Abstract
Kappa opioid receptor (KOR) agonists produce dysphoria and psychotomimesis. While KOR agonists produce pro-depressant-like effects, KOR antagonists produce anti-depressant-like effects in rodent models. The cellular mechanisms and downstream effector(s) by which KOR ligands produce these effects are not clear. KOR agonists modulate serotonin (5-HT) transmission in the brain regions implicated in mood and motivation regulation. Presynaptic serotonin transporter (SERT) activity is critical in the modulation of synaptic 5-HT and, subsequently, in mood disorders. Detailing the molecular events of KOR-linked SERT regulation is important for examining the postulated role of this protein in mood disorders. In this study, we used heterologous expression systems and native tissue preparations to determine the cellular signaling cascades linked to KOR-mediated SERT regulation. KOR agonists U69,593 and U50,488 produced a time and concentration dependent KOR antagonist-reversible decrease in SERT function. KOR-mediated functional down-regulation of SERT is sensitive to CaMKII and Akt inhibition. The U69,593-evoked decrease in SERT activity is associated with a decreased transport Vmax, reduced SERT cell surface expression, and increased SERT phosphorylation. Furthermore, KOR activation enhanced SERT internalization and decreased SERT delivery to the membrane. These data demonstrate that KOR activation decreases 5-HT uptake by altering SERT trafficking mechanisms and phosphorylation status to reduce the functional availability of surface SERT.
Keywords: kappa opioid receptor, serotonin, clearance, serotonin transporter, phosphorylation, trafficking
1. Introduction
The catalytic properties of serotonin transporters (SERT, SLC6A4) on serotonergic terminals regulate the synaptic availability of serotonin (5-HT) by rapid reuptake of vesicle-released 5-HT and are thus a key factor in the determination of serotonergic neurotransmission (Murphy et al., 1998; Ramamoorthy et al., 1993). Biochemical, pharmacological, and brain imaging studies pointed out the evidence for altered expression of SERT in depression, OCD, autism, anxiety, and addiction (Cannon et al., 2007; Cook et al., 1997; Hrdina, 1989; Insel et al., 1991; Murphy et al., 2004; Selvaraj et al., 2011). Therapeutic agents used to treat neuropsychiatric disorders, such as serotonin selective reuptake inhibitors (SSRIs), primarily inhibit SERT activity to modulate serotonergic signals in the CNS (Ramamoorthy et al., 1993). In addition, SERT is one of the major targets for drugs of abuse, such as cocaine and 3,4 –methylenedioxymethampetamine (MDMA or “Ecstasy”) (Bengel et al., 1998). Thus, SERT figures prominently in both psychotherapeutics and addiction biology. The functional regulation of SERT is dynamic and mediated by multiple regulatory pathways that control SERT-gene transcription, expression, catalytic activity, and trafficking. SERT activity is rapidly altered in response to several types of protein kinase activation and/or inhibition by modulating SERT phosphorylation, protein-protein interactions, and segregation into lipid raft domains (reviewed in (Ramamoorthy et al., 2011)). In addition, SERT-substrates and inhibitors can also influence kinase/phosphatase-mediated SERT regulation (Ramamoorthy and Blakely, 1999). Furthermore, recent studies have pointed out that single nucleotide polymorphisms (SNPs) within the SERT protein can impact normal regulation of SERT (Prasad et al., 2005; Veenstra-VanderWeele et al., 2012; Zhang et al., 2007).
Kappa Opioid Receptor (KOR) is a G-protein coupled receptor (GPCR) expressed on serotonergic neurons and neuronal terminals (Berger et al., 2006; Kalyuzhny and Wessendorf, 1999). Activation of KOR by the endogenous KOR ligand dynorphin or synthetic KOR agonists decreases 5-HT release (Grilli et al., 2009; Tao and Auerbach, 2002). As a GPCR, KOR-activation triggers several diverse intracellular signaling cascades (Bruchas and Chavkin, 2010). We hypothesized that KOR-linked signaling cascades might also be involved in the regulation of 5-HT reuptake via regulating SERT function. Our previous study has shown that while KOR activation increased dopamine transporter (DAT) activity, it decreased SERT activity and had no effect on norepinephrine transporter (NET) activity (Kivell et al., 2014). However, the molecular phenomenon underlying KOR-mediated SERT regulation is unknown. In an attempt to elucidate KOR-mediated SERT regulation in detail, we studied KOR-mediated SERT regulation in a cell model system where both KOR and SERT are coexpressed heterologously and in the rat brain striatum where both KOR and SERT are expressed endogenously. The present study revealed that acute activation of KOR downregulates SERT function via protein kinase B/Akt (Akt) and calcium calmodulin dependent kinase II (CaMKII) dependent signaling pathways. The KOR-mediated SERT downregulation is accompanied by a decrease in SERT plasma membrane delivery and an increase in SERT internalization and phosphorylation. The results indicate that these SERT regulatory mechanisms might contribute to decreased surface SERT levels and 5-HT transport.
2. Materials and Methods
2.1. Materials
Fetal bovine serum was obtained from Hyclone/GE Healthcare Life Sciences (Logan, UT), Lipofectamine™ 2000, DMEM and other cell culture media from Invitrogen/Life Technologies, (Grand Island, NY). AKT inhibitor Akt X, KN93, 4-(4-fluorophenyl)-2-(4-nitrophenyl)-5-(4-pyridyl)-1H-imidazole (PD169316), 2-(2-Amino-3-methoxyphenyl)-4H-1-benopyram—one (PD98059), 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazole (3,4,d)pyrimidine (PP2) and polyvinylidene difluoride membrane were purchased from EMD Milipore (Billerica, MA). 5-Hydroxy-[3H] tryptamine creatine sulphate ([3H]5-HT; 26.8 Ci/mmol), 32PO4 carrier-free orthophosphate and Optiphase Supermix, were purchased from PerkinElmer Inc. (Waltham, MA). U69,593, U50,488 and protease cocktail were obtained from Sigma-Aldrich (St. Louis, MO). Lipofectamine™ 2000, DMEM and all other cell culture media were purchased from Invitrogen/Life Technologies, (Grand Island, NY). SDS-polyacrylamide gel electrophoresis and Bradford protein assay were from Bio-Rad (Hercules, CA), ECL reagents, Supersignal West Pico Chemiluminscent substrate were from Thermo Fisher Scientific Inc., (Rockford, IL), Protein A Sepharose was obtained from GE Healthcare Life Sciences (Pittsburg, PA), Sulfosuccinimidyl-2-[biotinamido]ethyi-1,3-dithiopropionate (EZ link NHS-Sulfo-SS-biotin), Sulfo-NHS-Acetate and NeutrAvidin Agarose were purchased from Pierce (Rockford, IL), Anti-Calnexin was from BD Biosciences (San Jose CA), SR-12 SERT antibodies were generated by our laboratory. In our previous studies, the use and specificity of SR-12 SERT antibody were thoroughly characterized and published (Annamalai et al., 2012; Jayanthi et al., 2005; Samuvel et al., 2005). Anti-Phospho Akt1/1/2/3 (Ser473) was from Santa Cruz Biotechnology (Dallas, TX), Akt1 antibody (clone AW24) was from Millipore (Billerica, MA), HRP-conjugated secondary antibody from Jackson ImmunoResearch laboratories (West Grove, PA). Stock solutions of U69,593, PD169316, PD98059 and KN93 were prepared in ethanol, and U50,488 was prepared in KRH buffer. In all experiments, Vehicle-E contained 0.2–0.01% ethanol and Vehicle-B contained KRH buffer as needed.
2.2. Animals
Male Sprague-Dawley (200–250g Charles River Laboratories) rats were used. All procedures were approved by the Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide (NIH Publication No. 8023, revised 1978) for the Care and Use of Laboratory Animals. Rats were maintained in a temperature and humidity controlled room on a 12:12 h light/dark cycle. Food and water were supplied ad libitum. All efforts and care were taken to minimize animal suffering and to reduce the number of animals used. As alternatives to brain tissues, cell culture models were utilized.
2.3. Cell Culture and Transfection
HEK-293 (human embryonic kidney) cell line expressing a macrophage scavenger receptor to increase adherence to tissue culture plates (EM4 cells), were grown in Dulbecco’s Modified Eagle’s Media (DMEM) containing 10% fetal bovine serum, 2 mM glutamine, 100 μg/ml streptomycin and 100 units/ml penicillin at 37°C and in an atmosphere of 95% air and 5% CO2. Trypsinized cells were seeded in 24 well plates (100,000/well) for uptake assays or in 12 well plates (150,000/well) for biotinylation assay or in 35mm petri dishes (250,000/dish) for endocytosis and exocytosis assays. Cells were transiently co-transfected with cDNAs, 0.2μg His-hSERT plus 0.3μg myc-KOR or 0.3μg empty vector (pcDNA3) for uptake assays; 0.4μg His-hSERT plus 0.6μg myc-KOR or 0.6μg pcDNA3 for biotinylation assays; 0.6μg His-hSERT plus 0.9μg myc-KOR or 0.9μg pcDNA3 for exocytosis and endocytosis assays using Lipofectamine 2000 according to manufacturer’s instruction. Where indicated, cells were treated with different modulators and used 24 hr post transfection.
2.4. 5-HT Uptake in Transfected EM4 Cells
5-HT uptake was performed as described previously (Samuvel et al., 2005). The transfected cells were starved for 2 hours with serum free DMEM media at 37°C. Serum-starved cells were washed with warm Krebs-Ringer-HEPES (KRH) buffer (120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2 10 mM HEPES, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM D- glucose, pH 7.4) containing 0.1 mM ascorbic acid, and 0.1 mM pargyline and treated at 37°C with different modulators at indicated concentrations and times. To check the effect of kinase inhibitors, pre-starved cells were incubated with kinase inhibitors as indicated under legends, at 37°C for 45 min followed by U69,593 or U50,488 or appropriate vehicles for 15 min. The treated cells were incubated with 50 nM radiolabelled [3H]5-HT for 6 min and the radiolabelled cells were washed with KRH buffer twice. For saturation analysis, [3H]5-HT was mixed with unlabeled 5-HT from 10 nM to 2 μM. The cells were lysed in 400μl of Optiphase Supermix scintillant and the radioactivity was measured using MicroBeta2 LumiJET liquid scintillation counter (Perkin Elmer Inc., Waltham, MA). Nonspecific 5-HT uptake is defined as the accumulation in the presence of 0.05 μM fluoxetine and was subtracted from total 5-HT uptake. The experiments were repeated three times with different passages of EM4 cells and data are given as mean ± S.E.M. values. Maximal Velocity (Vmax) and substrate Km values were determined by nonlinear least square fits plotted as femtomoles of 5-HT uptake against the concentration of 5-HT using Michaelis-Menten equation.
2.5. Immunoblot Analysis
As indicated under legends, after the treatment, cells or synaptosomes were with lysed RIPA extraction buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate) supplemented with a mixture of protease inhibitors cocktail (100μM 4-(2-Aminoethyl)-Benzensesulfonyl Fluoride HCl, 0.8 μM aprotinin, 2 μM leupeptin, 1.5 μM pepstatin and 4 μM bestatin) and phosphatase inhibitors (10 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 μM okadaic acid). Equal quantity of proteins from the extracts were incubated with Laemmli buffer (62.5 mm Tris, pH 6.8, 20% glycerol, 2% SDS, 5% β-mercaptoethanol, and 0.01% bromophenol blue) and proteins were separated by 10% SDS-PAGE and transferred overnight onto polyvinylidene difluoride membrane at 4°C. Specific antibodies and appropriate secondary antibodies were used to visualize specific proteins as given under legends. The same blots were also stripped and reprobed with total Akt and calnexin (Samuvel et al., 2005). The intensity of protein band density was analyzed by utilizing NIH Image J software (1.48v).
2.6. Surface Protein Biotinylation Using EM4 Cells
Transiently transfected cells were pre-starved for 2 hours at 37°C and treated with U69,593 and Vehicle-E for 15 min at 37°C. Cells were washed with cold PBS/Ca/Mg solution (138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 9.6 mM Na2HPO4, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.3) and subjected to biotinylation as described earlier by us (Annamalai et al., 2012; Samuvel et al., 2005). Successively, the cells were lysed in RIPA buffer by shaking the petri dishes for one hour at 4°C and by passing through 25 Gauge needle 6 times. The samples were centrifuged at 25000 × g for 30 min and the protein concentration was estimated using Bradford protein assay. Equal amounts of proteins were incubated with 60 μl of NeutrAvidin agarose beads at 4°C overnight followed by RIPA buffer-wash thrice. Bound proteins were eluted with 45 μl of Laemmli buffer by shaking for 20 min at room temperature. Aliquots of total, unbound and eluted bound proteins were ran on 10% SDS-PAGE with protein marker and transferred onto polyvinylidene difluoride membrane overnight at 4°C. SR-12 antibody was used to detect the SERT. The blots were visualized with enhanced chemiluminescences reagents (Amersham Biosciences, GE Healthcare, Pittsburgh, USA). The proteins were quantified by utilizing NIH Image J software (1.48v) to ensure that the results were in linear range of film exposure. The blots were stripped and reprobed with anti-calnexin antibody to check for equal protein loading and to rule out contamination of biotinylated proteins with intracellular proteins.
2.7. Measurement of Endocytosed or Internalized SERT Using EM4 Cells
Serum-starved cells were washed with cold PBS/Ca/Mg solution and incubated with 0.5 mg/ml disulfide-cleavable biotin (sulfo-NHS-SS-Biotin) in cold PBS/Ca/Mg solution for 30 min on ice. The biotinylated cells were washed with cold PBS/Ca/Mg solution and biotinylation was terminated with 100 mM glycine in PBS/Ca/Mg solution for 15 min. The cells were washed with warm PBS/Ca/Mg solution and treated with U69,593 or Vehicle-E for 15 min at 37°C to induce endocytosis. The cells were brought back to ice and washed with cold PBS/Ca/Mg solution and incubated twice with 50 μM sodium 2-mercapto-ethanesulfonate (MesNa), a reducing agent in PBS/Ca-Mg buffer for 20 min to dissociate the biotin from cell surface-resident proteins via disulfide exchange. To define total biotinylated SERT, one dish of biotinylated cells was kept at 4°C and not subjected to reduction with MesNa, and directly processed for extraction. To define MesNa-accessible biotinylated SERT, another dish of biotinylated cells was treated with Vehicle-E for 15 min at 4°C and then treated with MesNa to reveal the quantity of surface SERT biotinylation that MesNa can reverse efficiently. The cells were lysed with RIPA buffer containing protease inhibitors (Jayanthi et al., 2006; Samuvel et al., 2005). The biotinylated proteins were isolated using NeutrAvidin beads and the biotinylated proteins were separated by SDS-PAGE and immunoblotting as described above.
2.8. Measurement of Plasma Membrane Delivery (Exocytosis) of SERT Using EM4 Cells
Serum-starved cells were washed with cold PBS/Ca/Mg solution and incubated with cell membrane impermeable sulfo-NHS acetate (1 mg/ml) for an hour on ice, followed by washing with ice-cold PBS/Ca/Mg solution. Then, the cells were biotinylated with sulfo-NHS-SS-Biotin in the presence of U69,593 or Vehicle-E for 15 min at 37°C to induce exocytosis and control cells with Vehicle-E were incubated on ice. To determine the effectiveness of sulfo-NHS acetate treatment to block all preexisting NHS-reactive biotinylation sites on the cell surface, another dish of cells containing Vehicle E was biotinylated at 4°C without sulfo-NHS acetate treatment. The treated cells were brought back on to ice and biotinylation was quenched with 100 mM glycine in cold PBS/Ca/Mg solution for 15 min twice. The cells were washed with cold PBS/Ca/Mg solution and lysed in RIPA buffer (Jayanthi et al., 2006). Biotinylated SERT proteins were detected and quantified as described above. Biotinylated transferrin receptor (TfR) was analyzed by stripping and reprobing the blot with TfR antibody.
2.9. SERT Phosphorylation in Transfected EM4 Cells
SERT phosphorylation in SERT plus KOR co-transfected EM4 cells was measured and quantified as described previously (Ramamoorthy and Blakely, 1999; Ramamoorthy et al., 1998). Briefly, cells were metabolically labeled with [32P]-orthophosphate in phosphate-free medium for 1 hour and incubated with Vehicle-E or U69,593 (10 μM) in one set, and with Vehicle-B or U50,488 (10 μM) in another set for 30 min at 37°C. The cells were washed and solubilized with RIPA buffer containing protease and phosphatase inhibitors (composition given above under Immunoblot Analysis). The SERT proteins were immunoprecipitated using SERT specific antibody SR-12. The immunoprecipitated proteins were isolated by the addition of protein A-Sepharose beads, washed three times with RIPA buffer and the proteins were eluted in 45 μl of Laemmli sample buffer for 30 min at 22°C. The eluted proteins were separated by SDS-PAGE (10%). Gels were dried and exposed to Kodak BioMax Ms-1 films and 32P-radiolabeled SERT was visualized by autoradiography. Multiple exposures (1, 2, 4 and 6 days) were evaluated by digital quantitation using NIH ImageJ (version 1.48j) software to ensure that results were within the linear range of the film (Ramamoorthy and Blakely, 1999; Ramamoorthy et al., 1998).
2.10. Synaptosomes Preparations and 5-HT Uptake
Synaptosomal preparations and 5-HT uptake were performed as described previously (Samuvel et al., 2005). Dorsal and ventral striatum was dissected from rat brain and immediately homogenized in cold 0.32 M sucrose. The homogenate was centrifuged at 1,000 g for 10 min at 4 °C and the resulting supernatant was centrifuged at 12,000 g for 20 min and the pellet was washed by resuspending in 0.32 M sucrose. The synaptosomes were suspended in 0.32 M sucrose saturated with 95%O2, 5%CO2. Protein concentration was determined by DC protein assay (Bio-Rad) using bovine serum albumin as standard. The synaptosomes (50 μg) were incubated with U69,593 or U50,488 or appropriate vehicle (Vehicle-E or Vehicle-B) for 15 min at 37°C in a total volume of 500 μl of KRH buffer. 5-HT uptake was measured by adding 50 nm of radiolabelled [3H]5-HT and continuing incubation at 37°C for 6 min. To check the effect of kinase inhibitors, synaptosomes were preincubated with kinase inhibitors as indicated under legends, at 37°C for 45 min followed by U69,593 or U50,488 or appropriate vehicle (Vehicle-E or Vehicle-B) for 15 min. The uptake was terminated by adding 100 μM of imipramine in ice-cold KRH buffer and filtered over 0.3% polyethylenimine coated GF-B filters using a Brandel Cell Harvester (Brandel Inc., Gaithersburg, MD). The filters were washed with 5 ml of cold PBS solution thrice and radioactivity retained to filter was detected by liquid scintillation counter. Non- specific uptake measured in the presence of 0.01 μM of fluoxetine was subtracted from total accumulation of 5-HT to provide SERT specific total 5-HT uptake.
2.11. Surface Protein Biotinylation Using Synaptosomes
Synaptosomes (300–500 μg) were incubated with U69,593 or U50,488 or appropriate vehicle (Vehicle-E or Vehicle-B) for 15 min at 37°C and biotinylated as described earlier by us (Samuvel et al., 2005). Biotinylated proteins were separated from nonbiotinylated proteins using avidin beads. Aliquots from total extracts (50 μl) and the entire avidin eluted fractions were separated by SDS-PAGE (10%) and transferred to membrane. The blots were probed with antibodies to SERT (SR12 antibody) and calnexin followed by quantifications as described earlier above.
2.12. SERT Phosphorylation in Synaptosomes
The protocol for assaying U69,593/KOR triggered SERT phosphorylation was similar as described previously (Samuvel et al., 2005). Synaptosomes prepared from ventral striatum (~0.750 mg) were incubated with 5.0 mCi [32P]-orthophosphate for 45 min prior to the addition of Vehicle-E and various of concentrations of U69,593 (μM: 0.5, 1, 5, 10) for 15 min at 37°C. Samples were then centrifuged and the pellet resuspended in RIPA buffer (containing protease and phosphatase inhibitors, composition given under Immunoblot Analysis) by passing through 25-gauge needle for 10 times. SERT immunoprecipitation with SR-12 antibody, SDS-PAGE followed by autoradiography to visualize phosphorylated SERT similar are to that described above under SERT Phosphorylation in Transfected EM4 Cells.
2.13. Statistical Analysis
GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA) was used for all data analyses and to generate graphical representations. Values are expressed as mean ± S.E.M. As noted in the figure legends and in the result section, one-way analysis of variance was used followed by post hoc testing (Bonferroni) for multiple comparisons. Two-tailed unpaired Student’s t test analysis was performed for comparisons between two groups. A value of p ≤ 0.05 was considered statistically significant.
3. Results
3.1. KOR activation inhibits SERT function
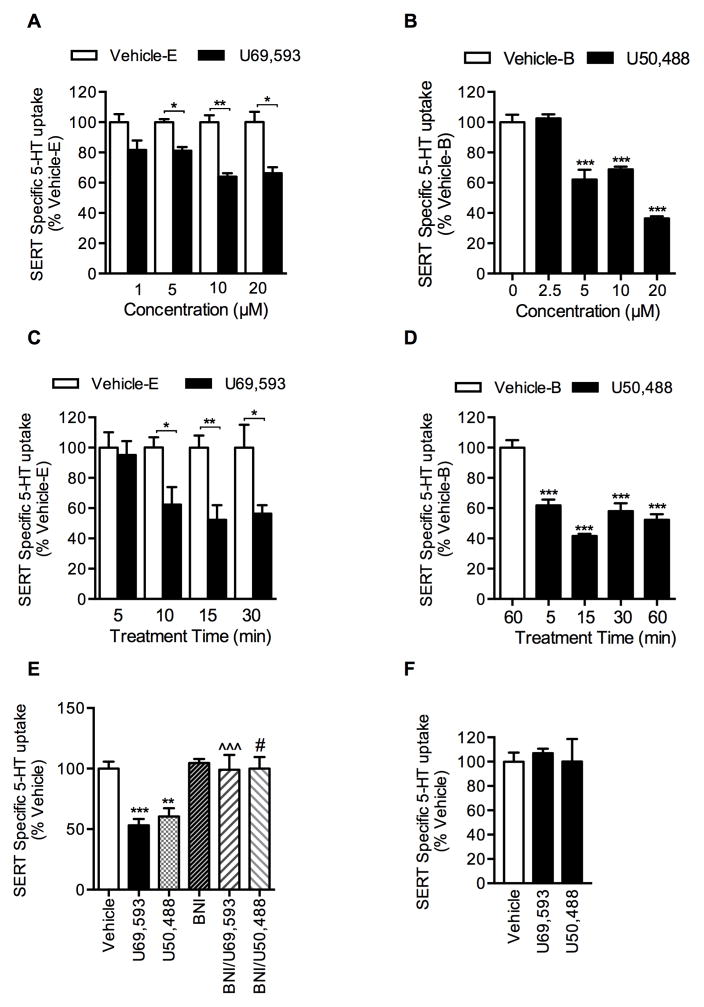
Pretreatment of EM4 cells co-transfected with SERT plus KOR with KOR agonists U69,593 or U50,488 reduced SERT mediated 5-HT uptake in a time and concentration dependent manner, as shown in Figures 1A–D. While a non-significant decrease (18%) in 5-HT uptake was evident following treatment with 1 μM U69,593, treatment with 5, 10, and 20 μM U69,593 produced significant inhibition (19%, 36%, and 34%, respectively) (Fig. 1A). Treatment with 5, 10, and 20 μM U50,488 also resulted in significant inhibition of SERT activity (38%, 32%, and 63%, respectively) (Fig. 1B). Results from the time course are shown in Figures 1C and 1D, where U69,593 and U50,488 at 10 μM produced significant reductions in 5-HT uptake following 10 and 5 min treatment times (38% and 38%, respectively). Comparison of dose and time course effects between U69,593 and U50,488 revealed comparable effects on SERT function, although U50,488 produced higher SERT inhibition in a shorter time period than U69,593. U69,593- or U50,488- induced SERT inhibition was completely abolished when the cells were pretreated with KOR antagonist nor-binaltorphimine (BNI) prior to U69,593 or U50,488 treatment (Fig. 1E); BNI alone did not produce any significant effect on SERT activity (Fig. 1E). Furthermore, U69,593 or U50,488 failed to produce any effect on SERT function in cells expressing only the SERT (in the absence of KOR coexpression) (Fig. 1F). These findings indicate that U69,593- or U50,488-induced SERT inhibition is mediated by KOR activation. U69,593 or U50,488 at 10 μM concentration was chosen for subsequent experiments. Several studies have used this concentration of U50,488 and U69,593 to delineate KOR-triggered signaling in heterologous transfected cell models as well as primary striatal neurons and astrocytes (Bruchas et al., 2006; Chen et al., 2016; Clayton et al., 2009; McLaughlin et al., 2003; Schmid et al., 2013).
Figure 1. Regulation of 5-HT uptake (SERT activity) by KOR ligands in EM4 cells.
EM4 cells were transfected with hSERT plus either pcDNA3 (empty vector) or KOR. After 24 hrs, cells were treated with KOR ligands or the vehicle as described under “Materials and Methods”. Non-specific 5-HT uptake in the presence of 0.1 μM fluoxetine was subtracted from the total 5-HT uptake to obtain SERT mediated 5-HT transport. The results were expressed as percentage of 5-HT uptake by vehicle-E or vehicle-B (100%) treated cells and provided as mean ± S.E.M of three independent experiments performed in duplicates or triplicates. A, B. Dose dependent effect of KOR-agonists U69,593 and U50,488: Cells were pre-incubated with indicated concentrations of U69,593 or U50,488 or corresponding vehicle (Vehicle-E or Vehicle-B) for 15 min at 37°C followed by 5-HT uptake assays. Both U69,593 and U50,488 decreased SERT activity (One-way ANOVA: F(7,34) = 12.12, P <0.0001 for U69,595 and F(7,48) = 120.1, P <0.0001 for U50,488). Bonferroni’s post hoc test: *p <0.05; **p < 0,01; ***p <0.001 U69,593 or U50,488 versus Vehicle-E (U69,593) or Vehicle-B (U50,488). C, D. Time dependent effect of KOR-agonists U69,593 and U50,488: Cells were pre-incubated with vehicle or 10 μM U69,593 or U50,488 for indicated times prior to 5-HT uptake assay. (One-way ANOVA: F(7,40) = 4.94, P <0.0004 for U69,595 and F(4,30) = 30.38, P <0.0001 for U50,488). Bonferroni’s post hoc test: *p <0.05; **p < 0,01; ***p <0.001 U69,593 or U50,488 versus control vehicle (Vehicle-E (U69,593) or Vehicle-B (U50,488). E, Effect of KOR antagonist BNI on U69,593 or U50,488-mediated down regulation of SERT function: Pre-incubation of BNI (1 μM, 60 min) prior to U69,593 or U50,488 (10 μM, 15 min) exposure prevents U69,593 or U50,488-mediated SERT down regulation (One-way ANOVA: F(5,47) = 8.16, P <0.0001). Bonferroni’s post hoc test: **p <0.01; ***p < 0,001 U69,593 or U50,488 versus control vehicle (contained both ethanol and buffer vehicle); ^^^p <0.001 verses U69,593; #p < 0.05 versus U50,488). F. U69,593 and U50,488 decreases SERT activity through KOR activation: EM4 cells expressing SERT with out the expression of KOR were treated with U69,593 or U50,488 (10 μM) or corresponding vehicle (contained both ethanol and buffer vehicle) for 15 min at 37°C followed by 6 min 5-HT uptake assays. In the absence of KOR coexpression, U69,593 and U50,488 failed to decrease SERT-mediated 5-HT uptake in cells expressing SERT. (One-way ANOVA: F(5=2,15) = 0.12, P <0.089).
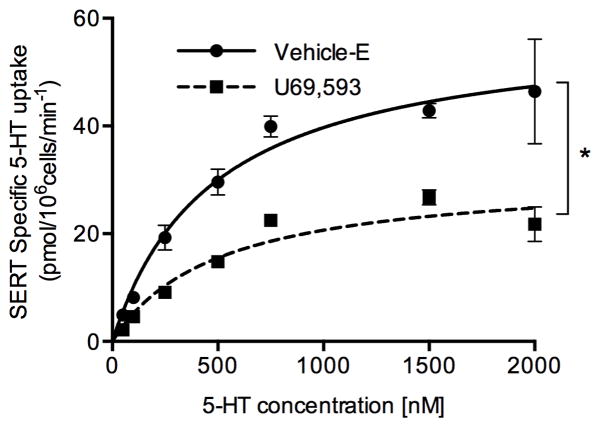
3.2. KOR agonist U69,593 alters SERT kinetic parameters
The kinetic analysis of SERT-mediated 5-HT uptake was determined in Vehicle-E and U69,593 treated EM4 cells coexpressing SERT and KOR (Fig. 2). U69,593 treatment decreased the maximal velocity (Vmax) significantly from 58.09 ± 5.21 (Vehicle-E) to 29.35 ± 1.23 (U69,593) pmol/min/106 cells. There was no significant change in SERT’s affinity for 5-HT following U69,593 treatment (Vehicle-E: Km = 866.9 ± 292.8 nM and U69,593: Km = 664.4 ± 120.7 nM).
Figure 2. Effect of KOR activation on SERT kinetics in EM4 cells.
EM4 cells coexpressing SERT and KOR were treated with 10 μM U69,593 or Vehicle-E for 15 min at 37°C. At the end of the treatments, SERT mediated 5-HT uptake (6 min) was measured over a concentration range of 0.025 to 2 μM 5-HT as described under “Materials and Methods” and in Figure 1 legend. Nonspecific uptake at each concentration of 5-HT used (in the presence of 0.01 μM fluoxetine) was subtracted from total uptake (measured in the absence of fluoxetine) to calculate SERT specific 5-HT uptake. Values represent the average ± S.E.M of 4–6 values. Nonlinear curve fits of uptake data (Michaelis-Menten equation) are given. *p<0.026 (Vmax U69,593 versus Vehicle-E, Student’s t test: t=5.368, df=2.21). Km: U69,593 versus vehicle, Student’s t test: t=0.639, df=2.66, p<0.574.
3.3. KOR-induced SERT downregulation is mediated by Akt and CaMKII
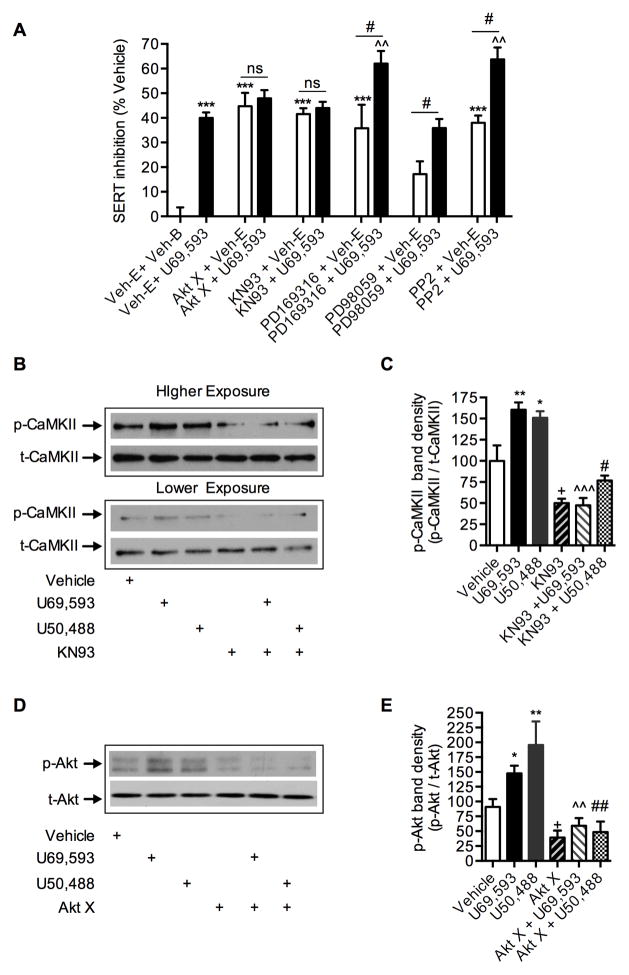
Our previous studies have established that Akt, CaMKII, p38 MAPK, PKG, and Src kinase inhibitors inhibit SERT activity (Annamalai et al., 2012; Rajamanickam et al., 2015; Samuvel et al., 2005). KOR activation triggers several downstream kinase pathways (Bruchas and Chavkin, 2010). To determine the signaling pathways downstream of KOR activation involved in mediating SERT downregulation, EM4 cells coexpressing SERT and KOR were treated with various kinase inhibitors at maximal efficacious concentrations prior to U69,593 treatment. Akt X (20 μM) for Protein Kinase B (Rajamanickam et al., 2015), KN93 (2 μM) for CaMKII (Annamalai et al., 2012), PD169316 (20 μM) for p38 MAPK (Samuvel et al., 2005), PP2 (20 μM) for Src Kinase (Annamalai et al., 2012) and PD98059 (20 μM) for Erk1/2 Kinases (Annamalai et al., 2012) were used as specific kinase inhibitors. Figure 3 shows the influence of kinase inhibitors on U69,593-mediated SERT inhibition. Consistent with previously reported studies (Annamalai et al., 2012; Rajamanickam et al., 2015; Samuvel et al., 2005), all kinase inhibitors, except Erk1/2 inhibitor PD98059, significantly inhibited SERT function to varying degrees (Fig. 3A). Treatment with U69,593 produced an additive inhibition of SERT function in cells pretreated with PD169316, PP2, or PD98059 (Fig. 3A). However, U69,593 treatment did not produce additive inhibition of SERT activity in cells pretreated with Akt X or KN93 (Fig. 3A). Furthermore, U69,593 treatment enhanced the activation of Akt and CaMKII, as evidenced by increased levels of both phospho-Akt and phospho-CaMKII in immunoblot analyses (Fig. 3B, 3C, 3D and 3E). Total expression levels of Akt and CaMKII were not altered upon U69,593 treatment. Pretreatment with Akt X or KN93 prior to U69,593 treatment prevented the U69,593-stimulated increase in phospho-Akt and phospho-CaMKII, respectively (Fig. 3D and 3E). These results strongly support the hypothesis that Akt and CaMKII play a crucial role in KOR-mediated downregulation of SERT function.
Figure 3. Effect of protein kinase inhibitors on KOR mediated down regulation of SERT activity and on the levels of active Akt and CaMKII in EM4 cells.
A. Additive and non-additive effects of kinase inhibitors on KOR- mediated down regulation of SERT activity: EM4 cells coexpressing SERT and KOR were pre-incubated with kinase inhibitors at maximal effective doses on the SERT activity (20 μM Akt X (Akt) or 2 μM KN93 (CaMKII) or 20 μM PD169316 (p38 MAPK) or 20 μM PD98059 (ERK1/2) or 20 μM PP2 (Src), see ref. (Annamalai et al., 2012; Rajamanickam et al., 2015; Samuvel et al., 2005)) or corresponding vehicles for 45 min prior to vehicle or U69,593 exposure (10 μM, 15 min). SERT mediated 5-HT uptake was determined as described under Figure 1 legend. The results were expressed as percentage of 5-HT uptake inhibition of Veh-B + Veh-E (Veh: Vehicle) treated cells and mean values ± S.E.M are given from three independent experiments performed in duplicates or triplicates. The percent inhibition of SERT was compared between vehicles and U69,593 in the presence and absence of pretreated kinase inhibitors. ***p <0.001 versus Veh-B + Veh-E; ^^p < 0.01 versus Veh-E + U69,593 (one-way ANOVA analysis of variance with Bonferroni’s multiple comparison test). #p <0.05 versus PD169316 + Veh-E (t=2.28, df=9) or PD98059 + Veh-E (t=2.92, df=8) or PP2 + Veh-E (t=4.56, df=4); ns: non-significant versus Akt X + Veh-E (p ≥0.63, t=0.50, df=10) or KN93 + Veh-E (p ≥0.05, t=0.69, df=10), two-tailed Student’s t test. B–E. KOR agonists U69,593 and U50,488 trigger CaMKII and Akt phosphorylation: KOR + SERT coexpressing EM4 cells were pretreated with KN93 (2 μM) or Akt X (20 μM) prior to U69,593 (10 μM) or U50,488 (10 μM) or the vehicle (contained both ethanol and buffer vehicles) for 15 min. Phosphorylated CaMKII (p-CaMKII), total CaMKII (t-CaMKII), phosphorylated Akt (p-Akt) and total Akt (t-Akt) were determined by immunoblotting with specific antibodies as described under “Materials and Methods”. B. Representative immunoblot shows p-CaMKII and t-CaMKII levels. C. For each samples, p-CaMKII was normalized to t-CaMKII and percent band density was given. Both U69,593 and U50,488 stimulated CaMKII phosphorylation and was sensitive to pretreatment with KN93. (One-way ANOVA: F(5,27) = 17.14, P <0.0001). Bonferroni’s post hoc test: *p < 0.05, **p <0.01 U69,593 or U50,488 versus control vehicle; +p < 0.05 KN93 versus control vehicle; ^^^p <0.001 KN93 + U69,593 verses U69,593; #p < 0.05 KN93 + U50,488 versus U50,488). D. Representative immunoblot shows p-Akt and t-Akt levels. E. For each samples, p-Akt was normalized to t-Akt and percent band density was given. Both U69,593 and U50,488 stimulated Akt phosphorylation, and was sensitive to pretreatment with Akt X. (One-way ANOVA: F(5,20) = 8.92, P <0.0001). Bonferroni’s post hoc test: *p < 0.05, **p <0.01 U69,593 or U50,488 versus control vehicle; +p < 0.05 Akt X versus control vehicle; ^^p <0.001 Akt X + U69,593 verses U69,593; #p < 0.05 Akt X + U50,488 versus U50,488).
3.4. KOR activation by U69,593 reduces SERT surface expression via decreased SERT exocytosis and enhanced SERT endocytosis and phosphorylation
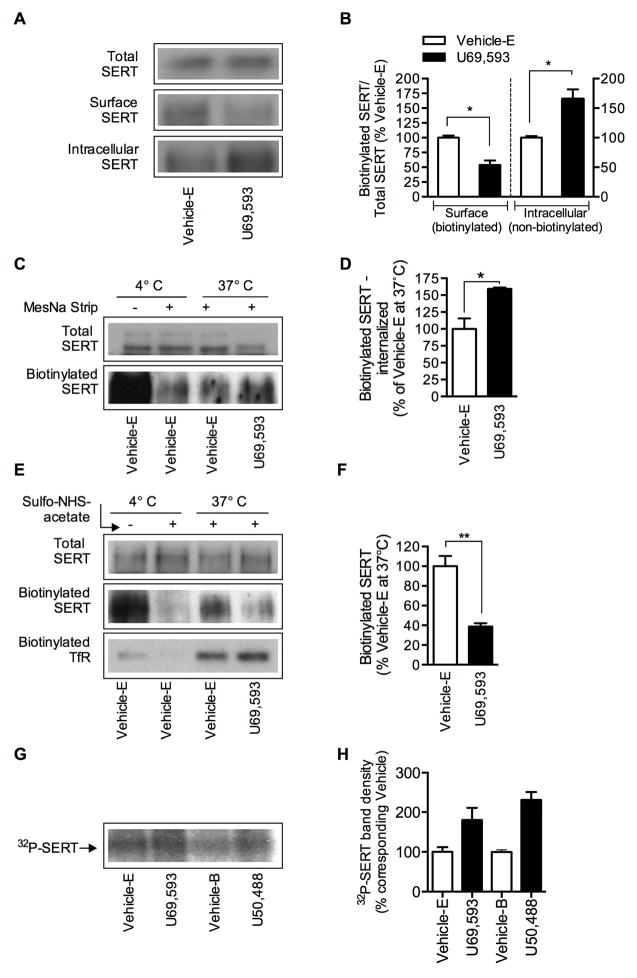
Because SERT functional expression is dynamically regulated at the level of SERT sequestration, trafficking and phosphorylation, SERT surface expression, SERT endocytosis/internalization, exocytosis and phosphorylation were examined in EM4 cells coexpressing SERT and KOR following Vehicle-E or U69,593 treatment. Consistent with our SERT functional assay results (5-HT uptake and kinetics shown in Fig. 1 and 2), cell surface biotinylation showed a significant (~47%) reduction in SERT surface expression (biotinylated) with concomitant increase (~65%) in intracellular SERT (non-biotinylated) following U69,593 treatment compared to Vehicle-E treatment (Fig. 4A and 4B). There was no change in the total SERT protein following U69,593 treatment compared to Vehicle-E treatment. Immunoblotting with calnexin antibody showed equal protein loading in the total blot and no intracellular calnexin protein is seen in the bound fractions, suggesting intact cell surface biotinylation (data not shown). The decrease in SERT surface expression following U69,593 treatment might arise from alterations in the endocytosis (internalization) or exocytosis (membrane delivery) of SERT protein “from” and “to” the plasma membrane. Reversible biotinylation strategy was employed to examine the internalization of SERT. The cells were incubated with sulfo-NHS-SS-biotin to biotinylate surface proteins and then treated with vehicle or U69,593 at 37°C to induce endocytosis. Next, cells were treated with cell membrane impermeable MesNa to dissociate the biotin from cell surface-resident proteins so that only internalized-biotinylated proteins are captured using avidin bead isolation. Figures 4C and 4D show that the treatment with U69,593 significantly increased the amount of biotinylated SERT internalization (~59%) compared to vehicle treatment. Following incubation with Vehicle-E at 4°C, less than 4% biotinylated SERT was evident after MesNa treatment, indicating that the removal of biotin from biotinylated surface proteins was effective. To examine the effects of U69,593 on SERT plasma membrane delivery, the cells were incubated with cell membrane impermeable sulfo-NHS acetate at 4°C to block all preexisting NHS-reactive biotinylation sites on the cell surface. Then the cells were incubated at 37°C for 15 min with Vehicle E or U69,593 to induce exocytosis in the presence of sulfo-NHS-SS-biotin, which allows biotinylation of proteins that are newly delivered to the cell surface. The biotinylated SERT, representing newly inserted plasma membrane SERT, was reduced significantly (~61%) in cells treated with U69,593 when compared to Vehicle E-treated cells (Fig. 4E & 4F). When compared to total biotinylated SERT from cells not pretreated with sulfo-NHS acetate (4°C, Vehicle-E), there was very little biotinylated SERT indicating that most of the biotinylating sites were successfully blocked by the NHS acetate pretreatment and the plasma membrane delivery was negligible at 4°C. Reprobing with transferrin antibody showed TfR bands only at the exocytosis-permissive temperature (37°C) but not at the exocytosis non-permissive temperature (4°C), as well as TfR bands showed no significant difference between Vehicle-E and U69,593 treated cells (Fig. 4E). Because Akt and CaMKII are involved in U69,593-mediated SERT downregulation and SERT is a known phospho-protein (Annamalai et al., 2012; Jayanthi et al., 2005; Ramamoorthy and Blakely, 1999; Ramamoorthy et al., 1998; Ramamoorthy et al., 2007; Samuvel et al., 2005), SERT phosphorylation was examined in cells metabolically labeled with 32P following Vehicle-E or U69,593 treatment in one set and Vehicle-B or U50,488 treatment in another set. The autoradiogram of 32P-labeled SERT is presented in Fig. 4G. Consistent with our previous observations (Ramamoorthy and Blakely, 1999; Ramamoorthy et al., 1998), the basal 32P-labeled SERT band (~96 kDa) was present in vehicle-treated cells. Following treatment with U69,593 or U50,488 (10 μM, 15 min), there was a significant increase in the 32P-labeled SERT band intensity (Fig. 4G and 4H). Together these findings reveal the capacity of KOR-linked downstream signals to regulate SERT function at various levels of cellular cascades involving SERT trafficking and phosphorylation.
Figure 4. Effect of KOR activation on SERT trafficking and phosphorylation in EM4 cells.
EM4 cells coexpressing SERT and KOR were used to determine SERT surface expression, endocytosis and exocytosis of SERT protein and SERT phosphorylation as described under “Materials and Methods”. A to H, cells were treated with vehicle or U69,593 (10 μM) or U50,488 (10 μM) for 15 min at 37°C. The effect of U69,593 was compared with Vehicle-E containing 0.1% ethanol and the effect of U50,488 was compared with Vehicle-B containing KRH buffer. A, B. SERT surface expression: A, representative SERT immunoblots show total, biotinylated (surface) and non-biotinylated (intracellular) SERT (~96 kDa). B, the relative band densities of biotinylated SERT and non-biotinylated SERT that was normalized with total SERT from four separate experiments are presented as mean ± S.E.M. *p <0.05 U69,593 versus Vehicle-E. C, D. SERT internalization/endocytosis: C, a representative immunoblot shows the total biotinylated (lane 1, with out MesNa strip) and internalized-biotin-tagged SERT in Vehicle-E treated cells at 4°C and 37°C, and U69,593 treated cells at 37°C following MesNa treatment. D, SERT band densities of internalized/biotinylated SERT (mean ± S.E.M.) are given. *p <0.05 U69,593 versus Vehicle-E (Student’s t test, N=3). In parallel experiments, cells treated with Vehicle-E were kept at 4°C and total surface biotinylated SERT (without MesNa) and after MesNa treatment (background) were determined to examine the effectiveness of MesNa to remove biotin from biotinylated surface proteins. Note that under nonpermissive endocytosis conditions (4°C) after MesNa treatment, only less than ~5–10% biotinylated SERT (background) was evident from total amount of biotinylated SERT at 4°C (C: first two lanes) E, F. SERT plasma membrane insertion (exocytosis): E, a representative immunoblot shows total SERT, the newly inserted or plasma membrane surface delivered-biotin-tagged SERT and transferrin (TfR). F, the bar graph shows the densities of newly inserted plasma membrane SERT from three experiments (Mean ± S.E.M.). **p <0,01 U69,593 versus Vehicle-E (Student’s t test, N=3). In parallel experiments, cells treated with Vehicle-E were kept at trafficking nonpermissive condition (4°C) and total biotinylated SERT (without sulfo-NHS-acetate treatment) and after sulfo-NHS-acetate treatment (background) were performed to determine the effectiveness of sulfo-NHS-acetate to block all biotinylatable free amino groups. Note that under trafficking nonpermissive condition (4°C) after sulfo-NHS-acetate treatment, only less than ~5 biotinylated SERT (background) was evident from total amount of biotinylated SERT at 4°C (E: first two lanes). G, H. SERT phosphorylation: G, a representative autoradiogram shows 32P-labelled SERT band (~96 kDa) in Vehicle-E, U69,593 and U50,488 treated cells. H, the bar graph shows the relative 32P-labelled SERT band intensity (N=2).
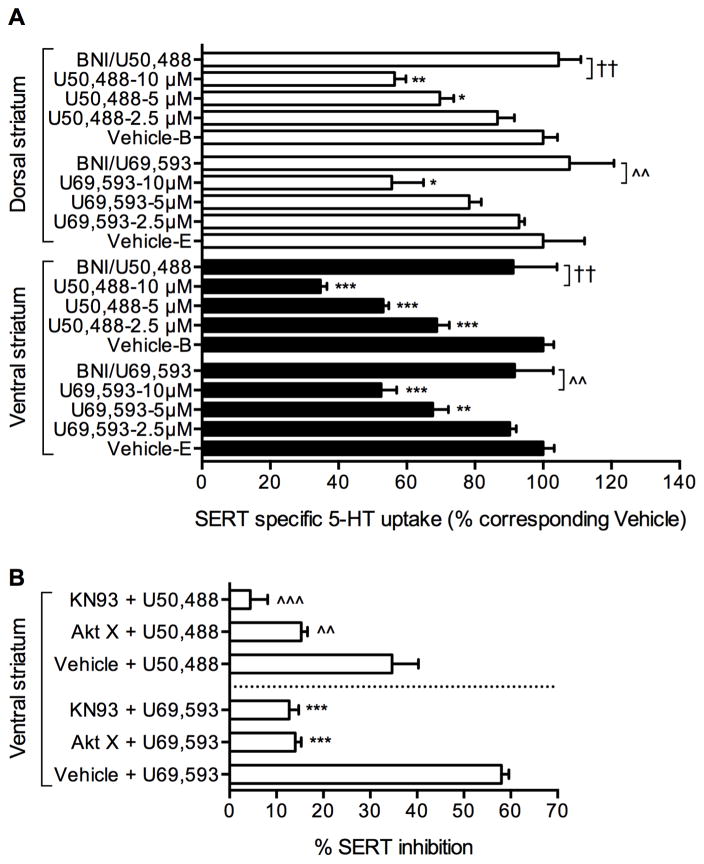
3.5. KOR activation regulates SERT in the rat dorsal and ventral striatum
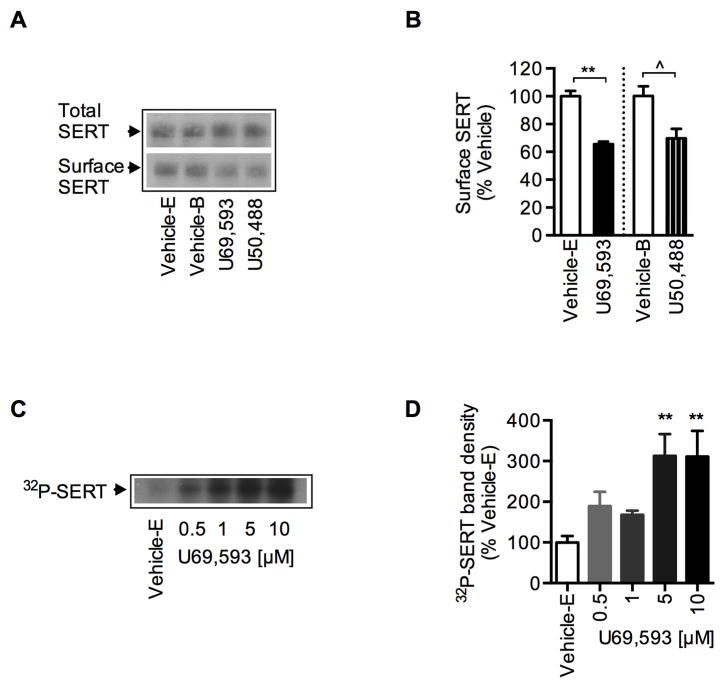
To examine whether native SERT is also regulated by KOR activation, synaptosomes from dorsal and ventral striatum (brain regions known to express both SERT and KOR endogenously) were treated with Vehicle-E or Vehicle-B or different concentrations of U69,593 or U50,488 for 15 min and 5-HT uptake was measured. U69,593 and U50,488 each significantly reduced 5-HT uptake in a dose dependent manner in both the ventral and dorsal striatum (Fig. 5A). The effect of U69,593 or U50,488 on SERT inhibition was abolished by KOR antagonist BNI, establishing the specific role of endogenous KOR in the regulation of native SERT-mediated 5-HT uptake (Fig. 5A). Furthermore, Akt inhibitor Akt X or CaMKII inhibitor KN93 pretreatment of ventral striatal synaptosomes attenuated the inhibitory effects of U69,593 and U50,488 on SERT function (Fig. 5B). Consistent with the reduced 5-HT uptake, treatment of ventral striatal synaptosomes with U69,593 or U50,488 significantly reduced surface SERT protein without changing the total SERT protein levels (Fig. 6A and 6B). Treatment of 32P-labeled ventral striatal synaptosomes with U69,593 resulted in increased 32P-labeled SERT protein (Fig. 6C and 6D). While U69,593 at 0.5 and 1 μM concentrations increased 32P-labeled SERT non-significantly, significantly elevated SERT phosphorylation was observed at 5 and 10 μM concentrations of U69,593. This elevated SERT phosphorylation at 5 and 10 μM U69,593 paralleled with decreased 5-HT uptake, as shown in Figure 5A. Thus, analogous to our results using a heterologous coexpression cell model (Figs. 1, 3, 4), KOR activation and perhaps subsequent activation of Akt and CaMKII resulted in the inhibition of SERT activity and reduced SERT surface expression while elevating SERT phosphorylation in rat striatal synaptosomes.
Figure 5. Effect of KOR activation on native SERT function and phosphorylation in ventral and dorsal striatal synaptosomes.
A. Dose dependent effect of KOR-agonists U69,593 and U50,488 on SERT activity: Synaptosomes prepared from ventral and dorsal striatum were preincubated with Vehicle-E or various concentrations of U69,593 (μM: 2.5, 5, 10) or Vehicle-B or U50,488 (μM: 2.5, 5, 10) for 15 min at 37°C before 5-HT uptake assays. Treatment with BNI (1 μM, 60 min) was carried out prior to U69,593 or U50,488 (10 μM, 15 min) treatment. 5-HT uptake was determined after 6-min incubation at 37°C using [3H]labeled 5-HT (40 nM) as described under “Materials and Methods”. SERT specific 5-HT uptake was determined by subtracting 5-HT accumulation in the presence of 0.1 μM fluoxetine from total 5-HT accumulation (in the absence of fluoxetine). The results were presented as percent change from corresponding vehicle treated controls. Both U69,593 and U50,488 decreased SERT activity (One-way ANOVA: U69,595/ventral striatum: F(3,8) = 33.01, P <0.01; U69,595/dorsal striatum: F(3,8) = 6.16, P <0.0001; U50,488/ventral striatum: F(3,8) = 105.7, P <0.0001 and U50,488/dorsal striatum: F(3,8) = 20.87, P <0.0004). Bonferroni’s post hoc test: *p <0.05; **p <0,01; ***p < 0.001 versus corresponding vehicles; ^^p < 0.001 versus U69,593 (10 μM); ††p <0.001 versus U50,488 (10 μM). B. The effects of Akt and CaMKII inhibition on KOR mediated reduction in SERT activity: Pretreatment of Akt X (20 μM) or KN93 (2 μM) for 45 min prior to U69,593 or U50,488 (10 μM, 15 min) treatment attenuated inhibitory effects of U69,593 or 50,488 on SERT activity (One-way ANOVA: F(5,46) = 18.57, P <0.0001). ***p <0.001 versus Vehicle + U69,593; ^^p <0,01, ^^^p <0.001 versus Vehicle + U50,488 (Bonferroni’s post hoc test). Vehicle contained both ethanol and buffer.
Figure 6. Effect of KOR activation on SERT expression and phosphorylation in ventral striatal synaptosomes.
A, B. Effect of U69,593 or U50,488 on SERT expression: Ventral striatal synaptosomes were treated with Vehicle-E, U69,593 or Vehicle-B or U50,488 (10 μM, 15 min) followed by surface protein biotinylation. Isolation of surface biotinylated proteins and SERT immunoblots were performed as described under “Materials and Methods”. A, representative SERT immunoblots and D, quantitation of biotinylated SERT band densities (~96 kDa, arbitrary units) **p <0,01 Vehicle-E versus U69,593 (t=8.02, df=4, N=3); ^p <0.05 Vehicle-B versus U50,488 (t=3.07, df=6; N=4) (Two-tailed Student’s t test). C, D. Dose dependent effect of U69,593 on native SERT phosphorylation: Synaptosomes (ventral striatum) were metabolically labeled with [32P]orthophosphate for 45 min at 37°C and then treated with Vehicle-E or various concentrations of U69,593 (μM: 0.5, 1, 5, 10) for 15 min at 37°C. Immunoprecipitation, SDS-PAGE and autoradiography were performed as given under “Materials and Methods”. C the autoradiogram shown is a representative of four independent experiments. D. The bar graph shows the percent average of 32P-labelled SERT band (~96 kDa) density from vehicle treated control (F(4,18) = 5.36, P <0.005; **p <0.01 versus Vehicle-E from one-way ANOVA analysis of variance with Bonferroni’s multiple comparison test).
4. Discussion
The present study demonstrates that acute activation of KOR reduces SERT-mediated 5-HT uptake and SERT surface expression in EM4 cells coexpressing SERT and KOR. In addition, KOR activation enhances SERT phosphorylation in this in vitro heterologous expression system. Similar effects were observed in neuronal preparations of rat striatal synaptosomes, which endogenously express native SERT and KOR. In addition, this study reveals the involvement of Akt and CaMKII as downstream signaling kinases involved in SERT regulation following KOR activation. Substantial evidence exists documenting that SERT function and surface expression are dynamically regulated by several cellular protein kinases, including Akt, CaMKII, PKC, p38 MAPK, PKG and Src (Jayanthi et al., 1994; Jayanthi et al., 2005; Qian et al., 1997; Rajamanickam et al., 2015; Ramamoorthy et al., 2007; Ramamoorthy et al., 2011; Samuvel et al., 2005). It has been postulated that autoreceptors and heteroreceptors present on serotonergic neurons play an important role in modulating intracellular kinase/phosphatase signaling cascades that in turn regulate SERT function and 5-HT signaling. Supportive to this notion is evidence that the autoreceptor 5-HT1B and heteroreceptors including adenosine-, atypical histamine-, α2 adrenergic-, and TrkB receptors regulate SERT activity (Benmansour et al., 2008; Daws et al., 2000; Launay et al., 1994; Matheus et al., 2009; Miller and Hoffman, 1994; Zhu et al., 2004). Expression of KOR as a heteroreceptor on serotonergic neurons and neuronal terminals is well documented (Berger et al., 2006; Kalyuzhny and Wessendorf, 1999). Activation of KOR by stress-induced endogenous KOR ligand dynorphin or synthetic KOR agonists decrease 5-HT release (Grilli et al., 2009; Tao and Auerbach, 2002). By using EM4 cells that coexpress KOR and SERT, we demonstrated that KOR agonists U69,593 and U50,488 decrease 5-HT uptake and this effect is prevented by KOR antagonist BNI. Furthermore, in the absence of KOR coexpression, U69,593 and U50,488 failed to decrease SERT-mediated 5-HT uptake in EM4 cells expressing SERT only. Thus the effects of KOR ligands on SERT activity are mediated by activation of KOR, and KOR agonists do not reduce SERT activity via direct interaction with the SERT protein.
KOR activation triggers multiple intracellular signaling cascades, including activating ERK1/2, p38 MAPK, PKC, Src, and Akt kinases and increasing intracellular calcium (Belcheva et al., 2005; Bohn et al., 2000; Bruchas and Chavkin, 2010; Gurwell et al., 1996; Jin et al., 1992; Pan, 2003; Schmid et al., 2013). Immunoblotting experiments revealed that treatment of KOR-SERT coexpressing EM4 cells with U69,593 or U50,488 resulted in increased levels of phosphorylated Akt and CaMKII. This KOR-mediated phosphorylation of Akt and CaMKII is sensitive to Akt X and KN93, the inhibitors of Akt and CaMKII, respectively, confirming that KOR stimulation activates Akt and CaMKII in our EM4 cell model. Pretreatment with Akt inhibitor Akt X or CaMKII inhibitor KN93 resulted in near complete prevention of U69,593-induced SERT inhibition. However, the inhibitory effect of U69,593 was not altered by pretreatment with inhibitors of p38 MAPK, ERK1/2, or Src kinase but produced additive inhibition at concentrations found to inhibit SERT activity maximally. These results suggest that while Akt and CaMKII are involved in KOR-mediated SERT downregulation, p38 MAPK, ERK1/2, and Src kinase are not. Since Akt or CaMKII inhibitors alone inhibit SERT activity, it is possible that the blocking effect of Akt X or KN93 on KOR-mediated SERT inhibition can be attributed to a maximal threshold of SERT inhibition. However, because U69,593 produced additive inhibition of SERT activity in the presence of p38 MAPK or Src inhibitors, it is unlikely that the blocking effect of Akt X or KN93 on KOR-mediated SERT inhibition reached the maximal inhibitory threshold. Previous study showed that U69,593 induces Akt phosphorylation in a cell culture model and in striatal neurons (Schmid et al., 2013). Furthermore, KOR activation increases intracellular calcium mobilization (Gurwell et al., 1996; Jin et al., 1992; Pan, 2003; Spencer et al., 1997), which might result in the activation of CaMKII. Together, these findings demonstrate the involvement of Akt and CaMKII in mediating KOR regulation of SERT.
The decrease in SERT activity following KOR activation is associated with decreased Vmax and no change in 5-HT affinity. This reduction in SERT activity occurs in parallel with a loss of surface SERT. Furthermore, KOR activation reduces SERT exocytosis and enhances SERT internalization. At present it is unclear why a cumulative effect of KOR activation on SERT internalization and exocytosis is not reflected in the observed net decrease in surface SERT expression or transport function. Phosphorylation assays revealed enhanced SERT phosphorylation following treatment with U69,593 or U50,488. Thus, our results point out that KOR-triggered signals target multiple sites of highly complex cellular regulatory pathways involving SERT exocytosis, endocytosis, recycling, and phosphorylation and that these regulatory mechanisms may act coordinately to reduce functional SERT protein levels on the surface without altering total SERT protein levels.
Results from the current study using rat ventral and dorsal striatum revealed a BNI-sensitive decrease in SERT-mediated 5-HT uptake following U69,593 or U50,488 exposure. Akt inhibitor Akt X and CaMKII inhibitor KN93 attenuate U69,593- and U50,488-mediated SERT inhibition. Furthermore, consistent with reductions in 5-HT uptake, U69,593 or U50,488 treatment reduces surface SERT and increases SERT phosphorylation. Analogous to results from the in-vitro cell culture model, the results from ex-vivo studies demonstrate that activation of endogenous KOR downregulates SERT activity by redistributing surface SERT and triggering SERT phosphorylation in the neuronal striatum. These results indicate a potential physiological role for dynorphin-KOR-mediated regulation of 5-HT clearance in the central nervous system.
Our previous studies established that inhibition of basal activity of Akt or CaMKII through Akt X or KN93 respectively decreases SERT function (Annamalai et al., 2012; Jayanthi et al., 1994; Rajamanickam et al., 2015). These SERT regulations have been studied under basal or unstimulated conditions and suggested that the constitutively active Akt- or CaMKII-mediated SERT regulation is involved in maintaining the normal-basal expression of SERT function. However, the endogenous receptor(s)-linked to Akt or CaMKII mediated SERT regulation and whether such regulations differ from basal Akt or CaMKII mediated SERT regulation are unknown. Thus, the current study, for the first time, reveals that KOR stimulated Akt and CaMKII lead to SERT inhibition in contrast to basal Akt or CaMKII mediated SERT regulation. Activation of the KOR has been linked to numerous molecular events (Bruchas and Chavkin, 2010) and SERT regulation is complex involving several regulatory modes (Bermingham and Blakely, 2016; Ramamoorthy et al., 2011). Future identification of downstream and upstream players in the activation of Akt and CaMKII following KOR stimulation, as well as identification of mechanisms such as phosphorylation and protein-protein interactions by which Akt and CaMKII modulate SERT functional expression, will enhance our understanding of the molecular basis of KOR-modulated 5-HT neurotransmission. Furthermore, future studies are certainly needed to understand how presynaptic receptor(s)- triggered multiple signaling pathways converge to regulate SERT in order to maintain serotonin homeostasis in milieu of various incoming external signals.
KOR-mediated SERT regulation may differ in certain models or under certain experimental conditions because various signaling pathways can be activated following KOR-activation depending on the cellular and environmental context. Thus, regulation of SERT activity and trafficking in response to KOR activation might differ from what we have observed in rat striatal synaptosomes and EM4-KOR-SERT coexpressing cells. For example, reported studies have documented that stress-induced KOR activation increases SERT activity and surface SERT levels via p38 MAPK-dependent pathway in mice (Bruchas et al., 2011; Schindler et al., 2012). Previously, we have demonstrated the requirement of p38 MAPK in constitutive expression of SERT and that p38 MAPK stimulation increases SERT activity, surface expression, and phosphorylation by influencing SERT membrane delivery (Samuvel et al., 2005). However, we are unable to reproduce the KOR-mediated increase in SERT activity in our EM4 cell model coexpressing SERT and KOR or in rat ventral and dorsal striatal preparations. Although different assays ([3H]5-HT uptake versus rotating disk electrode voltammetry) and species (mice versus rats) are employed, the discrepancies between our studies and others remain to be understood. Species differences in KOR-mediated functional signaling have been reported (DiMattio et al., 2015). In addition, there are many possible mechanisms that might be involved in KOR-mediated differential SERT regulation. It has been documented that KOR activation triggers differential neurochemical and behavioral outcomes based on short- and long-term KOR stimulation and the type of KOR ligands (reviewed in (Wee and Koob, 2010)). Short-term KOR agonist administrations decrease amine release, mediate aversive, antidepressant-like behavior, and suppress cocaine stimulatory and reward effects (Braida et al., 2009; Crawford et al., 1995; Gray et al., 1999; McLaughlin et al., 2006; Negus et al., 1997; Potter et al., 2011; Schenk et al., 1999; Shippenberg et al., 1996; Shippenberg et al., 1998; Spanagel et al., 1990; Tomasiewicz et al., 2008; Zhang et al., 2004). In contrast to short-term effects, repeated KOR agonist potentiate K+-triggered amine release, mediate pro-depressant like behavior, and augment drug seeking (Carlezon et al., 2006; Chartoff et al., 2008; Ebner et al., 2010; Fuentealba et al., 2006; Mague et al., 2003; McLaughlin et al., 2006; Potter et al., 2011). Nonetheless, unbiased and biased KOR agonists trigger differential signaling cascades and behaviors (Morgenweck et al., 2015; White et al., 2015). Therefore, it is possible that depending on the agonist and duration of KOR stimulation, KOR may trigger distinct signaling pathways that could induce differential regulation of SERT function. It has been shown that while short-term KOR activation stimulates DAT activity, long-term KOR agonist administration decreases DAT activity (Thompson et al., 2000). The neuronal mechanisms by which short- and long-term KOR stimulation modulates differential behavioral effects are largely unknown. We speculate that as a consequence of the differential effects of KOR stimulation on 5-HT release and synaptic clearance, the duration and concentration of synaptic 5-HT is altered, and thus altered 5-HT neurotransmission may be one neuronal mechanism that contributes to the behavioral effects of KOR ligands.
Previously, we have reported biphasic regulation of SERT activity dependent on the duration of kinase activation via differential SERT phosphorylation sites (Jayanthi et al., 2005). We postulated that the initial kinase-mediated phosphorylation of SERT may serve as a priming step to access or to not access additional SERT post-translational modifications in response to the next wave of signaling. Thus it is conceivable that the differences in SERT function observed in the current study and others may arise from differential SERT post-translational modifications in response to differential signaling downstream of KOR activation. Since acute KOR activation decreases 5-HT release, reduction in synaptic 5-HT could reduce SERT surface expression, as our prior report has shown that SERT activity (5-HT transport) can antagonize downregulation of SERT (Ramamoorthy and Blakely, 1999). Moreover, it is also possible that decreased synaptic 5-HT might also modify 5HT1B-mediated SERT regulation (Daws et al., 2000). However, future studies are needed to fully understand the complexities of cellular signaling pathways and post-translational modifications of SERT and KOR. Moreover, whether KOR-induced SERT modulation alters synaptic 5-HT and consequent downstream mediators of 5-HT signaling and whether altered SERT-linked serotonergic signaling pathways contribute to the behavioral effects of KOR activation remain to be defined.
5. Conclusions
The results of the present study demonstrate that in both the KOR-SERT coexpressing cell model and the endogenous KOR-SERT expressing native rat striatal preparations, acute stimulation of KOR decreases SERT mediated 5-HT uptake through KOR-linked Akt and CaMKII dependent signaling pathways. The decrease in SERT function is associated with decreased surface SERT and SERT exocytosis and increased SERT internalization and phosphorylation, implicating these cellular processes in KOR-mediated regulation of CNS serotonergic neurotransmission. Given that aberrant KOR, SERT, dynorphin, and 5-HT systems have been implicated in depression, anxiety, stress, and drug relapse, and that 5-HT has a close modulatory role on the KOR system (reviewed in (Bruchas et al., 2010; Bruijnzeel, 2009; D’Addario et al., 2007; Di Benedetto et al., 2004; Rudnick et al., 2014; Tejeda et al., 2012; Wee and Koob, 2010)), understanding the precise post-translational mechanisms regulating SERT functional expression in response to multiple branches of signaling cascades downstream of KOR activation will enhance our understanding of the physiological role of the KOR system in regulating monoaminergic neurotransmission. Moreover, the molecular characterization of biogenic amines-dynorphin/KOR interactions will increase the understanding of the neuronal mechanisms underlying KOR and SERT dysregulation in stress, mood, depression, and drug addiction and lead to the identification of novel molecular targets for the treatment of psychiatric illnesses.
Highlights.
K-opioid receptor agonists produce conditional aversive and pro-depressant-like effects
SERT is one of the major determinants of synaptic 5-HT and serotonergic neurotransmission
K-opioid receptor activation decreases 5-HT uptake through Akt and CaMKII pathways
K-opioid receptor activation decreases SERT surface expression and increases SERT phosphorylation and endocytosis
K-opioid receptor activation decreases serotonin clearance in the striatum
Acknowledgments
This work was supported by the National Institutes of Mental Health Grant MH083928, (S.R).
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- Akt X
Akt inhibitor X (10-(4′-(N-diethylamino)butyl)-2-chlorophenoxazine)
- Akt
protein kinase B/Akt
- BNI
nor-binaltorphimine
- CaMKII
Calcium calmodulin dependent kinase II
- DAT
dopamine transporter
- DMEM
Dulbecco’s modified Eagle’s medium
- GPCR
G-protein coupled receptor
- HEK-293
human embryonic kidney cells
- hSERT
human SERT
- KOR
kappa-opioid receptor
- KRH
Krebs-Ringer-HEPES buffer
- NAc
nucleus accumbens
- NET
norepinephrine transporter
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate buffered saline
- PKC
protein kinase C
- PKG
protein kinase G
- RIPA
radioimmunoprecipitation assay lysis buffer
- SERT
serotonin transporter
- SSRI
serotonin selective reuptake inhibitors
- TfR
transferrin receptor
Footnotes
Author Contributions
Participated in research design: Ramamoorthy, S., Jayanthi, L.D., Shippenberg, T.S.
Conducted experiments: Sundaramurthy, S., Annamalai, B., Samuvel, D.J.
Performed data analysis: Sundaramurthy, S., Ramamoorthy, S.
Wrote or contributed to the writing of the manuscript: Ramamoorthy, S., Jayanthi, L.D., Sundaramurthy, S.
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annamalai B, Mannangatti P, Arapulisamy O, Shippenberg TS, Jayanthi LD, Ramamoorthy S. Tyrosine phosphorylation of the human serotonin transporter: a role in the transporter stability and function. Mol Pharmacol. 2012;81:73–85. doi: 10.1124/mol.111.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Deltheil T, Piotrowski J, Nicolas L, Reperant C, Gardier AM, Frazer A, David DJ. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587:90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R. Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. Br J Pharmacol. 2006;148:795–806. doi: 10.1038/sj.bjp.0706782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham DP, Blakely RD. Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters. Pharmacol Rev. 2016;68:888–953. doi: 10.1124/pr.115.012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Belcheva MM, Coscia CJ. Mitogenic signaling via endogenous kappa-opioid receptors in C6 glioma cells: evidence for the involvement of protein kinase C and the mitogen-activated protein kinase signaling cascade. J Neurochem. 2000;74:564–573. doi: 10.1046/j.1471-4159.2000.740564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Capurro V, Zani A, Rubino T, Vigano D, Parolaro D, Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol. 2009;157:844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain research reviews. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chiu YT, Wu W, Huang P, Mann A, Schulz S, Liu-Chen LY. Determination of sites of U50,488H-promoted phosphorylation of the mouse kappa opioid receptor (KOPR): disconnect between KOPR phosphorylation and internalization. Biochem J. 2016;473:497–508. doi: 10.1042/BJ20141471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton CC, Xu M, Chavkin C. Tyrosine phosphorylation of Kir3 following kappa-opioid receptor activation of p38 MAPK causes heterologous desensitization. J Biol Chem. 2009;284:31872–31881. doi: 10.1074/jbc.M109.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 1997;2:247–250. doi: 10.1038/sj.mp.4000266. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- D’Addario C, Di Benedetto M, Izenwasser S, Candeletti S, Romualdi P. Role of serotonin in the regulation of the dynorphinergic system by a kappa-opioid agonist and cocaine treatment in rat CNS. Neuroscience. 2007;144:157–164. doi: 10.1016/j.neuroscience.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- Di Benedetto M, D’Addario C, Collins S, Izenwasser S, Candeletti S, Romualdi P. Role of serotonin on cocaine-mediated effects on prodynorphin gene expression in the rat brain. J Mol Neurosci. 2004;22:213–222. doi: 10.1385/JMN:22:3:213. [DOI] [PubMed] [Google Scholar]
- DiMattio KM, Ehlert FJ, Liu-Chen LY. Intrinsic relative activities of kappa opioid agonists in activating Galpha proteins and internalizing receptor: Differences between human and mouse receptors. Eur J Pharmacol. 2015;761:235–244. doi: 10.1016/j.ejphar.2015.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba JA, Gysling K, Magendzo K, Andres ME. Repeated administration of the selective kappa-opioid receptor agonist U-69593 increases stimulated dopamine extracellular levels in the rat nucleus accumbens. J Neurosci Res. 2006;84:450–459. doi: 10.1002/jnr.20890. [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Grilli M, Neri E, Zappettini S, Massa F, Bisio A, Romussi G, Marchi M, Pittaluga A. Salvinorin A exerts opposite presynaptic controls on neurotransmitter exocytosis from mouse brain nerve terminals. Neuropharmacology. 2009;57:523–530. doi: 10.1016/j.neuropharm.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Duncan MJ, Maderspach K, Stiene-Martin A, Elde RP, Hauser KF. kappa-opioid receptor expression defines a phenotypically distinct subpopulation of astroglia: relationship to Ca2+ mobilization, development, and the antiproliferative effect of opioids. Brain Res. 1996;737:175–187. doi: 10.1016/0006-8993(96)00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdina PD. Imipramine binding sites in brain and platelets: role in affective disorders. Int J Clin Pharmacol Res. 1989;9:119–122. [PubMed] [Google Scholar]
- Insel TR, Zohar J, Benkelfat C, Murphy DL. Serotonin in obsessions, compulsions, and the control of aggressive impulses. Annals New York Academy of Sciences. 1991:574–586. doi: 10.1111/j.1749-6632.1990.tb16911.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V. Calmodulin-dependent regulation of the catalytic function of the human serotonin transporter in placental choriocarcinoma cells. J Biol Chem. 1994;269:14424–14429. [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- Jin W, Lee NM, Loh HH, Thayer SA. Dual excitatory and inhibitory effects of opioids on intracellular calcium in neuroblastoma x glioma hybrid NG108-15 cells. Mol Pharmacol. 1992;42:1083–1089. [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. Serotonergic and GABAergic neurons in the medial rostral ventral medulla express kappa-opioid receptor immunoreactivity. Neuroscience. 1999;90:229–234. doi: 10.1016/s0306-4522(98)00376-5. [DOI] [PubMed] [Google Scholar]
- Kivell B, Uzelac Z, Sundaramurthy S, Rajamanickam J, Ewald A, Chefer V, Jaligam V, Bolan E, Simonson B, Annamalai B, Mannangatti P, Prisinzano TE, Gomes I, Devi LA, Jayanthi LD, Sitte HH, Ramamoorthy S, Shippenberg TS. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology. 2014;86:228–240. doi: 10.1016/j.neuropharm.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay JM, Bondoux D, Oset-Gasque MJ, Emami S, Mutel V, Haimart M, Gespach C. Increase of human platelet serotonin uptake by atypical histamine receptors. Am J Physiol. 1994;266:R526–536. doi: 10.1152/ajpregu.1994.266.2.R526. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Matheus N, Mendoza C, Iceta R, Mesonero JE, Alcalde AI. Regulation of serotonin transporter activity by adenosine in intestinal epithelial cells. Biochem Pharmacol. 2009;78:1198–1204. doi: 10.1016/j.bcp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Xu M, Mackie K, Chavkin C. Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem. 2003;278:34631–34640. doi: 10.1074/jbc.M304022200. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Hoffman BJ. Adenosine A3 receptors regulate serotonin transport via nitric oxide and cGMP. J Biol Chem. 1994;269:27351–27356. [PubMed] [Google Scholar]
- Morgenweck J, Frankowski KJ, Prisinzano TE, Aube J, Bohn LM. Investigation of the role of betaarrestin2 in kappa opioid receptor modulation in a mouse model of pruritus. Neuropharmacology. 2015;99:600–609. doi: 10.1016/j.neuropharm.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Andrews AM, Wichems CH, Li Q, Tohda M, Greenberg B. Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry. 1998;59(Suppl 15):4–12. [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Pan ZZ. Kappa-opioid receptor-mediated enhancement of the hyperpolarization-activated current (I(h)) through mobilization of intracellular calcium in rat nucleus raphe magnus. J Physiol. 2003;548:765–775. doi: 10.1113/jphysiol.2002.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. Repeated exposure to the kappa-opioid receptor agonist salvinorin A modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry. 2011;70:744–753. doi: 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamanickam J, Annamalai B, Rahbek-Clemmensen T, Sundaramurthy S, Gether U, Jayanthi LD, Ramamoorthy S. Akt-mediated regulation of antidepressant-sensitive serotonin transporter function, cell-surface expression and phosphorylation. Biochem J. 2015;468:177–190. doi: 10.1042/BJ20140826. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466:25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, Lemos JC, Chavkin NW, Hagan CE, Neumaier JF, Chavkin C. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J Neurosci. 2012;32:17582–17596. doi: 10.1523/JNEUROSCI.3220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, Bohn LM. Functional selectivity of 6′-guanidinonaltrindole (6′-GNTI) at kappa-opioid receptors in striatal neurons. J Biol Chem. 2013;288:22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Murthy NV, Bhagwagar Z, Bose SK, Hinz R, Grasby PM, Cowen PJ. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [11C]DASB. Psychopharmacology (Berl) 2011;213:555–562. doi: 10.1007/s00213-009-1660-y. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Thompson AC. Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol. 1998;345:27–34. doi: 10.1016/s0014-2999(97)01614-2. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spencer RJ, Jin W, Thayer SA, Chakrabarti S, Law PY, Loh HH. Mobilization of Ca2+ from intracellular stores in transfected neuro2a cells by activation of multiple opioid receptor subtypes. Biochem Pharmacol. 1997;54:809–818. doi: 10.1016/s0006-2952(97)00243-8. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:549–556. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69:857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Vaughan RA, Sharpe LG, Shippenberg TS. κ-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Gesmonde J, Ramamoorthy S, Rudnick G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J Neurosci. 2007;27:10878–10886. doi: 10.1523/JNEUROSCI.0034-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]