Abstract
The risk of hypertension is increased by intrauterine growth restriction (IUGR) and preterm birth. In the search for modifiable etiologies for this life-threatening cardiovascular morbidity, a number of pathways have been investigated, including excessive glucocorticoid exposure, nutritional deficiency, and aberration in sex hormone levels. As a neurotrophic hormone intimately involved in cardiovascular regulation whose levels are influenced by glucocorticoids, nutritional status and sex hormones, leptin has emerged as a putative etiologic and thus therapeutic agent. As a product of maternal and late fetal adipocytes as well as the placenta, circulating leptin typically surges late in gestation and declines following delivery until the infant consumes sufficient leptin-containing breast milk or accrues sufficient leptin-secreting adipose tissue to reestablish circulating levels. The leptin deficiency seen in IUGR infants is a multifactorial manifestation of placental insufficiency, exaggerated glucocorticoid exposure and fetal adipose deficit. The preterm infant suffers from the same cascade of events, including separation from the placenta, antenatal steroid exposure and persistently underdeveloped adipose depots. Preterm infants remain leptin deficient beyond term gestation, rendering them susceptible to neurodevelopmental impairment and subsequent cardiovascular dysregulation. This pathologic pathway is efficiently modeled by placing neonatal mice into atypically large litters, thereby recapitulating the perinatal growth restriction-adult hypertension phenotype. In this model, neonatal leptin supplementation restores the physiologic leptin surge, attenuates leptin-triggered sympathetic activation in adulthood and prevents leptin- or stress-evoked hypertension. Further pathway interrogation and clinical translation are needed to fully test the therapeutic potential of perinatal leptin supplementation.
Keywords: Adipose Tissue, Developmental Origins, Prematurity, Sex, Sympathetic Nervous System
Introduction
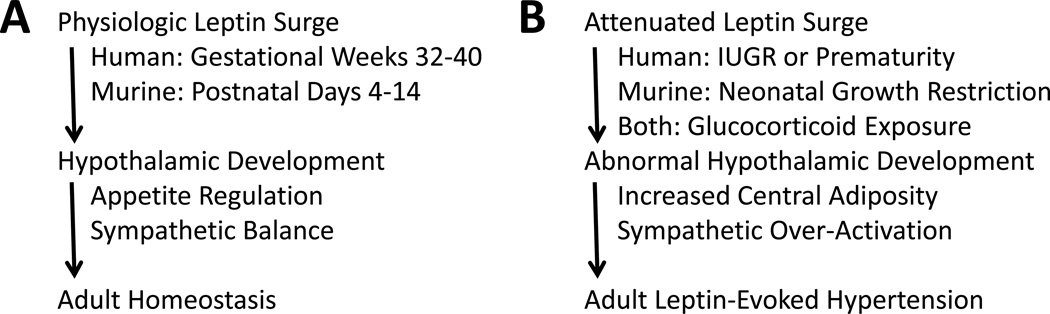
Leptin is a pleiotropic hormone with early neurotrophic effects on the hypothalamus (Bouret et al. 2004, Pinto et al. 2004). Later in life, leptin acts as a homeostatic agent by balancing appetite and sympathetic activity with energy stores such that increased adiposity increases circulating leptin levels, reducing appetite and increasing sympathetic tone. In addition to promoting energy expenditure, this sympathetic activation increases blood pressure (Rahmouni & Morgan 2007). According to the developmental origins of adult disease construct, physiologic challenges early in life can skew developmental processes during critical windows of susceptibility. This potentially can leave long-standing pathologic repercussions if the altered regulatory processes are rigidly established. This review presents the evidence of an attenuated late gestation leptin surge in intrauterine growth restricted (IUGR) or preterm infants. With IUGR and prematurity each affecting at least ten percent of all pregnancies, this perinatal leptin deficiency impacts nearly a quarter of all infants (Beck et al. 2010, WHO 1995), supporting the development of translational models. We review the clinical and preclinical data leading to murine models designed to test the hypothesis that neonatal leptin deficiency leads to permanent alterations in hypothalamic leptin signaling, predisposing to leptin-evoked hypertension in adulthood (Figure 1).
Figure 1.
The importance of the perinatal leptin surge as a stimulus for hypothalamic development. A: During normal human pregnancy, fetal leptin levels rapidly increase from gestational week 32 to delivery. Mice have a relative delay in adipogenesis and an analogous increase in leptin does not occur until postnatal days 4 to 14. In both species, the physiologic increase in perinatal leptin is critical for normal hypothalamic development. In adulthood, cardiometabolic homeostasis is facilitated by well-tuned appetite regulation and sympathetic tone. B: Perinatal leptin deficiency can occur following intrauterine growth restriction (IUGR) or prematurity in humans, neonatal growth restriction in mice, or exaggerated perinatal glucocorticoid exposure in both species. The attenuated leptin surge increases the risk of hypothalamic hypoplasia and dysregulation with the potential for hypersensitivity to leptin-triggered sympathetic activation in the presence of selective resistance to leptin-mediated anorexia. Individuals with obesity-related hyperleptinemia in the presence of heightened leptin-mediated sympathetic activation are at dramatically increased risk of adult hypertension.
I. Perinatal Growth Restriction, Prematurity and Adult Hypertension
A. Clinical Investigations
Intrauterine growth restriction is a strong, independent risk factor for adult cardiovascular disease. International investigations over the past 3 decades have reaffirmed the landmark epidemiological observations of Barker et al. (1989). In those seminal studies, an inverse relationship was noted between birth weight and ischemic heart disease. While dyslipidemia and obesity often coexist, hypertension remains a modifiable risk factor for life-threatening heart disease. Subsequent studies have thus focused on the association between IUGR and adult hypertension.
Consistent with the earlier investigations, a number of studies over the following decades demonstrated an inverse correlation between birth weight and adult blood pressure (Curhan et al. 1999, Eriksson et al. 2007). In a large twin cohort study, Bergvall et al. (2007) found decreased birth weight associated with increased risk of hypertension independent of genetic factors, shared familial environment, and adult body mass index. Although relatively few human studies have been powered for sex-specific effects, the available data are consistent with sex differences in the developmental origins of hypertension (Intapad et al. 2014). Jones et al. (2008) and Miles et al. (2011) have each shown that former IUGR men but not women have increased baseline blood pressure that is exacerbated by psychological stress, and this sexual dimorphism has been a consistent theme in preclinical animal models (Intapad et al. 2014).
Clinical investigations into the potential etiology of IUGR-associated hypertension have consistently demonstrated increased sympathetic tone in formerly or persistently growth restricted adults, including heightened peroneal nerve traffic, urinary catecholamine excretion and stress-evoked cardiovascular alterations (Boguszewski et al. 2004, Johansson et al. 2007, Jones et al. 2008). Intriguingly, the stress hypertension seen in IUGR infants correlates with a lack of salivary cortisol response to painful procedures, suggesting programmed alterations in hypothalamic function (Schaffer et al. 2009). This contrasts with the increased cortisol response typically seen in adults with stress-evoked hypertension (Grant et al 2009). Studies on autonomic blood pressure regulation in adults generally do not report birth weight or gestational age at delivery. Longitudinal assessment of premature or growth restricted populations will be necessary to identify the temporal relationship between sympathoadrenal activation and the development of hypertension.
The impact of IUGR on adult blood pressure is significantly influenced by the rate of postnatal growth. Central adiposity is seen in a subpopulation of growth restricted individuals which otherwise remain smaller than their normal birth weight peers (Crume et al. 2014, Hack et al. 2003). Neonatal growth acceleration increases the risk of adult obesity and hypertension (Ben-Shlomo et al. 2008), while continued growth restriction throughout infancy increases the risk of hypertension and ischemic heart disease more than that seen after IUGR alone (Barker et al. 1989, Eriksson et al. 2007). More so than term infants, premature infants are at substantial risk for neonatal growth restriction, with 90% of preterm infants developing postnatal weights below the 10th percentile by the time of hospital discharge (Dusick et al. 2003). Extremely low birth weight children remain smaller than their peers in regards of height and weight at age 8 years and by 14 years, boys catch up but girls continue to remain smaller (Hack et al. 2014). In part related to this near universal prematurity-associated neonatal growth restriction, preterm birth trumps IUGR as a risk factor for hypertension (Johansson et al. 2005).
As seen in IUGR infants, preterm infants have additional cardiovascular risk factors consistent with sympathetic over-activation, including increased catecholamine excretion, increased resting heart rates, and exaggerated pressor responses to psychological stress (Johansson et al. 2007, Jones et al. 2007, Pyhala et al. 2009, Ward et al. 2004). Up to 70% of preterm infants demonstrate elevated systolic blood pressure as early as 1 year of age and hypertension remains a significant health concern into adulthood, especially in the presence of adult obesity (Dagle et al. 2011, Duncan et al. 2011, Johansson et al. 2005, Pyhala et al. 2009, Sipola-Leppanen et al. 2015). The fact that prematurity-related hypertension persists into adulthood puts significant afterload on the heart for decades (de Jong et al. 2012, Kerkhof et al. 2012), potentially contributing to the abnormal cardiac dimensions seen even in infancy (Lewandowski et al. 2013, Sehgal et al. 2013). Unfortunately, this hypertensive phenotype is present among infants born as late as 37 weeks gestation (Gunay et al. 2014), and unlike the sexual dimorphism seen in IUGR infants, both preterm boys and girls appear to be at a heightened risk of adult hypertension (Bonamy et al. 2012). Notably, the same neurodevelopmental consequences that follow preterm delivery have been increasingly reported in IUGR infants, including reduced brain volumes and impaired neuronal connectivity (Miller et al. 2016), suggesting a potential common etiology linking neurodevelopment and the neuronal regulation of blood pressure.
B. Murine Model
Animal models have been indispensable in elucidating the physiologic and cellular processes that are altered by perinatal growth restriction. Interspecies differences are important considerations in identifying appropriate preclinical models to test critical hypotheses. We continue to collaborate with others in the pursuit of answers in sheep, rats and mice with notable sex-specific phenotypes observed (Katkhuda et al. 2012, Roghair & Aldape 2007, Roghair et al. 2009). Important investigations in rats have already shown late gestation uterine artery ligation elicits intrauterine growth restriction, increased sympathetic tone and post-menopausal hypertension that can be normalized by estrogen replacement or bilateral renal denervation (Intapad et al. 2013, Ojeda et al. 2007). Likewise, maternal malnutrition-induced IUGR programs sympathetic nerve overactivity in response to physical stress (Mizuno et al. 2013). Interestingly, it has been shown that the effects of uterine artery ligation may be mediated, in part, by placental hypoxia-related perinatal leptin deficiency (Nüsken et al. 2016). We in turn developed a mouse model to test the mechanistic hypothesis that central neuronal pathways are involved in the perpetuation of programmed hypertension.
As a foundation for the mechanistic studies to come, we assessed the physiologic implications of natural variation in perinatal growth. Those initial studies replicated human observations that IUGR with persistent neonatal growth restriction increases the risk of hypertension and neurodevelopmental impairment (Hermann et al. 2009), and that estrogen replacement mitigates the hypertensive phenotype otherwise seen in postmenopausal growth restricted mice (Haskell et al. 2016). Our follow-up investigations revealed that neonatal leptin supplementation prevents the neuropsychiatric impairment and hypertensive response to psychological stress that otherwise follow neonatal growth restriction (Erkonen et al. 2011, Meyer et al. 2014). This therapeutic use of leptin was informed by clinical data showing both IUGR and preterm infants undergo critical phases of neuromaturation in the presence of low circulating levels of the neurotrophic hormone leptin.
II. Perinatal Growth Restriction, Prematurity and Circulating Leptin Levels
A. Clinical Investigations
Leptin is primarily secreted by mature adipocytes and circulating leptin levels highly correlate with body mass index. In the fetus, adipose development begins in the 2nd trimester, and by 28 weeks gestation, adipose tissue has appeared in the major deposition areas (Poissonnet et al. 1988). Considering the key role leptin plays in organogenesis, it is understandable that redundant systems are in place to ensure the adequacy of circulating leptin levels throughout gestation. Among the contributors to fetal plasma leptin levels, the mother and the placenta play a major role in the first and second trimesters, and the fetus itself becomes a main contributor in the final trimester.
Studies describing the strong association between maternal adiposity, maternal leptin levels and cord blood leptin levels support an important role for transplacental (maternal-to-fetal) leptin delivery (Ho et al. 2010, Luo et al. 2013, Toprak et al. 2004, Varvarigou et al. 1999). Arguing for alternative leptin sources to support fetal development, other investigations have not observed the same association (Clapp & Kiess 1998, Hassink et al. 1997, Helland et al. 1998, Hytinantti et al. 1999, Pighetti et al. 2003, Sooranna et al. 2001, Yildiz et al. 2002), and direct maternal to fetal leptin transfer has not been identified.
Many investigators have demonstrated a direct correlation between placental weight and cord blood leptin levels (Clapp & Kiess 1998, Jaquet et al. 1998, Koistinen et al. 1997, Valuniene et al. 2007, Varvarigou et al. 1999). Hassink et al. (1997) signaled the potential of the placenta as a contributor to fetal leptin by showing that placental trophoblastic cells secrete leptin, and Yura et al. (1998) demonstrated higher leptin levels in umbilical venous blood compared to umbilical arterial blood. Lea et al. (2000) subsequently demonstrated that leptin mRNA and protein can be found in the placenta as early as the 1st trimester. In the same study, leptin mRNA and protein were localized to the syncytiotrophoblast and the villous vascular endothelium (Lea et al. 2000). These data suggest that placental leptin is released into both the fetal and maternal circulations. Laperecq et al. (2001) later showed that nearly 95% of placental leptin is secreted into maternal circulation with only 5% delivered to the fetus. Evidence supporting the physiologic relevance of placental leptin production includes the increased plasma leptin levels seen in pregnant versus non-pregnant women (Matsuda et al. 1999, Schubring et al. 1999, Yokota 2003), and the positive correlation of maternal leptin levels with advancing gestational age (Helland et al. 1998, Visentin et al. 2014). While the increase in plasma leptin level during pregnancy often correlates with gestational weight gain (Castellano Filho et al. 2013, Marino-Ortega et al. 2015), that association is not observed in obese women (Franco-Sena et al. 2015, Misra & Trudeau 2011), suggesting that increased fat mass is not the only contributor to the rise in maternal leptin levels during pregnancy. Further support for an adipose-independent source for leptin is found in the studies by Highman et al. (1998) showing the increase in maternal leptin precedes pregnancy-related weight gain, and maternal leptin levels promptly decrease after delivery (Highman et al. 1998, Lage et al. 1999, Schubring et al. 1998).
Placental leptin production and release appear to be regulated by factors including synthetic and endogenous glucocorticoid exposure. Antenatal betamethasone acutely increases maternal leptin but decreases fetal leptin (Marinoni et al. 2008). Those results were replicated by third trimester dexamethasone administration to pregnant rats (Sugden et al. 2001), supporting glucocorticoid-induced reduction in maternal to fetal and placental to fetal leptin passage (Smith & Waddell 2003). Consistent with those data, growth-restricted fetuses that are exposed to increased endogenous glucocorticoids typically have reduced leptin levels with decreased placental leptin expression, while maternal plasma leptin levels are increased (Lepercq et al. 2001, Nezar et al. 2009, Pighetti et al. 2003, Visentin et al. 2014). Similar changes are seen in twin pregnancies with lower placental leptin expression in IUGR affected twin placenta compared to normal growth twin placenta (Lea et al. 2000). It is notable that other studies have failed to demonstrate a consistent relationship between antenatal glucocorticoid exposure and fetal leptin levels (Ng et al. 2001, Shekhawat et al. 2000, Spear et al. 2001). Beyond adrenal steroids, sex steroids appear to influence leptin levels with male infants having lower levels than females (Chiesa et al. 2008, Ertl et al. 1999, Hassink et al. 1997, Helland et al. 1998, Jaquet et al. 1999, Matsuda et al. 1999, Ng et al. 2000, Ng et al. 2001, Ong et al. 1999, Toprak et al. 2004, Valuniene et al. 2007, Yokota 2003) and an indirect relationship has been observed between testosterone and leptin levels (Ertl et al. 1999, Ng et al. 2001, Su et al. 2002). Of note, this sexual dimorphism does not emerge until the final 6 weeks of gestation (Jaquet et al. 1998, Koistinen et al. 1997, Spear et al. 2001, Stoll-Becker et al. 2003). The dependence of leptin levels on maternal-independent factors suggests the fetus helps regulate its own leptin level in the later stages of gestation. It has not been determined clinically whether growth restriction itself reduces adipocyte leptin production independent of fetal fat mass accrual.
While fetal adipocytes produce leptin as early as 20 weeks gestation (Lepercq 2001), fetal fat mass more than doubles from 32 to 41 weeks gestation (Lapillonne et al. 1997). Umbilical cord blood leptin levels thus slowly increase from 18 to 32 weeks gestation and then increase rapidly until birth (Cetin et al. 2000, Ertl et al. 1999, Fonseca et al. 2004, Jaquet et al. 1998, Lo et al. 2002, Matsuda et al. 1999, Park et al. 2001, Stoll-Becker et al. 2003, Yokota 2003). The importance of fetal fat mass accumulation is supported by the fact that neonatal fat mass has a stronger association with newborn leptin levels than birth weight (Clapp & Kiess 1998, Fonseca et al. 2004, Jaquet et al. 1998, Toprak et al. 2004). Not surprisingly, by 34 weeks gestation, intrauterine growth restricted (IUGR) infants tend to have lower cord blood leptin levels than appropriate for gestational age infants (Cetin et al. 2000, Chiesa et al. 2008, Jaquet et al. 1998, Koistinen et al. 1997, Lapillonne et al. 1997, Martos-Moreno et al. 2009, Nezar et al. 2009, Pighetti et al. 2003, Valuniene et al. 2007, Yildiz et al. 2002), although some investigations have not observed the same results (Aydin et al. 2015, Geary et al. 1999, Stoll-Becker et al. 2003). Furthermore, leptin levels do not vary between appropriate for gestational age and small for gestational age early preterm infants (Hellgren et al. 2015, Jaquet et al. 1998, Ohkawa et al. 2010, Stoll-Becker et al. 2003). Overall, depending on the population and gestational stage of assessment, cord blood leptin positively correlates with a mix of factors including maternal weight gain during pregnancy, maternal BMI, gestational age, birth weight, ponderal index, infant skin fold thickness, and ultrasound-measured adiposity (Brynhildsen et al. 2013, Cetin et al. 2000, Karakosta et al. 2013, Mantzoros et al. 2009, Su et al. 2002, Yildiz et al. 2002). The finding that infant BMI has a greater association with infant plasma leptin levels than birth weight (Fonseca et al. 2004, Toprak et al. 2004) again supports the premise that the development of adipose tissue and the accumulation of fat mass drive the late gestation surge in serum leptin.
Consistent with the importance of transplacental leptin passage even in the later stages of gestation, the physiologic leptin surge is abruptly terminated by delivery for both preterm and term infants (Helland et al. 1998, Hytinantti et al. 1999, Jaquet et al. 1999, Kawamata et al. 2014, Valuniene et al. 2007). Following this initial drop, leptin levels remain low for the first week of life (Ertl et al. 1999, Harigaya et al. 1997, Matsuda et al. 1999, Ng et al. 2000, Park et al. 2001, Valuniene et al. 2007, Yokota 2003). Not surprisingly, this postnatal leptin decline does not correlate with the extent of neonatal weight loss (Hytinantti et al. 1999, Jaquet et al. 1999, Matsuda et al. 1999, Park et al. 2001, Valuniene et al. 2007) since a majority of initial weight loss reflects fluid shifts rather than changes in fat mass.
Premature infants face the challenge to grow and develop in the presence of profound leptin deficiency. They are born prior to the third trimester leptin surge and suffer from a lack of placental and transplacental leptin delivery. There are reports showing a correlation between infant plasma leptin levels and infant gestational age and anthropometric measures (Chiesa et al. 2008, Lo et al. 2002, Ng et al. 2000, Ng et al. 2001, Toprak et al. 2004, Valuniene et al. 2007), but there are also reports suggesting a lack of correlation in preterm infants (Hellgren et al. 2015, Ho et al. 2010, Jaquet et al. 1998, Ohkawa et al. 2010, Spear et al. 2001). The correlation between preterm infant leptin and weight typically becomes significant after infants begin to accrue adipose tissue mass (Enzi et al. 1981). Depending on the gestational age at birth, leptin levels remain below term infant values for 3 to 8 weeks (Ertl et al. 1999, Ohkawa et al. 2010). Ng et al. (2001) studied 61 premature infants and did not observe a statistically significant leptin level increase through day of life 35. Likewise, Toparak et al. (2004) showed that an increase in body weight and thickness of subcutaneous adipose tissue eventually led to an increase in leptin levels with adipose tissue appearing to be the main contributor to leptin levels after 30 days of life.
To further quantify the leptin deficiency of prematurity, Hellgren et al. (2015) compared 4 week-old preterm infants at 32 weeks adjusted gestational age to newborn infants at 32 weeks gestation and noted that former 28 week preterm infants had leptin levels far below the reference cohort. To our knowledge, this was the first study comparing postnatal leptin levels of preterm infants to “reference fetus” levels. Those data highlight the concern that preterm infants develop persistent postnatal leptin deficiency during a window of developmental vulnerability with potential neurodevelopmental implications. The duration of the postnatal leptin deficiency has not been fully quantified, but recent investigations have confirmed the persistent growth deficiency consistently reported in longitudinal studies (Rochow et al. 2016). For example, Spear et al. (2001) followed weekly leptin levels for infants born before 32 weeks gestation until discharge and did not observe leptin level increase in these infants, and their levels remained significantly below those seen in term infants, even after the preterm cohort reached 40 weeks corrected gestational age. Likewise, Toporak et al (2004) showed that infants born on average at 34 weeks gestation needed three months to reach serum leptin levels near those of full-term infants. This prolonged low leptin state might represent functional immaturity, a lack of sufficient adipose tissue or the suboptimal nutrition that is frequently seen in premature infants. Further studies are needed to define postnatal leptin changes and the cause-effect relationship between circulating leptin and postnatal growth.
In addition to preterm infants, IUGR infants have decreased postnatal leptin levels (Harigaya et al. 1997, Jaquet et al. 1999). Twin studies by Sooranna et al. (2001) and Lea et al. (2000) have shown that leptin levels are lower in the growth restricted twin when compared to the normal growth twin, further supporting the theory that leptin is either a cause or consequence of growth deficiency. Similar to the abrupt postnatal leptin decline observed in preterm infants, leptin levels decrease below already low fetal levels in IUGR infants throughout the first week of life (Duncan et al. 2011, Jaquet et al. 1999). With many IUGR infants showing significant catch-up growth during the first year of life, Jaquet et al. (1999) evaluated their leptin level changes over 2 years, and observed a peak at 6 months.
Beyond caloric intake, the nutritional source influences postnatal leptin levels. Parenteral nutrition and infant formulas do not contain leptin (O’Connor et al. 2003, Resto et al. 2001), but breast milk does (Casabiell et al. 1997, Miralles et al. 2006, Resto et al. 2001). Savino et al. (2013) evaluated leptin levels among healthy term infants and then performed follow-up at a median age of 8.8 years. They reported that the infants who were breast fed had higher leptin levels than formula fed infants. At follow up, breast fed infants had lower BMI contributing to the inverse relationship seen between leptin levels in infancy and childhood BMI. The encouragement of breast feeding may help curtail transgenerational obesity as maternal BMI and maternal serum leptin correlates with breast milk leptin levels, and increased breast milk leptin is associated with reduced neonatal growth velocity (Schuster et al. 2011). Studies in rats have confirmed absorption of enterally administered leptin (Casabiell et al. 1997, Sanchez et al. 2005). Unfortunately, at 15 days of life, breast milk leptin is decreased in mothers of growth restricted infants in comparison to the breast milk provided by mothers of appropriately grown infants (Dundar et al. 2005). Table 1 summarizes the risk factors in common for perinatal leptin deficiency and programmed adult hypertension.
Table 1.
Factors that increase the risk of perinatal leptin deficiency and programmed hypertension
| Decreased breast milk intake |
| Male sex |
| Perinatal growth restriction |
| Perinatal glucocorticoid exposure |
| Placental insufficiency or hypoxia |
| Preterm birth |
B. Murine Model
Consistent with their relative short gestation, mice are born with a paucity of adipose tissue and thus have low leptin levels throughout the first 3 to 4 postnatal days; with increasing adipose deposition and advancing breast milk intake, leptin levels then rise and peak between day 4 and 14 (Ahima et al. 1998, Delahaye et al. 2008, Yura et al. 2005). In the initial investigation of Ahima et al. (1998) female mice with normal birth weights were fostered in litters of 5 pups and leptin levels surged between days 4 and 16. When Yura et al. (2005) instead fostered normal birth weight mice into mixed sex litters of 8 or 9 pups, they observed a smaller leptin surge spanning from day 8 through 16. In that study, intrauterine growth restriction was induced in additional mice by maternal caloric restriction, and the postnatal leptin surge was skewed towards higher values on days 8 to 10 with decreasing values on day16, leading to the conclusion that IUGR may provoke a “premature leptin surge”. However, the peaked leptin levels seen in IUGR mice on days 8 to 10 mirrored the timing of the leptin surge in the study by Ahima et al.(1998), as well the investigations by Delahaye et al. (2008) showing a postnatal peak on days 4 to 10 in control male rats fostered in litters of 8 pups. In that latter study, maternal perinatal undernutrition did not alter the timing of the leptin surge, but did significantly decrease neonatal leptin levels with parallel interference with hypothalamic development (Delahaye et al. 2008).
III. Leptin’s Role in Adult Hypertension
A. Clinical Investigations
The first insights into the hemodynamic effects of leptin emerged from epidemiological investigations showing that individuals with rare leptin gene mutations were morbidly obese but paradoxically normotensive (Montague et al. 1997, Ozata et al. 1999, Rahmouni et al. 2005). Controversy ensued because early clinical trials of leptin in patients with obesity or lipodystrophy primarily evaluated metabolic outcomes but cursorily reported an absence of leptin-induced cardiovascular manifestations (Heymsfield et al. 1999). The concept of leptin-mediated hypertension gained traction when leptin levels were shown to independently correlate with blood pressure in both postmenopausal women and otherwise healthy men (Allison et al. 2013, Ma et al. 2009, Smith et al. 2015). Elegant preclinical and clinical investigations finally demonstrated retention of sympathetic activation and hypertension despite selective obesity-related resistance to leptin-induced anorexia (Simonds et al. 2014). Further investigations continue to uncover down-stream pathways that can be exploited to target the various effects leptin elicits in the normal physiologic state. For example, hypothalamic leptin receptor expression is found to play a critical role in leptin-induced sympathetic activation and hypertension (Harlan et al. 2011, Rahmouni & Morgan 2007) with signal transducer and activator of transcription 3 (STAT3) phosphorylation within proopiomelanocortin neurons particularly important in leptin- or stress-evoked hypertension (do Carmo et al. 2011, Dubinion et al. 2013).
B. Murine Models
We developed a murine model to specifically test the hypothesis that leptin is involved in both the inception and the propagation of perinatal growth restriction-related hypertension. After validating the model by demonstrating adult hypertension in intrauterine and/or neonatal growth restricted mice (Hermann et al. 2009), we went on to test leptin’s therapeutic potential. Given the relative developmental immaturity of newborn mice, the critical third trimester of human adipogenesis and neurodevelopment is analogous to the first two postnatal weeks in mice (Grove et al. 2005, Romijn et al. 1991). Likewise, the leptin surge that occurs in the final months of human gestation coincides with the postnatal day 4 to 14 leptin surge that occurs in well-grown mice. To focus on that critical window of vulnerability, we developed a neonatal growth restriction model by placing pups into litters of 12 to elicit neonatal growth restriction, defined by a weanling weight below the 10th percentile in a manner consistent with WHO guidelines. Mice with incipient growth restriction then receive daily parental leptin supplementation from day 4 to 14 at a dose (80 ng/g) that recapitulates the leptin levels measured in control mice that are fostered in typical litters of 6 pups. This is a multifactorial model with likely contributions from the same factors operating in preterm humans, including neonatal stress as well as relative neonatal undernutrition. Beyond leptin’s neurodevelopmental effects, exogenous leptin can attenuate the adrenal response to neonatal stress (Salzmann et al. 2004). Future studies are needed to determine the acute effect of neonatal leptin supplementation on adrenal function and glucocorticoid levels to test the hypothesis that leptin protects against the hypertensive effects of glucocorticoids.
In adulthood, mice with a history of neonatal growth restriction displayed psychological stress-exacerbated hypertension, neuropsychiatric impairment and altered brain morphology (Erkonen et al. 2011, Meyer et al. 2014). Neonatal leptin supplementation exerted trophic effects on the hypothalamus and normalized all of those findings (Erkonen et al. 2011, Meyer et al. 2014). Mechanistically, neonatal leptin normalization corrected the exaggerated pressor response seen following intracerebroventricular leptin administration, normalized the heightened renal sympathetic nerve response seen following parenteral leptin administration, and normalized the increased leptin receptor expression seen in the arcuate nucleus of the hypothalamus (ARC) (Peotta et al. 2016).
Regarding the potential effects of neonatal leptin on adult cardiovascular outcomes; the dose and timing of the intervention are critically important. When neonatal leptin is administered to well grown mice and rats at supraphysiologic doses (2.5 to 6 mg/kg/d), long-term impairment in leptin-triggered anorexia is observed (Samuelsson et al. 2013, Yura et al. 2005). Vickers et al. (2005 and 2008) have shown that supraphysiologic leptin administration (2.5 mg/kg/d) leads to selective leptin resistance in adult mice, with loss of leptin’s anorectic effects but retention of leptin-induced hypertension. This inverse correlation between leptin sensitivity and neonatal leptin levels is consistent with the investigations of Rahmouni et al. (2005) showing obesity-related (supraphysiologic) hyperleptinemia is associated with selective resistance to leptin’s metabolic effects. Finally, the timing of the leptin replacement must match the phase of leptin-sensitive neurodevelopment. For example, physiologic leptin administration to lactating rats confers protection from diet-induced obesity in association with favorable alterations in hypothalamic gene expression (Palou et al. 2011, Pico et al. 2007, Stocker et al. 2007). Likewise, placing pups in large litters to elicit neonatal undernutrition leads to a lean, leptin-responsive adult phenotype; while placement in small litters to elicit overnutrition predisposes to obesity in association with impaired leptin-triggered hypothalamic STAT3 signaling (Rodrigues et al. 2009). Complementary models once again show that intrauterine growth restriction followed more importantly by accelerated neonatal growth, suppresses adult leptin signaling (Coupe et al. 2012).
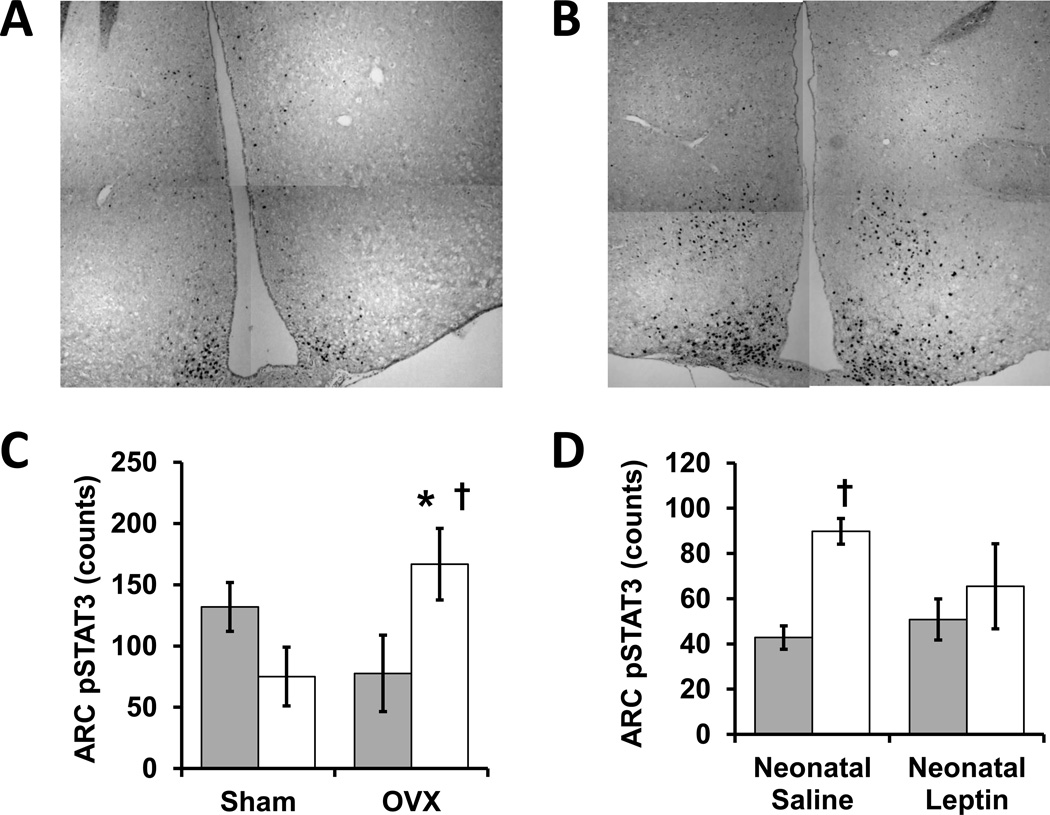
Further investigations are required to dissect relevant signaling partners with leading candidates including leptin-induced co-activation of the renin-angiotensin system (Hilzendeger et al. 2012). There are also known interactions between estrogen and leptin, with both hormones acting in part through phosphorylation of STAT3 (Clegg et al. 2006, Gao et al. 2007, Shi & Brooks 2015). Regarding brain-site specific regulation, the ARC is exquisitely sensitive to environmental modulation of leptin sensitivity and sympathetic tone increases after ARC-targeted leptin microinjections (Harlan et al. 2011, Münzberg et al. 2004, Tanida et al. 2015). Our initial investigations have confirmed interactions between neonatal growth restriction, ovarian function, and neonatal leptin administration in the establishment of leptin-induced STAT3 signaling in the ARC (Figure 2).
Figure 2.
The influence of perinatal growth, ovarian status and neonatal leptin administration on leptin-stimulated hypothalamic signaling in adult mice. These original experiments with C57BL/6J mice (Jackson Laboratory) were approved by the University of Iowa Office of Animal Resources. Neonatal growth restriction (GR) was induced by placing mice into litters of 12 rather than 6 pups on postnatal day 1, and GR was confirmed by weanling weights below the 10th percentile for the colony on postnatal day 20. At 2 to 4 months, female mice underwent bilateral ovariectomy (OVX) or sham surgery (incisions without ovary removal) under inhaled isoflurane anesthesia (4%, Phoenix Scientific). After 2 to 3 months of postoperative recovery, a 3 hour fast was followed by intraperitoneal injection of murine leptin (1 mg/kg, Biomyx Technology) one hour prior to transcardiac perfusion fixation during anesthesia with intraperitoneal ketamine (262.5 mg/kg, Sigma-Aldrich) and xylazine (37.5 mg/kg, Sigma-Aldrich). Coronal cryosections were obtained though the arcuate nucleus of the hypothalamus (ARC) and pSTAT3 immunoreactivity was quantified with anti-phospho-STAT3 (9145, Cell Signaling) followed by detection with ImmPRESS/DAB (MP-7401, Vector Laboratories). Representative immunostains (A, Control-OVX; B, GR-OVX) are composites of 4 higher magnification images. C, Leptin-stimulated pSTAT3-positive cells counts were influenced by a significant interaction between GR and adult ovarian status (control: closed bars, GR: open bars, N = 4 or 5). OVX significantly increased leptin-evoked pSTAT3 signaling in GR mice (†P<0.05 by ANOVA) such that GR-OVX mice had significantly more pSTAT3 reactive nuclei than control-OVX mice (*P<0.05 by ANOVA), suggesting adult ovarian function suppresses GR-associated leptin responsiveness. Compared to control-OVX mice, GR-OVX mice also tended to have more pSTAT3 reactive nuclei in the ventral medial hypothalamus, but the difference did not reach statistical significance (control-OVX 11+/−6, GR-OVX 31+/−7). D, Unlike the significant increase in ARC pSTAT3 seen in control (neonatal saline) mice that received leptin (open bars) versus saline (closed bars) prior to perfusion fixation (†P<0.05 by ANOVA), neonatal leptin-treated mice did not have significantly different responses to pre-fixation injection of leptin versus saline, suggesting neonatal leptin administration decreased adult leptin responsiveness (N= 3 or 4).
Conclusion
In conclusion, the cumulative evidence indicates that leptin plays a critical role in perinatal development and that perinatal leptin levels are insufficient to optimize neuroregulatory pathways in both IUGR and preterm infants. In translational mouse models, leptin supplementation targeted to otherwise leptin deficient growth restricted mice is sufficient to prevent the development of neurocardiovascular dysfunction and normalize the neural control of renal sympathetic activity and arterial pressure. In addition to mice, leptin supplementation elicits neurotrophic effects in growth restricted piglets as well as genetically leptin-deficient humans (Attig et al. 2008, Matochik et al. 2005). Although the neuroanatomic and molecular interactions in the pathways leading to programmed adult hypertension are incompletely understood, the important role leptin plays in key developmental and regulatory pathways support ongoing investigations into the impact of leptin on the inception and perpetuation of perinatal growth restriction or prematurity-related hypertension.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant number HL007485).
We thank our families and colleagues for their invaluable support.
Footnotes
Declaration of interest
There are no conflicts of interest that could be perceived as prejudicing the impartiality of this review or the research reported.
Author contributions
Baiba Steinbrekera and Robert Roghair drafted and revised the article. Robert Roghair was involved in the design, analysis and interpretation of the primary experiments.
References
- Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. Journal of Clinical Investigation. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison MA, Ix JH, Morgan C, McClelland RL, Rifkin D, Shimbo D, Criqui MH. Higher leptin is associated with hypertension: the Multi-Ethnic Study of Atherosclerosis. Journal of Human Hypertension. 2013;27:617–622. doi: 10.1038/jhh.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attig L, Djiane J, Gertler A, Rampin O, Larcher T, Boukthir S, Anton PM, Madec JY, Gourdou I, Abdennebi-Najar L. Study of hypothalamic leptin receptor expression in low-birth-weight piglets and effects of leptin supplementation on neonatal growth and development. American Journal of Physiology: Endocrinology and Metabolism. 2008;295:E1117–E1125. doi: 10.1152/ajpendo.90542.2008. [DOI] [PubMed] [Google Scholar]
- Aydin HI, Eser A, Kaygusuz I, Yildirim S, Celik T, Gunduz S, Kalman S. Adipokine, adropin and endothelin-1 levels in intrauterine growth restricted neonates and their mothers. Journal of Perinatal Medicine. 2015;44:669–676. doi: 10.1515/jpm-2014-0353. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization. 2010;88:31–8838. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y, McCarthy A, Hughes R, Tilling K, Davies D, Smith GD. Immediate postnatal growth is associated with blood pressure in young adulthood: the Barry Caerphilly Growth Study. Hypertension. 52:638–644. doi: 10.1161/HYPERTENSIONAHA.108.114256. [DOI] [PubMed] [Google Scholar]
- Bergvall N, Iliadou A, Johansson S, de Faire U, Kramer MS, Pawitan Y, Pedersen NL, Lichtenstein P, Cnattingius S. Genetic and shared environmental factors do not confound the association between birth weight and hypertension: a study among Swedish twins. Circulation. 2007;115:2931–2938. doi: 10.1161/CIRCULATIONAHA.106.674812. [DOI] [PubMed] [Google Scholar]
- Boguszewski MC, Johannsson G, Fortes LC, Sverrisdóttir YB. Low birth size and final height predict high sympathetic nerve activity in adulthood. Journal of Hypertension. 2004;22:1157–1163. doi: 10.1097/00004872-200406000-00017. [DOI] [PubMed] [Google Scholar]
- Bonamy AK, Källén K, Norman M. High blood pressure in 2.5-year-old children born extremely preterm. Pediatrics. 2012;129:e1199–e1204. doi: 10.1542/peds.2011-3177. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Brynhildsen J, Sydsjö G, Blomberg M, Claesson IM, Theodorsson E, Nyström F, Sydsjö A, Josefsson A. Leptin and adiponectin in cord blood from children of normal weight, overweight and obese mothers. Acta Paediatrica. 2013;102:620–624. doi: 10.1111/apa.12202. [DOI] [PubMed] [Google Scholar]
- Casabiell X, Piñeiro V, Tomé MA, Peinó R, Diéguez C, Casanueva FF. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. Journal of Clinical Endocrinology & Metabolism. 1997;82:4270–4273. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- Castellano Filho DS, do Amaral Correa JO, Dos Santos Ramos P, de Oliveira Montessi M, Aarestrup BJ, Aarestrup FM. Body weight gain and serum leptin levels of non-overweight and overweight/obese pregnant women. Medical Science Monitor. 2013;19:1043–1049. doi: 10.12659/MSM.884027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin I, Morpurgo PS, Radaelli T, Taricco E, Cortelazzi D, Bellotti M, Pardi G, Beck-Peccoz P. Fetal plasma leptin concentrations: relationship with different intrauterine growth patterns from 19 weeks to term. Pediatric Research. 2000;48:646–651. doi: 10.1203/00006450-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Chiesa C, Osborn JF, Haass C, Natale F, Spinelli M, Scapillati E, Spinelli A, Pacifico L. Ghrelin, leptin, IGF-1, IGFBP-3, and insulin concentrations at birth: is there a relationship with fetal growth and neonatal anthropometry? Clinical Chemistry. 2008;54:550–558. doi: 10.1373/clinchem.2007.095299. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Clapp JF3rd, Kiess W. Cord blood leptin reflects fetal fat mass. Journal of the Society for Gynecologic Investigation. 1998;5:300–303. [PubMed] [Google Scholar]
- Coupé B, Grit I, Hulin P, Randuineau G, Parnet P. Postnatal growth after intrauterine growth restriction alters central leptin signal and energy homeostasis. PLoS ONE. 2012;7:e30616. doi: 10.1371/journal.pone.0030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crume TL, Scherzinger A, Stamm E, McDuffie R, Bischoff KJ, Hamman RF, Dabelea D. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 2014;22:608–615. doi: 10.1002/oby.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Prevention of cardiovascular disease: birthweight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1999;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- Dagle JM, Fisher TJ, Haynes SE, Berends SK, Brophy PD, Morriss FH, Jr, Murray JC. Cytochrome P450 (CYP2D6) genotype is associated with elevated systolic blood pressure in preterm infants after discharge from the neonatal intensive care unit. Journal of Pediatrics. 2011;159:104–109. doi: 10.1016/j.jpeds.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinion JH, do Carmo JM, Adi A, Hamza S, da Silva AA, Hall JE. Role of proopiomelanocortin neuron Stat3 in regulating arterial pressure and mediating the chronic effects of leptin. Hypertension. 2013;61:1066–1074. doi: 10.1161/HYPERTENSIONAHA.111.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AF, Heyne RJ, Morgan JS, Ahmad N, Rosenfeld CR. Elevated systolic blood pressure in preterm very-low-birth-weight infants ≤3 years of life. Pediatric Nephrology. 2011;26:1115–1121. doi: 10.1007/s00467-011-1833-x. [DOI] [PubMed] [Google Scholar]
- Dundar NO, Anal O, Dundar B, Ozkan H, Caliskan S, Büyükgebiz A. Longitudinal investigation of the relationship between breast milk leptin levels and growth in breast-fed infants. Journal of Pediatric Endocrinology and Metabolism. 2005;18:181–187. doi: 10.1515/jpem.2005.18.2.181. [DOI] [PubMed] [Google Scholar]
- Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Seminars in Perinatology. 2003;27:302–310. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Enzi G, Zanardo V, Caretta F, Inelmen EM, Rubaltelli F. Intrauterine growth and adipose tissue development. American Journal of Clinical Nutrition. 1981;34:1785–1790. doi: 10.1093/ajcn/34.9.1785. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsén TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–1421. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- Erkonen GE, Hermann GM, Miller RL, Thedens DL, Nopoulos PC, Wemmie JA, Roghair RD. Neonatal leptin administration alters regional brain volumes and blocks neonatal growth restriction-induced behavioral and cardiovascular dysfunction in male mice. Pediatric Research. 2011;69:406–412. doi: 10.1203/PDR.0b013e3182110c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl T, Funke S, Sárkány I, Szabó I, Rascher W, Blum WF, Sulyok E. Postnatal changes of leptin levels in full-term and preterm neonates: their relation to intrauterine growth, gender and testosterone. Biology of the Neonate. 1999;75:167–176. doi: 10.1159/000014093. [DOI] [PubMed] [Google Scholar]
- Fonseca VM, Sichieri R, Moreira ME, Moura AS. Early postnatal growth in preterm infants and cord blood leptin. Journal of Perinatology. 2004;24:751–756. doi: 10.1038/sj.jp.7211188. [DOI] [PubMed] [Google Scholar]
- Franco-Sena AB, Rebelo F, Pinto T, Farias DR, Silveira GE, Mendes RH, Henriques VT, Kac G. The effect of leptin concentrations and other maternal characteristics on gestational weight gain is different according to pre-gestational BMI: results from a prospective cohort. British Journal of Obstetrics and Gynaecology. 2015 Dec 10; doi: 10.1111/1471-0528.13826. 2015. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Medicine. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Geary M, Herschkovitz R, Pringle PJ, Rodeck CH, Hindmarsh PC. Ontogeny of serum leptin concentrations in the human. Clinical Endocrinology (Oxford) 1999;51:189–192. doi: 10.1046/j.1365-2265.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- Grant N, Hamer M, Steptoe A. Social isolation and stress-related cardiovascular, lipid, and cortisol responses. Annals of Behavioral Medicine. 2009;37:29–37. doi: 10.1007/s12160-009-9081-z. [DOI] [PubMed] [Google Scholar]
- Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS. Development of metabolic systems. Physiology & Behavior. 2005;86:646–660. doi: 10.1016/j.physbeh.2005.08.063. [DOI] [PubMed] [Google Scholar]
- Gunay F, Alpay H, Gokce I, Bilgen H. Is late-preterm birth a risk factor for hypertension in childhood? European Journal of Pediatrics. 2014;173:751–756. doi: 10.1007/s00431-013-2242-x. [DOI] [PubMed] [Google Scholar]
- Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003;112:e30–e38. doi: 10.1542/peds.112.1.e30. [DOI] [PubMed] [Google Scholar]
- Hack M, Schluchter M, Margevicius S, Andreias L, Taylor HG, Cuttler L. Trajectory and correlates of growth of extremely-low-birth-weight adolescents. Pediatric Research. 2014;75:358–366. doi: 10.1038/pr.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya A, Nagashima K, Nako Y, Morikawa A. Relationship between concentration of serum leptin and fetal growth. Journal of Clinical Endocrinology and Metabolism. 1997;82:3281–3284. doi: 10.1210/jcem.82.10.4321. [DOI] [PubMed] [Google Scholar]
- Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circulation Research. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell SE, Peotta V, Reinking BE, Zhang C, Zhu V, Kenkel EJ, Roghair RD. Oral oestrogen reverses ovariectomy-induced morning surge hypertension in growth-restricted mice. Clinical Science (London) 2016;130:613–623. doi: 10.1042/CS20150693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassink SG, de Lancey E, Sheslow DV, Smith-Kirwin SM, O’Connor DM, Considine RV, Opentanova I, Dostal K, Spear ML, Leef K, et al. Placental leptin: an important new growth factor in intrauterine and neonatal development? Pediatrics. 1997;100:E1. doi: 10.1542/peds.100.1.e1. [DOI] [PubMed] [Google Scholar]
- Helland IB, Reseland JE, Saugstad OD, Drevon CA. Leptin levels in pregnant women and newborn infants: gender differences and reduction during the neonatal period. Pediatrics. 1998;101:E12. doi: 10.1542/peds.101.3.e12. [DOI] [PubMed] [Google Scholar]
- Hellgren G, Engström E, Smith LE, Löfqvist C, Hellström A. Effect of Preterm Birth on Postnatal Apolipoprotein and Adipocytokine Profiles. Neonatology. 2015;108:16–22. doi: 10.1159/000381278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GM, Miller RL, Erkonen GE, Dallas LM, Hsu E, Zhu V, Roghair RD. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatric Research. 2009;66:53–58. doi: 10.1203/PDR.0b013e3181a7c5fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM. Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. American Journal of Obstetrics and Gynecology. 1998;178:1010–1015. doi: 10.1016/s0002-9378(98)70540-x. [DOI] [PubMed] [Google Scholar]
- Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. American Journal of Physiology: Heart and Circulatory Physiology. 303:H197–H206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SP, Wang LJ, Cheng I, Chen YL, Sung TC, Jow GM, Mu SC. Association of plasma leptin levels with maternal body weight and body mass index in premature and term newborns. Pediatrics and Neonatology. 2010;51:19–25. doi: 10.1016/S1875-9572(10)60005-8. [DOI] [PubMed] [Google Scholar]
- Hytinantti T, Koistinen HA, Koivisto VA, Karonen SL, Andersson S. Changes in leptin concentration during the early postnatal period: adjustment to extrauterine life? Pediatric Research. 1999;45:197–201. doi: 10.1203/00006450-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension. 2013;61:828–834. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiology (Bethesda) 2014;29:122–132. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. Journal of Clinical Endocrinology and Metabolism. 1998;83:1243–1246. doi: 10.1210/jcem.83.4.4731. [DOI] [PubMed] [Google Scholar]
- Jaquet D, Leger J, Tabone MD, Czernichow P, Levy-Marchal C. High serum leptin concentrations during catch-up growth of children born with intrauterine growth retardation. Journal of Clinical Endocrinology and Metabolism. 1999;84:1949–1953. doi: 10.1210/jcem.84.6.5744. [DOI] [PubMed] [Google Scholar]
- Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation. 2005;112:3430–3436. doi: 10.1161/CIRCULATIONAHA.105.540906. [DOI] [PubMed] [Google Scholar]
- Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpée M. Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. Journal of Internal Medicine. 2007;261:480–487. doi: 10.1111/j.1365-2796.2007.01776.x. [DOI] [PubMed] [Google Scholar]
- Jones A, Beda A, Ward AM, Osmond C, Phillips DI, Moore VM, Simpson DM. Size at birth and autonomic function during psychological stress. Hypertension. 2007;49:548–555. doi: 10.1161/01.HYP.0000257196.13485.9b. [DOI] [PubMed] [Google Scholar]
- Jones A, Beda A, Osmond C, Godfrey KM, Simpson DM, Phillips DI. Sex-specific programming of cardiovascular physiology in children. European Heart Journal. 2008;29:2164–2170. doi: 10.1093/eurheartj/ehn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakosta P, Georgiou V, Fthenou E, Papadopoulou E, Roumeliotaki T, Margioris A, Castanas E, Kampa M, Kogevinas M, Chatzi L. Maternal weight status, cord blood leptin and fetal growth: a prospective mother-child cohort study (Rhea study) Paediatric Perinatology Epidemiology. 2013;27:461–471. doi: 10.1111/ppe.12074. [DOI] [PubMed] [Google Scholar]
- Katkhuda R, Peterson ES, Roghair RD, Norris AW, Scholz TD, Segar JL. Sex-specific programming of hypertension in offspring of late-gestation diabetic rats. Pediatric Research. 2012;72:352–361. doi: 10.1038/pr.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata R, Suzuki Y, Yada Y, Koike Y, Kono Y, Yada T, Takahashi N. Gut hormone profiles in preterm and term infants during the first 2 months of life. Journal of Pediatric Endocrinology and Metabolism. 2014;27:717–723. doi: 10.1515/jpem-2013-0385. [DOI] [PubMed] [Google Scholar]
- Kerkhof GF, Breukhoven PE, Leunissen RW, Willemsen RH, Hokken-Koelega AC. Does preterm birth influence cardiovascular risk in early adulthood? Journal of Pediatrics. 2012;161:390–396. doi: 10.1016/j.jpeds.2012.03.048. [DOI] [PubMed] [Google Scholar]
- Koistinen HA, Koivisto VA, Andersson S, Karonen SL, Kontula K, Oksanen L, Teramo KA. Leptin concentration in cord blood correlates with intrauterine growth. Journal of Clinical Endocrinology and Metabolism. 1997;82:3328–3330. doi: 10.1210/jcem.82.10.4291. [DOI] [PubMed] [Google Scholar]
- Lage M, Garcia-Mayor RV, Tomé MA, Cordido F, Valle-Inclan F, Considine RV, Caro JF, Dieguez C, Casanueva FF. Serum leptin levels in women throughout pregnancy and the postpartum period and in women suffering spontaneous abortion. Clinical Endocrinology (Oxford) 1999;50:211–216. doi: 10.1046/j.1365-2265.1999.00637.x. [DOI] [PubMed] [Google Scholar]
- Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatrica. 1997;86:196–200. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Lea RG, Howe D, Hannah LT, Bonneau O, Hunter L, Hoggard N. Placental leptin in normal, diabetic and fetal growth-retarded pregnancies. Molecular Human Reproduction. 2000;6:763–769. doi: 10.1093/molehr/6.8.763. [DOI] [PubMed] [Google Scholar]
- Lepercq J, Challier JC, Guerre-Millo M, Cauzac M, Vidal H, Hauguel-de Mouzon S. Prenatal leptin production: evidence that fetal adipose tissue produces leptin. Journal of Clinical Endocrinology and Metabolism. 2001;86:2409–2413. doi: 10.1210/jcem.86.6.7529. [DOI] [PubMed] [Google Scholar]
- Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, McCormick K, Wilkinson AR, Singhal A, Lucas A, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127:197–206. doi: 10.1161/CIRCULATIONAHA.112.126920. [DOI] [PubMed] [Google Scholar]
- Lo HC, Tsao LY, Hsu WY, Chen HN, Yu WK, Chi CY. Relation of cord serum levels of growth hormone, insulin-like growth factors, insulin-like growth factor binding proteins, leptin, and interleukin-6 with birth weight, birth length, and head circumference in term and preterm neonates. Nutrition. 2002;18:604–608. doi: 10.1016/s0899-9007(01)00811-5. [DOI] [PubMed] [Google Scholar]
- Luo ZC, Nuyt AM, Delvin E, Fraser WD, Julien P, Audibert F, Girard I, Shatenstein B, Deal C, Grenier E, et al. Maternal and fetal leptin, adiponectin levels and associations with fetal insulin sensitivity. Obesity (Silver Spring) 2013;21:210–216. doi: 10.1002/oby.20250. [DOI] [PubMed] [Google Scholar]
- Ma D, Feitosa MF, Wilk JB, Laramie JM, Yu K, Leiendecker-Foster C, Myers RH, Province MA, Borecki IB. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. 2009;53:473–479. doi: 10.1161/HYPERTENSIONAHA.108.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino-Ortega LA, Molina-Bello A, Polanco-García JC, Muñoz-Valle JF, Salgado-Bernabé AB, Guzmán-Guzmán IP, Parra-Rojas I. Correlation of leptin and soluble leptin receptor levels with anthropometric parameters in mother-newborn pairs. International Journal of Clinical and Experimental Medicine. 2015;8:11260–11267. [PMC free article] [PubMed] [Google Scholar]
- Marinoni E, Letizia C, Ciardo F, Corona G, Moscarini M, Di Iorio R. Effects of prenatal betamethasone administration on leptin and adiponectin concentrations in maternal and fetal circulation. American Journal of Obstetrics and Gynecology. 2008;199:141.e1-e6. doi: 10.1016/j.ajog.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Martos-Moreno GA, Barrios V, Sáenz de Pipaón M, Pozo J, Dorronsoro I, Martínez-Biarge M, Quero J, Argente J. Influence of prematurity and growth restriction on the adipokine profile, IGF1, and ghrelin levels in cord blood: relationship with glucose metabolism. European Journal of Endocrinology. 2009;161:381–389. doi: 10.1530/EJE-09-0193. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. Journal of Clinical Endocrinology and Metabolism. 2005;90:2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Yokota I, Iida M, Murakami T, Yamada M, Saijo T, Naito E, Ito M, Shima K, Kuroda Y. Dynamic changes in serum leptin concentrations during the fetal and neonatal periods. Pediatric Research. 1999;45:71–75. doi: 10.1203/00006450-199901000-00012. [DOI] [PubMed] [Google Scholar]
- Meyer LR, Zhu V, Miller A, Roghair RD. Growth restriction, leptin, and the programming of adult behavior in mice. Behavioural Brain Research. 2014;275:131–135. doi: 10.1016/j.bbr.2014.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles KL, McDonnell BJ, Maki-Petaja KM, Yasmin, Cockcroft JR, Wilkinson IB, McEniery CM Enigma Study investigators. The impact of birth weight on blood pressure and arterial stiffness in later life: the Enigma Study. Journal of Hypertension. 2011;29:2324–2331. doi: 10.1097/HJH.0b013e32834d0ca1. [DOI] [PubMed] [Google Scholar]
- Miller SL, Huppi PS, Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. Journal of Physiology. 2016;594:807–823. doi: 10.1113/JP271402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles O, Sanchez J, Palou A, Pico C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring) 2006;14:1371–1377. doi: 10.1038/oby.2006.155. [DOI] [PubMed] [Google Scholar]
- Misra VK, Trudeau S. The influence of overweight and obesity on longitudinal trends in maternal serum leptin levels during pregnancy. Obesity (Silver Spring) 2011;19:416–421. doi: 10.1038/oby.2010.172. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Siddique K, Baum M, Smith SA. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension. 2013;61:180–186. doi: 10.1161/HYPERTENSIONAHA.112.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Nezar MA, el-Baky AM, Soliman OA, Abdel-Hady HA, Hammad AM, Al-Haggar MS. Endothelin-1 and leptin as markers of intrauterine growth restriction. Indian Journal of Pediatrics. 2009;76:485–488. doi: 10.1007/s12098-009-0079-0. [DOI] [PubMed] [Google Scholar]
- Ng PC, Lam CW, Lee CH, Wong GW, Fok TF, Chan IH, Ma KC, Wong E. Leptin and metabolic hormones in preterm newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2000;83:F198–F202. doi: 10.1136/fn.83.3.F198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Lam CW, Lee CH, Wong GW, Fok TF, Wong E, Chan IH, Ma KC. Changes of leptin and metabolic hormones in preterm infants: a longitudinal study in early postnatal life. Clinical Endocrinology (Oxford) 2001;54:673–680. doi: 10.1046/j.1365-2265.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- Nüsken E, Wohlfarth M, Lippach G, Rauh M, Schneider H, Dötsch J, Nüsken KD. Reduced perinatal leptin availability may contribute to adverse metabolic programming in a rat model of utero-placental insufficiency. Endocrinology. 2016;157:1813–1825. doi: 10.1210/en.2015-1898. [DOI] [PubMed] [Google Scholar]
- O’Connor D, Funanage V, Locke R, Spear M, Leef K. Leptin is not present in infant formulas. Journal of Endocrinological Investigation. 2003;26:490. doi: 10.1007/BF03345207. [DOI] [PubMed] [Google Scholar]
- Ohkawa N, Shoji H, Kitamura T, Suganuma H, Yoshikawa N, Suzuki M, Lee T, Hisata K, Shimizu T. IGF-I, leptin and active ghrelin levels in very low birth weight infants during the first 8 weeks of life. Acta Paediatrica. 2010;99:37–41. doi: 10.1111/j.1651-2227.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, Dunger DB. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Journal of Clinical Endocrinology and Metabolism. 1999;84:1145–1148. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. Journal of Clinical Endocrinology and Metabolism. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Palou M, Picó C, McKay JA, Sánchez J, Priego T, Mathers JC, Palou A. Protective effects of leptin during the suckling period against later obesity may be associated with changes in promoter methylation of the hypothalamic pro-opiomelanocortin gene. British Journal of Nutrition. 2011;106:769–778. doi: 10.1017/S0007114511000973. [DOI] [PubMed] [Google Scholar]
- Park MJ, Namgung R, Kim JN, Kim DH. Serum leptin, IGF-I and insulin levels in preterm infants receiving parenteral nutrition during the first week of life. Journal of Pediatric Endocrinology and Metabolism. 2001;14:429–433. doi: 10.1515/jpem.2001.14.4.429. [DOI] [PubMed] [Google Scholar]
- Peotta V, Rahmouni K, Segar JL, Morgan DA, Pitz KM, Rice OM, Roghair RD. Neonatal growth restriction-related leptin deficiency enhances leptin-triggered sympathetic activation and central angiotensin II receptor-dependent stress-evoked hypertension. Pediatric Research. 2016 Apr 6; doi: 10.1038/pr.2016.64. 2016 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó C, Oliver P, Sánchez J, Miralles O, Caimari A, Priego T, Palou A. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. International Journal of Obesity. 2007;31:1199–1209. doi: 10.1038/sj.ijo.0803585. [DOI] [PubMed] [Google Scholar]
- Pighetti M, Tommaselli GA, D’Elia A, Di Carlo C, Mariano A, Di Carlo A, Nappi C. Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstetrics and Gynecology. 2003;102:535–543. doi: 10.1016/s0029-7844(03)00668-9. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Poissonnet CM, LaVelle M, Burdi AR. Growth and development of adipose tissue. Journal of Pediatrics. 1988;113:1–9. doi: 10.1016/s0022-3476(88)80520-1. [DOI] [PubMed] [Google Scholar]
- Pyhälä R, Räikkönen K, Feldt K, Andersson S, Hovi P, Eriksson JG, Järvenpää AL, Kajantie E. Blood pressure responses to psychosocial stress in young adults with very low birth weight: Helsinki study of very low birth weight adults. Pediatrics. 2009;123:731–734. doi: 10.1542/peds.2008-0277. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- Resto M, O’Connor D, Leef K, Funanage V, Spear M, Locke R. Leptin levels in preterm human breast milk and infant formula. Pediatrics. 2001;108:E15. doi: 10.1542/peds.108.1.e15. [DOI] [PubMed] [Google Scholar]
- Rochow N, Raja P, Liu K, Fenton T, Landau-Crangle E, Göttler S, Jahn A, Lee S, Seigel S, Campbell D, et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatric Research. 2016;79:870–879. doi: 10.1038/pr.2016.15. [DOI] [PubMed] [Google Scholar]
- Rodrigues AL, de Moura EG, Passos MC, Dutra SC, Lisboa PC. Postnatal early overnutrition changes the leptin signalling pathway in the hypothalamic-pituitary-thyroid axis of young and adult rats. Journal of Physiology. 2009;587:2647–2661. doi: 10.1113/jphysiol.2009.169045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghair RD, Aldape G. Naturally occurring perinatal growth restriction in mice programs cardiovascular and endocrine function in a sex- and strain-dependent manner. Pediatric Research. 2007;62:399–404. doi: 10.1203/PDR.0b013e31813cbf16. [DOI] [PubMed] [Google Scholar]
- Roghair RD, Segar JL, Volk KA, Chapleau MW, Dallas LM, Sorenson AR, Scholz TD, Lamb FS. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. American Journal of Physiology: Regulatory Integrative Comprehensive Physiology. 2009;296:R651–R662. doi: 10.1152/ajpregu.90756.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Human Development. 1991;26:61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Salzmann C, Otis M, Long H, Roberge C, Gallo-Payet N, Walker CD. Inhibition of steroidogenic response to adrenocorticotropin by leptin: implications for the adrenal response to maternal separation in neonatal rats. Endocrinology. 2004;145:1810–1822. doi: 10.1210/en.2003-1514. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Clark J, Rudyk O, Shattock MJ, Bae SE, South T, Pombo J, Redington K, Uppal E, Coen CW, et al. Experimental hyperleptinemia in neonatal rats leads to selective leptin responsiveness, hypertension, and altered myocardial function. Hypertension. 2013;62:627–633. doi: 10.1161/HYPERTENSIONAHA.111.00691. [DOI] [PubMed] [Google Scholar]
- Sánchez J, Oliver P, Miralles O, Ceresi E, Picó C, Palou A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology. 2005;146:2575–2582. doi: 10.1210/en.2005-0112. [DOI] [PubMed] [Google Scholar]
- Savino F, Liguori SA, Benetti S, Sorrenti M, Fissore MF, Cordero di Montezemolo L. High serum leptin levels in infancy can potentially predict obesity in childhood, especially in formula-fed infants. Acta Paediatrica. 2013;102:e455–e459. doi: 10.1111/apa.12354. [DOI] [PubMed] [Google Scholar]
- Schäffer L, Müller-Vizentini D, Burkhardt T, Rauh M, Ehlert U, Beinder E. Blunted stress response in small for gestational age neonates. Pediatric Research. 2009;65:231–235. doi: 10.1203/PDR.0b013e318191fb44. [DOI] [PubMed] [Google Scholar]
- Schubring C, Englaro P, Siebler T, Blum WF, Demirakca T, Kratzsch J, Kiess W. Longitudinal analysis of maternal serum leptin levels during pregnancy, at birth and up to six weeks after birth: relation to body mass index, skinfolds, sex steroids and umbilical cord blood leptin levels. Hormone Research. 1998;50:276–283. doi: 10.1159/000023290. [DOI] [PubMed] [Google Scholar]
- Schubring C, Prohaska F, Prohaska A, Englaro P, Blum W, Siebler T, Kratzsch J, Kiess W. Leptin concentrations in maternal serum and amniotic fluid during the second trimenon: differential relation to fetal gender and maternal morphometry. European Journal of Obstetrics and Gynecology and Reproductive Biology. 1999;86:151–157. doi: 10.1016/s0301-2115(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatric Research. 2011;70:633–637. doi: 10.1203/PDR.0b013e31823214ea. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Doctor T, Menahem S. Cardiac function and arterial biophysical properties in small for gestational age infants: postnatal manifestations of fetal programming. Journal of Pediatrics. 2013;163:1296–1300. doi: 10.1016/j.jpeds.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Shekhawat PS, Garland JS, Alex C, Sasidharan P, Mick G, McCormick KL. Cord blood and postnatal serum leptin and its relationship to steroid use and growth in sick preterm infants. Journal of Pediatric Endocrinology and Metabolism. 2000;13:1571–1576. doi: 10.1515/jpem.2000.13.9.1571. [DOI] [PubMed] [Google Scholar]
- Shi Z, Brooks VL. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: role of oestrogen. Journal of Physiology. 2015;593:1633–1647. doi: 10.1113/jphysiol.2014.284638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipola-Leppänen M, Vääräsmäki M, Tikanmäki M, Matinolli HM, Miettola S, Hovi P, Wehkalampi K, Ruokonen A, Sundvall J, Pouta A, et al. Cardiometabolic risk factors in young adults who were born preterm. American Journal of Epidemiology. 2015;181:861–873. doi: 10.1093/aje/kwu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144:3024–3030. doi: 10.1210/en.2003-0145. [DOI] [PubMed] [Google Scholar]
- Smith W, Schutte R, Huisman HW, Van Rooyen JM, Ware LJ, Fourie CM, Mels CM, Kruger R, McCarthy N, Schutte AE. Leptin is positively associated with blood pressure in african men with a low body mass index: the SAfrEIC study. Hormone and Metabolic Research. 2015;47:145–151. doi: 10.1055/s-0034-1389926. [DOI] [PubMed] [Google Scholar]
- Sooranna SR, Ward S, Bajoria R. Fetal leptin influences birth weight in twins with discordant growth. Pediatric Research. 2001;49:667–672. doi: 10.1203/00006450-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Spear ML, Hassink SG, Leef K, O’Connor DM, Kirwin SM, Locke R, Gorman R, Funanage VL. Immaturity or starvation? Longitudinal study of leptin levels in premature infants. Biology of the Neonate. 2001;80:35–40. doi: 10.1159/000047117. [DOI] [PubMed] [Google Scholar]
- Stocker CJ, Wargent E, O’Dowd J, Cornick C, Speakman JR, Arch JR, Cawthorne MA. Prevention of diet-induced obesity and impaired glucose tolerance in rats following administration of leptin to their mothers. American Journal of Physiology: Regulatory Integrative Comprehensive Physiology. 2007;292:R1810–R1818. doi: 10.1152/ajpregu.00676.2006. [DOI] [PubMed] [Google Scholar]
- Stoll-Becker S, Kreuder J, Reiss I, Etspüler J, Blum WF, Gortner L. Influence of gestational age and intrauterine growth on leptin concentrations in venous cord blood of human newborns. Klinische Padiatrie. 2003;215:3–8. doi: 10.1055/s-2003-36892. [DOI] [PubMed] [Google Scholar]
- Su PH, Wang SL, Chen JY, Lai CP, Jian SH. Serum leptin levels in preterm, healthy and sick-term newborns. Acta Paediatrica Taiwanica. 2002;43:249–254. [PubMed] [Google Scholar]
- Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. European Journal of Endocrinology. 2001;145:529–539. doi: 10.1530/eje.0.1450529. [DOI] [PubMed] [Google Scholar]
- Tanida M, Yamamoto N, Morgan DA, Kurata Y, Shibamoto T, Rahmouni K. Leptin receptor signaling in the hypothalamus regulates hepatic autonomic nerve activity via phosphatidylinositol 3-kinase and AMP-activated protein kinase. Journal of Neuroscience. 2015;35:474–484. doi: 10.1523/JNEUROSCI.1828-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprak D, Gökalp AS, Hatun S, Zengin E, Arisoy AE, Yumuk Z. Serum leptin levels of premature and full-term newborns in early infancy: metabolic catch-up of premature babies. Turkish Journal of Pediatrics. 2004;46:232–238. [PubMed] [Google Scholar]
- Valūniene M, Verkauskiene R, Boguszewski M, Dahlgren J, Lasiene D, Lasas L, Wikland KA. Leptin levels at birth and in early postnatal life in small- and appropriate-for-gestational-age infants. Medicina (Kaunas) 2007;43:784–791. [PubMed] [Google Scholar]
- Varvarigou A, Mantzoros CS, Beratis NG. Cord blood leptin concentrations in relation to intrauterine growth. Clinical Endocrinology (Oxford) 1999;50:177–183. doi: 10.1046/j.1365-2265.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- Visentin S, Lapolla A, Londero AP, Cosma C, Dalfrà M, Camerin M, Faggian D, Plebani M, Cosmi E. Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother-child couple. BioMed Research International. 2014;2014:401595. doi: 10.1155/2014/401595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Moore VM, Steptoe A, Cockington RA, Robinson JS, Phillips DI. Size at birth and cardiovascular responses to psychological stressors: evidence for prenatal programming in women. Journal of Hypertension. 2004;22:2295–2301. doi: 10.1097/00004872-200412000-00011. [DOI] [PubMed] [Google Scholar]
- WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization Technical Report Series. 1995;854:1–452. [PubMed] [Google Scholar]
- Yildiz L, Avci B, Ingeç M. Umbilical cord and maternal blood leptin concentrations in intrauterine growth retardation. Clinical Chemistry and Laboratory Medicine. 2002;40:1114–1117. doi: 10.1515/CCLM.2002.195. [DOI] [PubMed] [Google Scholar]
- Yokota I. Roles of leptin and ghrelin during the perinatal period. Clinical Pediatric Endocrinology. 2003;12:57–65. [Google Scholar]
- Yura S, Sagawa N, Mise H, Mori T, Masuzaki H, Ogawa Y, Nakao K. A positive umbilical venous-arterial difference of leptin level and its rapid decline after birth. American Journal of Obstetrics and Gynecology. 1998;178:926–930. doi: 10.1016/s0002-9378(98)70525-3. [DOI] [PubMed] [Google Scholar]
- Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metabolism. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]