubMed] [Google Scholar]
- Tyshenko MG, Walker VK. Towards a reconciliation of the introns early or late views: triosephosphate isomerase genes from insects. Biochim Biophys Acta. 1997;1353:131–136. doi: 10.1016/s0167-4781(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Uvarov B 1977 Grasshopper and Locusts, a Handbook of General Acridology, vol 2. Centre of Overseas Pest Research, London. [Google Scholar]
- Weis-Fogh T. Biology and physics of locust flight. II. Flight performance of the desert locust (Schistocerca gregaria) Philos Trans R Soc Lond B. 1956;239:459–510. [Google Scholar]
- Whyard S, Wyatt GR, Walker VK. The heat shock response in Locusta migratoria. J Comp Physiol B. 1986;156:813–817. [Google Scholar]
- Wu B, Hunt C, Morimoto RI. Structure and expression of the human gene encoding major heat shock protein hsp70. Mol Cell Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Walker VK, Robertson RM. Heat shock-induced thermotolerance of action potentials in locust flight system. J Neurobiol. 2001;49:188–199. doi: 10.1002/neu.1074. [DOI] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S. Translation of unspliced transcripts after heat shock. Science. 1988;242:1544–1548. doi: 10.1126/science.3201243. [DOI] [PubMed] [Google Scholar]
- Zatsepina OG, Velikodvorskaia VV, and Molodtsov VB. et al. 2001 A Drosophila melanogaster strain from sub-equatorial Africa has exceptional thermotolerance but decreased hsp70 expression. J Exp Biol. 204:1869–1881. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zhang J, Wyatt GR, and Walker VK 2002 Sequences and juvenile hormone regulation of elongation factors −1α and −γ in Locusta migratoria. Insect Biochem Mol Biol, in press. [DOI] [PubMed] [Google Scholar]