Abstract
For T cells to recognize foreign antigens, the latter must be processed into peptides and associated to major histocompatibility complex (MHC) class II molecules by antigen-presenting cells (APC). APCs frequently operate under stress conditions induced by tissue damage, antigens, or inflammatory reactions. We analyze the effects of oxidative stress on intracellular processing using APC B cell lines. Before being tested for APC function, B cells (IIA1.6) were exposed for 2 hours to hydrogen peroxide (H2O2), a treatment that impairs their capacity to stimulate specific T cell clones. Because paraformaldehyde-fixed H2O2-treated B cells can still present extracellular peptides to T cell clones, the intracellular events of processing were investigated. Purified lysosomes from H2O2-treated B cells show increased proteolytic activity and increased generation of antigenic peptides. In addition, H2O2 treatment targets antigens to compartments that express low levels of MHC II and proteins (H-2M, H-2O) required for peptide loading onto this molecule. Finally, we suggest that impairment of antigen processing by oxidative stress reduces the induction of a T cell's response because H2O2 decreases the activation of naive T lymphocytes by dendritic cells. Together, these data indicate that oxidative stress inhibits the capacity of APCs to process antigens and to initiate a primary T cell response. The role of such modifications on the outcome of the specific immune response is discussed.
INTRODUCTION
To be recognized by T lymphocytes, foreign antigens must be processed into peptides by antigen-presenting cells (APC) (B lymphocytes, dendritic cells [DC], and macrophages) to be associated to major histocompatibility complex (MHC) II molecules. Antigens are first internalized by APC and then degraded in endosomes and lysosomes by proteases such as cathepsins or aspartyl endopeptidases (Diment, 1990; Fineschi and Miller 1997; Manoury et al 1998). Some of the resulting peptides are loaded onto MHC II molecules and then transported to the cell surface for presentation to specific T cells.
In B lymphocytes, two pathways of antigen (Ag) processing and presentation by MHC II molecules have been described (Pinet et al 1995; Watts 1997): The first or classical pathway uses newly synthesized class II molecules (αβ dimers) that are assembled in the endoplasmic reticulum with the invariant chain (Ii) to form (αβIi)3 heterononameric complexes (Roche et al 1991). These complexes are transported to the Golgi and then to the endocytic compartments (Roche et al 1991; Pinet et al 1995; Watts 1997; Bakke and Nordeng 1999; Neefjes 1999), where Ii is sequentially proteolyzed into peptides. One of these, named class II–associated invariant chain peptide (CLIP), remains associated within the binding site of MHC II (Ceman and Sant 1995) before its substitution by an antigenic peptide. This exchange is catalyzed by the interaction of the MHC II–CLIP complex with H-2M (Denzin and Cresswell 1995; Jensen et al 1999a, 1999b). Another protein, H-2O, was shown to interfere in the peptide loading by limitation of the pH range where H-2M is active (Denzin et al 1997; Liljedahl et al 1998; Alfonso et al 1999; Kropshofer et al 1999). After their formation, the MHC II–peptide complexes are targeted at the cell surface, where the interaction with specific CD4+ T cells occurs (Watts 1997). The second or alternative pathway involves intracellular recycling of mature MHC II molecules already expressed at the cell surface. In endosomes, they become available for antigenic peptide binding, and the newly formed αβ–antigenic peptide complexes are then transported to the plasma membrane (Pinet et al 1995; Griffin et al 1997). This recycling pathway requires neither protein synthesis nor Ii and H-2M participation.
Stress, such as physical or biochemical hyperthermia, oxidation, anoxia, radiation, or inflammatory stimuli, induces modifications of cell functions. Oxidative stress is a change in the intracellular redox state (Martinez-Cayuela 1995) after exposure to an excess of oxidant species (peroxides, free radicals, etc) or from a deficit in their antioxidant activities such as glutathione. Glutathione in its reduced state (GSH) acts as a scavenger of free-radical species and is regenerated through transient formation of the oxidized form GSSG (Hedley and Chow 1994). Oxidative stress is also known to modify cellular metabolism (Martinez-Cayuela 1995) or physiology through structural perturbations of proteins, lipids, or nucleic acids.
At inflammatory sites, phagocytic cells produce peroxides and free-radical species that facilitate pathogen destruction. At the same time, T lymphocytes and APC such as B lymphocytes, macrophages, and DC migrate to inflammatory sites to contribute to the immunological response. Little is known about the consequences of oxidative stress on immune responses. Hydrogen peroxide and other pro-oxidant species have been shown to decrease the capacity of T lymphocytes to be activated (Flescher et al 1994, 1998; Tatla et al 1999), whereas antigen oxidation by OH· radicals increases specific T cell proliferation (Pernollet et al 1993).
APC can be exposed to oxidative stress at inflammatory sites or when infected. In fact, prion protein (Burthem et al 2001; Li et al 2001; Sugaya et al 2002) or human immunodeficiency virus (Muller et al 1986) was described to induce oxidative stress in infected APC (Israel and Gougerot-Pocidalo 1997; Milhavet et al 2000). The effects of oxidative stress on APC functions are unknown, and previous results suggest that antigen processing could be modified (Rees et al 1991). We analyzed the effects of hydrogen peroxide (H2O2) on the capacity of APC to process and present antigens to specific T cell clones and demonstrate that antigen processing is impaired by oxidative stress through modifications of intracellular events involved in the generation of MHC II–peptide complexes.
MATERIALS AND METHODS
Cell lines and oxidative stress
Experiments were performed using 2 murine MHC II–positive B hybridomas, IIA1.6 (H-2d), an FcR-defective variant of A20 (Jones et al 1986), and 2A4 (H-2k), and three T cell hybridomas, CAB II4.3, G28, and TS12 specific for the peptides 7–31 and 106–116 of hen egg lysozyme (HEL) and 42–56 of ribonuclease (RNase), respectively. Cells were cultured at 37°C in 5% CO2 in air, in Dulbecco modified Eagle medium (DMEM) supplemented with 10% inactivated fetal calf serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 1% nonessential amino acids, 5 × 10−5 β-mercaptoethanol, 50 μg/mL penicillin and streptomycin (all from Life Technologies, Grand Island, NY, USA).
Oxidative stress was performed by incubation of APC (106 cells/mL) for 2 hours at 37°C in the culture medium supplemented with 0.5 mM H2O2 (Sigma, St Louis, MO, USA) (B cell hybridomas) or 0.2 mM H2O2 (DC) in 5% CO2.
Antigen presentation assay
APC were incubated with HEL or RNase for the indicated time period at 37°C. After fixation (0.15% paraformaldehyde for 10 minutes), APC (1 × 105) were incubated with T cells (1 × 105) at 37°C for 17 hours. Interleukin 2 (IL-2) secretion was measured in the culture supernatant by its capacity to stimulate the growth of the IL-2–dependent CTLL-2 cell line (5 × 103 cells/well) as assessed by incorporation of [3H]-thymidine.
Peptide presentation by paraformaldehyde-fixed B cells
Paraformaldehyde-fixed IIA1.6 cells (5 × 104) were incubated with peptides (HEL 7–31 or HEL 106–116) and the corresponding specific T cell clone: CAB II4.3 or G28. After 17 hours incubation, supernatants were collected and IL-2 secretion quantified as described above.
Flow cytometry
For cell cycle analysis, IIA1.6 cells (106) were stained for 10 minutes with Hoechst 33342 (Sigma) 2 μg/mL after cell fixation (3.7% paraformaldehyde) and permeabilization (0.5% Triton X-100) (Calbiochem, San Diego, CA, USA) in phosphate-buffered saline (PBS). The suspension was then analyzed using a FACStar flow cytometer (Becton Dickinson, Meylan, France) under UV excitation.
For intracellular reduced glutathione measurement, IIA1.6 cells were washed twice with PBS and stained with 50 μM monochlorobimane (Molecular Probes, Eugene, OR, USA) in PBS. After 5 minutes on ice, the fluorescence of the cell suspension was analyzed by flow cytometry under UV excitation.
For the analysis of membrane protein expression, cells were incubated with fluorescein isothiocyanate (FITC)–labeled monoclonal antibodies (anti–I-Aβd or anti-CD40) (all from Pharmingen, San Diego, CA, USA). After 10 minutes, cells were analyzed with a FACSCalibur flow cytometer (Becton Dickinson).
Measurement of proteolytic activity in lysosomes
IIA1.6 cells (1 × 108) were disrupted in the homogenization buffer (0.25 M sucrose, 1 mM ethylenediaminetetraacetic acid, 1 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid pH 7.2), using a cell disrupter at 350 bars (Constant System Ltd., Warwick, UK). After centrifugation at 2800 × g for 10 minutes, 1 mL postnuclear supernatant was loaded on 11 mL of 17% Percoll (Pharmacia, Uppsala, Sweden) in homogenization buffer. After centrifugation (90 minutes at 22 000 × g at 4°C), gradient was recovered in 19 fractions. Endosomes (fractions 12–15) and lysosomes (fractions 3–5) were characterized as previously described (Villiers et al 1996). Broken lysosomes were then incubated for 1 hour at 37°C with 10 μg/mL BODIPY-casein (Molecular Probes), and the fluorescence was measured at different points of time (excitation 370 nm, emission 450 nm).
Subcellular generation of the immunodominant peptides HEL 106–116
IIA1.6 cells (500 × 106) were incubated for 30 minutes at 37°C with 75 mg/mL HEL, washed, and further incubated for 2 hours at 37°C in DMEM before subcellular fractionation. After purification on Percoll gradient in the presence of the protease inhibitors (leupeptin, 10 μg/mL; pepstatin A, 10 μg/mL; and Pefabloc, 1 mM), endosomes and lysosomes were broken by 3 cycles of freezing and thawing and then incubated with paraformaldehyde-fixed B cells (1 × 105) in 200 μL of PBS. After 4 hours incubation at 37°C, cells were washed and further cultured with T cells (1 × 106) for 17 hours before measuring IL-2 secretion as described above.
Magnetic fractionation
Magnetic fractionation was performed as previously described (Perrin-Cocon et al 1999). In brief, cells were incubated for 15 minutes at 37°C with ferromagnetic microbeads at a final concentration of 2 mg/mL of iron. After washing, cells were incubated in DMEM at 37°C for the indicated time period and then broken by cell disrupter, and the compartments containing magnetic beads were isolated by magnetic sorting followed by centrifugation at 10 000 × g for 40 minutes. The vesicles were kept frozen at −80°C.
Western blot
Magnetic fractions were dissolved in 0.2 M Tris–HCl, 8 M urea, 2% sodium dodecyl sulfate (SDS), pH 8.0. Proteins were separated by SDS–polyacrylamide gel electrophoresis (12.5% acrylamide) and transferred to polyvinyliden difluoride membrane at 100 V (250 mA) for 1.5 hours. Membranes were revealed by rabbit polyclonal antibodies specific for the cytoplasmic tail of I-Aβ, H-2Oβ, or H-2Mβ (all are generous gifts from N. Barois) and β-actin. Immune detection was performed using horseradish peroxidase–conjugated goat anti-rabbit IgG and the enhanced chemiluminescence kit.
Primary immune response
Naive T lymphocytes were isolated by magnetic cell sorting from 3DO11.10 transgenic mice (generously given by Nicolas Glaichenhaus). These cells express a T cell receptor specific for the peptides 326–337 of ovalbumin linked to I-Ad molecules. DC purified from spleen progenitors (Berthier et al 2000) and naive T cells were cocultured at 37°C for 72 hours in the presence of ovalbumin in complete medium. After addition of [3H]-thymidine (1 μCi/well), cells were further incubated at 37°C for 16 hours, and cell proliferation was quantified after harvesting by measurement of the radioactivity incorporated. Interferon (IFN)–γ secretion in the supernatants was determined by enzyme-linked immunosorbent assay, using the OptEIA set for mouse cytokine detection from Pharmingen, according to the procedure recommended by the manufacturer.
RESULTS
Characterization of stress
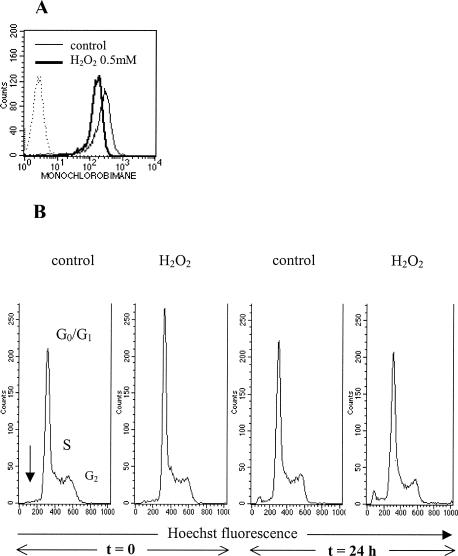
To induce oxidative stress, B cell hybridoma IIA1.6 cells were incubated with 0.5 mM H2O2 for 2 hours at 37°C. After incubation, the amount of the reduced form of intracellular glutathione is decreased, as shown by the diminution of the fluorescence of monochlorobimane (Fig 1A). This result indicates that our experimental conditions modify the intracellular redox state of cells as a consequence of oxidative stress. These conditions do not induce apoptosis or modifications of cell cycle, either immediately or 24 hours after stress (Fig 1B). Cell viability is preserved according to trypan blue exclusion, even 48 hours and 72 hours after stress (data not shown). Furthermore, protein neosynthesis is not modified after stress (data not shown).
Fig 1.
Peroxide stress induction was performed by incubation of the B cells IIA1.6 for 2 hours at 37°C with hydrogen peroxide (H2O2) 0.5 mM. (A) Analysis of the redox state of IIA1.6 cells by measuring intracellular reduced glutathione immediately after exposure to H2O2. (B) Flow cytometric analysis of cell cycle immediately or 24 hours after exposure to H2O2. Phases G0/G1, S, G2 and apoptosis (an arrow) are observed as indicated
The ability of a B cell hybridoma to stimulate specific T cell clones after antigen processing is decreased after treatment with H2O2
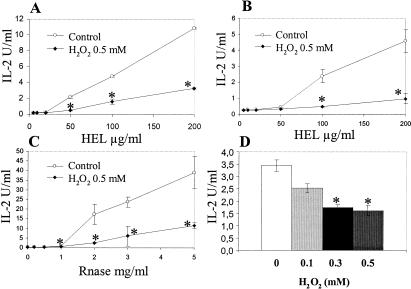
To evaluate the effect of H2O2 on the ability of APC to process antigens and express MHC II–peptide complexes, B cells (IIA1.6 and 2A4) were incubated with antigens (HEL or RNase, respectively) for the indicated time-period, fixed with paraformaldehyde, and then incubated with T cell clones: CAB II4.3, G28, or TS12, which are specific for the peptides HEL7–31, HEL106–116, and RNase42–56, respectively. The generation of RNase 42–56 was described to be dependent on the alternative pathway (Griffin et al 1997) and HEL 106–116 upon the classical pathway. As shown in Figure 2 A–C, the stimulation of these T cell clones is significantly reduced after stress, as assessed by the inhibition of IL-2 secretion. These results indicate that the inhibition is not related to the peptide tested or to the antigen used and that both classical and alternative pathways are impaired by stress. Furthermore, as shown in Figure 2D, the inhibition of T cells activation is dependent on H2O2 concentration.
Fig 2.
IIA1.6 (A, B) or 2A.4 (C) cells were exposed to hydrogen peroxide (H2O2) 0.5 mM for 2 hours at 37°C before incubation with hen egg lysozyme (HEL) (A, B) or RNase (C) for 4 hours. Then, specific T cell clones were added. Interleukin 2 secretion by T cells was measured. T cell clones used were CAB II4.3, specific for HEL 7–31 (A); G28 specific for HEL 106–116 (B); and TS12 specific for ribonuclease 42–56 (C). (D) The same experiment as described in (A) was performed using different concentrations of H2O2 and 200 μg/mL of HEL. Results correspond to the mean value of 3 experiments. Statistical analysis was performed according to Student's t-test. *, P < 0.05.
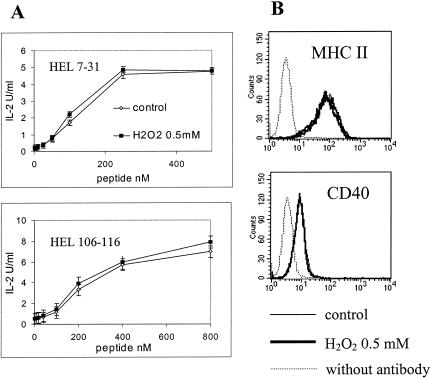
Extracellular peptide presentation by paraformaldehyde-fixed IIA1.6 cells is not modified after treatment with H2O2
We showed that H2O2 decreases the ability of IIA1.6 cells to stimulate specific T cell clones after antigen internalization. Then, we investigated whether this reduction is because of modifications of the extracellular presentation of MHC II–peptide complexes to T cells. Immediately after stress, IIA1.6 cells were fixed with paraformaldehyde and then incubated with soluble peptides HEL7–31 or HEL106–116 before the addition of the corresponding specific T cell clones CABII4.3 or G28. As shown in Figure 3A, H2O2 does not impair the capacity of IIA1.6 cells to present peptides after direct binding on the cell surface by MHC II molecules. Furthermore, cell surface molecules involved in peptide presentation (MHC II [I-Ad], CD40) are not modified after stress (Fig 3B). These results suggest that the inhibition of T cell clones activation by H2O2 is likely because of modifications of the intracellular events of antigen processing involved in the generation of MHC–peptide complexes. Then, we analyzed the effects of H2O2 on intracellular events involved in antigen processing: antigen internalization, proteolysis, and generation of antigenic peptides and loading onto MHC II molecules.
Fig 3.
(A) After treatment with hydrogen peroxide, IIA1.6 cells were fixed with paraformaldehyde and incubated with the peptides hen egg lysozyme (HEL) 7–31 or HEL 106–116. The corresponding T cell clones (CAB II4.3 and G28, respectively) were then added, and interleukin 2 secretion was measured. (B) Flow cytometric analysis of stressed IIA1.6 cells phenotype using anti–I-Ad fluorescein isothiocyanate (FITC) and anti-CD40 FITC antibodies
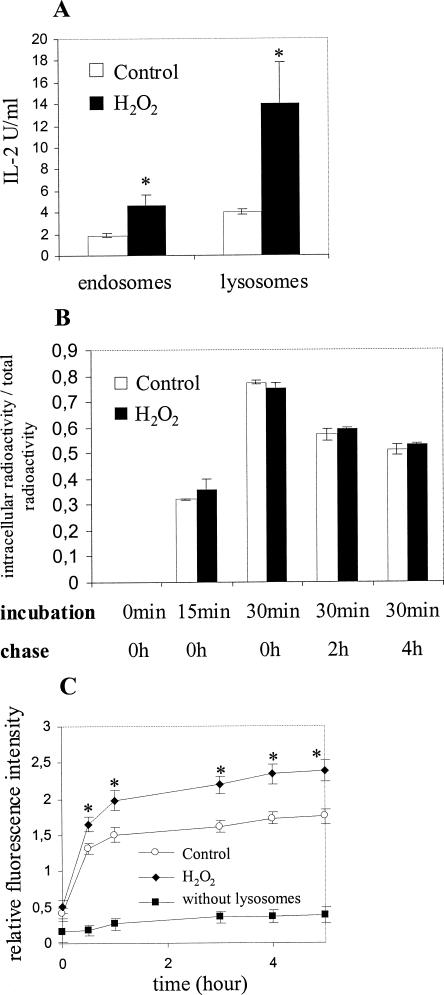
Increased generation of antigenic peptides in lysosomes and endosomes of H2O2-treated IIA1.6 cells
After internalization, antigens are proteolytically cleaved in endosomes and lysosomes by different proteases to generate antigenic peptides. To measure the lysosomal and endosomal generation of antigenic peptides, H2O2-treated IIA1.6 cells were incubated for 1 hour with HEL. Endosomes and lysosomes were then purified by subcellular fractionation, their membranes were broken, and lysates were incubated with paraformaldehyde-fixed IIA1.6 cells before addition of the corresponding T cell clone. We focused our analysis on the generation of the immunodominant peptides HEL 106–116 (Gapin et al 1998). As shown in Figure 4A, the response of T lymphocytes assessed by IL-2 secretion is significantly increased when the vesicles were purified from stressed cells, indicating that lysosomes purified from H2O2-treated IIA1.6 cells contain more antigenic peptides than lysosomes purified from control cells. This result indicates that oxidative stress enhances subcellular generation of immunogenic peptides.
Fig 4.
(A) IIA1.6 cells were incubated with hen egg lysozyme (HEL) for 30 minutes at 37°C and then fractionated on a Percoll gradient. Lysosomes and endosomes were located, collected, broken by freezing and thawing, and incubated for 4 hours at 37°C with paraformaldehyde-fixed IIA1.6 cells. T cell clone specific for HEL 106–116 (G28) was then added and interleukin 2 secretion measured after 17 hours incubation. (B) IIA1.6 cells were incubated with radio-labeled HEL (125I*), and cell radioactivity was measured after 15 minutes and 30 minutes incorporation and a 2-hour or 4-hour chase at 37°C. Results are expressed as the percentage of total radioactivity: cell radioactivity/(radioactivity of supernatant + cell radio activity). For (A) and (B), results correspond to the mean value of three experiments. Statistical analysis was performed according to Student's t-test. *, P < 0.05. (C) Lysosomes of hydrogen peroxide–treated IIA1.6 cells were broken by freezing and thawing and incubated with BODIPY-casein, and protease activity was assayed by the measurement of the fluorescence. Each value is standardized by the measurement of the N-acetyl-β-glucosaminidase activity, which is specific for lysosomes. Results correspond to the mean value of 4 experiments. Statistical analysis was performed according to Student's t-test. *, P < 0.05
As the upregulation of subcellular peptide generation might be the consequence of an increased uptake of HEL, we measured the capacity of IIA1.6 cells to internalize HEL after H2O2 treatment. As shown in Figure 4B, HEL internalization and its release after a 2-hour or 4-hour chase are not modified after treatment with H2O2.
Then, the effect of H2O2 treatment on proteolytic activities in lysosomes of IIA1.6 cells was measured. Lysosomes were purified immediately after stress, their membrane was broken, and the lysates were assayed for their capacity to degrade BODIPY-casein (Molecular Probes), which becomes fluorescent when proteolyzed. As shown in Figure 4C, the kinetic of variation of fluorescence is increased when lysosomes were purified from H2O2-treated IIA1.6 cells, corresponding to a significant stimulation of BODIPY-casein degradation. Similar results are obtained when the fluorescent probe BODIPY-ovalbumin is used (data not shown). Because of a very low amount of proteases in endosomes, this method was unable to detect proteolytic activities in these vesicles (data not shown).
Taken together, these results indicate that treatment of APC by H2O2 increases proteolytic activities in lysosomal vesicles, which is likely the cause of the increased generation of antigenic peptide formation.
After H2O2 treatment, antigens are targeted to vesicles, which contain less MHC II, H-2M, and H-2O molecules
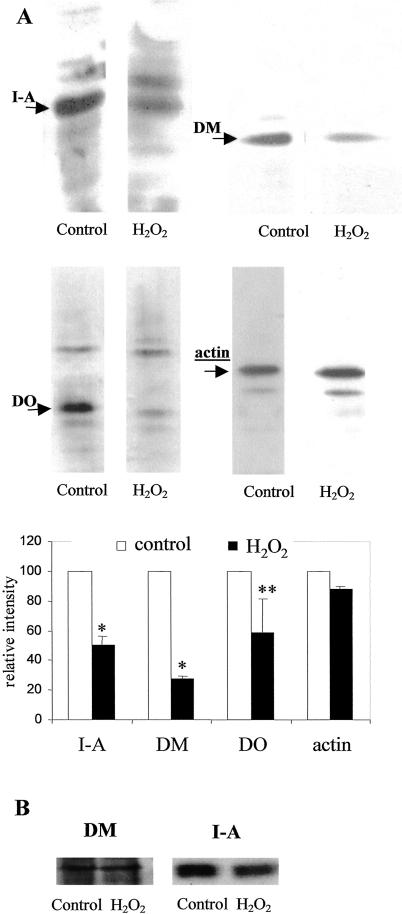
Our data indicate that treatment of IIA1.6 cells with H2O2 increases antigenic peptide production but decreases antigen presentation to T cells; this may result from an impairment of peptide loading onto MHC II molecules. To investigate this issue, antigen-containing vesicles were purified by magnetic sorting, and molecules involved in peptide loading on MHC II molecules were analyzed by Western blotting: MHC II, H-2M, and H-2O. As shown in Figure 5A, purified vesicles from H2O2-treated IIA1.6 cells contain lower amount of MHC II, H-2M, and H-2O molecules than vesicles purified from untreated cells. The same analysis performed on overall cells indicates that the total amount of MHC II and H-2M is not modified by stress (Fig 5B). Because of its very low concentration, it was not possible to visualize the overall amount of H-2O (data not shown). In conclusion, these results suggest that internalized antigens are targeted into compartments, which are deficient for molecules involved in peptide loading onto MHC II molecules.
Fig 5.
(A) IIA1.6 cells were incubated with ferromagnetic microbeads for 15 minutes at 37°C, and compartments containing these beads were isolated by magnetic sorting. Analysis of I-A, H-2M, and H-2O molecules in these compartments was performed by Western blotting. Results correspond to the mean value of 3 experiments. Statistical analysis was performed according to Student's t-test. *, P < 0.05; **, P < 0.1. (B) IIA1.6 cells were treated with hydrogen peroxide and analyzed for their expression of I-A and H-2M molecules by Western blotting
H2O2 reduces the capacity of APC to induce a primary T cell response
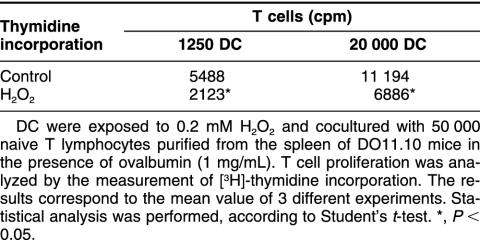
Because H2O2 impairs the intracellular events involved in antigen processing, we next investigated the effects of H2O2 on the capacity of APC to initiate a primary T cell response. Because B cells are unable to initiate such a response, DC were generated from BALB/c mouse and incubated with spleen-derived naive T cells in the presence of antigen. As shown in Table 1, H2O2-treated DC are more than 2-fold less efficient than control DC in inducing T cell proliferation in response to a given Ag concentration. To analyze the stimulation of T cells by stressed DC, the resulting secretion of IFN-γ was measured in the supernatant after 72 hours coculture (time selected from preliminary experiments). We found that the amount of secreted IFN-γ is significantly reduced (P < 0.05) after H2O2 treatment: 14 014 pg/mL for control cells vs 5 863 pg/mL for stressed cells. Taken together, these results indicate that stressed DC have a reduced capacity to stimulate naive T cells and are likely to induce a primary T cell response.
Table 1.
Hydrogen peroxide reduces the capacity of dendritic cells (DC) to induce the proliferation of spleen-derived naive T cellsa
DISCUSSION
In the present study, oxidative stress is induced by the addition of H2O2, a molecule that crosses the cell membrane and induces the generation of the highly reactive hydroxyl radical OH· (Martinez-Cayuela 1995). The concentration of H2O2 used in our experiments is comparable with what is measured in the inflammatory sites. Furthermore, in agreement with the definition of cellular stress, the amount of H2O2 does not modify cell viability, even when measurements were performed 72 hours after stress. It is noteworthy that similar conditions were previously used to analyze the effect of stress on APC (Rees et al 1991).
Using different antigenic models, we show that oxidative stress impairs the capacity of B cells to process and present peptides to specific T cell clones. Interestingly, both classical and alternative pathways are impaired by stress, indicating that B cells cannot compensate the effects of stress by an unaffected pathway. The amount of antigen internalized is not modified by stress, but its proteolysis is increased as shown by the generation of a large amount of the antigenic peptides HEL 106–116 in both endosomes and lysosomes compared with vesicles of unstressed cells. This phenomenon may be because of an upregulation of protease activities or a direct oxidation of antigens that can break peptide chains or disulfide bridge (Wolff and Dean 1986), thus facilitating proteolysis. To understand why stressed cells are deficient for antigen presentation, whereas their capacity to generate antigenic peptides is enhanced, we analyzed the proteins involved in antigen loading to MHC II molecules. After stress, we show a diminution of the amount of MHC II molecules in antigen-containing compartments. Interestingly, H-2M and H-2O, which regulate peptide loading onto MHC II molecules (Denzin and Cresswell 1995; Jensen et al 1999b), are also downregulated in these compartments. These results indicate that antigens are targeted into compartments deficient for proteins that are necessary for peptide loading onto MHC II molecules. Taken together, stress may reduce the capacity of APC to process antigens and stimulate T cells through impairment of the mechanisms involved in antigenic peptide loading onto MHC II molecules. It has been shown previously using transgenic mice that a deficit for H-2M (Liljedahl et al 1998) or for H-2O (Wolf et al 1998) reduces the efficiency of antigenic presentation and the initiation of specific T cell responses. Such modifications of the content of intracellular compartments may be explained either by perturbation of the targeting of H-2M, H-2O, and MHC II to antigen-containing compartments or by a modification of intracellular antigen trafficking.
Oxidative stress was previously described to modify intracellular trafficking. It decreases internalization of epidermal growth factor (De Wit et al 2001) or transferrin receptors (Malorni et al 1998), with modifications, in the latter case, of the intracellular distribution. It was also shown that oxidative stress modifies endocytosis and intracellular trafficking of vesicles by increasing activation of Rab5 (Cavalli et al 2001). In the present work, the internalization of antigens was because of pinocytosis and was not affected by oxidative stress, but the ensuing intracellular traffic is Rab5-dependent (Gorvel et al 1991) and is likely modified by stress.
All together, our results indicate that stress induces an upregulation of antigenic peptides generation that is counterbalanced by a limitation of their capacity to be loaded onto the MHC II molecule. Interestingly, our findings are similar to those of a previous study that evaluated the effect of heat stress on B cell antigen presentation (Pepin et al 1996). This work demonstrates that heat shock markedly affects the ability of these cells to process and present tetanus toxin to T cell clones, although the antigenic peptide generation in subcellular compartments is increased. These data suggest that this stress induces a failure in the intracellular peptide loading onto MHC class II molecules. During inflammatory reactions, the cellular environment is modified, such as temperature elevation and oxidant species production. Free oxygen radicals generated in inflammatory sites react with intrinsic proteins and yield protein modifications such as oxidation of thiols in cysteine and methionine, disulfide bridge cleavage, or hydroxylation of tyrosine (Martinez-Cayuela 1995). These modifications can enhance antigenicity, as shown for tetanus toxin, which displays an increased processing and presentation to specific T cells after exposure to OH· (Pernollet et al 1993). Such modifications of antigenicity may also occur for intrinsic proteins, therefore affecting self-tolerance. Consequently, inflammation would act as an inducer of autoimmunity against various self-proteins that are modified by oxidant species. However, our data suggest that oxidative stress reduces initiation of specific immune response by abrogating antigen processing and APC capacity to stimulate T cells. This downregulation may prevent inappropriate specific immune response against modified self-proteins and autoimmunity. It is noteworthy that H2O2 produced by granulocytes was shown to be the major cause of severe systemic T cell immunity suppression in advanced cancer patients (Schmielau and Finn 2001), suggesting that oxidative stress on APC functions may play an important role in tumor escape.
In conclusion, the evaluation of antigen processing, presentation, and the resulting issue of the specific immune response are highly linked to the redox status of APC.
Acknowledgments
The authors thank Dr Rhodri Ceredig and Dr Marie-Bernadette Villiers for their critical reading of the manuscript. This work was supported in part by specific grants from the Délégation Générale pour l'Armement (ref. DSP 99-34-038) and the MENRT (Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires).
REFERENCES
- Alfonso C, Liljedahl M, Winqvist O, Surh CD, Peterson PA, Fung-Leung WP, Karlsson L. The role of H2-O and HLA-DO in major histocompatibility complex class II-restricted antigen processing and presentation. Immunol Rev. 1999;172:255–266. doi: 10.1111/j.1600-065x.1999.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Bakke O, Nordeng TW. Intracellular traffic to compartments for MHC class II peptide loading: signals for endosomal and polarized sorting. Immunol Rev. 1999;172:171–187. doi: 10.1111/j.1600-065x.1999.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Berthier R, Martinon-Ego C, Laharie AM, Marche PN. A two-step culture method starting with early growth factors permits enhanced production of functional dendritic cells from murine splenocytes. J Immunol Methods. 2000;239:95–107. doi: 10.1016/s0022-1759(00)00186-1. [DOI] [PubMed] [Google Scholar]
- Burthem J, Urban B, Pain A, Roberts DJ. The normal cellular prion protein is strongly expressed by myeloid dendritic cells. Blood. 2001;98:3733–3738. doi: 10.1182/blood.v98.13.3733. [DOI] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Ceman S, Sant AJ. The function of invariant chain in class II-restricted antigen presentation. Semin Immunol. 1995;7:373–387. doi: 10.1006/smim.1995.0042. [DOI] [PubMed] [Google Scholar]
- De Wit R, Makkinje M, Boonstra J, Verkleij AJ, Post JA. Hydrogen peroxide reversibly inhibits epidermal growth factor (EGF) receptor internalization and coincident ubiquitination of the EGF receptor and Eps15. FASEB J. 2001;15:306–308. doi: 10.1096/fj.00-0454fje. [DOI] [PubMed] [Google Scholar]
- Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Denzin LK, Sant'Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–109. doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- Diment S. Different roles for thiol and aspartyl proteases in antigen presentation of ovalbumin. J Immunol. 1990;145:417–422. [PubMed] [Google Scholar]
- Fineschi B, Miller J. Endosomal proteases and antigen processing. Trends Biochem Sci. 1997;22:377–382. doi: 10.1016/s0968-0004(97)01116-x. [DOI] [PubMed] [Google Scholar]
- Flescher E, Ledbetter JA, Schieven GL, Vela-Roch N, Fossum D, Dang H, Ogawa N, Talal N. Longitudinal exposure of human T lymphocytes to weak oxidative stress suppresses transmembrane and nuclear signal transduction. J Immunol. 1994;153:4880–4889. [PubMed] [Google Scholar]
- Flescher E, Tripoli H, Salnikow K, Burns FJ. Oxidative stress suppresses transcription factor activities in stimulated lymphocytes. Clin Exp Immunol. 1998;112:242–247. doi: 10.1046/j.1365-2249.1998.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapin L, Bravo de Alba Y, Casrouge A, Cabaniols JP, Kourilsky P, Kanellopoulos J. Antigen presentation by dendritic cells focuses T cell responses against immunodominant peptides: studies in the hen egg-white lysozyme (HEL) model. J Immunol. 1998;160:1555–1564. [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523–1532. [PubMed] [Google Scholar]
- Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- Israel N, Gougerot-Pocidalo MA. Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci. 1997;53:864–870. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PE, Weber DA, Thayer WP, Chen X, Dao CT. HLA-DM and the MHC class II antigen presentation pathway. Immunol Res. 1999a;20:195–205. doi: 10.1007/BF02790403. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Weber DA, Thayer WP, Westerman LE, Dao CT. Peptide exchange in MHC molecules. Immunol Rev. 1999b;172:229–238. doi: 10.1111/j.1600-065x.1999.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Jones B, Tite JP, Janeway CA Jr.. Different phenotypic variants of the mouse B cell tumor A20/2J are selected by antigen- and mitogen-triggered cytotoxicity of L3T4- positive, I-A-restricted T cell clones. J Immunol. 1986;136:348–356. [PubMed] [Google Scholar]
- Kropshofer H, Hammerling GJ, Vogt AB. The impact of the non-classical MHC proteins HLA-DM and HLA-DO on loading of MHC class II molecules. Immunol Rev. 1999;172:267–278. doi: 10.1111/j.1600-065x.1999.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Li R, Liu D, and Zanusso G. et al. 2001 The expression and potential function of cellular prion protein in human lymphocytes. Cell Immunol. 207:49–58. [DOI] [PubMed] [Google Scholar]
- Liljedahl M, Winqvist O, and Surh CD. et al. 1998 Altered antigen presentation in mice lacking H2-O. Immunity. 8:233–243. [DOI] [PubMed] [Google Scholar]
- Malorni W, Testa U, Rainaldi G, Tritarelli E, Peschle C. Oxidative stress leads to a rapid alteration of transferrin receptor intravesicular trafficking. Exp Cell Res. 1998;241:102–116. doi: 10.1006/excr.1998.4020. [DOI] [PubMed] [Google Scholar]
- Manoury B, Hewitt EW, Morrice N, Dando PM, Barrett AJ, Watts C. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature. 1998;396:695–699. doi: 10.1038/25379. [DOI] [PubMed] [Google Scholar]
- Martinez-Cayuela M. Oxygen free radicals and human disease. Biochimie. 1995;77:147–161. doi: 10.1016/0300-9084(96)88119-3. [DOI] [PubMed] [Google Scholar]
- Milhavet O, McMahon HE, and Rachidi W. et al. 2000 Prion infection impairs the cellular response to oxidative stress. Proc Natl Acad Sci U S A. 97:13937–13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H, Falk S, Stutte HJ. Accessory cells as primary target of human immunodeficiency virus HIV infection. J Clin Pathol. 1986;39:1161. doi: 10.1136/jcp.39.10.1161-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. CIIV, MIIC and other compartments for MHC class II loading. Eur J Immunol. 1999;29:1421–1425. doi: 10.1002/(SICI)1521-4141(199905)29:05<1421::AID-IMMU1421>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Pepin E, Villiers CL, Gabert FM, Serra VA, Marche PN, Colomb MG. Heat shock increases antigenic peptide generation but decreases antigen presentation. Eur J Immunol. 1996;26:2939–2943. doi: 10.1002/eji.1830261220. [DOI] [PubMed] [Google Scholar]
- Pernollet M, Villiers C, Gabert F, Drouet C, Colomb M. OH· treatment of tetanus toxin reduces its susceptibility to limited proteolysis with more efficient presentation to specific T cells. Mol Immunol. 1993;30:1639–1646. doi: 10.1016/0161-5890(93)90437-g. [DOI] [PubMed] [Google Scholar]
- Perrin-Cocon LA, Marche PN, Villiers CL. Purification of intracellular compartments involved in antigen processing: a new method based on magnetic sorting. Biochem J. 1999;338:123–130. [PMC free article] [PubMed] [Google Scholar]
- Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- Rees AD, Donati Y, Lombardi G, Lamb J, Polla B, Lechler R. Stress-induced modulation of antigen-presenting cell function. Immunology. 1991;74:386–392. [PMC free article] [PubMed] [Google Scholar]
- Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- Sugaya M, Nakamura K, and Watanabe T. et al. 2002 Expression of cellular prion-related protein by murine Langerhans cells and keratinocytes. J Dermatol Sci. 28:126–134. [DOI] [PubMed] [Google Scholar]
- Tatla S, Woodhead V, Foreman JC, Chain BM. The role of reactive oxygen species in triggering proliferation and IL-2 secretion in T cells. Free Radic Biol Med. 1999;26:14–24. doi: 10.1016/s0891-5849(98)00133-6. [DOI] [PubMed] [Google Scholar]
- Villiers MB, Villiers CL, Jacquier-Sarlin MR, Gabert FM, Journet AM, Colomb MG. Covalent binding of C3b to tetanus toxin: influence on uptake/internalization of antigen by antigen-specific and non-specific B cells. Immunology. 1996;89:348–355. doi: 10.1046/j.1365-2567.1996.d01-747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- Wolf PR, Tourne S, Miyazaki T, Benoist C, Mathis D, Ploegh HL. The phenotype of H-2M-deficient mice is dependent on the MHC class II molecules expressed. Eur J Immunol. 1998;28:2605–2618. doi: 10.1002/(SICI)1521-4141(199809)28:09<2605::AID-IMMU2605>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wolff SP, Dean RT. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986;234:399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]