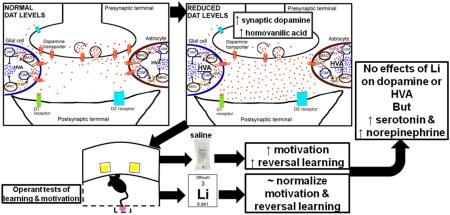
Abstract
Background
Bipolar disorder (BD) mania patients exhibit poor cognition and reward-seeking/hypermotivation, negatively impacting a patient’s quality of life. Current treatments (e.g., lithium), do not treat such deficits. Treatment development has been limited due to a poor understanding of the neural mechanisms underlying these behaviors. Here, we investigated putative mechanisms underlying cognition and reward-seeking/motivational changes relevant to BD mania patients using two validated mouse models and neurochemical analyses.
Methods
The effects of reducing dopamine transporter (DAT) functioning via genetic (knockdown vs. wild-type littermates), or pharmacological (GBR12909- vs. vehicle-treated C57BL/6J mice) means were assessed in the probabilistic reversal learning task (PRLT), and progressive ratio breakpoint (PRB) test, during either water or chronic lithium treatment. These tasks quantify reward learning and effortful motivation, respectively. Neurochemistry was performed on brain samples of DAT mutants ± chronic lithium using high performance liquid chromatography.
Results
Reduced DAT functioning increased reversals in the PRLT, an effect partially attenuated by chronic lithium. Chronic lithium alone slowed PRLT acquisition. Reduced DAT functioning increased motivation (PRB), an effect attenuated by lithium in GBR12909-treated mice. Neurochemical analyses revealed that DAT knockdown mice exhibited elevated homovanillic acid levels, but that lithium had no effect on these elevated levels.
Conclusions
Reducing DAT functioning recreates many aspects of BD mania including hypermotivation and improved reversal learning (switching), as well as elevated homovanillic acid levels. Chronic lithium only exerted main effects, impairing learning and elevating norepinephrine and serotonin levels of mice, not specifically treating the underlying mechanisms identified in these models.
Keywords: DAT knockdown, GBR12909, cognition, HPLC, HVA, breakpoint
Graphical abstract
1.1 INTRODUCTION
Bipolar disorder (BD) is a debilitating life-long illness, affecting approximately 2% of the global population (Merikangas et al., 2011). Mania is a cardinal feature of BD and is characterized by complex and multifaceted symptoms. Symptoms of BD mania include heightened risk-taking, impaired decision-making, and increased hedonistic (reward-directed) behavior (DSM-V, 2013). Patients with BD exhibit impaired cognitive functioning including impaired decision making in the Iowa Gambling Task (IGT) (Christodoulou et al., 2006; Jollant et al., 2007; van Enkhuizen et al., 2014b), a probabilistic reversal learning task (PRLT) that also includes reward and punishments. There is also evidence of impaired simplistic probabilistic reversal learning in youths at risk of BD (Dickstein et al., 2010), in addition to other learning deficits (Duek et al., 2014; Pizzagalli et al., 2008). Increased reward-directed behaviors have not been as readily quantified in patients but are commonly measured in rodents using a progressive ratio breakpoint (PRB) schedule of reinforcement (Young and Markou, 2015). These dysfunctional behaviors negatively affect a patient’s quality of life and no medications have been approved for their treatment. Currently approved BD treatments are ineffective at treating such cognitive deficits (Burdick et al., 2012; Joffe et al., 1988; Mora et al., 2013). In fact, one of the most commonly approved treatments, the mood stabilizer lithium, adversely affects certain aspects of cognition including learning and memory in healthy subjects (Stip et al., 2000). Identifying treatments that can improve, or at least not impair, cognition in patients is, therefore, an important target for therapeutic development. These targets would more readily be identified with a better understanding of underlying mechanisms and the effects of current treatments on such abnormal behavior
Some neurobiological mechanisms for BD have been proposed. For example, altered dopaminergic homeostasis may contribute to mania symptoms (van Enkhuizen et al., 2014a). The dopamine transporter (DAT) regulates synaptic dopamine levels and polymorphisms in this gene have been associated with BD (Greenwood et al., 2006; Pinsonneault et al., 2011). This polymorphism may result in reduced DAT levels (Horschitz et al., 2005), as is observed in positron emission tomography imaging of unmedicated BD patients (Anand et al., 2011). Reduced DAT levels slow the clearance of synaptic dopamine, allowing for greater metabolism of dopamine to HVA by monoamine oxidase (MOA) and catechol-O-methyl transferase (COMT) (Best et al., 2009). This mechanism likely drives the elevated homovanillic acid (HVA) levels seen in the cerebrospinal fluid (CSF) of mania patients (Gerner et al., 1984). To-date, it is unclear what effect chronic lithium has on neurotransmitter levels of organisms with reduced DAT function exhibiting mania-like behaviors. Although, in studies of rats with normal levels of DAT expression, lithium did not affect basal dopamine levels, but impaired dopamine release (Ferrie et al., 2005, 2006).
In support of a possible mechanistic contribution of DAT to mania-relevant behaviors, mice with reduced DAT functioning exhibit abnormal exploratory profiles consistent with BD mania patients (Perry et al., 2009). Specifically, mice treated with the selective DAT inhibitor GBR12909 (GBR) or with a genetic knockdown (KD) of DAT exhibited hyperactivity, increased specific exploration, and straighter movement through space consistent with BD mania patients in the cross-species behavioral pattern monitor (BPM) (Perry et al., 2009; Young et al., 2010a, b). Chronic administration of the standard BD treatments valproate and lithium, at clinically therapeutic levels, to DAT KD and GBR-treated mice attenuated the hyperactivity of mice, without affecting their specific exploration, or straight-line movement (Queiroz et al., 2015; van Enkhuizen et al., 2013a; van Enkhuizen et al., 2015a, b). These effects were similar to treatment-induced reduction of activity of mania patients (Minassian et al., 2011). Furthermore, GBR treatment or genetic reduction of DAT increased risk taking (van Enkhuizen et al., 2013b; van Enkhuizen et al., 2014b), motivation (Young and Geyer, 2010), and motor impulsivity-like behavior (Loos et al., 2010) similar to aberrations observed in BD patients (van Enkhuizen et al., 2014b). Apart from the reduced DAT in BD with links to similar behavioral abnormalities, the construct validity of this model has rarely been assessed. Hence, testing the behavior, treatment-reactivity, and neurochemistry of mice with reduced DAT function could provide predictive, and construct validity for this model of altered cognition and behavior that is relevant to mania. This assessment could then lead to the development of targeted therapeutics.
Here, we assessed the effect of the pharmacological (GBR) and genetic (KD) DAT inhibition on probabilistic reversal learning and effortful motivation in the PRLT and PRB test, respectively. We also determined the effects of chronic high doses of lithium on these behaviors. Additionally, we assessed the neurochemical profile of these mice treated with water or chronic lithium. We hypothesized that: (a) Chronic lithium would impair learning in the PRLT irrespective of DAT inhibition; (b) GBR-treated mice or DAT KD would exhibit impaired performance in the PRLT and increased reward-seeking/motivation-like behavior in the PRB; (c) Lithium would synergistically normalize BD-relevant behaviors induced by DAT reduction; and (d) DAT KD mice would exhibit elevated HVA consistent with mania patients (Gerner et al., 1984), an effect remediated by lithium treatment.
2.1 METHODS
2.2 Animals
C57BL/6J male mice (n=45) were purchased from Jackson Laboratories at 3 months old and male DAT KD mice and their WT littermates (n=55) aged 13 months and weighing between 22 - 29 g were used in this study. DAT heterozygous breeders backcrossed onto a C57BL/6 background for more than 10 generations were sent to the University of California San Diego (UCSD) from the University of Chicago (Zhuang et al., 2001). All DAT KD and WT mice used in this study resulted from heterozygous breeding pairs performed at UCSD. Mice were group housed (maximum 4/cage) and maintained in a temperature-controlled vivarium (21±1 °C) on a reversed day-night cycle (lights on at 19:00 h, off at 07:00 h) and tested during the dark phase between 08:00 h and 16:00 h. All mice had ad libitum access to water and were food-restricted to 85% of their free-feeding weight during periods of training and testing. All of the behavioral testing procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
2.3 Drug treatment
GBR12909 dihydrochloride (Sigma-Aldrich, St Louis, USA) was dissolved in 0.9% saline vehicle after sonicating for 2-4 h at 40 °C as described previously (Young et al., 2010a). GBR12909 at 16 mg/kg (dose based on previous publications (Loos et al., 2010; van Enkhuizen et al., 2013a; van Enkhuizen et al., 2013c; van Enkhuizen et al., 2015a, b) was administered by intraperitoneal injection 10 min prior to testing in a volume of 10 ml/kg. Lithium chloride (Sigma-Aldrich, St Louis, USA) was dissolved into the drinking water at 0.6 or 1.0 g/l and given for 10 days and 7 or 8 days for the DAT KD study PRLT and PRB respectively based on previous studies generating clinical therapeutic levels (Dehpour et al., 1995; Roybal et al., 2007; van Enkhuizen et al., 2015a, b).
2.4 Training and testing
Training and testing took place in 15 five-hole operant chambers (25 × 25 × 25 cm; Med Associates, St. Albans, USA). During the first training phase (Hab1), mice were required to recognize magazine illumination and delivery of 30 μl strawberry milkshake as a reward and collect it every 15 s for 10 min (criterion was 30 collection responses per session for two consecutive days). During the second training phase (Hab2), mice were trained to holepoke into one of two lit holes to obtain the reward (criterion was 70 correct holepokes per session for two consecutive days). Once responding consistently, mice were baseline-matched on total responses prior to treatment and testing in PRLT or PRB.
2.5 Probabilistic reversal learning test (PRLT)
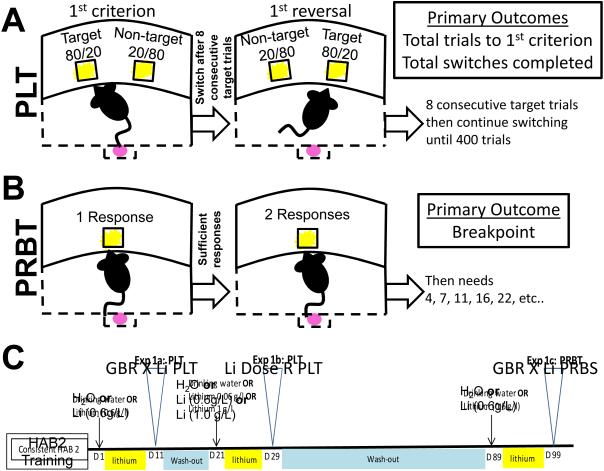
During the 60 min probabilistic learning test, the same two stimulus locations as during Hab2 training were presented, but with altered contingencies (Fig. 1A). The target hole provided a high probability of reward (80%) and low probability (20%) of punishment (house light illumination for 4 s). The non-target hole provided a low probability of reward (20%) and high probability of punishment (80%). After 8 consecutive responses at the target hole, criterion was met and the target hole became the non-target hole and vice versa (reversals). The primary outcome measures of the task were total trials to criterion and number of reversals. Secondary outcome measures included % premature responses (% of times responding in a hole prior to stimuli presentation), and latency measures (target and non-target mean response latencies). Other secondary measures are described in Table 1.
Figure 1. Task schematics for Probabilistic Learning, Progressive Ratio Breakpoint, and the timeline of testing using C57BL/6 mice.
Schematic representation of the probabilistic reversal learning paradigm (A). Mice can nose poke in one of two holes and will either be rewarded with strawberry milkshake or punished with a time-out period of 4s with the house light illuminated. Schematics describing the progressive ratio paradigm (B). Timeline of GBR 12909 (GBR) experiments using C57BL/6 mice (C). One group of mice (n=45) were tested twice on the PRLT, once in a lithium(Li)/GBR study, once in a Li dose response study, and once on the PRB in a Li/GBR study. Mice were trained on HAB2 in between indicated testing days, simply responding in either of the two lit holes for food rewards. Before each study, mice were baseline-matched based on their HAB2 scores.
Table 1.
Description of the behavioral measures used in the Probabilistic Reversal Learning Task.
| Measures | Description |
|---|---|
| Reversals | Number of successful reversals throughout the course of the session. |
| Total trials to criterion | Number of trials needed to reach the initial criterion (8 consecutive responses at the target site) |
| Target Win Stay Ratio | Probability of choosing the target after being rewarded from choosing the target |
| Non Target Win Stay Ratio | Probability of choosing the non-target after being rewarded from choosing the non-target |
| Target Lose Shift Ratio | Probability of choosing the non-target after being punished from choosing the target |
| Non Target Loose shift Ratio | Probability of choosing the target after being punished from choosing the non-target |
| Mean Target Latency | Mean latency to holepoke at the target hole |
| Mean Non Target Latency | Mean latency to holepoke at the non-target hole |
| Mean Reward Latency | Mean latency to collect food reinforcer after being rewarded |
| % Premature Responses | % of responses in a hole prior to stimuli presentation |
| % Reward Perseveration | % of continued responses in a hole after being rewarded |
| % Punishment Perseveration | % of continued responses in a hole after being punished |
| Total trials | Total number of trials performed during the session (60 min) |
2.6 Progressive ratio breakpoint test (PRB)
During the 60 min progressive ratio breakpoint test, the mice had to make increasingly more holepokes in the central lit stimulus aperture in order to get a food reward. The number of holepokes required to gain a reward increased according to the following progression: 1, 2, 4, 7, 11, 16, 22, 29, 37, 46, 56 and 67 [as described previously (Young and Geyer, 2010). To maintain responding, the mice had to respond three times at each ratio before moving to the next, receiving one reward each time (Fig. 1B). The primary outcome measure of this task was the ‘breakpoint’, defined as the last ratio to be completed before the end of the session.
2.7.1 Effects of lithium on probabilistic reversal learning in GBR-treated C57BL/6 mice
Once the C57BL/6 mice were fully trained on Hab2, a series of studies were conducted (Fig. 1C). The mice were treated with either normal drinking water (vehicle; n=22) or drinking water with 0.6 g/l lithium (n=23). Mice continued Hab2 training until they were tested in the PRLT on day 10, at which point half of each group was injected with GBR 12909 (16 mg/kg) 10 min prior to testing. For a detailed overview of sample sizes, refer to Table 2.
Table 2.
Overview of sample sizes by treatment for each experiment performed.
| Study | Groups | |||
|---|---|---|---|---|
| 2.7.1 PRLT - Lithium (0.6 g/l) + GBR |
Water + vehicle | Water + GBR | Lithium + vehicle | Lithium + GBR |
| 11 | 11 | 11 | 12 | |
| 2.7.2 PRLT – Lithium (various) |
Water | Lithium 0.6 g/l | Lithium 1.0 g/l | |
| 15 | 15 | 15 | ||
| 2.7.3 PRBT - Lithium (0.6 g/l) + GBR |
Water + vehicle | Water + GBR | Lithium + vehicle | Lithium + GBR |
| 11 | 11 | 11 | 12 | |
| 2.8.1 PRLT – Lithium (1.0 g/l) + DAT |
Water + DAT WT | Water + DAT KD | Lithium + DAT WT | Lithium + DAT KD |
| 13 | 13 | 17 | 16 | |
| 2.8.2 PRBT – Lithium (1.0 g/l) + DAT |
Water + DAT WT | Water + DAT KD | Lithium + DAT WT | Lithium + DAT KD |
| 13 | 13 | 17 | 16 | |
| 2.9 Neurochemistry |
Water + DAT WT | Water + DAT KD | Lithium + DAT WT | Lithium + DAT KD |
| 8 | 8 | 8 | 8 | |
DAT=dopamine transporter; GBR=GBR12909 (13 mg/kg); KD=knockdown; PRBT=progressive ratio breakpoint; PLT=probabilistic reversal learning task; WT=wildtype.
2.7.2 Effects of varied lithium doses on probabilistic reversal learning in C57BL/6 mice
After 10 days of Hab2 training without drug exposure, mice were baseline-matched into groups (based on Hab2 score and previous treatment), receiving vehicle (n=15), or lithium drinking water at 0.6 g/l (n=15), or 1.0 g/l (n=15), and tested in the PRLT on day 8. For a detailed overview of sample sizes, refer to Table 2.
2.7.3 Effects of chronic lithium on motivation (PRB) in GBR-treated C57BL/6 mice
Finally, after 60 days without drug exposure and intermittent Hab2 training, the mice were again baseline-matched into groups receiving vehicle (n=22), or lithium 0.6 g/l (n=23), drinking water (based on Hab2 scores and previous treatments). Mice were tested in the PRB on day 10 during which half of each group received either vehicle, or GBR12909 (16 mg/kg) 10 min prior to testing. For a detailed overview of sample sizes, refer to Table 2.
2.8.1 Effects of chronic lithium on probabilistic reversal learning of DAT mutant mice
After training DAT KD (n=27), and WT (n=28), mice to Hab2 criterion, they were baseline-matched on the number of training days to criterion. Half of these mice were then treated with lithium (1.0 g/l) or water for 7 days prior to testing in the PRLT. For a detailed overview of sample sizes, refer to Table 2.
2.8.2 Effects of chronic lithium on motivation (PRB) of DAT mutant mice.
After testing in the PRLT, DAT KD and WT mice were tested in the PRB the following day. For a detailed overview of sample sizes, refer to Table 2.
2.9 Brain neurochemistry from High Performance Liquid Chromatography (HPLC)
Fourteen-week-old DAT KD, WT male mice that were used in the previous experiment (n= 32, Table 2) received lithium treatment at 1.0 g/l for 10 days before tissue collection. Mice were removing the frontal lobes (FL), caudate putamen (CPu), nucleus accumbens (NAcc), and hypothalamus (Hth). Tissue samples were frozen on dry ice and stored at -80°C. Catecholamines were measured by high performance liquid chromatography with electrochemical detection (Kesby et al., 2009). Brain tissues were homogenized in 0.1 M perchloric acid with 50 ng/mL deoxyepinephrine (internal standard) using probe sonication (Vibra-Cell, Sonics & Materials, CT, USA) and centrifuged at 13,000 rpm for 5 min. The supernatant was filtered by a 4 mm 0.22 μM nylon syringe filter (MicroSolv Technology Corporation, NJ, USA).
15 μ L of sample was injected into the HPLC system, which consisted of an autosampler (Dionex UltiMate 3000, Thermo Scientific, CA, USA), an isocratic HPLC pump (Model 584, ESA Laboratories, MA, USA), a Sunfire C18 column, (4.6 mm × 100 mm, 3 um; Waters Corporation, MA, USA) and a Coulochem III (ESA Laboratories) electrochemical detector. The mobile phase consisted of a 12% acetonitrile/50 mM citric acid and 25 mM potassium dihydrogen phosphate buffer containing 1 mM EDTA and 1.4 mM octane sulfonic acid adjusted to pH 4.3 with phosphoric acid. Flow rate was 0.5 ml/min. An analytical cell (Model 5014B, ESA Laboratories) with the first and second electrodes maintained at −150 and +300 mV, respectively, was used for detection. All data was stored and processed with Dionex Chromeleon software (version 7.2, Thermo Scientific). Data were quantified by calculating peak-area ratios of each compound compared to the internal standard and expressed as pg/mg wet tissue. The following neurotransmitters were identified and quantified: norepinephrine (NE), dopamine (DA), dihydroxyphenylacetic acid (DOPAC), HVA, 3-methoxytyramine (3MT), serotonin (5HT), and 5-hydroxyindoleacetic acid (5HIAA). Ratios of these were used to assess conversion of neurotransmitters and metabolites.
2.10 Statistical Analyses
We first confirmed that all data was distributed normally and displayed equal variances. Stable performance during training was assessed using a repeated measure analysis of variance (ANOVA) with days as a within-subject factor. The primary measures for each experiment were analyzed using a one- or two-way ANOVA, with lithium treatment, GBR12909, or genotype as between-subject variables. For the PRB analysis, mice were excluded from analyses if total trials completed were 0 or if the reaction times were more than 2 times the standard deviation away from the mean. HPLC studies were examined using a two-way ANOVA with genotype and lithium treatment as between-subjects factors for neurotransmitter and metabolite levels in each brain region. Tukey post hoc analyses of statistically significant main and interaction effects or a priori planned comparisons were performed where applicable, with Bonferroni corrections conducted for multiple comparisons. All data are reported as mean and standard error of the mean (S.E.M.). The level of probability for statistical significance was set at 0.05. All statistics were performed using SPSS (22.0, Chicago, USA).
3.1 RESULTS
3.2 Reward Associated Learning
3.2.1 Effects of chronic lithium alone
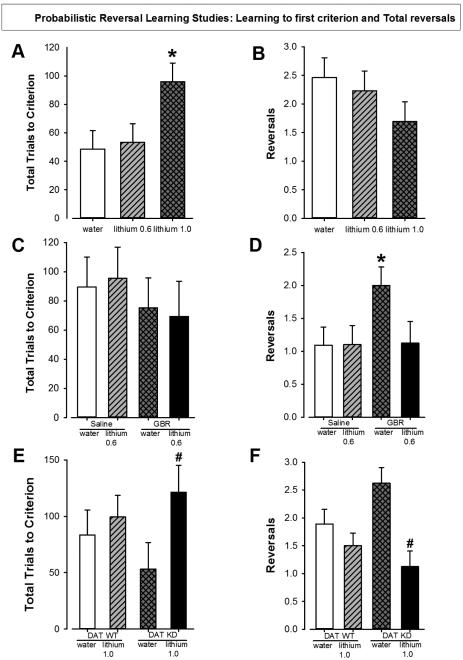
To determine the effects of varying doses of chronic lithium on probabilistic reversal learning, C57BL/6 mice were pre-treated with lithium (0.6 or 1.0 g/l) for 8 days prior to testing in the PRLT (Fig. 1C). There was a main effect of lithium for total trials to criterion (F(2,36)=5.0, p<0.05; Fig. 2A). No effect of lithium was observed for the number of reversals (F(2,36)=1.3, ns; Fig. 2B). There was also a trend towards an effect of lithium on percentage premature responses (F(2,34)=2.6, p=0.09; Supplemental Table 1). Post hoc analyses revealed that lithium at 1.0 g/l increased trials to criterion compared to both vehicle and lithium at 0.6 g/l (p<0.05), while those treated with 0.6 g/l did not differ from those given water (p>0.05). Mice treated with lithium at 1.0 g/l exhibited a higher percentage of premature responses compared to mice treated with water (p<0.05) but did not differ from mice treated with lithium at 0.6 g/l (p>0.1). There was no effect of chronic lithium on the animals’ weight (F<1, ns) or general health appearance. For secondary measurements such as punishment or reward perseverance, see Supplemental Table 1.
Figure 2. Effects of lithium alone and in combination with GBR12909 in C57BL/6 mice or in DAT WT and KD mice in the Probabilistic Reversal Learning Task.
Lithium at 1 g/l increased the number of trials needed to reach criterion in C57BL/6 mice while lithium at 0.6 g/l did not change the number of trials to criterion compared to water treated mice (A). Lithium at 0.6g/l and 1 g/l did not significantly reduced the number reversals made by C57BL/6 mice compared to water treated mice (B). GBR12909 (13 mg/kg) nor lithium had any significant effects on the number of trials to criterion (C). The number of reversals was greatly increased by GBR12909 treatment in water pre-treated mice. Lithium (0.6 g/l) blocked this increase in reversals (D). Treatment with lithium increased the number of trials needed to reach initial criterion in DAT KD mice but not in WT mice (E). Lithium treatment significantly lowered reversals in DAT KD mice compared to those treated with water but this effect was not seen in WT mice (F).
Data are presented as mean ± S.E.M. * p<0.05 when compared to vehicle. # p<0.05 when compared to DAT KD with vehicle treatment.
3.2.2 Effects of chronic lithium with or without GBR12909
In order to determine whether acute reduced DAT function interacted with lithium treatment, we pretreated C57BL/6 mice with lithium (0.6 g/l) or water for 10 days prior to GBR treatment and testing in the PRLT. There was no main effect of lithium, GBR, or an interaction between both on total trials to criterion (F<1, ns; Fig. 2C). For the number of reversals, there was a trend effect of lithium (F(1,39)=4.4, p=0.09) and a trend towards a lithium by GBR interaction (F(1,39)=3.2, p<0.1; Fig. 2D), but no main effect of GBR (F≤2, ns). Post hoc planned comparison analyses revealed that GBR-treatment increased reversals compared to vehicle-treated mice when given water (p<0.05) but this effect was blocked when pretreated with lithium (p<0.05). There was a trend towards an effect of lithium on non-target latency (F(1, 39)=3.0, p=0.1) but no effect of GBR or any interaction for this measure (F<1.4, ns.). There was a main effect of lithium on the mean reward latency (F(1,39)=4.6, p<0.05) but no effect of GBR (F<1, ns) or any interaction for this measure (F<2.3, ns). For secondary measurements such as punishment or reward perseverance, see Supplemental Table 2.
3.2.3 Effects of chronic lithium in DAT KD and WT mice
Mice with a chronic reduction in DAT levels (KD mice) were pretreated with lithium to determine whether either factor alone or interactively affected learning. There was no main effect of genotype on total trials to criterion (F<1, ns), although there was a trend towards an effect of lithium on this measure (F(1,33)=3.6, p=0.07; Fig. 2E) and there was no interaction between genotype and drug (F<1, ns). For the number of reversals, there was also no effect of genotype (F<1, ns) but a main effect of lithium was observed (F(1, 33)=12.8, p<0.01; Fig. 2F) as was an interaction between genotype and drug (F(1,33)=4.4, p<0.05). Post hoc planned comparison analyses revealed that DAT KD mice treated with lithium needed more trials to reach criterion compared to DAT KD mice treated with vehicle (p<0.05). This effect was not seen in WT mice (p>0.1). Although KD mice exhibited more reversals than WT mice when treated with water, this effect was not significant. Likely as a result of higher reversals, lithium treatment significantly lowered reversals in KD mice compared to those treated with water (p<0.05) but this effect was not seen in WT mice (p>0.1). For secondary measurements such as punishment or reward perseverance, or latencies, see Supplemental Table 3.
3.3 Effortful Motivation
3.3.1 Effects of chronic lithium with or without GBR12909
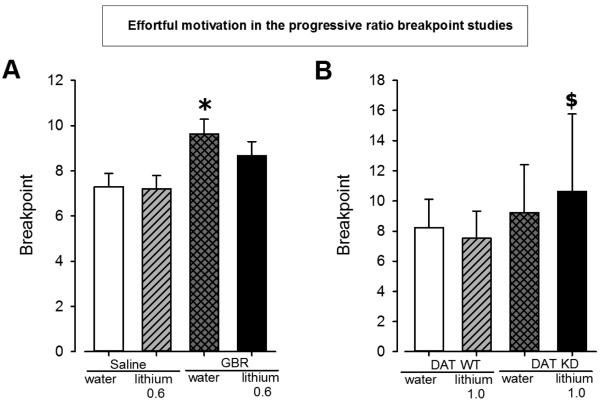
Mice were pretreated with lithium prior to GBR12909-induced acute reduction of DAT function to determine whether their interaction affected motivation. A main effect of GBR was observed for breakpoint (F(1,33)=9.6, p<0.01; Fig. 3A), without an effect of lithium or interaction between both (F<1, ns). A priori predicted analyses revealed that GBR treatment increased breakpoint in mice given water (p<0.05), but not significantly in mice given lithium (p>0.1).
Figure 3. Effect of lithium pre-treatment in combination with GBR12909 treatment in C57BL/6 mice or in DAT WT and KD mice in the Progressive Ration Breakpoint Task.
GBR12909 increased the breakpoint of water treated mice while lithium at 0.6g/l attenuated this increase in breakpoint (A). Breakpoint was increased in DAT KD receiving lithium treatment (1.0 g/l for 8 days) compared to WT mice receiving the same treatment (B).
Data are presented as mean ± S.E.M. * p<0.05 when compared to vehicle. $ p<0.05 when compared to DAT WT mice with lithium treatment.
3.3.2 Effects of chronic lithium in DAT KD and WT mice
The effects of lithium pretreatment on the motivation of mice with chronically reduced DAT levels were assessed using the PRB. A main effect of genotype was observed for breakpoint (F(1,50)=5.8, p<0.05; Fig. 3B) but no effect of lithium was observed (F<1, ns) or interaction between genotype and drug (F<1, ns). There was no effect of genotype on the cumulative reward latency (F<1, ns) but there was a main effect of drug on this measure (F(1,50)=4.7, p<0.05) and no interaction between genotype and drug (F<1, ns). Although no interaction was observed a priori predicted analyses revealed that DAT KD mice exhibited a higher breakpoint when treated with lithium compared to WT mice treated with lithium (p<0.05). This effect was not observed with water-treated mice however (p<0.05). Lithium slowed the cumulative reward latency in both DAT KD and WT.
3.4 Brain neurochemistry
To determine possible mechanisms underlying the altered performance of DAT KD mice and the effects of lithium, we examined the neurotransmitter levels and metabolite ratios of DAT WT and KD mice treated with water or lithium. Specifically, we measured NE, dopamine, DOPAC, HVA, 3MT, 5HT, and 5HIAA in the FL, NAcc, CPu, and Hth.
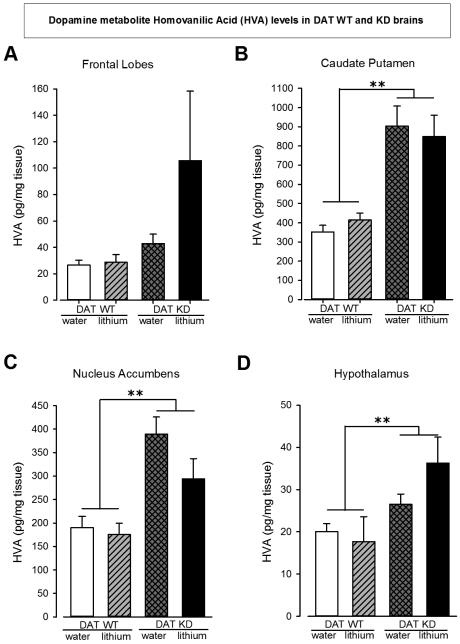
Across almost every brain region, elevated levels of HVA were observed in DAT KD mice compared to WT mice: FL (F(1,27)=3.0, p=0.1), CPu (F(1,27)=39.6, p<0.0001), NAcc (F(1,28)=23.5, p<0.0001), and Hth (F(1,27)=8.4, p<0.01). Additionally, decreased DOPAC/HVA ratios in DAT KD mice compared to DAT WT mice were observed in each brain region: FL (F(1,27)=26.3, p<0.001), CPu (F(1,27)=43.8, p<0.0001), NAcc (F(1,28)=18.3, p<0.0001), and Hth (F(1,26)=4.3, p<0.05). See Fig. 4 A - D.
Figure 4. Neurochemistry values for HVA levels in four different brain regions of brains of DAT WT and KD mice.
The HVA level is not significantly different in the Frontal Lobes of DAT KD mice compared DAT WT mice (A). It is significantly higher in DAT KD mice compared to WT mice irrespective of lithium treatment in the Caudate Putamen (B). Similarly, the HVA levels are significantly higher in the Nucleus Accumbens and Hypothalamus regions of DAT KD mice compared to WT mice (C and D resp.). Data are presented as mean ± S.E.M. **p<0.01 when compared to DAT WT mice.
Interestingly, lithium treatment did not interact with genotype in any region (F<1, ns), although lithium treatment increased 5-HT (F(1,27)=4.7, p<0.05) and NE (F(1,27)=4.7, p<0.05) levels alone in the CPu. DOPAC/dopamine ratios were only elevated in the CPu (F(1,27)=13.0, p<0.001) irrespective of drug treatment, likely due to the denser dopaminergic innervation in those regions compared with others. Other non-significant values and the mean ±SEM are detailed in Supplemental Table 4.
4.1 DISCUSSION
Consistent with our hypothesis and previous observations (Young and Geyer, 2010), acute DAT inhibition by GBR12909 administration increased the reward-seeking behavior (motivation) of mice, as measured in the progressive ratio schedule of reinforcement and reflected in the breakpoint. This effect was modestly attenuated by lithium treatment. Overall, DAT KD mice also exhibited higher breakpoints compared to WT mice, although surprisingly this effect was potentiated by lithium treatment. In contrast to our expectations, GBR12909 increased the number of successful reversals achieved within a probabilistic reversal learning session reflective of enhanced cognitive performance. Chronic reduction of DAT, as seen in DAT KD mice, also resulted in increased number of completed reversals, although non-significantly. Interestingly, lithium moderately attenuated both DAT KD- and GBR12909-induced increases in reversal learning at clinically relevant treatment doses as reported in earlier studies (van Enkhuizen et al., 2015a, b). Reducing DAT function also elevated HVA and reduced dopamine levels (DAT KD mice), an effect not blocked by lithium treatment, which elevated norepinephrine and serotonin levels. Importantly, lithium at its highest clinically relevant mania dose (1.0 g/l) impaired cognition (trials to criterion). These data support the interpretation that chronic lithium exerts main effects, impairing cognition in addition to attenuating the enhanced reversal learning of mice with reduced DAT functioning.
4.2 Probabilistic reversal learning in reduced DAT functioning mice
BD patients exhibit a variety of learning deficits (Dickstein et al., 2010; Pizzagalli et al., 2008). We hypothesized that reducing DAT function via pharmacological (GBR12909) or genetic (DAT KD) means would impair probabilistic reversal learning performance. Indeed, patients with BD in a euthymic state exhibit poor PRLT performance (Dickstein et al., 2010; Gorrindo et al., 2005; McKirdy et al., 2009). In contrast to our expectation however, acute, and to a degree, chronic DAT inhibition improved reversal learning in mice. Interestingly, in a probabilistic switching task, BD mania patients exhibited hypersensitivity to error rates during choice switching resulting in more frequent switches (Minassian et al., 2004), and arguably better performance compared to healthy controls. These data may therefore reflect reduced DAT functioning that would induce a hyperdopaminergic state (Zhuang et al., 2001), and could more rapidly induce adaptive shifting of behavior from non-rewarding to rewarding stimuli.
Amphetamine, a psychostimulant with combined norepinephrine transporter and DAT inhibition, enhanced learning in the PRLT (Young et al., 2015). GBR12909 alone slightly increased the number of target wins stay ratios compared to vehicle treated animals in the current study while DAT KD mice did not exhibit any differences in win stay ratios (see Supplemental Tables 2 & 3). In an effort-based decision-making task, low doses of amphetamine increased motivation to gain high rewards in rats, while higher doses disrupted such decision making (Floresco et al., 2008). Alternatively, amphetamine reduced cognitive effort in hard-working rats, but had no effect on low-working rats (Cocker et al., 2012). The history of the effects of such dopamine-based psychostimulants on decision making is complex and often task-dependent (Orsini et al., 2015). An ‘inverted U-shaped’ function of effect likely exists whereby small increases in dopamine can enhance cognition, whereas large increases in dopamine impair performance, termed the Yerkes-Dodson principle (Cohen, 2011). The smaller pro-cognitive effect in DAT KD mice compared to GBR12909-treated mice could reflect the modestly elevated extracellular dopamine (~25%) in rats at the dose of GBR 12909 used here (Weikop et al., 2007), as opposed to DAT KD mice (~45%) compared to WT levels (Zhuang et al., 2001). Further examination of dose response effects on PRLT combined with extracellular dopamine level analyses are required to test this hypothesis.
Previous studies using a Pavlovian association learning task also observed that learning for food was not enhanced nor impaired in DAT KD mice (Cagniard et al., 2006; Yin et al., 2006). Cagniard and colleagues also observed that DAT KD mice learned operant tasks at the same or faster rates than their WT littermates (Cagniard et al., 2006; Hironaka et al., 2004). When complex high punishment and reward rules are included such as in the mouse IGT however, mice with reduced DAT exhibited impaired learning (van Enkhuizen et al., 2014b). Similarly, patients with BD also exhibit deficient learning in the IGT (Anand et al., 2011; van Enkhuizen et al., 2014b), driven by a preference for high rewards irrespective of punishment (van Enkhuizen et al., 2014b), yet BD patients exhibit enhanced learning in simpler paradigms (Higier et al., 2014). These studies reflect therefore, that enhanced simpler learning, yet impaired complex feedback-based decision making with varied reward/punishments seen in BD patients, can be recreated by reducing DAT function in mice.
4.3 Motivation in reduced DAT functioning mice
In addition to altered learning rates, patients with BD also exhibit increased motivation and reward-seeking/goal-oriented behaviors. In fact, increased goal-seeking activity is used as diagnostic criterion for mania (Fulford et al., 2015). Multiple studies have shown that reducing DAT functioning and hence elevating striatal dopamine levels by pharmacological or genetic means increases rodents’ effortful motivation for food (Cagniard et al., 2006; Sommer et al., 2014). In contrast, reduced motivation is observed during periods of depression in patients compared with healthy subjects (Treadway and Zald, 2011), and may result from reduced striatal dopamine levels (Salamone and Correa, 2012). The present findings confirm that reducing DAT function increases breakpoint in a progressive ratio breakpoint study (Cagniard et al., 2006; Young and Geyer, 2010). Increased levels of dopamine, particularly in the nucleus accumbens, elevate motivation for food (Pecina et al., 2003), and micro-infusion of amphetamine into the nucleus accumbens in non-food-deprived rats elevated the rats' breakpoint for food (Zhang et al., 2003). Interestingly, amphetamine only increased the breakpoint of rats trained to respond for food rewards, indicating that amphetamine did not immediately induce learning but increased motivation, with the increased dopamine increasing the salience of stimuli previously associated with reward (Hanlon et al., 2004). Such reward-associative behaviors could be important for cognitive training in psychiatric patients (Acheson et al., 2013), and may be mediated by dopamine D1 receptors in the nucleus accumbens (or prefrontal cortex) because a reduction in breakpoint for cocaine reward was observed when D1 antagonists were directly administered in these regions (McGregor and Roberts, 1993, 1995; Nicola and Deadwyler, 2000). Considering that reduced dopamine D1 receptor expression attenuated the hyper-motivating effects of DAT inhibition (Young and Geyer, 2010), future studies could examine whether antagonism of dopamine D1 receptors could also block DAT inhibition-induced hyper-motivation.
4.4. The interactive effects of reduced DAT function and lithium pretreatment
Cognitive dysfunction in patients with BD correlates closely with their functional outcome, that is their ability to live independently (Green, 2006). Unfortunately, most studies are conducted in individuals who are on medications such as lithium, with few cognitive studies conducted in medication-free patients (Chandler et al., 2009; Soeiro-de-Souza et al., 2012). It is therefore difficult to dissociate causal from confounding effects. Rodent studies enable the examination of the effects of chronic lithium treatment alone and in combination with putative mechanisms underlying the disease. In the present study, lithium at 1.0 g/l exerted deleterious effects on probabilistic reversal learning performance, increasing the number of trials taken to attain initial criterion. This dose resulted in desired serum levels for the clinical treatment of BD mania (van Enkhuizen et al., 2015a, b). It is unlikely that putative toxic effects at this high serum level interacted with performance given that the body weight of mice was unaffected. These data contrast with other studies whereby giving chronic lithium chow to rats enhanced simpler aspects of learning such as spatial holeboard and delayed alternation learning (Nocjar et al., 2007; Sharifzadeh et al., 2007). In that study the given lithium dose resulted in lower serum levels (low end of clinical maintenance) that are usually not used in mania treatment, which did not affect performance alone in the present study (0.6 g/l). Furthermore, reduced weight in the rats may have encouraged their learning of appetitive tasks, whereas lithium did not affect weight in the current study. Hence, at therapeutic doses, lithium impairs normal probabilistic reversal learning in mice.
Although lower lithium treatment dose (0.6 g/l) did not affect learning, it did attenuate GBR-induced enhancement of reversal learning. Indeed, lithium can negatively affect cognitive performance in patients with BD (Pachet and Wisniewski, 2003; Wingo et al., 2009), as well as healthy subjects (Judd et al., 1977; Stip et al., 2000). These results highlight the possible major limitations of current BD treatments in that; a) dysfunctional cognitive performance often remains untreated; b) current treatments may impair cognition alone; and c) these treatments could block any baseline enhanced performance patients experience, decreasing treatment compliance which is a major problem. The long-term cognitive effects of such treatments have yet to be tested, but such studies are required in combination with mechanistic studies that might remediate the effects of reduced DAT functioning.
Chronic lithium treatment also exerted some effects on motivational behavior. Similar to lithium blockade of GBR-induced enhancement of learning, lithium only exerted attenuating effects in GBR-induced increased motivation. This limitation of effect, not affecting saline-treated mice, is likely why no significant interaction between GBR and lithium treatments was observed. These data provide some support for the potential pharmacological predictive validity of this model for hedonic/hyper-motivated behavior seen in BD mania patients, consistent with its effects of attenuating mania-like exploratory behavior of GBR-treated mice (van Enkhuizen et al., 2014b).
The precise mechanism(s) of the apparent effect of lithium on DAT-manipulated mice remains unclear. Lithium treatment can suppress phasic dopamine release (Fortin et al., 2015), which could be a result from lithium chloride-induced cation leakage from the DAT (Borre et al., 2014). It is perhaps this mechanism of lithium-induced leakage that results in the long-term increase in DAT levels. Few interactive studies between reduced DAT functioning and chronic lithium effects have been conducted to-date however, and our current study did not reveal any specific interactions on neurochemistry.
4.5 Brain neurochemistry in DAT mutants treated with lithium or water
While recreating the mania-like profile of mice – and the effects of BD treatments – in cross-species tasks remain important, delineating the neural mechanisms that underlie altered behavior is also vital for treatment development. Here, we observed elevated HVA levels of DAT KD compared to WT mice were observed across all four brain regions assessed (FL, CPu, NAc, and Hth - significantly in the latter 3 regions), consistent with elevated CSF of BD mania patients. Additionally, the HVA/dopamine ratio was significantly higher in KD mice compared to WT in all four brain regions while dopamine levels and the DOPAC/HVA ratio were significantly lower in striatal regions (CPu and NAc). Considering that the vast majority of dopamine in the brain is located intra-neuronally (Best et al., 2009), the lower dopamine levels of DAT KD mice likely arose from altered homeostatic control of dopamine production given reduced DAT clearance mechanisms. These data support the importance of evaluating multiple levels of dopamine clearance.
With reduced dopamine clearance via DAT, KD mice generate HVA at higher rates than WT mice. These findings are consistent with elevated HVA levels in the cerebro-spinal fluid of BD mania, but not schizophrenia or depressed patients (Gerner et al., 1984; Koslow et al., 1983), providing construct validity for reduced DAT functioning as a model of mania (Young et al., 2011). HVA is generated as a result of COMT- and/or MAO-mediated degradation of dopamine to DOPAC or 3-MT, then to HVA (Best et al, 2009). Elevated levels of HVA after DAT KD are understandable since dopamine is not scavenged back into the neuron as readily by the DAT. This heightened HVA generation in DAT KD mice could also subsequently cause an increase in the amount of toxic oxygen radicals that are produced during the conversion of dopamine into DOPAC by MAO, and of 3-MT into HVA by MAO. It is possible therefore, that the oxidative stress in the brains of DAT KD mice could deleteriously affect brain function and contribute to the behavioral differences observed between DAT KD and WT mice. Lithium did not reverse this DAT KD-induced elevation of HVA. Instead, lithium treatment elevated 5-HT and NE in the CPu (Supplemental Table 4). These data therefore support our behavioral evidence here and elsewhere (van Enkhuizen et al., 2015a, b), that chronic lithium may not be the optimal strategy for reversing behavioral deficits that result from reduced DAT function, exerting only modest effects altering behavior.
4.6 CONCLUSIONS
Reducing DAT function in mice recreates behaviors relevant to BD mania, including increased motivation and more adaptive reversal learning between choices. Additionally, lithium treatment impaired learning of mice at clinically relevant doses and exerted only modest treatment effects on mice with reduced DAT functioning, i.e., lithium attenuated the enhanced motivation and reversal learning of mice with reduced DAT functioning. Lithium did not attenuate the elevated HVA of DAT KD mice however. Hence, lithium-induced changes in behavior may have been driven by elevating serotonin and norepinephrine levels. Additionally, these data provide further construct validity for the DAT KD mice as a model of mania given that elevated HVA levels are also observed in BD mania patients. Considering pro-cognitive therapeutics have yet to be developed for BD, reducing DAT function and assessing the consequences of putative therapeutics in cross-species relevant cognitive tests could be a useful model in the future.
Supplementary Material
Highlights.
Reduced dopamine transporter levels improved reversal learning and motivation
Such reductions also resulted in elevated homovanilic acid levels in mice
Chronic lithium alone impaired probabilistic learning in mice
Lithium remediated behavioral effects of reduced dopamine transporter levels
Lithium exerted only main, not synergistic effects, on neurotransmitter levels
5.1 ACKNOWLDGEMENTS
We thank Drs. William Perry, Brook Henry, Johan Garssen and Lucianne Groenink, Kerin Higa, Xia Li, and David MacQueen, as well as Ms. Mahalah Buell, and Mr. Richard Sharp for their support.
6.1 FUNDING SUPPORT
This work was supported NIH grants R01-MH071916, R01-MH104344, R21-MH101579, and the Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acheson DT, Twamley EW, Young JW. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: a roadmap for preclinical development. Front Neurosci. 2013;7:103. doi: 10.3389/fnins.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, Hutchins GD, Normandin MD, Yoder KK. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disorder. 2011;13:406–413. doi: 10.1111/j.1399-5618.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Best JA, Nijhout HF, Reed MC. Homeostatic mechanisms in dopamine synthesis and release: a mathematical model. Theor Biol Med Model. 2009;6:21. doi: 10.1186/1742-4682-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre L, Andreassen TF, Shi L, Weinstein H, Gether U. The second sodium site in the dopamine transporter controls cation permeation and is regulated by chloride. J Biol Chem. 2014;289:25764–25773. doi: 10.1074/jbc.M114.574269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Braga RJ, Nnadi CU, Shaya Y, Stearns WH, Malhotra AK. Placebo-controlled adjunctive trial of pramipexole in patients with bipolar disorder: targeting cognitive dysfunction. J Clin Psychiatry. 2012;73:103–112. doi: 10.4088/JCP.11m07299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Chandler RA, Wakeley J, Goodwin GM, Rogers RD. Altered risk-aversion and risk-seeking behavior in bipolar disorder. Biol Psychiatry. 2009;66:840–846. doi: 10.1016/j.biopsych.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Christodoulou T, Lewis M, Ploubidis GB, Frangou S. The relationship of impulsivity to response inhibition and decision-making in remitted patients with bipolar disorder. Eur Psychiatry. 2006;21:270–273. doi: 10.1016/j.eurpsy.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 2012;37:1825–1837. doi: 10.1038/npp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. Yerkes–Dodson Law. In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. Springer; New York: 2011. pp. 2737–2738. [Google Scholar]
- Dehpour AR, Farsam H, Azizabadi-Farahani M. Inhibition of the morphine withdrawal syndrome and the development of physical dependence by lithium in mice. Neuropharmacology. 1995;34:115–121. doi: 10.1016/0028-3908(94)00121-8. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010;40:1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-V . Diagnostic and statistical manual of mental health disorders: DSM-5. 5th American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- Duek O, Osher Y, Belmaker RH, Bersudsky Y, Kofman O. Reward sensitivity and anger in euthymic bipolar disorder. Psychiatry Res. 2014;215:95–100. doi: 10.1016/j.psychres.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Ferrie L, Young AH, McQuade R. Effect of chronic lithium and withdrawal from chronic lithium on presynaptic dopamine function in the rat. J Psychopharmacol. 2005;19:229–234. doi: 10.1177/0269881105051525. [DOI] [PubMed] [Google Scholar]
- Ferrie L, Young AH, McQuade R. Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int J Neuropsychopharmacol. 2006;9:729–735. doi: 10.1017/S1461145705006243. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Fortin SM, Chartoff EH, Roitman MF. The Aversive Agent Lithium Chloride Suppresses Phasic Dopamine Release Through Central GLP-1 Receptors. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford D, Eisner LR, Johnson SL. Differentiating risk for mania and borderline personality disorder: The nature of goal regulation and impulsivity. Psychiatry Res. 2015;227:347–352. doi: 10.1016/j.psychres.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Gerner RH, Fairbanks L, Anderson GM, Young JG, Scheinin M, Linnoila M, Hare TA, Shaywitz BA, Cohen DJ. CSF neurochemistry in depressed, manic, and schizophrenic patients compared with that of normal controls. Am J Psychiatry. 1984;141:1533–1540. doi: 10.1176/ajp.141.12.1533. [DOI] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006;11:125–133. doi: 10.1038/sj.mp.4001764. 115. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology (Berl) 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- Higier RG, Jimenez AM, Hultman CM, Borg J, Roman C, Kizling I, Larsson H, Cannon TD. Enhanced neurocognitive functioning and positive temperament in twins discordant for bipolar disorder. Am J Psychiatry. 2014;171:1191–1198. doi: 10.1176/appi.ajp.2014.13121683. [DOI] [PubMed] [Google Scholar]
- Hironaka N, Ikeda K, Sora I, Uhl GR, Niki H. Food-reinforced operant behavior in dopamine transporter knockout mice: enhanced resistance to extinction. Ann N Y Acad Sci. 2004;1025:140–145. doi: 10.1196/annals.1316.018. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, Schloss P. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Mol Psychiatry. 2005;10:1104–1109. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Joffe RT, MacDonald C, Kutcher SP. Lack of differential cognitive effects of lithium and carbamazepine in bipolar affective disorder. J Clin Psychopharmacol. 1988;8:425–428. [PubMed] [Google Scholar]
- Jollant F, Guillaume S, Jaussent I, Bellivier F, Leboyer M, Castelnau D, Malafosse A, Courtet P. Psychiatric diagnoses and personality traits associated with disadvantageous decision-making. Eur Psychiatry. 2007;22:455–461. doi: 10.1016/j.eurpsy.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard B, Janowsky DS, Huey LY, Takahashi KI. The effect of lithium carbonate on the cognitive functions of normal subjects. Arch Gen Psychiatry. 1977;34:355–357. doi: 10.1001/archpsyc.1977.01770150113013. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Cui X, Ko P, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett. 2009;461:155–158. doi: 10.1016/j.neulet.2009.05.070. [DOI] [PubMed] [Google Scholar]
- Koslow SH, Maas JW, Bowden CL, Davis JM, Hanin I, Javaid J. CSF and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis. Arch Gen Psychiatry. 1983;40:999–1010. doi: 10.1001/archpsyc.1983.01790080081011. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993;624:245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behav Brain Res. 1995;67:75–80. doi: 10.1016/0166-4328(94)00106-p. [DOI] [PubMed] [Google Scholar]
- McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med. 2009;39:1289–1293. doi: 10.1017/S0033291708004935. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PLoS One. 2011;6:e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Paulus MP, Perry W. Increased sensitivity to error during decision-making in bipolar disorder patients with acute mania. Journal of Affective Disorders. 2004;82:203–208. doi: 10.1016/j.jad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol Med. 2013;43:1187–1196. doi: 10.1017/S0033291712001948. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Hammonds MD, Shim SS. Chronic lithium treatment magnifies learning in rats. Neuroscience. 2007;150:774–788. doi: 10.1016/j.neuroscience.2007.09.063. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 2015 doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachet AK, Wisniewski AM. The effects of lithium on cognition: an updated review. Psychopharmacology (Berl) 2003;170:225–234. doi: 10.1007/s00213-003-1592-x. [DOI] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK, Gu HH, Sadee W. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36:1644–1655. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry. 2008;64:162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz AI, de Araujo MM, da Silva Araujo T, de Souza GC, Cavalcante LM, de Jesus Souza Machado M, de Lucena DF, Quevedo J, Macedo D. GBR 12909 administration as an animal model of bipolar mania: time course of behavioral, brain oxidative alterations and effect of mood stabilizing drugs. Metab Brain Dis. 2015 doi: 10.1007/s11011-015-9697-6. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr., McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh M, Aghsami M, Gholizadeh S, Tabrizian K, Soodi M, Khalaj S, Ranjbar A, Hosseini-Sharifabad A, Roghani A, Karimfar MH. Protective effects of chronic lithium treatment against spatial memory retention deficits induced by the protein kinase AII inhibitor H-89 in rats. Pharmacology. 2007;80:158–165. doi: 10.1159/000103265. [DOI] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Machado-Vieira R, Soares Bio D, Do Prado CM, Moreno RA. COMT polymorphisms as predictors of cognitive dysfunction during manic and mixed episodes in bipolar I disorder. Bipolar Disord. 2012;14:554–564. doi: 10.1111/j.1399-5618.2012.01030.x. [DOI] [PubMed] [Google Scholar]
- Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W. The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol. 2014;17:2045–2056. doi: 10.1017/S1461145714000996. [DOI] [PubMed] [Google Scholar]
- Stip E, Dufresne J, Lussier I, Yatham L. A double-blind, placebo-controlled study of the effects of lithium on cognition in healthy subjects: mild and selective effects on learning. J Affect Disord. 2000;60:147–157. doi: 10.1016/s0165-0327(99)00178-0. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Halberstadt AL, Zhuang X, Young JW. Dopamine depletion attenuates some behavioral abnormalities in a hyperdopaminergic mouse model of bipolar disorder. J Affect Disord. 2014a;155:247–254. doi: 10.1016/j.jad.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int J Neuropsychopharmacol. 2013a;16:1021–1031. doi: 10.1017/S1461145712001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task : Relevance to mania. Psychopharmacology. 2013b;225:661–674. doi: 10.1007/s00213-012-2854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task: relevance to mania. Psychopharmacology (Berl) 2013c;225:661–674. doi: 10.1007/s00213-012-2854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Henry BL, Minassian A, Perry W, Milienne-Petiot M, Higa KK, Geyer MA, Young JW. Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder. Neuropsychopharmacology. 2014b;39:3112–3122. doi: 10.1038/npp.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Milienne-Petiot M, Geyer MA, Young JW. Modeling bipolar disorder in mice by increasing acetylcholine or dopamine: chronic lithium treats most, but not all features. Psychopharmacology (Berl) 2015a doi: 10.1007/s00213-015-4000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Milienne-Petiot M, Geyer MA, Young JW. Modeling bipolar disorder in mice by increasing acetylcholine or dopamine: chronic lithium treats most, but not all features. Psychopharmacology (Berl) 2015b;232:3455–3467. doi: 10.1007/s00213-015-4000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikop P, Kehr J, Scheel-Kruger J. Reciprocal effects of combined administration of serotonin, noradrenaline and dopamine reuptake inhibitors on serotonin and dopamine levels in the rat prefrontal cortex: the role of 5-HT1A receptors. J Psychopharmacol. 2007;21:795–804. doi: 10.1177/0269881107077347. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Wingo TS, Harvey PD, Baldessarini RJ. Effects of lithium on cognitive performance: a meta-analysis. J Clin Psychiatry. 2009;70:1588–1597. doi: 10.4088/JCP.08r04972. [DOI] [PubMed] [Google Scholar]
- Yin HH, Zhuang X, Balleine BW. Instrumental learning in hyperdopaminergic mice. Neurobiol Learn Mem. 2006;85:283–288. doi: 10.1016/j.nlm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of modafinil--increased motivation via the dopamine transporter inhibition and D1 receptors? Biol Psychiatry. 2010;67:784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010a;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol Biochem Behav. 2010b;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kamenski ME, Higa KK, Light GA, Geyer MA, Zhou X. GlyT-1 Inhibition Attenuates Attentional But Not Learning or Motivational Deficits of the Sp4 Hypomorphic Mouse Model Relevant to Psychiatric Disorders. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Markou A. Translational Rodent Paradigms to Investigate Neuromechanisms Underlying Behaviors Relevant to Amotivation and Altered Reward Processing in Schizophrenia. Schizophr Bull. 2015;41:1024–1034. doi: 10.1093/schbul/sbv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J Psychopharmacol. 2011;25:934–943. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.