Abstract
Alcoholism is a chronic relapsing disorder characterized by periods of heavy alcohol consumption and unsuccessful attempts at abstinence. Relapse is one of the most problematic aspects in the treatment of alcoholism and is triggered by ethanol-associated cues. Extinction-based cue exposure therapies have proven ineffective in the treatment of alcoholism. However, positive allosteric modulation of mGlu5 with CDPPB enhances the extinction learning of alcohol-seeking behavior. The current study investigated the impact of chronic alcohol exposure on the extinction of ethanol-seeking behavior. Adult Wistar rats were trained to self-administer alcohol with a light/tone stimulus serving as the alcohol cue. After training, one group of rats was exposed to chronic intermittent ethanol (CIE) daily for a period of 2 weeks to induce ethanol dependence. Control rats were exposed to air for the same period of time. Both groups were then retrained to self-administer ethanol and subsequently tested for changes in extinction learning. CIE exposed rats consumed more ethanol compared to their pre-CIE levels and to control rats. During extinction training, CIE rats responded significantly more on the previously active lever and required more sessions to reach extinction criteria compared to control rats. Treatment with CDPPB facilitated extinction in control rats and attenuated the increased resistance to extinction in CIE-exposed rats. These results demonstrate that chronic ethanol exposure not only alters ethanol intake, but also the extinction of ethanol-seeking behaviors. The ability to attenuate deficits through modulation of mGlu5 provides a potential target for pharmacological manipulation that could ultimately reduce relapse in alcoholics.
Keywords: alcohol, extinction, glutamate, mGlu5, PFC
1. Introduction
Theories of drug addiction have incorporated various concepts from the fields of learning and memory and suggest that classical and operant conditioning principles underlie the compulsiveness of addictive behaviors. Thus, certain aspects of addiction result from “overlearning” of the associations between drugs and discrete cues that predict drug availability1. As such, alcohol use disorder (AUD) is characterized by periods of heavy alcohol consumption followed by unsuccessful attempts at abstinence. This chronic relapsing disorder is associated with numerous brain changes that include neuronal circuits, neurotransmitters systems, and cellular mechanisms that overlap with those that mediate normal learning and memory processes2-5.
During the initial stages of the addiction, associative learning processes mediate the conditioning between the reinforcing effects of a drug and environmental cues and contexts. With repeated drug use, a “drug” memory forms that remains even after drug use ceases. The extinction of drug-seeking behavior involves repeated presentations of drug-related cues without the previously associated reinforcing effects of the drug (reviewed in6). This process does not appear to remove the previously formed drug memory, but instead creates a new “inhibitory” memory that competes with the drug memory for control over behavior7-9. Because the drug memory remains, it can motivate drug-seeking behavior and relapse. While behavioral extinction training can reduce drug-seeking behavior, the persistence of the drug memory likely contributes to the ineffectiveness of current extinction-based behavioral therapies for addiction. However, the use of pharmacological compounds to facilitate the extinction of drug-seeking behaviors theoretically creates a more persistent extinction memory that can reduce drug-seeking and ultimately relapse behavior10.
In clinical studies, the use of exposure therapy to treat addiction has for the most part been ineffective1, 11. This has lead to the suggestion that the facilitation of extinction learning and the neural mechanisms involved could serve as a novel therapeutic approach to increase the effectiveness of extinction learning in addiction treatment and highlight potential drug targets. Previous studies have shown that extinction of conditioned fear12 and cocaine-seeking13 behavior is facilitated by the NMDA receptor partial agonist D-cycloserine (DCS). It has also been shown that the mGlu5 positive allosteric modulator (PAM) 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) enhances the extinction of cocaine memories14, 15. Importantly, using a rat non-dependence model of alcohol consumption, it has been shown that the extinction of ethanol-seeking behavior can be facilitated with the mGlu5 PAM CDPPB10. This facilitation is associated with differential changes in plasticity in subregions of the medial prefrontal cortex (PFC) and nucleus accumbens (NAc). These observations are consistent with the suggestion that facilitating glutamatergic transmission during extinction training strengthens acquisition of new memories formed during the extinction process. Furthermore, areas of the PFC mediate extinction learning, and chronic ethanol exposure is associated with structural and functional changes in the PFC16-19. Importantly, evidence indicates that these cognitive deficits contribute to relapse and decrease the probability of successful treatment outcomes in alcoholics20-23. While it has been shown that the ability to facilitate extinction of ethanol-seeking following moderate ethanol consumption, the effects of chronic ethanol exposure on the subsequent consumption of ethanol and the extinction learning of ethanol-related behaviors has not been investigated. Therefore, the goal of the current studies was two fold: 1) determine if the induction of ethanol dependence alters alcohol consumption and the subsequent extinction of ethanol-seeking behaviors, and 2) investigate whether CDPPB administration can attenuate deficits in the extinction of cue-maintained ethanol-seeking behavior.
2. Materials and Methods
2.1 Drugs
3-cyano-N-(1,3-diphenyl-1H-pyrazol-5- yl)benzamide (CDPPB) was custom synthesized by Chemir Analytical Services (Maryland Heights, MO) according to previously published methods24, 25, purified to >95% purity by liquid chromatography-mass spectrometry, and suspended in 10% v/v Tween-80 (Sigma-Aldrich).
2.2 Animals
Male Wistar rats (250-275 g upon arrival, Harlan, Indianapolis, IN) were housed individually in standard polycarbonate cages. Access to food and water in the home cage was continuous throughout the experiment except during behavioral testing. The animal colony room was maintained on a 12:12 reverse light-dark cycle with lights off at 09:00 am, and experimental testing was performed during the dark portion of the cycle. All experimental procedures were conducted with the approval of the Institutional Animal Care and Use Committee at the Medical University of South Carolina, and within guidelines set forth by the National Research Council's Guideline for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003).
Of the 46 rats trained to self-administer ethanol in an operant task and then subsequently exposed to extinction training, 6 were removed from the study prior to the commencement of extinction training because they failed to successfully acquire ethanol self-administration. The criteria for successful acquisition of ethanol self-administration was <20% variation in the number of active lever presses across 3 consecutive sessions. A minimum of 30 reinforcers per session and at least 12 sessions with 10% ethanol as the reinforcer (post CIE or air exposure) were also required. There were a total of 10 rats per group (n = 10) used for statistical analysis.
2.3 Ethanol Self-Administration Apparatus and Procedures
Ethanol self-administration and extinction test sessions were conducted in Plexiglas chambers (32 cm W × 25 cm D × 11 cm H, Med Associates, model ENV-008, St. Albans, VT) located in melamine sound attenuating cubicles. Each cubicle was equipped with an exhaust fan to provide air circulation and mask external noise. Mounted on one wall of the self-administration chamber were two response levers that flanked a liquid receptacle connected to a single speed syringe infusion pump with polyethylene tubing. Responses on one lever, designated the active lever, resulted in delivery of the liquid reinforcer (see below), while responses on the other (designated inactive) lever had no programmed consequences. Located above the active lever was a 2.5 cm diameter stimulus light, which was illuminated for 1.5 sec during each reinforcer delivery. Located atop the chambers was a house light to provide general illumination, and a Sonalert speaker that emitted a tone (2900 Hz, ~65 dB) for 1.5 sec during each reinforcer delivery. Chambers were interfaced to a PC computer that controlled experimental sessions and recorded data using commercially available software (MED Associates, MED-PC IV).
Training in the self-administration paradigm was based on our previously published methods10, 26. Beginning at approximately postnatal day (PD) 65, all rats were initially trained to consume ethanol by first exposing them to an intermittent two-bottle choice drinking initiation paradigm for a period of two weeks. Three days per week, two bottles - one containing water and the other containing 20% ethanol - were placed on the cage. Twenty-four hours later the ethanol bottles were removed and replaced with water-only bottles. The purpose of this procedure was to introduce the rats to the sensory components (e.g. taste, smell) of the 20% ethanol solution. Following the two bottle choice procedure, rats were then trained to lever press on the active lever to receive a reinforcer on an FR1 schedule of reinforcement. Each active lever press activated the syringe pump to deliver ~ 45 μL of a 20% ethanol solution over a 1.5 second period. During reinforcer delivery, the stimulus light above the active lever was illuminated and the tone was presented. Following each reinforcer delivery, a 4-sec timeout period was initiated during which additional active lever presses were recorded but had no programmed consequences. After stable responding for 20% ethanol (approximately 10-12 sessions) was reached, the concentration of ethanol was reduced to 10% for the remaining sessions (10-12 sessions). Rats were then assigned to either chronic intermittent ethanol (CIE) exposure or air exposure (see below). Following CIE or air exposure, all rats were given a 7-day “withdrawal” period before being placed back in the operant chambers and again allowed to self-administer 10% ethanol for 8-12 sessions. Following stabilization of responding for 10% ethanol (both prior to and following CIE or air exposure), blood samples (20 μL) were taken from the tail vein using heparin-coated borosilicate capillary tubes immediately following a 30 min self-administration session for subsequent analysis of blood ethanol levels (see below). After an additional week of daily self-administration sessions with less than 15% variability in active lever responding and at least 30 reinforcers averaged over the last two days, extinction training procedures commenced.
2.4 Chronic Intermittent Ethanol (CIE) Exposure Model
Following the acquisition of ethanol self-administration, rats assigned to CIE exposure group began ethanol vapor exposure procedures as previously described17. In brief, rats were single housed under a 12 hr reverse light/dark cycle, with continuous access to food and water. All rats began the CIE exposure paradigm at approximately PD90. During CIE exposure rats were exposed to ethanol vapor or air in inhalation chambers for 14 hr/d for 15 consecutive days (from 18:00 to 08:00) with 10 hr periods of withdrawal separating each vapor exposure period. Blood samples were collected from all animals by tail vein puncture on exposure days 2, 6, 10, and 15 for measurement of blood ethanol concentration (BEC; target range of 250 – 300 mg%). Intoxication ratings were assessed using a 5 point intoxication rating scare27. Rats were scored according to the following behaviors: 1, No signs of intoxication; 2, Slightly intoxicated; 3, Moderately intoxicated (obvious motor impairment but able to walk); 4, Highly intoxicated (dragging abdomen, loss of righting reflex); 5, Extremely intoxicated (loss of righting reflex and loss of eye blink reflex). Following completion of the CIE procedure and a 7-day withdrawal period, rats were again allowed to self-administer a 10% ethanol solution in an operant chamber until stable responding was maintained as described above.
2.5 Blood Ethanol Determination
Blood samples were taken from rats following the final self-administration session prior to CIE or air exposure and on the final day of self-administration that occurred post-CIE/air exposure (~21 days following CIE/air treatment). Immediately following blood sample collection, samples were centrifuged at 10,000 × g for 10 min to obtain a plasma supernatant, which was then stored at 4°C for a maximum of 24 hr. Ten μL of plasma was used for determination of blood ethanol levels using a colormetric enzymatic assay as previously described17, 28.
2.6 Extinction Procedures
Extinction procedures and treatment with the mGlu5 positive allosteric modulator CDPPB commenced after maintenance criteria for the ethanol-only solution was reached (less than 15% variability in active lever responding and at least 30 reinforcers averaged over the last two days of self-administration). Extinction training was conducted in 30 min daily sessions in the presence of ethanol-associated cues (e.g., presentation of the light/tone stimulus complex for 1.5 sec following each active lever press, followed by a 4 sec timeout), since it has been observed that such procedures produce drug-seeking behavior that is more resistant to extinction than that observed during extinction in the absence of drug-associated cues29, 30. No liquid solution was given during extinction sessions, and presses on the inactive lever during extinction were recorded but produced no programed consequences.
Twenty min prior to each extinction training session, rats were administered Vehicle (10% Tween 80) or CDPPB (30 mg/kg s.c.) according to their group assignment and returned to their home cages. The dose of CDPPB was based on previous studies that showed facilitation of extinction of ethanol seeking behavior in non-dependent rats10, 26 and cocaine-seeking behavior14, 15. Rats were placed in the self-administration apparatus 20 min following treatment and lever-pressing behavior was recorded. Extinction criteria were met when the number of active lever presses exhibited by an individual rat was <20% (for two consecutive days) of those observed on the average of the last two days of active drug self-administration for that particular rat. Rats in the forced abstinence groups were brought into the testing area, given injections based on their group assignment and then placed back into their home cages.
2.7 Statistical Analyses
Behavioral data were analyzed using SPSS version 22.0 software (SPSS Inc., Chicago, IL) and Prism version 6 (GraphPad software, La Jolla, CA). A p value of less than 0.05 was considered statistically significant for all tests and all ANOVAs included post-hoc tests (e.g. Tukey) to determine the nature of the group differences. For experiments involving analysis of ethanol consumption before and after vapor (or air) exposure, a two-way ANOVA with treatment (CIE or air) and time (before or after vapor/air exposure) as the variables was used. For experiments involving analysis of extinction behavior, lever presses on the last two days of active self-administration of 10% ethanol (i.e., maintenance) were averaged and compared to each day of extinction training using a mixed two-way repeated-measures ANOVA with treatment (control+CDPPB, control+saline, CIE+CDPPB, CIE+saline) as the between-subjects factor and experimental phase (maintenance/extinction session) as the within-subjects factor. An Independent Samples t-test was used to analyze the number of sessions required to reach extinction criteria. Separate ANOVAs were performed on the number of both active and inactive lever presses. Results from these analyses provided a statistical basis for determination of extinguished responding.
3. Results
3.1 CIE vapor exposure
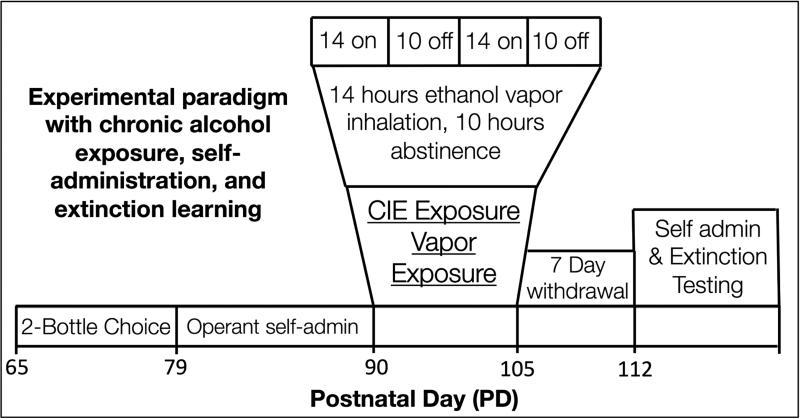
This study utilized a well-characterized ethanol vapor exposure paradigm that induces alcohol dependence through daily episodes of ethanol vapor exposure and withdrawal over a period of two weeks (Figure 1). To monitor the level of intoxication after each 14 hr ethanol exposure period, a 5-point behavioral intoxication rating scale27 was utilized, and a target level of slight-to-moderate intoxication (corresponding to an intoxication rating of 2-3, respectively) was used. The intoxication ratings were averaged for each rat across all exposure periods. The intoxication score averages across all days and all rats were 2.2 ± 0.05. As a complementary measure to the behavioral rating of intoxication, BECs were obtained from tail vein puncture following exposure periods on days 2, 6, 10, and 15. The target range for BECs were between 200 – 300 mg%. Our analysis revealed average BEC values (measured in mg%) of 247.6 ± 22.9 (Day 2), 226.0 ± 18.9 (Day 6), 247.8 ± 20.52 (Day 10), and 238.6 ± 23.6 (Day 15), and an overall grand average across all 4 days of 240.1 ± 21.5.
Figure 1. The CIE & extinction experimental design.
Rats were first trained to self-administer alcohol in paradigm that began at ~ PD60. Rats were initially exposed to a two-bottle choice drinking procedure in their home cage for two weeks. They were then trained to self-administer a 10% alcohol solution in an operant setting (lever pressing). Next, rats were exposed to ethanol vapor or air via inhalation chambers for 14 h/d for 15 consecutive days at PD90. Following CIE and a 7-day withdrawal period, rats were re-trained in the operant alcohol self-administration paradigm, and once stabilized, extinction testing began.
3.2 Ethanol self-administration
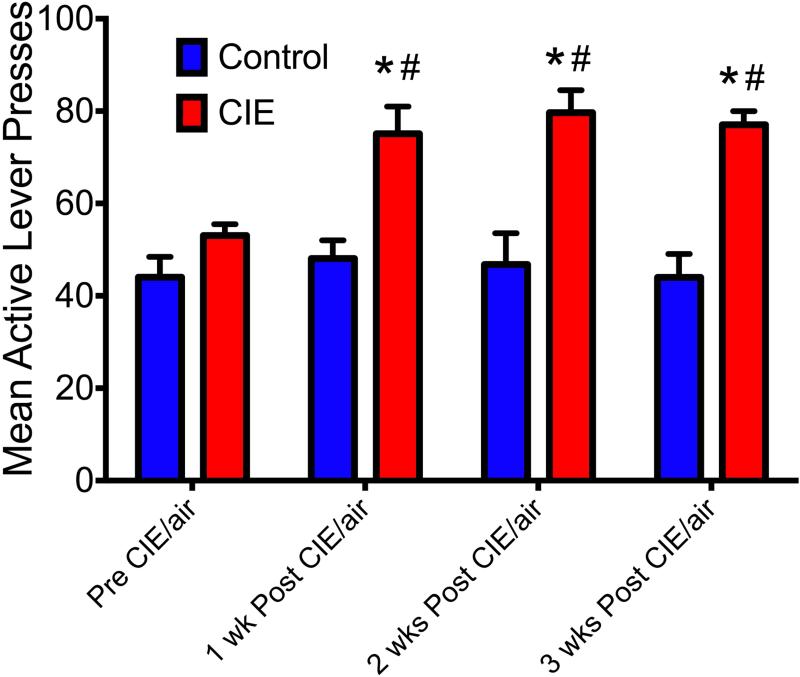
To examine the effects of CIE on ethanol self-administration, rats were trained to self-administer a 10% ethanol solution and then exposed to either CIE or air for a period of 15 days, and again allowed to self-administer the 10% ethanol solution for a period of 3 weeks. Analysis of lever responding before and for 3 weeks following CIE exposure & withdrawal lead to a significant Treatment × Period interaction [F(3,146) = 2.9, p = 0.03] (n=20). As shown in Figure 2, post-hoc analyses revealed that CIE exposure increased active lever responding during ethanol self-administration compared to pre-vapor exposure responding (indicated by #, p < 0.05) and compared to air-exposed rats post air exposure (indicated by *, p < 0.05). Additionally, the increase in ethanol self-administration remained significantly greater than air-exposed controls when the groups were compared during each of the 3 weeks following CIE/air exposure (indicated by *, p < 0.05). Similarly, on the final day of self-administration training, CIE-exposed rats received more reinforcements (59.05 ± 1.97) compared to air-exposed controls (43.55 ± 2.38) [F(1,73) = 5.7, p = 0.019].
Figure 2. The effects of CIE on operant responding for ethanol.
Exposure to CIE lead to a significant increase in active lever presses for ethanol for a period of 3 weeks following CIE exposure. # indicates a significant difference compared to pre exposure responding; * indicates a significant difference compared to the air-exposed controls following treatment (#,* p < 0.05).
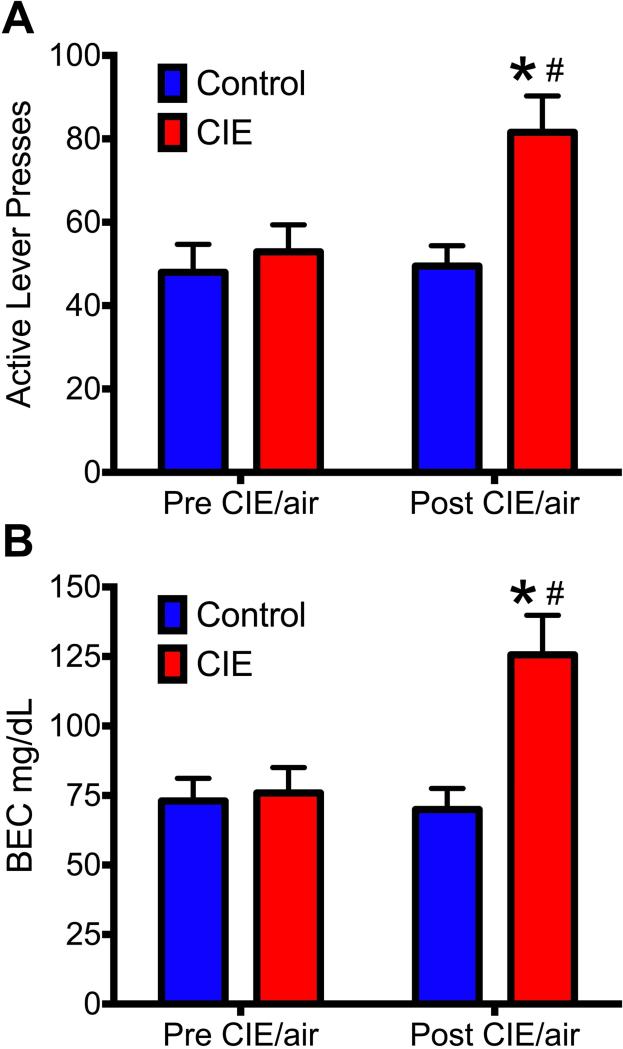
As previously described, blood samples were obtained after the final self-administration session prior to CIE/air exposure and after the final self-administration following CIE/air exposure (approximately 21 days following the withdrawal period (see Figure 1). During these time periods, tail-vein blood was collected in order to assess the impact of CIE exposure on the BEC following a 30 min ethanol self-administration session. As shown in Figure 3A, rats that had been exposed to CIE had significantly increased responding when compared to pre CIE levels (indicated by *) and compared to air-treated controls (indicated by #) [F(1,60) = 4.0, p = 0.041] (post-hoc p values < 0.05). Additionally, rats exposed to CIE exhibited increased BECs during self-administration compared to pre-CIE exposure levels (indicated by *) that was consistent with an increase in ethanol-seeking behavior (as shown in Figure 2) and when compared to air-exposed controls (indicated by #) [F(1,60) = 7.1, * p = 0.009]. Post-hoc analysis revealed that while the BECs in the two groups (CIE vs Air) did not differ during the pre-exposure period, there was a significant increase in the BECs during post the exposure period in the CIE group following a 30 min self-administration session (* p = <0.05).
Figure 3. A history of chronic alcohol exposure leads to increased BECs obtained during operant self-administration.
A) Exposure to CIE significantly increased active lever responding and B) significantly increased BEC levels during a normal self-administration session. # indicates a significant difference compared to pre-exposure responding; * indicates a significant difference compared to the air-exposed controls following treatment (#,* p < 0.05).
3.3 Extinction Learning
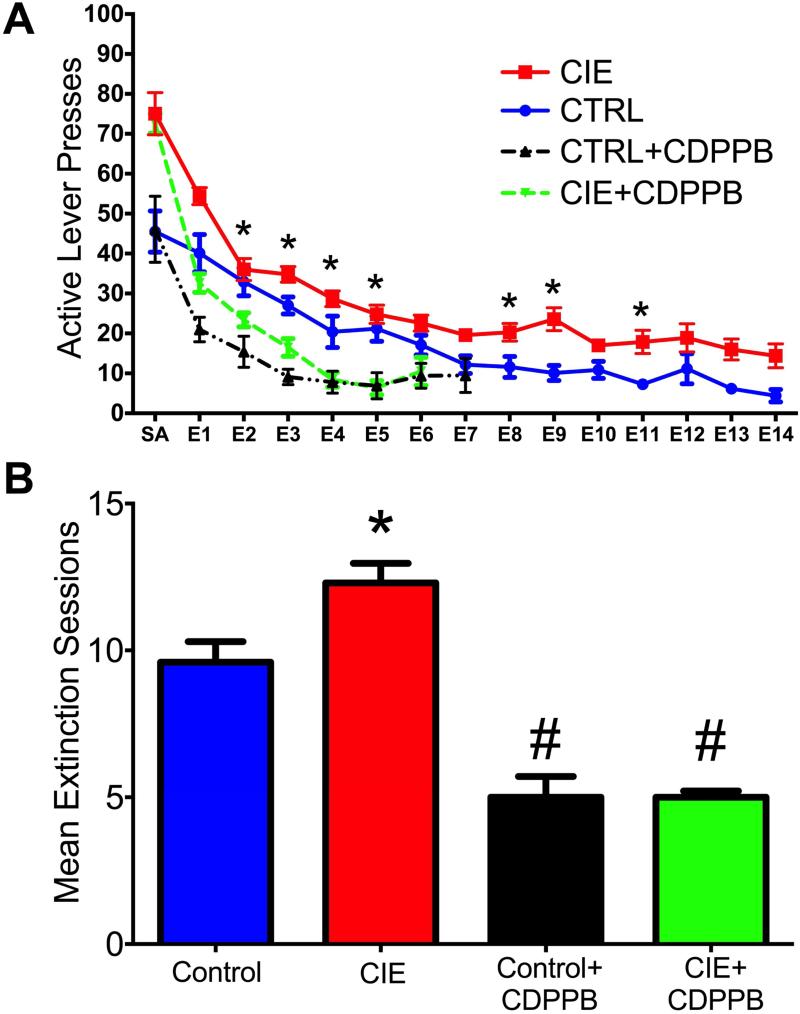
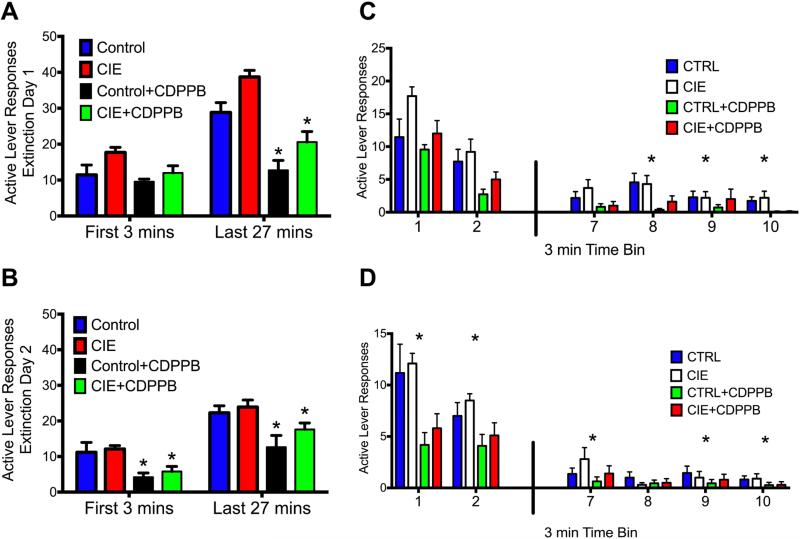
The next set of studies investigated the impact of CIE on the extinction of ethanol-seeking behavior, and the ability of the mGlu5 positive allosteric modulator (PAM) CDPPB to facilitate the extinction learning of ethanol-seeking and to attenuate deficits in extinction learning following CIE exposure. The results indicated a significant interaction between Treatment and Extinction session. As shown in Figure 4A, exposure to CIE lead to deficits in the extinction of ethanol seeking behavior [F(42,504) = 5.0, p = 0.001]. Furthermore, rats exposed to CIE required significantly more extinction training days to reach criteria [F(3,35) = 3.8, p = 0.001; indicated by *] (Figure 4B). To assess the ability of CDPPB treatment to attenuate the CIE-induced deficits in extinction learning, CDPPB was administered 20 min prior to each daily extinction session. As shown in Figure 4A, CDPPB resulted in a significant Treatment × Session interaction [F(42,504) = 5.0, p = 0.001]. Post-hoc analyses revealed that rats treated with CDPPB had significantly fewer responses on the previously active lever compared to control-treated rats on multiple extinction sessions (indicated by *; p values < 0.05). Furthermore, rats treated with CDPPB also required significantly fewer extinction sessions to reach extinction criteria when compared to air exposed control rats (indicated by #, p values < 0.05) (Figure 4B). To further compare the extinction behavior among the groups we performed a linear regression analysis of the extinction data to determine the slope/rate of extinction. The results indicated that both CDPPB-treated groups had significantly different rates of extinction compared to their respective controls [F(3,37) = 4.5, p = 0.008] (data not shown). Additionally, we analyzed the slope of the extinction rates between CIE-treated rats and controls on the last day of operant self-administration and the first day of extinction. The results indicated there was not a significant difference between the groups in the rate of extinction (slope) between the last day of self-administration and the first day of extinction training [F(1,26) = 0.4, p = 0.546] (data not shown).
Figure 4. CDPPB attenuated CIE-induced effects in extinction learning.
A) CIE exposure resulted in delayed extinction of ethanol-seeking behavior. Treatment with CDPPB prior to each extinction session reduced active lever responding on multiple days in both CIE-treated rats and in air-exposed controls. B) While CIE exposure increased the number of sessions to reach extinction criteria, CDPPB attenuated this deficit. Furthermore, CDPPB treatment also facilitated extinction learning in control (non-CIE) rats (#,* p < 0.05; * indicates a significant increase from controls; # indicates a significant decrease compared to respective controls).
To assess whether facilitation of ethanol-seeking behavior with CDPPB represents new learning due to a failure to receive ethanol in response to pressing the previously active lever, we examined the within-session time-course of responding in 3-min time bins during the first day of extinction. As shown in Figure 5, this analysis revealed a significant treatment × time period interaction [F(3,76) = 6.3, p = 0.001]. CDPPB treatment did not alter responding on the previously active lever during the initial 3-min of the extinction session (Figure 5A). However, there was a significant reduction of responding on the previously active lever during the remainder of the extinction session (indicated by *; p values < 0.05). Conversely, analysis of the second day of extinction learning revealed a significant treatment × time period interaction [F(3,76) = 4.1, p = 0.03] indicating that CIE-exposed rats responded significantly less than controls during both time periods (p values < 0.05) (Figure 5B). To further examine responding among the groups during the first two sessions of extinction training, we investigated previously active lever pressing in 3 min bins during these sessions. As also shown in Figure 5, the groups did not differ in responding during the first two time bins (Figure 5C) [F(15, 190) = 2.3, p = 0.01] (post-hoc p values < 0.05). However, rats treated with CDPPB differed from their respective controls (e.g. CIE+CDPPB vs. CIE) during the latter bins of the extinction session. Conversely, on Day 2 of extinction learning, groups treated with CDPPB significantly differed from their respective controls during both the initial time bins and the latter time bins of the session (Figure 5D) [F(15, 190) = 2.6, p = 0.002] (post-hoc p values < 0.05).
Figure 5. CDPPB facilitation of extinction reflects new learning.
A) Comparison of the binned responses during the first day of extinction training revealed that CDPPB did not alter responding on the previously active lever during the initial 3 min of the extinction session, but did significantly reduce responding on this lever during the remainder of the extinction session (*p < 0.01). B) Furthermore, analysis of the second day of extinction training revealed significant group differences as rats treated with CDPPB responded significantly less during both time periods (*p < 0.01). When 3-min time bins were compared, a similar significant difference in responding was observed between the groups on Day 1 (C) and Day 2 (D) of extinction training (*p < 0.05; indicates a significant difference when compared to their respective controls).
4. Discussion
The results of the present study demonstrate that exposure to chronic ethanol has lasting affects on several characteristics associated with ethanol seeking behaviors. We observed that, in a rodent model of alcohol “dependence,” exposure to CIE results in a significant and lasting increase in subsequent ethanol consumption (evidenced by both increased operant responding for ethanol and increased BECs). Furthermore, CIE disrupts the extinction learning of ethanol-seeking behavior (evidenced by increased responding on multiple days of extinction training compared to controls and significantly more sessions required to reach extinction criteria). A particularly important observation was that modulation of mGlu5 with the PAM CDPPB not only facilitated extinction learning in control (non-CIE) rats, it also attenuated CIE-induced deficits in extinction learning.
Through creation of a new inhibitory memory trace that is different from the original association memory, extinction learning can lessen the impact of the original drug memory on relapse31. As extinction training progresses, strengthening of this new inhibitory association is observed as a reduction in conditioned responding. Our observation that potentiation of mGlu5 attenuated CIE-induced deficits in extinction of ethanol-seeking behavior adds to a growing body of literature demonstrating that mGlu5 and glutamatergic transmission are key targets for pharmacological intervention in the treatment of drug-seeking behavior10, 14, 15, 26, 32-38. It is important to note that CDPPB's effects on extinction learning in CIE-exposed rats was not due to a blunting effect on motor behavior (as shown in15) and, in fact, appears to represent experience-dependent learning. This is supported by the observation that CDPPB did not alter responding during initial 3 mins of the first extinction session (when response rate is the highest), but did significantly reduce responding during the rest of the session compared to control animals. Furthermore, on the second day of extinction training, rats treated with CDPPB showed a significant reduction in previously active lever responding during the initial 3 min block as well as blocks of the session suggesting that learning from previous day was reflected during day two of extinction training. In combination with our previous studies using CDPPB to facilitate extinction learning10, 26, these results are consistent with the idea that modulation of mGlu5 to enhance extinction learning reflects new learning that occurs due to the lack of ethanol delivery during the initial period of the extinction session. Importantly, these results also indicate CDPPB's effects are not due to non-specific motor effects, which are in agreement with previously published findings using CDPPB to facilitate learning in extinction15 and memory39 paradigms.
It could be argued that treatment with CDPPB altered the rats’ interoceptive state. Theoretically, this could have altered the behavior of CDPPB-treated rats by producing an interoceptive state that was different from the state that is typically experienced during ethanol self-administration sessions. However, this interpretation is unlikely since others have shown that CDPPB treatment does not alter the interoceptive state of a rat32.
There is a substantial body of evidence from studies of human alcoholics and animals that chronic alcohol exposure results in structural and functional deficits of PFC16, 40. Therefore, the deficits in extinction of ethanol self-administration after CIE exposure may relate, at least in part, to alterations in PFC activity. Previous studies have shown that CIE exposure results in deficits in behavioral flexibility that are consistent with PFC dysfunction. These include deficits in operant tasks designed to assess cognitive flexibility17, 41, 42. Extinction learning can also be considered a measure of behavioral flexibility, as the animal has to ignore previously formed contingencies and discontinue behaviors that lead to reinforcement (e.g. active lever pressing) because they are no longer advantageous.
An important observation in the present study is that CIE exposure leads to a significant increase in subsequent voluntary ethanol intake that lasted for at least 3 weeks and occurred after the withdrawal period had ended (1 week after the CIE procedure). While Koob and collegues have previously shown a similar effect, their findings were restricted to measuring alcohol intake during the withdrawal period43. Our results reveal that increased intake following CIE is not constrained to the withdrawal period nor is it a transient effect as it was still present at 3 weeks following discontinuation of CIE exposure. Disruption of normal PFC function from CIE exposure may have also contributed to the increased ethanol consumption observed in CIE-treated rats. Given that the PFC plays a particularly important role in inhibitory control over impulsive behaviors6, 16, 40, 44, exposure to chronic ethanol may have altered the ability of the PFC to regulate volitional ethanol intake. Because abstinent alcoholics who display deficits in cognitive/inhibitory control are more likely to relapse45, 46, this suggests that restoration of normal PFC function, possibly through an mGlu5 mechanism, could reduce increased ethanol intake as well as deficits in extinction of ethanol-seeking behaviors.
One aspect of addiction that was not addressed in the present study was the effect of CIE exposure on cue-induced ethanol-seeking behavior (a rodent model of relapse-like behavior). While our previous work has shown that CDPPB attenuates cue-induced reinstatement following moderate ethanol self-administration10, the impact of alcohol dependence on reinstatement has yet to be investigated. If the propensity of cue-induced reinstatement of ethanol-seeking behavior is increased following CIE exposure, it is important to determine if CDPPB's ability to attenuate deficits in extinction learning following CIE also helps reduce the ability of ethanol related cues to induce relapse-like behavior.
The goal of extinction-based therapies is to suppress drug seeking elicited by drug-associated memories. The neurocircuitry that underlies extinction of conditioned behaviors includes many of the same brain regions that are involved in normal learning and memory processes. For instance, subregions of the PFC that are involved in fear conditioning are also implicated in drug conditioning47, 48. Animal studies have shown the prelimbic (PrL) cortex is a crucial region that drives cocaine-49-52 and heroin-seeking behaviors53, 54, while the infralimbic (IfL) cortex mediates both the acquisition and expression of extinction behaviors55-57. Extinction training also involves a number of neurochemical and molecular processes within the striatum58-60. Glutamatergic projections from the PrL cortex to the nucleus accumbens (NAc)61 core subregion may regulate drug-seeking while glutamatergic projections from the IfL cortex to the NAc shell subregion are suggested to mediate extinction behavior7, 53, 56, 62. These converging lines of evidence suggest that the PrL cortex serves as an on-switch for drug-seeking behavior while the IfL cortex functions as an off-switch to mediate extinction learning7. In support of this view we have shown that both extinction learning and mGlu5-mediated facilitation of extinction are associated with changes in glutamate receptor signaling within the PrL and IfL cortices10.
In the present study, the reversal of behavioral deficits following CDPPB treatment in CIE-exposed rats suggests the involvement of glutamatergic signaling, likely through mGlu5, in extinction learning. One possible mechanism by which CDPPB facilitates extinction learning is through indirect stimulation of the NMDA receptor63, which has been shown to be involved in various aspects of learning. Our previous findings show that CDPPB treatment can attenuate CIE-induced deficits in cognitive tasks17, 26 and facilitate extinction learning through mGlu5 activation in the IfL cortex. When combined with the findings from the present study, these observations provide further support that this subregion of the PFC is a potential therapeutic target for treating deficits associated with chronic ethanol exposure.
It is important to note that effects of CDPPB on extinction learning can be interpreted in an alternative way. For example, these behavioral changes could instead reflect a disruption of memory reconsolidation64. When a memory is evoked, a temporary labile state is initiated during which changes to the original memory can occur, thus allowing the memory to be updated with new information. As a result, CDPPB treatment during this state may have disrupted the original memory and ultimately affected conditioned ethanol-seeking behavior. Since our extinction behavioral paradigm involves repeated exposures to the ethanol-related cues and reconsolidation studies typically require short presentations of the cues65, this interpretation is unlikely. Furthermore, CDPPB treatment has been shown to have mnemonic benefits14, 35, 66, suggesting that disruption of memory reconsolidation was not the primary cause of the observed effects. However, it is possible that a combination of extinction learning and disruption of memory consolidation contributed to the effects CDPPB on CIE-induced deficits in extinction learning.
In summary, the results of the present study demonstrate that CIE exposure results in increased alcohol consumption and deficits in the extinction of ethanol-seeking behavior. Treatment with the mGlu5 PAM CDPPB attenuated the CIE-induced deficits in extinction learning and facilitated the extinction of ethanol-seeking behavior in non-CIE rats. Previously published findings indicate that facilitated extinction learning is associated with both functional and structural changes in plasticity within the IfL cortex10. Combined with our current observations, chronic ethanol exposure may alter the neurocircuitry of drug-seeking and extinction behaviors. Furthermore, the ability of mGlu5 modulation to reverse deficits following chronic ethanol exposure suggests that compounds such as CDPPB may help reduce the high incidence rates of relapse in AUD by serving as a pharmacological enhancement to current exposure-based therapies.
Highlights.
Chronic intermittent ethanol (CIE) increased voluntary alcohol consumption.
CIE exposure resulted in delayed extinction of alcohol-seeking behavior.
CDPPB treatment attenuated CIE-induced deficits in extinction learning.
Acknowledgements
*Supported by NIH grants: R00AA020537 (JTG), R01AA010983 (LJC), and U01AA019967 (LJC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conklin CA, Tiffany ST. Cue-exposure treatment: time for change. Addiction. 2002;97(9):1219–21. doi: 10.1046/j.1360-0443.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 3.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, et al. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78(3):610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 5.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5(1):9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Gass JT, Chandler LJ. The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Front Psychiatry. 2013;4:46. doi: 10.3389/fpsyt.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16(5):279–88. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phelps EA, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gass JT, et al. Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci. 2014;34(22):7562–74. doi: 10.1523/JNEUROSCI.5616-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Childress AR, et al. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 12.Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99(2):217–28. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172(1):173–8. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65(8):717–20. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleva RM, et al. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci. 2011;125(1):10–9. doi: 10.1037/a0022339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol. 2010;91:289–320. doi: 10.1016/S0074-7742(10)91009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci. 2014;34(10):3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24(5):611–21. [PubMed] [Google Scholar]
- 19.Tedstone D, Coyle K. Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug Alcohol Depend. 2004;75(3):277–86. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Finn PR, et al. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology (Berl) 1999;146(4):465–72. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- 21.Campanella S, et al. How cognitive assessment through clinical neurophysiology may help optimize chronic alcoholism treatment. Neurophysiol Clin. 2011;41(3):115–23. doi: 10.1016/j.neucli.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Campanella S, et al. Chronic alcoholism: insights from neurophysiology. Neurophysiol Clin. 2009;39(4-5):191–207. doi: 10.1016/j.neucli.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Myrick H, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221–7. doi: 10.1111/j.1530-0277.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 24.Lindsley CW, et al. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H- pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J Med Chem. 2004;47(24):5825–8. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- 25.Kinney GG, et al. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther. 2005;313(1):199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- 26.Gass JT, et al. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014;39(11):2570–83. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83(5):1087–93. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 28.Prencipe L, Iaccheri E, Manzati C. Enzymic ethanol assay: a new colorimetric method based on measurement of hydrogen peroxide. Clin Chem. 1987;33(4):486–9. [PubMed] [Google Scholar]
- 29.Ranaldi R, Roberts DC. Initiation, maintenance and extinction of cocaine self-administration with and without conditioned reward. Psychopharmacology (Berl) 1996;128(1):89–96. doi: 10.1007/s002130050114. [DOI] [PubMed] [Google Scholar]
- 30.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174(1):1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 31.Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11(5):501–9. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- 32.Besheer J, et al. Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology. 2014;39(10):2376–86. doi: 10.1038/npp.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleva RM, et al. Glutamatergic targets for enhancing extinction learning in drug addiction. Curr Neuropharmacol. 2010;8(4):394–408. doi: 10.2174/157015910793358169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2(1):83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, et al. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29(12):3676–84. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cozzoli DK, et al. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36(9):1623–33. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cozzoli DK, et al. Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2014;39(2):435–44. doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cozzoli DK, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29(27):8655–68. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichel CM, et al. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36(4):782–92. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience. 2014;277:139–51. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu W, et al. Effects of acamprosate on attentional set-shifting and cellular function in the prefrontal cortex of chronic alcohol-exposed mice. Alcohol Clin Exp Res. 2015;39(6):953–61. doi: 10.1111/acer.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroener S, et al. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5):e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Dell LE, et al. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28(11):1676–82. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charney DA, Zikos E, Gill KJ. Early recovery from alcohol dependence: factors that promote or impede abstinence. J Subst Abuse Treat. 2010;38(1):42–50. doi: 10.1016/j.jsat.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Wicks S, et al. Factors Affecting the Short-Term Prognosis of Alcohol Dependent Patients Undergoing Inpatient Detoxification. Subst Abus. 2001;22(4):235–245. doi: 10.1080/08897070109511465. [DOI] [PubMed] [Google Scholar]
- 47.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217(2):454–62. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 49.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capriles N, et al. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168(1-2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 51.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526(1-3):140–6. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24(11):3285–98. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 53.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–88. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci Lett. 2008;444(1):52–5. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 56.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters J, et al. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008;197(2):319–26. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt EF, et al. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21(7):RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Self DW, et al. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11(5):648–57. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate putamen. J Neurosci. 2006;26(13):3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6(6):339–44. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- 62.LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17(4):168–75. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor. Curr Drug Targets CNS Neurol Disord. 2002;1(1):1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- 64.Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev. 2012;36(5):1400–17. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10(3):224–34. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 66.Ayala JE, et al. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34(9):2057–71. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]