Abstract
Recent studies revealed that mutations in SPOP (Speckle-type POZ protein) occur in up to 15% of patients with prostate cancer. However, the physiological role of SPOP in regulating prostate tumorigenesis remains elusive. Here, we identified the Cdc20 oncoprotein as a novel ubiquitin substrate of SPOP. As such, pharmacological inhibition of Cullin-based E3 ligases by MLN4924 could stabilize endogenous Cdc20 in cells. Furthermore, we found that Cullin 3, and, to a less extent, Cullin 1, specifically interacted with Cdc20. Depletion of Cullin 3, but not Cullin 1, could upregulate the abudance of Cdc20 largely via prolonging Cdc20 half-life. Moreover, SPOP, the adaptor protein of Cullin 3 family E3 ligase, specifically interacted with Cdc20, and promoted the poly-ubiquitinaton and subsequent degradation of Cdc20 in a degron-dependent manner. Importantly, prostate cancer-derived SPOP mutants failed to interact with Cdc20 to promote its degradation. As a result, SPOP-deficient prostate cancer cells with elevated Cdc20 expression became resistant to a pharmacological Cdc20 inhibitor. Therefore, our results revealed a novel role of SPOP in tumorigenesis in part by promoting the degradation of the Cdc20 oncoprotein.
Keywords: Cdc20, SPOP, degradation, ubiquitination, cancer
1. Introduction
Ubiquitination is characterized as a critical type of post-translational modification that governs various important cellular processes including cell cycle and apoptosis, primarily through regulation of the protein abundance of key cell fate determining factors [1, 2]. Thus far, two related, multi-subunit E3 ubiquitin ligases, the Anaphase Promoting Complex (APC) and the Skp1-Cullin1-F-box complex (SCF) have been considered as the major driving forces governing cell cycle regulation and tumorigenesis [1–4]. In doing so, APC forms two functional sub-complexes APCCdc20 and APCCdh1 [5], by associating with two substrate adaptor proteins Cdc20 or Cdh1, respectively, to play critical roles in regulating both the M and G1 phases [6]. Cdc20 contains seven WD40 repeats for protein binding, serving as the substrate recognizing subunit of APC, recruiting substrates with the Destruction Box (D-box) motif [7]. However, besides Cdh1 [8], the upstream regulator(s) for APCCdc20 remain largely elusive.
Recent studies began to reveal that Cdc20 might possess oncogenic activity [9] and genetic ablation of Cdc20 blocks in vivo tumorigenesis, largely due to elevated cellular apoptosis [10]. Furthermore, depleting Cdc20 in various cancer cell lines also led to mitotic arrest followed by cell death [11]. These studies suggested that inhibiting APCCdc20 might lead to elevated cellular apoptosis, which advocates for Cdc20 as a novel anti-cancer therapeutic target. In keeping with this notion, inactivating APC by an IR-motif-mimetic inhibitor, pro-TAME, induced cell death in multiple cancer cell lines [12, 13]. Furthermore, a more specific APCCdc20 E3 ligase inhibitor, Apcin, inhibits its oncogenic function through directly interfering with the binding of Cdc20 to its substrates and causes blockade of mitotic exit in human cancer cells [14]. Consistent with its oncogenic role, Cdc20 is highly expressed in many human tumors including prostate [15], breast [16], cervical [17], glioblastoma [18] and ovarian tumors [19]. Notably, high expression of Cdc20 was tightly associated with advanced clinical stages and poor prognosis in human cancers including prostate cancer [15]. These findings thus suggest that Cdc20 expression may be used as a prognostic marker and therapeutic target in treating various human cancers.
The Cullin-Ring ligases (CRLs) are the largest family of ubiquitin E3 ligases, which govern a plethora of vital cellular processes including cell cycle progression [20]. Based on Cullin scaffold proteins (Cullin1, 2, 3, 4A, 4B, 5 and 7), CRLs can be categorized into seven subfamilies (termed CRL1 through CRL7, respectively) [2, 20]. Among these CRLs, the CRL1 complex (also named SCF), is well studied at both biochemical and physiological levels [21, 22]. Recently, the emerging CRL3 subfamily complex is identified as major regulators for different cellular processes and disruption of this degradation pathway has been linked to various human diseases, including nerve degeneration and cancers [23]. Structurally, CRL3 is composed of the scaffold protein Cullin 3, the RING protein RBX1, and one of numerous BTB domain adaptor proteins, which recruit its protein substrates for poly-ubiquitination [23].
Recently, it has been reported that Speckle-type POZ (pox virus and zinc finger protein) protein (SPOP), a substrate-interacting adaptor protein of CRL3, is one of most frequently mutated genes, with up to 15% mutation rate in primary human prostate cancers [24–26]. SPOP comprises two conserved domains: an N-terminal meprin and TRAF homology (MATH) domain that is primarily involved in substrate recognition and a C-terminal bric-a-brac, tramtrack and broad complex (BTB)/POZ domain that binds the CRL scaffold protein, Cullin 3 [27]. The ongoing list of SPOP substrates includes MacroH2A [28], Ci/Gli [29], androgen receptor (AR) [30, 31], steroid receptor coactivator 3 (SRC-3) [32], DEK [33], TRIM24 [33], ERG [34, 35] and SENP7 [36]. As most of these characterized substrates are well-known oncogenic proteins that are frequently overexpressed in human prostate cancers, SPOP probably functions largely as a tumor suppressor in PrCa that negatively regulates the stability of these oncogenic proteins. In keeping with this model, SPOP is frequently inactivated by genetic mutations in human prostate cancers [24, 25]. However, in other cancer settings including kidney cancer and breast cancer, on the other hand, SPOP is overexpressed and displays a possible oncogenic role in part by promoting the degradation of PTEN [37]. Thus, the physiological role of SPOP in tumorigenesis might also be tissue or context dependent. In support of this notion, the transcription factor E2Fs also can function as an oncogene or tumor suppressor in different cellular context [38]. In this circumstance, this study mainly focuses on understanding the tumor suppressor role of SPOP in the prostate cancer setting. To this end, even though known substrates of SPOP are well documented for their oncogenic roles in prostate cancer development and progression, the exact molecular mechanisms by which SPOP suppresses cancer formation in part by regulating cell cycle progression or chromosomal segregation have not yet been fully elucidated. To further clarify the physiological role of SPOP in regulating tumorigenesis, we identified oncoprotein Cdc20 as a novel ubiquitin substrate of SPOP in the prostate cancer setting, which offers further molecular insights into its tumor suppressive role in PrCa development.
2. Materials and Methods
2.1 Cell Culture
PC3 and DU145 cells were cultured in RPMI 1640 medium (Corning, NY) with 10% FBS, 100 units of penicillin and 100 µg/ml streptomycin. 293T, HeLa, and U2OS cells were cultured in DMEM medium (Life Technologies, CA) supplemented with 10% FBS, penicillin and streptomycin.
2.2 Plasmids
Myc-Cullin 1, Cullin 2, Cullin 3, Cullin 4A, Cullin 4B, Cullin 5, Flag-SPOP WT, Y87C, F102C, W131G, pGEX-4T-1-SPOP, Flag-Keap1, Flag-Cop1, shScramble, shCullin 1, shCullin 3, shSPOP, and His-ubiquitin constructs were described previously [35]. The construct of HA-Cdc20 was also described previously [13]. HA-Cdc20 deletion degron (GKSSS) mutant was generated using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene).
2.3 Cell transfection and viral infection
For cell transfection, cells with 80% confluence were transfected using Lipofectamine (Invitrogen) in Opti-MEM medium (Invitrogen). 48 hours post-transfection, cells were harvested for immunoprecipation or immunoblot analysis. For viral infection, cells with 50% confluence were infected with lentivirus vector with 4 µg/mL polybrene (Sigma-Aldrich). 48 hours post-infection, the cells were passaged and selected using 1 µg/mL puromycin (Sigma-Aldrich) for 72 hours to eliminate the uninfected cells before harvesting for western blot analysis.
2.4 Antibodies and Reagents
Anti-Cdc20 (p55 CDC, sc-5296 and sc-13162), anti-Cdh1 (Fzr, sc-56312), anti-p27 (SC-527), anti-HA antibody (SC-805), anti-c-Myc (sc-40), and anti-p27 (SC-527) antibodies were purchased from Santa Cruz. Anti-SPOP antibody (16750-1-AP) was purchased from Proteintech. Mouse monoclonal anti-Myc-Tag (2276), rabbit polyclonal anti-Myc-Tag antibody (2278), anti-Cullin 3 (2759), and anti-GST (2625) antibodies were purchased from Cell Signaling. Polyclonal anti-Flag antibody (F-2425), monoclonal anti-Flag antibody (F-3165, clone M2), anti-vinculin antibody (V-4505), anti-tubulin antibody (T5168), peroxidase-conjugated anti-mouse secondary antibody (A-4416), peroxidase-conjugated anti-rabbit secondary antibody (A-4914), anti-HA agarose beads (A-2095) and anti-Flag agarose beads (A-2220) were purchased from Sigma. All antibodies were used in 1:1000 dilutions in 5% non-fat milk for western blot. MLN4924 was a kind gift from Dr. William Kaelin (Dana-Farber cancer institute). MG132 (BML-PI102-0005) was purchased from Enzo life science. Apcin (I-444) was purchased from BostonBiochem.
2.5 Immunoprecipitation and Western Blotting
Cells were lysed in EBC buffer (50 mM Tris pH 7.5, 120 mM NaCl, 0.5% NP40) supplemented with protease inhibitors (Roche) and phosphatase inhibitors (Calbiochem). The protein concentrations of lysates were measured by Beckman Coulter DU-800 spectrophotometer (Beckman Coulter) using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, CA). For immunoprecipitation assays, 1 mg total lysates were incubated with the appropriate antibody-conjugatd beads (1–2 µg) for 4 hours or overnight at 4°C. Immunocomplexes were washed four times with NETN buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, and 0.5% NP-40) before being resolved by SDS-PAGE and immunoblotted with indicated antibodies.
2.6 Cell viability and apoptosis assays
For cell viability assays, 3000 cells per well were plated in 96-well plates, and incubated with complete DMEM medium containing different concentrations of Cdc20 inhibitor for 48 h. Assays were performed with the Cell Titer-Glo Luminescent Cell Viability Assay Kit according to the manufacturer’s instructions (Promega). For detection of apoptosis, cells treated with indicated concentration of Apcin for 48 h were co-stained with Annexin-V-PE and 7-AAD (Annexin V-PE Apoptosis Detection Kit I, BD Bioscience) and analysed by FACS according to the manufacturer’s instructions.
2.7 Colony Formation Assays
PC3 cells with stably expressing shScramble or shSPOP were plated in 6-well culture dishes (1000 cells per well). The second day after seeding cells, 50 µM Apcin was added in and allowed to grow undisturbed for one week. Cells were stained with crystal violet and the colony number were counted.
2.8 FACS Analysis
SPOP+/+ and SPOP−/− MEFs were digested with trypsin, washed twice with phosphate buffered saline (PBS). The cells were then fixed with 70% ice cold Ethanol. Approximately 2 hours before FACS analysis, cells were washed with PBS and subsequently re-suspended in 1 ml PBS with 1 µg/ml RNase A. Samples were then incubated for 30 minutes at 37 °C. Afterwards, cells were stained with 0.05 mg/ml propidium iodide on ice for 1 hour before being analyzed through flow cytometry (BD FACSCalibur, San Jose, CA, USA).
2.9 Statistical Analyses
Student t tests were used to evaluate significance between groups, and p-values indicated. Error bars represent standard deviation. * p < 0.05 indicates significance.
Results
3.1 Inhibition of the Cullin-based E3 ligases stabilizes endogenous Cdc20 in cells
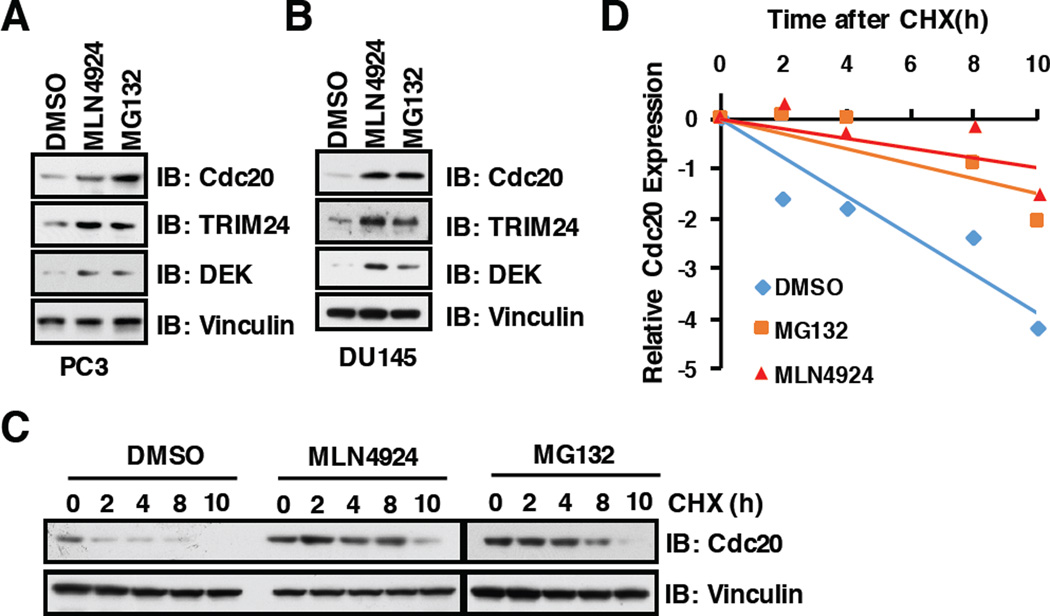
MLN4924 is a specific inhibitor of the NEDD8-activating enzyme (NAE) and first-in-class anti-cancer drug, which has entered Phase-I trials for cancer therapy [39, 40]. Mechanistically, MLN4924 could inactivate CRLs E3 ligases through blocking Cullin Neddylation, an essential step in activating CRLs [41], which subsequently led to upregulation of downstream substrates of CRLs [40]. Importantly, we found that, in addition to the proteasome inhibitor MG132, MLN4924 could also stabilize the key cell cycle regulatory protein, Cdc20, at endogenous level in prostate cancer cell lines PC3 (Figure 1A) and DU145 (Figure 1B). Furthermore, we demonstrated that stabilization of Cdc20 by MLN2924 or MG132 was achieved largely through prolonging the half-life of endogenous Cdc20 in multiple cell lines including PC3 (Figure1C and 1D), HeLa (Supplementary Figure S1A and S1B) and U2OS (Supplementary Figure S1C and S1D).
Figure 1. Inhibition of the Cullin-based E3 ligases or 26S proteasome by MLN4924 and MG132, respectively, stabilize endogenous Cdc20.
A–B) Immunoblot (IB) analysis of whole cell lysates derived from PC3 and DU145 cells, which were treated with 1 µM MLN4924 or 10 µM MG132 for 12 h before harvesting.
C) PC3 cells were harvested at indicated time points after 100 µg/ml cycloheximide (CHX) addition with/without 10 µM MG132 treatment for 12h. Blots were probed with indicated antibodies.
(D) Quantification of Cdc20 band intensities. Cdc20 immunoblot bands were normalized to Vinculin, then normalized to the t = 0 time point.
3.2 Cdc20 stability is controlled by Cullin 3 family E3 ligases
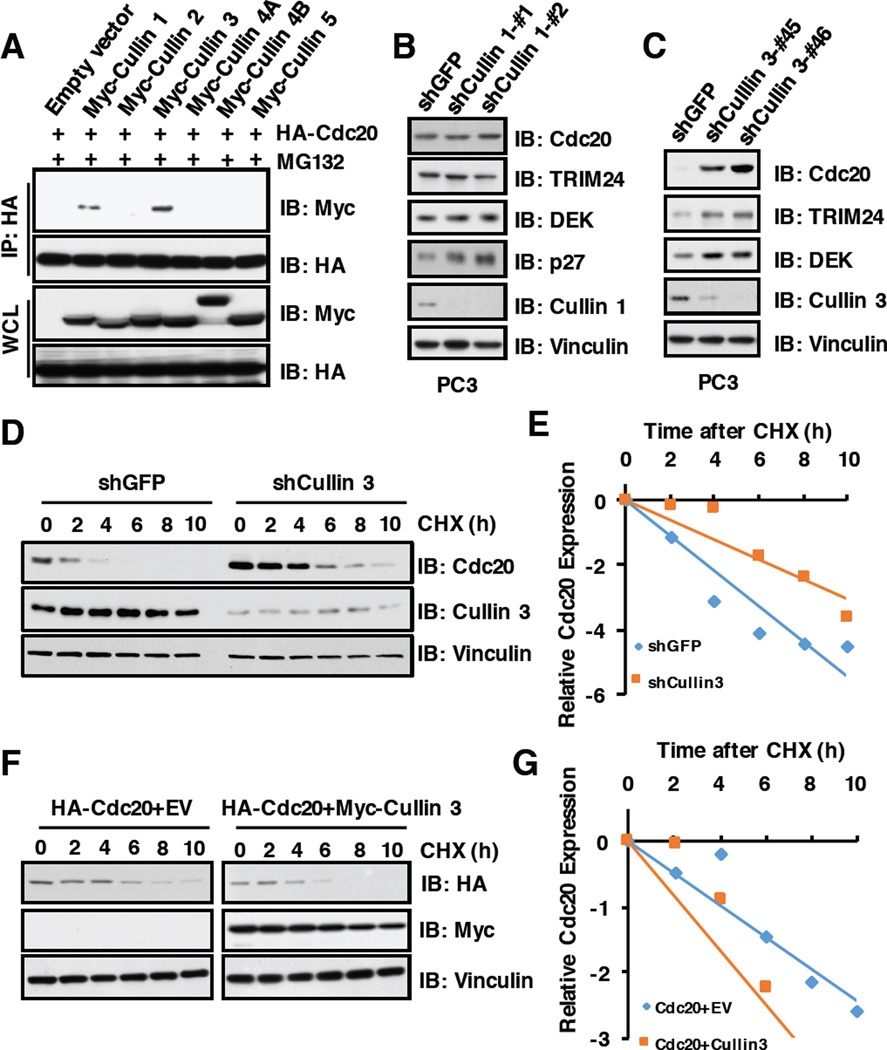
As MLN4924 could stabilize endogenous Cdc20, we speculate that Cullin-Ring family E3 ligase(s) may be the upstream regulator(s) to control Cdc20 stability. In keeping with this notion, Cullin 3, and to a much lesser extent, Cullin 1, but not other Cullin family members specifically interacted with Cdc20 in cells (Figure 2A). Furthermore, depletion of Cullin 3, but not Cullin 1, in multiple cancer cell lines including PC3 (Figure2B and 2C), HeLa (Supplementary Figure S2A and S2B) and U2OS cells (Supplementary Figure S2C and S2D), could dramatically upregulate the protein abundance of endogenous Cdc20. These results therefore argue that Cullin 3, but not Cullin 1, subfamily of E3 ligases, is the primary CRL type of E3 ligase that negatively regulates Cdc20 stability in cells. Consistently, depletion of Cullin 3 prolonged the half-life of Cdc20 (Figure2D and 2E), whereas ectopic expression of Cullin 3 shortened the half-life of Cdc20 in cells (Figure2F and 2G). These results together demonstrate that in addition to the reported APCCdh1 E3 ligase [8, 42], Cullin 3-based E3 ligase also play a critical role in governing Cdc20 protein stability in cells.
Figure 2. Cdc20 stability is controlled by the Cullin 3 family E3 ligase.
A) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with indicated constructs. 36 hours post-transfection, cells were pretreated with 10 µM MG132 for 10 hours before harvesting.
B) IB analysis of WCL derived from PC3 cells infected with the indicated lentiviral shRNAs against Cullin 1. The infected cells were selected with 1 µg/ml puromycin for 72 hours to eliminate the non-infected cells before harvesting.
C) IB analysis of WCL derived from PC3 cells infected with the indicated lentiviral shRNAs against Cullin 3. The infected cells were selected with 1 µg/ml puromycin for 72 hours to eliminate the non-infected cells before harvesting.
D) PC3 cells stably infected with the indicated lentiviral shRNAs were treated for indicated times with 100 µg/ml CHX. WCL were prepared and immune-blotted with indicated antibodies.
E) Quantification of western blots shown in D using ImageJ software. Cdc20 immunoblot bands were normalized to Vinculin, then normalized to the t = 0 time point.
F) 293T cells transfected with indicated constructs were treated with 100 µg/ml CHX. WCL were analyzed with indicated antibodies.
G) Quantification of western blots shown in F using ImageJ software. Cdc20 immunoblot bands were normalized to Vinculin, then normalized to the t = 0 time point.
3.3 SPOP, the adaptor protein of Cullin 3 family E3 ligase, specifically interacts with Cdc20 through its N-terminal MATH domain
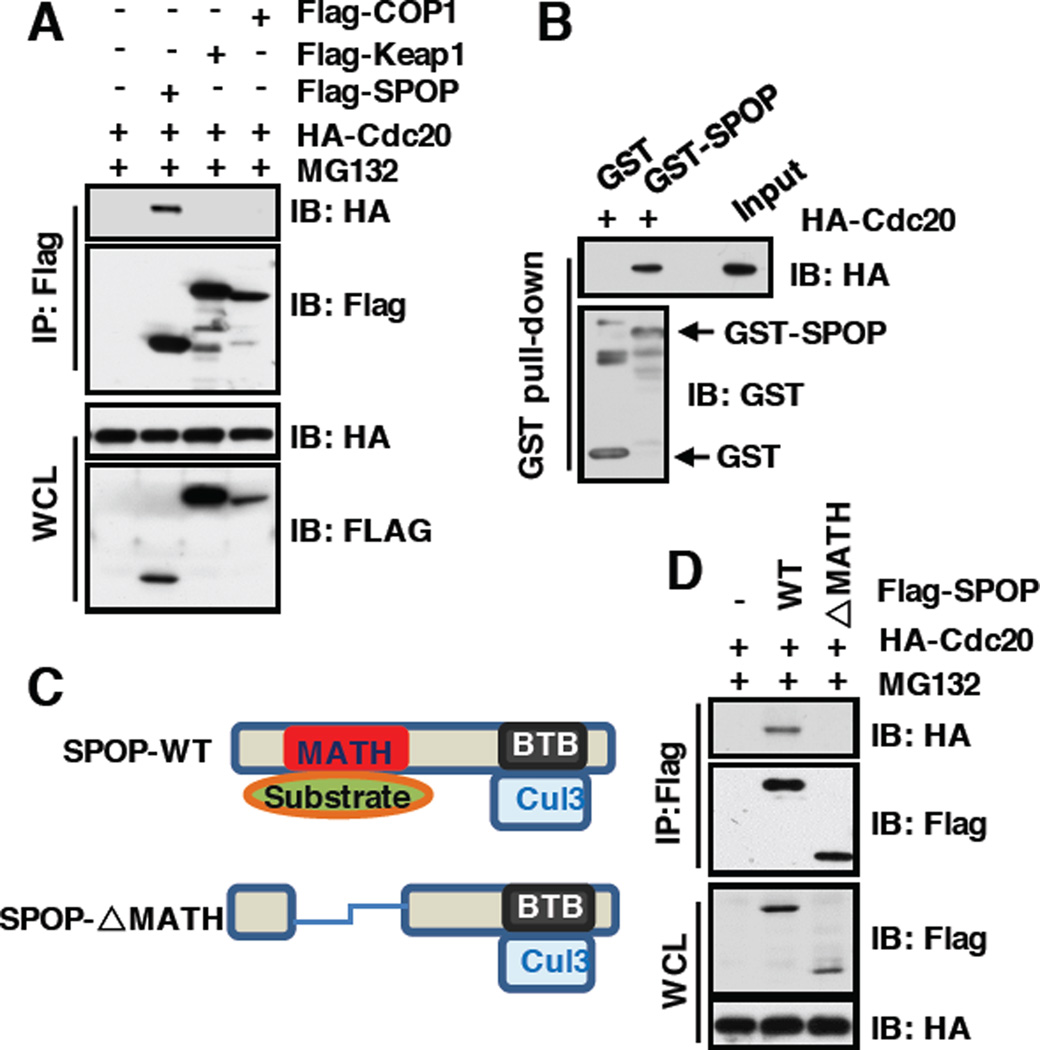
Cullin 3 exerts its E3 ubiquitin ligase activity largely through recruiting one of several adaptors with BTB/POZ domain, such as SPOP and Keap1 [23]. We further explored which adaptor protein can specifically bind and promote Cdc20 poly-ubiquitination and degradation. Notably, we found that Cdc20 specifically interacted with SPOP, but not Keap1 or Cop1, a Cullin 4-based E3 ligase substrate adaptor protein (Figure 3A). Furthermore, bacterially purified GST-SPOP, but not GST, specifically interacted with Cdc20 in vitro (Figure 3B), further advocating the specific interaction between SPOP and Cdc20. As SPOP recognizes its ubiquitin substrate largely through its N-terminal conserved MATH domain and binds CRL scaffold protein Cullin 3 through its C-terminal BTB domain [27], we hypothesized that deletion of MATH domain in SPOP may disrupt its binding with Cdc20. In keeping with this notion, we found that SPOP mutant deleting the MATH domain failed to interact with Cdc20 in cells (Figure3C and 3D). Together, these results demonstrate that SPOP specifically interacts with Cdc20 through its N-terminal substrate recognizing MATH domain.
Figure 3. SPOP, the adaptor protein of Cullin3 family E3 ligase, specifically interacts with Cdc20 through its N-terminal MATH domain.
A) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with indicated constructs. 36 hours post-transfection, cells were pretreated with 10 µM MG132 for 10 hours before harvesting.
B) IB analysis of GST pull-down precipitates from 293T cell lysate with overexpression of HA-Cdc20 using bacterially purified GST and GST-SPOP proteins.
C) Schematic of SPOP with MATH and BTB domain.
D) IB analysis of WCL and anti-Flag IP derived from 293T cells transfected with indicated constructs. 36 hours post-transfection, cells were pretreated with 10 µM MG132 for 10 hours before harvesting.
3.4 SPOP promotes the poly-ubiquitinaton and subsequent degradation of Cdc20
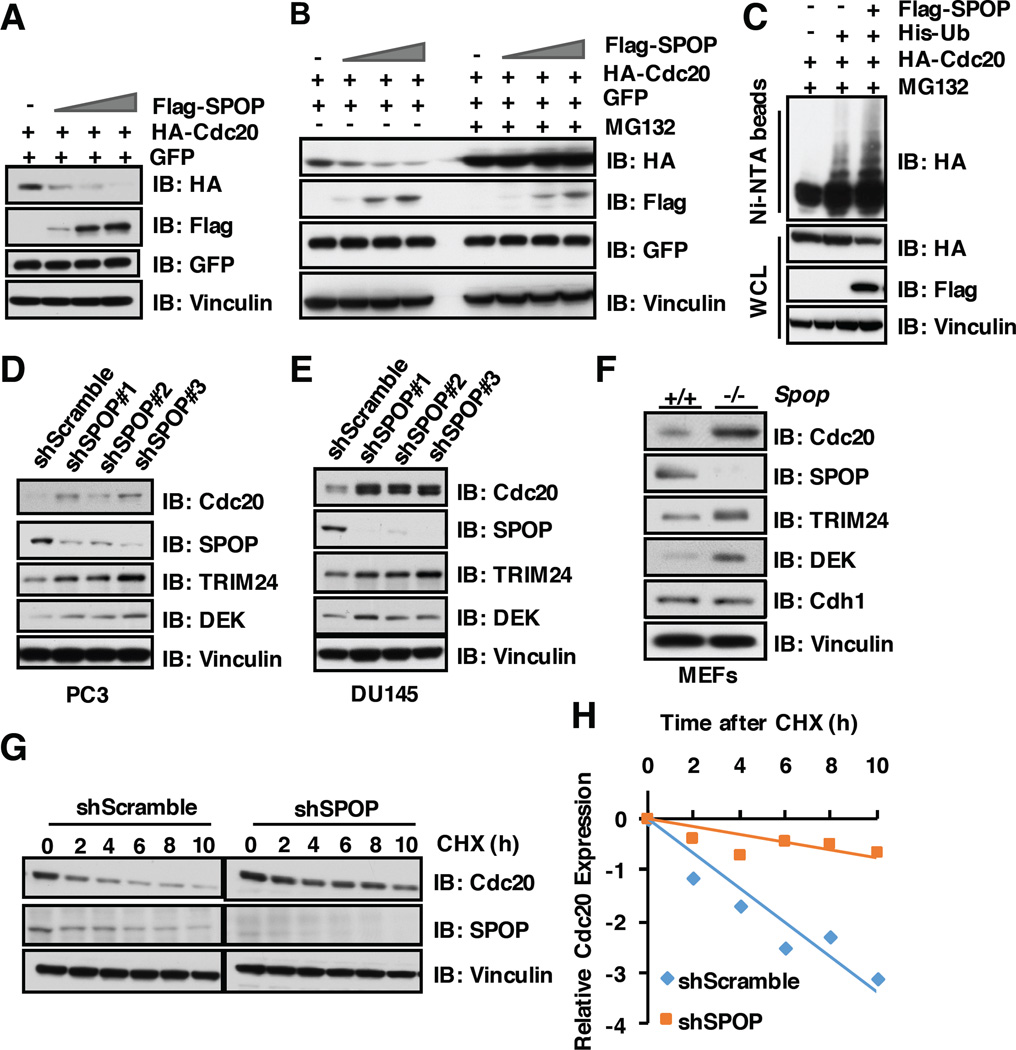
As SPOP interacts with Cdc20 through its substrate recognition MATH domain, we further explored whether SPOP can promote Cdc20 degradation in a proteasome-dependent manner. To this end, we found that ectopic expression of SPOP could markedly decrease the protein abundance of Cdc20, but not its close homologue, Cdh1, in a dose-dependent manner (Figure 4A and Supplementary Figure S3A). Moreover, SPOP-dependent degradation of Cdc20 could be blocked by the proteasome inhibitor MG132 (Figure 4B), which indicates that SPOP-mediated degradation of Cdc20 through the 26S proteasome pathway. In keeping with this notion, SPOP could promote Cdc20 poly-ubiquitination in cells (Figure 4C). On the other hand, depleting SPOP using several different shRNAs dramatically elevated Cdc20 protein abundance in multiple cell lines including PC3 (Figure 4D), DU145 (Figure 4E) and HeLa cells (Supplementary Figure S3B). Moreover, Cdc20 protein level was also elevated in SPOP knock out MEFs, while the protein abudnace of Cdh1, the close homologue of Cdc20, did not change significantly (Figure 4F). Consistently, the half-life of Cdc20 is prolonged in SPOP-deficient cells (Figure 4G–H and Supplementary Figure S3C–D). However, the cell cycle profile did not change significantly after depletion of endogenous SPOP in MEFs (Figure S4A–C). These results together show that the Cullin 3SPOP E3 ligase functions as a novel negative regulator for Cdc20 in part through promoting Cdc20 poly-ubiquitination and subsequent 26S proteasome-dependent degradation.
Figure 4. SPOP promotes the poly-ubiquitinaton and subsequent degradation of Cdc20.
A) Immunoblot (IB) analysis of whole cell lysates (WCL) derived from 293T cells transfected with the indicated constructs.
B) 293T cells transfected with indicated constructs were pretreated with/without 10 µM MG132 for 10 hours before harvesting for IB analysis.
C) IB of WCL and His pull-down of PC3 cells transfected with the indicated constructs. Cells were treated with 30 µM MG132 for 6 hours and lysed with denature buffer.
D) IB analysis of WCL derived from PC3 cells infected with the indicated lentiviral shRNAs against SPOP. Infected cells were selected with 1 µg/ml puromycin for 72 hours to eliminate the non-infected cells before harvesting.
E) IB analysis of WCL derived from DU145 cells infected with the indicated lentiviral shRNAs against SPOP. Infected cells were selected with 1 µg/ml puromycin for 72 hours to eliminate the non-infected cells before harvesting.
F) IB analysis of WCL derived from SPOP WT and SPOP knock out MEFs, respectively.
G) IB analysis of WCL derived from PC3 cells infected with indicated shRNAs, 100 µg/ml cycloheximide (CHX) was used to measure Cdc20 half-life.
H) Quantification of western blots shown in G using ImageJ software. Cdc20 immunoblot bands were normalized to Vinculin, then normalized to the t = 0 time point.
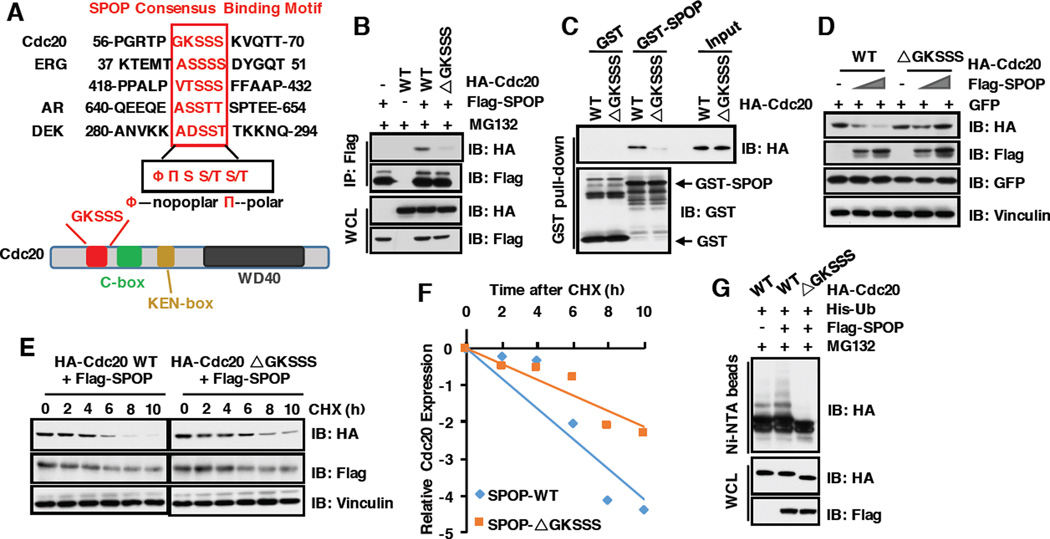
3.5 SPOP promotes Cdc20 degradation in a degron-dependent manner
Several identified SPOP substrates including Ci/Gli [43], Daxx [44], MacroH2A [28], AR [30, 31], PTEN [37] SRC-3) [32], DEK [33], TRIM24 [33], ERG [34, 35] and SENP7 [36] share a SPOP-binding consensus motif Φ-Π-S-S/T-S/T (Φ-nonpolar; Π, polar) (Figure 5A) [27]. Notably, we identified a putative motif 61GKSSS65 located in the N-terminus of Cdc20 (Figure 5A), and further showed that deleting this putative motif (Cdc20-ΔGKSSS) impaired Cdc20 interaction with SPOP both in cells (Figure 5B) and in vitro (Figure 5C). Consistently, the Cdc20-ΔGKSSS mutant became resistant to SPOP-mediated destruction (Figure 5D–5F) and poly-ubiquitination (Figure 5G) in cells. Taken together, these results demonstrate that the 61GKSSS65 motif is required for SPOP to interact with Cdc20, and subsequently triggers poly-ubiquitination and degradation of Cdc20.
Figure 5. SPOP promotes Cdc20 degradation in a degron-dependent manner.
A) Sequence comparison of putative SPOP binding motif in Cdc20 with known SPOP substrates and the schematic of Cdc20 protein.
B) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) from 293T cells transfected with indicated constructs. 36 hours post-transfection, cells were pretreated with 10 µM MG132 for 10 hours before harvesting.
C) IB analysis of GST pull-down precipitates from 293T cell lysate with overexpression of HACdc20 WT and deletion mutants (ΔGKSSS).
D) IB analysis of WCL derived from 293T cells transfected with the indicated constructs.
E) 293T cells transfected with indicated constructs were treated with 100 µg/ml CHX for different time course. WCL were prepared and western blotted with indicated antibodies.
F) Quantification of western blots shown in E using ImageJ software. Cdc20 immunoblot bands were normalized to Vinculin, then normalized to the t = 0 time point.
G) IB of WCL and His pull-down of PC3 cells transfected with the indicated constructs. Cells were treated with 30 µM MG132 for 6 hours and lysed with denature buffer.
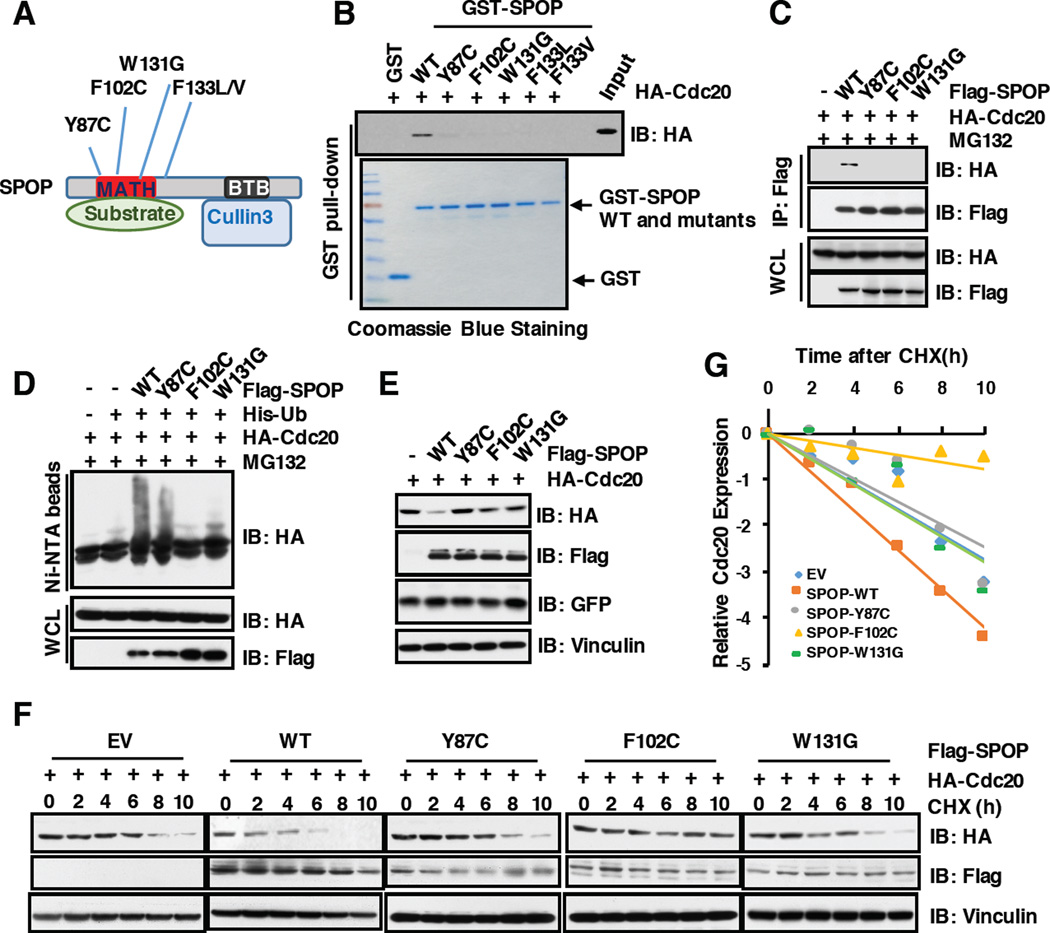
3.6 Prostate cancer-associated SPOP mutants are defective in promoting Cdc20 poly-ubiquitination and degradation
Recent large scale sequencing studies showed that SPOP, encoding a CRL3-based E3 ligase adaptor protein, is one of the most frequently mutated genes in human prostate cancer [24–26]. To date, most of identified SPOP somatic mutations in prostate cancer including Y87C, F102C and W131G are clustered in the MATH domain, presumably impairing substrate binding (Figure 6A). In keeping with this finding, we found that prostate cancer-derived SPOP mutants including Y87C, F102C and W131G failed to interact with Cdc20 both in vitro (Figure 6B) and in cells (Figure 6C). Consistently, compared to SPOP wild type, these PrCa derived SPOP mutants were incapable of promoting the ubiquitination (Figure 6D) and subsequent degradation of Cdc20 (Figure 6E–6G) in cells. These results demonstrate that prostate cancer derived mutations of SPOP abolish its ability to interact with, and promote poly-ubiquitination and degradation of Cdc20.
Figure 6. Prostate cancer-associated SPOP mutants are defective in promoting Cdc20 degradation and poly-ubiquitination.
A) A schematic illustration of SPOP with MATH and BTB domain and cancer-associated mutations.
B) Immunoblot (IB) analysis of GST pull-down precipitates from 293T cell lysate with overexpression of HA-Cdc20.
C) IB analysis of whole cell lysates (WCL) and immunoprecipitates (IP) from 293T cells transfected with indicated constructs. 36 hours post-transfection, cells were treated with 10 µM MG132 for 12 hours before harvesting.
D) IB of WCL and His pull-down from PC3 cells transfected with the indicated constructs. Cells were treated with 10 µM MG132 for 12 hours and lyzed with denature buffer.
E) IB analysis of WCL from 293T cells transfected with indicated constructs.
F) 293T cells were transfected with indicated constructs. 36 hours post-transfection, cells were treated for indicated times with 100 µg/ml CHX. WCL were prepared and western blotted with indicated antibodies.
G) Quantification of western blots shown in F using ImageJ software. Cdc20 immunoblot bands were normalized to Vinculin, then normalized to the t = 0 time point.
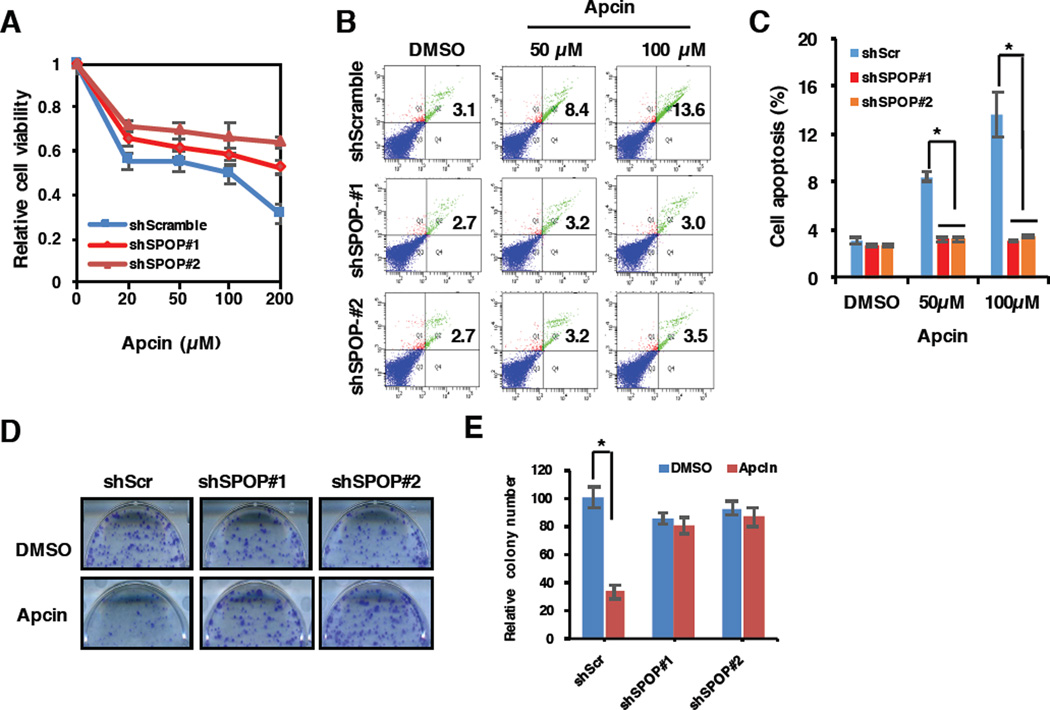
3.7 SPOP-deficient prostate cancer cells are resistant to the pharmacological Cdc20 inhibitor, Apcin
Recently, the King group reported that a small molecule, Apcin (APC inhibitor), specifically binds the D-box-binding pocket within the WD40 domain of Cdc20 to competitively inhibit the degradation of D-box-containing substrates [14, 45]. As our previous report showed Cdc20 suppressed cell apoptosis largely through targeting Bim for poly-ubiquitination and degradation [13], we next explored whether Cdc20 specific inhibitor, Apcin, affects cell viability and apoptosis in prostate cancer. To this end, we found that Apcin could reduce the cell viability and apoptosis of PC3 cells in a dose-dependent manner (Figure 7A–7C). More importantly, compared to shScramble treatment cells, cells depleted of SPOP using shRNAs and with elevated Cdc20 expression (Figure 4D) became more resistant to the Cdc20 inhibitor, Apcin (Figure 7A–7C). Consistently, Apcin could dramatically reduce the oncogenic transformation of shScramble-treated PC3 cells, but not SPOP-depleted cells (Figure 7D–7E). Together, these results suggest that the Cdc20 pharmacological inhibitor, Apcin, might be clinically used to treat SPOP-WT PrCa patients, but not SPOP-deficient prostate cancers.
Figure 7. SPOP-deficient prostate cancer cells become more resistant to the pharmacological Cdc20 inhibitor, Apcin.
A) PC3 cells stably infected with virus expressing shRNAs against SPOP as well as shScramble as control were treated with indicated concentration of Cdc20 inhibitor, Apcin, for 48 h before performing a cell viability assay. Data are shown as mean ± SD from three independent experiments. *p<0.05
B) PC3 cells stably expressing shSPOP or shScramble were treated with indicated concentration of Apcin for 48 h before performing a cell apoptosis assay.
C) Quantification of results for Fig. 7B. Data were shown as mean ± SD from three independent experiments. *p<0.05
D) 1000 PC3 cells with stably expressing shScramble or shSPOP were plated in each well of 6-well culture dishes with/without 50 µM Apcin treatment. After one week, cells were stained with crystal violet and the colony number were counted. E) Quantification of results for Fig. 7D. Data were shown as mean ± SD from three independent experiments. *p<0.05
3. Discussion
Here we have identified Cullin 3SPOP as a novel upstream E3 ubiquitin ligase complex that governs Cdc20 stability and oncogenic functions through promoting the ubiquitination and subsequent destruction of Cdc20. Our results showed that although Cdc20 interacts specifically with Cullin 1 and Cullin 3 of the Cullin-based E3 ligase family members, only Cullin 3 is largely responsible for the stability control of Cdc20 in cells (Figure 2). More importantly, we also showed that SPOP, one of substrate interaction modules of Cullin 3 family, specifically binds and promotes the degradation of Cdc20 in a poly-ubiquitinaion dependent proteolysis. However, previous studies have revealed that phosphorylation of certain proteins such as SRC-3 is required for SPOP-mediated interaction and degradation [46], while phosphorylation of Cdc20 is required for its timely activation at M phase [7, 47]. Thus, it is unclear whether similar modifications are needed for Cullin 3SPOP in recognition of Cdc20 for poly-ubiquitination and degradation, which warrants further investigation.
Previous studies have reported that Cdc20 was degraded by APCCdh1 in early G1 phase, which is required of Plk1-dependent phosphorylation of Cdc20 [8, 42]. Our results presented here indicated that the Cullin 3SPOP regulation of Cdc20 appears to function independently of APCCdh1. Of particular interest in light of these results presented here as well as results from previous studies [8, 42], it remains elusive how these two E3 ligases, Cullin 3SPOP and APCCdh1 are controlling Cdc20 degradation. It is plausible that each E3 ligase is working in different cell cycle phase, or in a temporal or spatial specificity to provide a timely control of the Cdc20 stability.
Increasing evidence showed that Cdc20 exhibits an oncogenic function and targeting Cdc20 could be a novel strategy for combating human cancers [9, 48]. Notably, our results showed that prostate cancer-associated SPOP mutants including Y87C, F102C and W131G, which are clustered in its substrate-recruiting MATH domain, have lost the ability to bind and promote Cdc20 poly-ubiquitination and degradation (Figure 6). Consequently, Cdc20 expression levels are elevated in SPOP-deficient prostate cancer cells, which become more resistant to the pharmacological Cdc20 inhibitor, Apcin.
Therefore, our studies delineated the molecular mechanism of upstream regulator Cullin 3SPOP in controlling the stability of the oncoprotein Cdc20 through promoting Cdc20 poly-ubiquitination and subsequent degradation in prostate cancer. As personalized targeted therapy utilizing individual tumor genetic status becomes more readily available in cancer diagnostics and treatment, we envision that our studies will provide the rationale for developing novel therapeutic strategies to treat SPOP-WT PrCa patients with the Cdc20 inhibitor, Apcin.
Supplementary Material
Research highlights.
-
➢
Cullin 3 negatively regulates Cdc20 stability in prostate cancer.
-
➢
SPOP promotes Cdc20 poly-ubiquitination and degradation.
-
➢
Prostate cancer-associated mutants are defective in interaction with Cdc20.
-
➢
SPOP-deficient prostate cancer cells become resistant to Cdc20 inhibitor.
Acknowledgments
This work was supported by grants from the National Institute of Health (R01CA177910 and R01GM094777 to W.W.), National Basic Research Program of China (2015CB943003) and National Natural Science Foundation of China (81370753). F.W. received financial support from the China Scholarship Council (CSC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- 1.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14:233–247. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Wan L, Dai X, Sun Y, Wei W. Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis. Biochim Biophys Acta. 2014;1845:277–293. doi: 10.1016/j.bbcan.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 6.King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 7.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Huang JN, Park I, Ellingson E, Littlepage LE, Pellman D. Activity of the APC(Cdh1) form of the anaphase-promoting complex persists until S phase and prevents the premature expression of Cdc20p. J Cell Biol. 2001;154:85–94. doi: 10.1083/jcb.200102007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidokoro T, Tanikawa C, Furukawa Y, Katagiri T, Nakamura Y, Matsuda K. CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene. 2008;27:1562–1571. doi: 10.1038/sj.onc.1210799. [DOI] [PubMed] [Google Scholar]
- 10.Manchado E, Guillamot M, de Carcer G, Eguren M, Trickey M, Garcia-Higuera I, Moreno S, Yamano H, Canamero M, Malumbres M. Targeting mitotic exit leads to tumor regression in vivo: Modulation by Cdk1, Mastl, and the PP2A/B55alpha,delta phosphatase. Cancer Cell. 2010;18:641–654. doi: 10.1016/j.ccr.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16:347–358. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh DC, Hathaway N, Dimova N, Cuny GD, King RW. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18:382–395. doi: 10.1016/j.ccr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan L, Tan M, Yang J, Inuzuka H, Dai X, Wu T, Liu J, Shaik S, Chen G, Deng J, Malumbres M, Letai A, Kirschner MW, Sun Y, Wei W. APC(Cdc20) suppresses apoptosis through targeting Bim for ubiquitination and destruction. Dev Cell. 2014;29:377–391. doi: 10.1016/j.devcel.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sackton KL, Dimova N, Zeng X, Tian W, Zhang M, Sackton TB, Meaders J, Pfaff KL, Sigoillot F, Yu H, Luo X, King RW. Synergistic blockade of mitotic exit by two chemical inhibitors of the APC/C. Nature. 2014 doi: 10.1038/nature13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Y, Li K, Lu L, Si-Tu J, Lu M, Gao X. Overexpression of Cdc20 in clinically localized prostate cancer: Relation to high Gleason score and biochemical recurrence after laparoscopic radical prostatectomy. Cancer Biomark. 2016;16:351–358. doi: 10.3233/CBM-160573. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Jedinak A, Sliva D. Ganodermanontriol (GDNT) exerts its effect on growth and invasiveness of breast cancer cells through the down-regulation of CDC20 and uPA. Biochem Biophys Res Commun. 2011;415:325–329. doi: 10.1016/j.bbrc.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar T, Sabitha K, Vijayalakshmi N, Shirley S, Bose MV, Gopal G, Selvaluxmy G. Identification and validation of genes involved in cervical tumourigenesis. BMC Cancer. 2011;11:80. doi: 10.1186/1471-2407-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marucci G, Morandi L, Magrini E, Farnedi A, Franceschi E, Miglio R, Calo D, Pession A, Foschini MP, Eusebi V. Gene expression profiling in glioblastoma and immunohistochemical evaluation of IGFBP-2 and CDC20. Virchows Arch. 2008;453:599–609. doi: 10.1007/s00428-008-0685-7. [DOI] [PubMed] [Google Scholar]
- 19.Ouellet V, Guyot MC, Le Page C, Filali-Mouhim A, Lussier C, Tonin PN, Provencher DM, Mes-Masson AM. Tissue array analysis of expression microarray candidates identifies markers associated with tumor grade and outcome in serous epithelial ovarian cancer. Int J Cancer. 2006;119:599–607. doi: 10.1002/ijc.21902. [DOI] [PubMed] [Google Scholar]
- 20.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nature reviews. Molecular cell biology. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 21.Lee AE, Castaneda CA, Wang Y, Fushman D, Fenselau C. Preparing to read the ubiquitin code: a middle-out strategy for characterization of all lysine-linked diubiquitins. J Mass Spectrom. 2014;49:1272–1278. doi: 10.1002/jms.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, Wei W, Sun Y. Genetically engineered mouse models for functional studies of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases. Cell research. 2013;23:599–619. doi: 10.1038/cr.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genschik P, Sumara I, Lechner E. The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular functions and disease implications. The EMBO journal. 2013;32:2307–2320. doi: 10.1038/emboj.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger D, Sougnez C, Onofrio R, Carter SL, Park K, Habegger L, Ambrogio L, Fennell T, Parkin M, Saksena G, Voet D, Ramos AH, Pugh TJ, Wilkinson J, Fisher S, Winckler W, Mahan S, Ardlie K, Baldwin J, Simons JW, Kitabayashi N, MacDonald TY, Kantoff PW, Chin L, Gabriel SB, Gerstein MB, Golub TR, Meyerson M, Tewari A, Lander ES, Getz G, Rubin MA, Garraway LA. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, Nickerson E, Chae SS, Boysen G, Auclair D, Onofrio RC, Park K, Kitabayashi N, MacDonald TY, Sheikh K, Vuong T, Guiducci C, Cibulskis K, Sivachenko A, Carter SL, Saksena G, Voet D, Hussain WM, Ramos AH, Winckler W, Redman MC, Ardlie K, Tewari AK, Mosquera JM, Rupp N, Wild PJ, Moch H, Morrissey C, Nelson PS, Kantoff PW, Gabriel SB, Golub TR, Meyerson M, Lander ES, Getz G, Rubin MA, Garraway LA. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.s.c.m.o. Cancer Genome Atlas Research Network. Electronic address, N. Cancer Genome Atlas Research, The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuang M, Calabrese MF, Liu J, Waddell MB, Nourse A, Hammel M, Miller DJ, Walden H, Duda DM, Seyedin SN, Hoggard T, Harper JW, White KP, Schulman BA. Structures of SPOP-substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol Cell. 2009;36:39–50. doi: 10.1016/j.molcel.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Munoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E, Nusinow DA, Panning B, Marahrens Y, van Lohuizen M. Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proc Natl Acad Sci U S A. 2005;102:7635–7640. doi: 10.1073/pnas.0408918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Shi Q, Chen Y, Yue T, Li S, Wang B, Jiang J. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2009;106:21191–21196. doi: 10.1073/pnas.0912008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An J, Wang C, Deng Y, Yu L, Huang H. Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants. Cell reports. 2014;6:657–669. doi: 10.1016/j.celrep.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng C, Rajapakshe K, Shah SS, Shou J, Eedunuri VK, Foley C, Fiskus W, Rajendran M, Chew SA, Zimmermann M, Bond R, He B, Coarfa C, Mitsiades N. Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer Res. 2014;74:5631–5643. doi: 10.1158/0008-5472.CAN-14-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, Zimmermann M, Bond R, Shou J, Li C, Blattner M, Lonard DM, Demichelis F, Coarfa C, Rubin MA, Zhou P, O'Malley BW, Mitsiades N. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci U S A. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theurillat JP, Udeshi ND, Errington WJ, Svinkina T, Baca SC, Pop M, Wild PJ, Blattner M, Groner AC, Rubin MA, Moch H, Prive GG, Carr SA, Garraway LA. Prostate cancer. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP-mutant prostate cancer. Science. 2014;346:85–89. doi: 10.1126/science.1250255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An J, Ren S, Murphy SJ, Dalangood S, Chang C, Pang X, Cui Y, Wang L, Pan Y, Zhang X, Zhu Y, Wang C, Halling GC, Cheng L, Sukov WR, Karnes RJ, Vasmatzis G, Zhang Q, Zhang J, Cheville JC, Yan J, Sun Y, Huang H. Truncated ERG Oncoproteins from TMPRSS2-ERG Fusions Are Resistant to SPOP-Mediated Proteasome Degradation. Mol Cell. 2015;59:904–916. doi: 10.1016/j.molcel.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Gan W, Dai X, Lunardi A, Li Z, Inuzuka H, Liu P, Varmeh S, Zhang J, Cheng L, Sun Y, Asara JM, Beck AH, Huang J, Pandolfi PP, Wei W. SPOP Promotes Ubiquitination and Degradation of the ERG Oncoprotein to Suppress Prostate Cancer Progression. Mol Cell. 2015;59:917–930. doi: 10.1016/j.molcel.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Ren S, Bitler BG, Aird KM, Tu Z, Skordalakes E, Zhu Y, Yan J, Sun Y, Zhang R. SPOP E3 Ubiquitin Ligase Adaptor Promotes Cellular Senescence by Degrading the SENP7 deSUMOylase. Cell Rep. 2015;13:1183–1193. doi: 10.1016/j.celrep.2015.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G, Ci W, Karmakar S, Chen K, Dhar R, Fan Z, Guo Z, Zhang J, Ke Y, Wang L, Zhuang M, Hu S, Li X, Zhou L, Li X, Calabrese MF, Watson ER, Prasad SM, Rinker-Schaeffer C, Eggener SE, Stricker T, Tian Y, Schulman BA, Liu J, White KP. SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer. Cancer Cell. 2014;25:455–468. doi: 10.1016/j.ccr.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evangelou K, Havaki S, Kotsinas A. E2F transcription factors and digestive system malignancies: how much do we know? World J Gastroenterol. 2014;20:10212–10216. doi: 10.3748/wjg.v20.i29.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nawrocki ST, Griffin P, Kelly KR, Carew JS. MLN4924: a novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin Investig Drugs. 2012;21:1563–1573. doi: 10.1517/13543784.2012.707192. [DOI] [PubMed] [Google Scholar]
- 40.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 41.Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyun SY, Sarantuya B, Lee HJ, Jang YJ. APC/C(Cdh1)-dependent degradation of Cdc20 requires a phosphorylation on CRY-box by Polo-like kinase-1 during somatic cell cycle. Biochem Biophys Res Commun. 2013;436:12–18. doi: 10.1016/j.bbrc.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Zhang L, Wang B, Ou CY, Chien CT, Jiang J. A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Dev Cell. 2006;10:719–729. doi: 10.1016/j.devcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH, Baek SH, Chiba T, Tanaka K, Bang OS, Joe CO, Chung CH. BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. J Biol Chem. 2006;281:12664–12672. doi: 10.1074/jbc.M600204200. [DOI] [PubMed] [Google Scholar]
- 45.Verma R, Peters NR, D'Onofrio M, Tochtrop GP, Sakamoto KM, Varadan R, Zhang M, Coffino P, Fushman D, Deshaies RJ, King RW. Ubistatins inhibit proteasome-dependent degradation by binding the ubiquitin chain. Science. 2004;306:117–120. doi: 10.1126/science.1100946. [DOI] [PubMed] [Google Scholar]
- 46.Li C, Ao J, Fu J, Lee DF, Xu J, Lonard D, O'Malley BW. Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene. 2011;30:4350–4364. doi: 10.1038/onc.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Wan L, Zhong J, Inuzuka H, Liu P, Sarkar FH, Wei W. Cdc20: a potential novel therapeutic target for cancer treatment. Current pharmaceutical design. 2013;19:3210–3214. doi: 10.2174/1381612811319180005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Zhang J, Wan L, Zhou X, Wang Z, Wei W. Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacol Ther. 2015;151:141–151. doi: 10.1016/j.pharmthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.