Abstract
Magnetic fields (MFs) from domestic power sources have been implicated as being a potential risk to human health. A number of epidemiological studies have found a significant link between exposure to MFs and increased rates of cancers. There have also been a number of in vivo and in vitro studies reporting effects of MFs in animal disease models and on the expression or activity of a range of proteins. In the past decade, our group proposed that atherosclerosis may have an autoimmune component, with heat shock protein 60 (Hsp60) expressed in endothelial cells as the dominant autoantigen. A number of stressors have been shown to induce the expression of Hsp60, including the classical risk factors for atherosclerosis. We were interested to see if the exposure of endothelial cells to an MF elicited increased expression of Hsp60, as has been reported previously for Hsp70. The present work describes the exposure of endothelial cells to domestic power supply (50 Hz) MFs at an intensity of 700 μT. The results from our system indicate that cultured endothelial cells exposed to a high intensity of MF either alone or in combination with classical heat stress show no effects on the expression of Hsp60 at either the messenger ribonucleic acid or the protein level. As such, there is no evidence that exposure to extremely low–frequency MF would be expected to increase the expression of Hsp60 and therefore the initiation or progression of atherosclerosis.
INTRODUCTION
The possible effects on human health of exposure to extremely low–frequency magnetic fields (ELF-MFs) produced by every electrical power source and appliance at 50–60 Hz has been a topic of heated debate for more than 2 decades. The first epidemiological study linking exposure to higher levels of ELF-MF with an increased rate of leukemia was published by Wertheimer and Leeper (1979). Since then, there have been a large number of epidemiological studies on various cancers along with a range of other diseases, extensively reviewed in Ahlbom et al (2001). Exposure to ELF-MFs has also been reported to be a contributing factor to the onset of Alzheimer disease (Sobel et al 1996). Some have supported the original finding of a link between increased exposure and increased tumor incidence (for example, Feychting and Ahlbom 1993; Olsen et al 1993), whereas others have found no increased risk from ELF-MF exposure (for example Linet et al 1997; McBride et al 1999).
One of the main problems associated with epidemiological studies is the difficulty in accurately standardizing and measuring exposure levels to ELF-MFs and in adequately defining the control groups. Because of the ubiquitous nature of ELF-MFs in modern society, the intensity of exposure varies throughout the day and from day to day, further complicating the analysis. To avoid these problems, there have been extensive laboratory-based studies both in vitro and in vivo, where exposure regimes can be strictly controlled (reviewed in Loscher and Liburdy 1998). Many of the in vivo studies have focused on the possible role of ELF-MFs in inducing or copromoting tumor development or reproductive defects in mice or rats (Fam and Mikhail 1996; Mevissen et al 1996; Juutilainen et al 1997; Yasui et al 1997). Further publications have reported on possible effects on a range of other parameters including function of the immune system, blood chemistry, and sleeping patterns in a range of species including humans (Graham et al 1996, 1997; Selmaoui et al 1996, 1997; Fernie and Bird 2001).
The in vitro studies have been conducted using a range of transformed and primary cell lines. A number of genes have been reported to be influenced by exposure of various cell lines to varying intensities of ELF-MFs, along with the intracellular Ca2+ levels (Walleczek and Liburdy 1990; Liburdy 1992, 1993a). Some genes that are reported to have been affected include factors implicated in tumor development or progression, for example, the transcription factors c-myc and AP-1 (Goodman et al 1989; Wei et al 1990; Phillips et al 1992; Liburdy 1993a; Jin et al 2000), ornithine decarboxylase (Byus et al 1987; Litovitz et al 1991), and heat shock proteins (Hsps) (Goodman and Henderson 1988; Goodman et al 1994; Goodman and Blank 1998; Pipkin et al 1999). Because of the reported ability of ELF-MFs to induce expression of Hsp70, this has even been suggested as a possible cytoprotective treatment for cells exposed to ischemia (DiCarlo et al 1998; Han et al 1998). Unfortunately, like the epidemiological studies, the interpretation of the in vivo and in vitro data is made difficult by the lack of agreement and reproducibility of results.
In addition to the uncertainty surrounding the possible effects of ELF-MF exposure, there is a lack of a plausible mechanism for their actions in biological systems. A number of hypotheses have been made, including a reduction in the circulating levels of the potent, free-radical scavenger melatonin as a possible pathway to increased tumor formation (Reiter 1992) or a direct effect through interaction with electrons in the deoxyribonucleic acid (DNA) backbone (Blank and Goodman 1997, 1999).
The heat shock family of proteins is found in all organisms, fulfilling a variety of physiological roles—most importantly, protein folding and transport, along with chaperoning activities of new or denatured proteins, and cellular signaling and protein degradation (Lindquist and Craig 1988; Hightower 1991; Gething and Sambrook 1992). Furthermore, they have been implicated as playing a role in a number of diseases such as cancer (Morimoto 1991; Fuller et al 1994) as well as in a number of autoimmune diseases such as arthritis (Res et al 1988; van Eden et al 1989), type I diabetes (Elias et al 1990), Crohn disease and ulcerative colitis (Elsaghier et al 1992; Stevens et al 1992), multiple sclerosis (Salvetti et al 1992), and systemic lupus erythematosus (Deguchi et al 1987; Minota et al 1988a, 1988b).
During the past decade, a great deal of evidence has emerged that shows that atherosclerosis is predominantly an inflammatory disease, with a contributing immune component (Ross 1999; Hansson 2001). During this time, our group developed a hypothesis that atherosclerosis is indeed an immune-mediated disease and that the main antigen may be a member of the Hsp60 family (recently reviewed by Wick et al 2001). Humans are continuously exposed to infectious microbes, and Hsps (particularly Hsp60) constitute a major antigenic component recognized by the immune system (Kaufmann 1990, 1992). To protect the body from infection, the immune system develops a response to the Hsps. Because of the high level of conservation of Hsps across all species (indeed there is 34% identity and 71% homology at the protein level between human Hsp60 and the homologue from Escherichia coli GroEL), we believe that this may in turn lead to an autoimmune response against the human Hsp60. It has been shown that antibodies in human serum against bacterial Hsp60 proteins are capable of cross-reacting with human Hsp60 (Mayr et al 1999), leading to the possibility that we may “pay” for immunity against microbes with the chance of autoimmunity against self-Hsp60 by cross-reactive antibodies.
The induction of Hsps is a well-known phenomenon after exposure of cells to stressful conditions, the most well known and studied being heat shock (Morimoto 1993). Many publications have shown that a number of other stressors can also lead to increased expression of Hsp60, and we believe that this may be particularly important for atherogenesis when Hsp60 expression is highly and chronically induced in endothelial cells. These stressors include the classical atherosclerosis risk factors, such as modified low-density lipoprotein (Amberger et al 1997), toxins from cigarette smoke, oxidative or free-radical sources (reviewed recently in Cai and Harrison 2000), cytokines, and, most important, mechanical shear stress (Hochleitner et al 2000). Previous work from our group has also shown Hsp60 to be present on the surface of stressed cells and that only stressed and not unstressed cells are lysed by cytotoxic anti-Hsp 60 antibodies (Xu et al 1994; Schett et al 1995; Soltys and Gupta 1997).
A positive correlation of both the level of antibodies to mycobacterial Hsp65 and the concentration of soluble Hsp60 present in the blood to the incidence of atherosclerotic lesions has been observed (Xu et al 2000; Kiechl et al 2001). This appears to be a robust and reliable indicator for morbidity and mortality independent of all other factors associated with atherosclerosis except for age. This finding has also been reported independently in other studies (Pockley et al 2000; Zhu et al 2001).
There have been a large number of publications citing an effect on the expression of Hsps (mostly Hsp70) in cells after exposure to ELF-MFs (including Goodman and Henderson 1988; Lin et al 1997, 1998; Goodman and Blank 1998; Pipkin et al 1999; Chow and Tung 2000a, 2000b; Di Carlo et al 2002). In contrast, there has been only a single paper reporting an increase in the expression of the E coli Hsp60 homologue GroEL after ELF-MF exposure (Nakasono and Saiki 2000). Given the significant role of Hsp60 in a number of human diseases, we felt it was important to investigate the possible influence of ELF-MFs on the expression of Hsp60 in primary (nontransformed) human cells, under strictly controlled conditions. We were also interested to see if a normal physiological heat-stress response in human endothelial cells was influenced by exposure to MF either before or after heat stress, as has been reported in the case of Caenorhabditis elegans (Junkersdorf et al 2000). An increase in the expression level of Hsp60 could, from our hypothesis, implicate a possible role of ELF-MFs in the development and progression of atherosclerosis.
MATERIALS AND METHODS
Cell culture conditions and general reagents
Human umbilical venous endothelial cells (HUVECs) were isolated from umbilical cords kindly donated by the Gynaecology and Obstetrics Department, University Clinic of Innsbruck, Austria. The cells were isolated by enzymatic detachment using collagenase, as described elsewhere (Amberger et al 1997). The cells were routinely passaged in 0.2% gelatin-coated (2% solution, Sigma, Steinheim, Germany) polystyrene culture flasks (Becton Dickinson, Meylan Cedex, France) in endothelial cell basal medium (CC-3121, BioWhittaker Inc., Walkersville, MD, USA) supplemented with EGM SingleQuots supplements and growth factors (CC-4133, BioWhittaker), in a humidified atmosphere containing 5% CO2. For experiments, 1 × 106 HUVECs were seeded onto fibronectin (1 mg/mL, Sigma)–coated cell culture petri dishes (100 mm, Becton Dickinson) and grown to confluence under the same conditions used for routine cell culture. Medium was changed 48 hours before the cells were harvested. Chemical reagents were purchased from Merck (Darmstadt, Germany) unless otherwise stated and were of analytical grade quality.
Exposure conditions
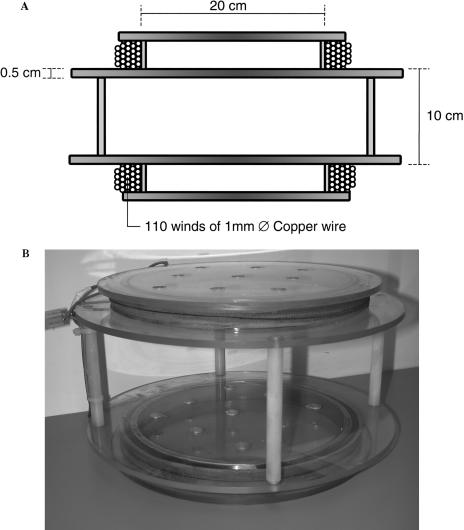
Exposure of the cells to a defined intensity of MF at 50 Hz was achieved by construction of a Helmholtz coil (Fig 1). This consisted of an identical pair of circular coils (200-mm internal diameter) made from 110 winds of 1-mm-diameter copper wire separated by 110 mm, powered by standard mains electricity (50 Hz). The frame was constructed from Perspex to ensure no interference with the magnetic field. The intensity of the field was measured using a CA 40 Gaussmeter (Chauvin Arnoux, Paris, France), before and after each experimental exposure. The earth's static MF in Innsbruck is 20 μT. The control cells (designated near control [NC]) were placed in the opposite corner of the same incubator to exclude environmental differences. As such, NC was exposed to an MF of 1% (6 μT) of that of the MF-exposed cells.
Fig 1.
Helmholtz coil used for magnetic field (MF) exposure. (A) Schematic cross section of the Helmholtz coil to supply the 50-Hz MF. Each of the 2 coils was formed from 110 winds of 1-mm-diameter copper wire around a Perspex frame. The field intensity was regulated using a separate rheostat connected to the domestic power supply. (B) The Helmholtz coil as constructed and used in the experiments
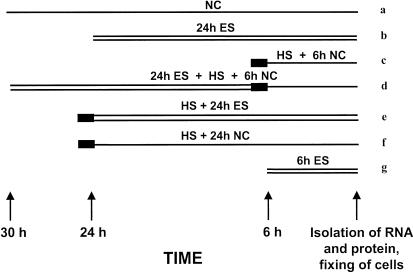
The experimental protocol included exposure of cells to MF alone or in combination with heat stress, a standard positive control. The MF exposure was set to a relatively high intensity at 700 μT, which is slightly higher than the guideline of 500 μT issued by the International Nonionizing Radiation Committee of the International Radiation Protection Association but well within the range of possible occupational exposures. Cells were exposed to a 700 μT field for either 24 or 6 hours or to heat stress (30 minutes, 42°C) followed by either 24 or 6 hours of recovery at 37°C (Fig 2). Combination experiments consisted of exposure to 700 μT for 24 hours immediately followed by heat stress (30 minutes, 42°C) and recovery for 6 hours at 37°C out of the MF or heat stress (30 minutes, 42°C) followed by 24 hours exposure to 700 μT MF. All cells were harvested at the same time as the negative (near) control.
Fig 2.
Graphical representation of the exposure protocol used. Samples were exposed to a 700 μT magnetic field (MF) (“ES,” double thin line) for 24 (b), or 6 hours (g), and for 24 hours immediately before (d), or after (e) 30 minutes heat stress at 42°C (“HS,” thick line). After heat stress was performed, cells were allowed to recover for 6 (c and d) or 24 hours (f) away from the MF (near control [NC], single thin line), with the negative control (a), except for (e), which was exposed to the MF for 24 hours
RNA and protein isolation and Northern and Western blots
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) before being scraped from the surface of the dish with a rubber policeman in PBS. The cells were collected by centrifugation before being suspended in TriReagent (Sigma). Isolation of total ribonucleic acid (RNA) and total protein was done according to the manufacturer's instructions. Total RNA was resuspended in ribonuclease-free water, whereas the protein was rehydrated in 1% sodium dodecyl sulfate (SDS). The concentration of RNA present in each sample was determined by absorbance at 260 nm, assuming 1.0 optical density equals 40 μg/mL RNA. Protein concentrations were measured by the detergent-compatible protein assay (Bio-Rad Laboratories, Hercules, CA, USA), using bovine serum albumin (BSA) in 1% SDS as a standard.
Total RNA (10 μg) was separated by denaturing formaldehyde-agarose electrophoresis. The RNA was then transferred to Zeta-bind membrane (Bio-Rad Laboratories) by capillary action and subsequently cross-linked to the membrane by ultraviolet light (UV Stratalinker, Stratagene, La Jolla, CA, USA). The Northern blotting protocol for hsp60 was performed as described (Amberger et al 1997), using a PhosphorImager (Fuji Photo Co Ltd, Tokyo, Japan) to image the blot. Quantification of the bands was performed using the supplied software (ImageQuant, Fuji), and loading was normalized by the intensity of the ethidium bromide staining of the 28S and 18S ribosomal bands.
For Western blotting, total protein (10 or 20 μg) was resolved on a 10% denaturing polyacrylamide gel. Protein was transferred to a nitrocellulose membrane (Protran, Schleicher and Schuell, Dassel, Germany) at 1200 mA/h in 25 mM Tris, 192 mM glycine, and 20% methanol (pH 8.3). The membrane was blocked by incubation in 5% skim milk for at least 1 hour, before being exposed for 60 minutes to an anti-human Hsp60 antibody (clone II-13 [Singh and Gupta 1992] or LK-1, both 1:10 000 dilution in 5% skim milk). After incubation with the secondary antibody (horseradish peroxidase (HRP)–conjugated rabbit anti-mouse Ig, Dako, Glostrup, Denmark), diluted 1:1000 in 5% skim milk for 60 minutes at room temperature, the bands were visualized using the enhanced chemiluminescence (ECL) Western-blotting detection system (Amersham Pharmacia Biotech, Buckinghamshire, UK) and by exposure to BioMax film (Eastman Kodak Ltd, Rochester, NY, USA). Bands were quantified with Imagequant software (Fuji), using total protein staining with ponceau S (Serva, Heidelberg, Germany) or Western blotting of β-actin (clone AC-15, Sigma) as a loading control, with NC samples set to a relative value of 1.0.
Immunofluorescence and confocal microscopy
Cells for confocal immunofluorescence microscopy were grown in 4-chamber Labtek slides (Nalge Nunc, Naperville, IL, USA) for 48 hours before they were fixed. Exposure of the cells to ELF-MF for confocal microscopy was always performed in parallel with samples for Northern and Western blot analyses. The medium was removed, and the cells were fixed using precooled acetone–methanol (50:50) at −20°C for 2 minutes for Hsp60 and CD31 observation. After drying, the cells were routinely stored at −20°C before immunofluorescence staining was performed. All subsequent steps were performed at room temperature. The samples were blocked with 1% BSA in PBS for 60 minutes before being incubated with monoclonal antibodies against Hsp60 (clone II-13) or CD31/PECAM-1 as endothelial specific marker (1:20 in PBS, clone JC/70A, Dako) for a further 60 minutes. The cells were then incubated with a fluorescein isothiocyanate (FITC)–labeled rabbit anti-mouse secondary antibody (Dako) for 60 minutes. Cytoskeleton staining was performed using tetramethyl rhodamine iso-thiocyanate (TRITC)–labeled phalloidin (10 μg/mL, Sigma) for 60 minutes. DNA was labeled using TOPRO-3 (Molecular Probes, Eugene, OR, USA) according to the manufacturer's instructions. Optimal working dilutions of all antibodies and conjugates were determined in pilot experiments. Confocal microscopy analysis was performed using a μ-Radiance confocal scanning system (Bio-Rad Laboratories) attached to a Zeiss Axiophot microscope (Carl Zeiss, Göttingen, Germany), using the Laser Sharp 3.1 software supplied by Bio-Rad.
RESULTS
RNA expression levels of hsp60
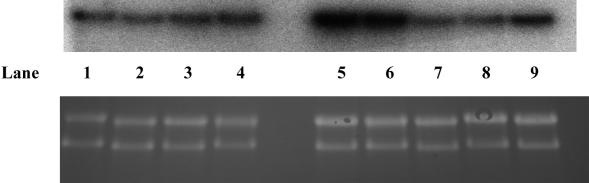
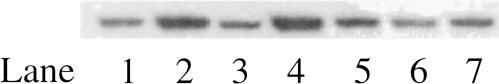
Northern blot analysis of hsp60 revealed no increase in expression after exposure of the cells to 700 μT ELF-MF for either 6 hours or 24 hours compared with the NC (Fig 3). We generally saw a response of ∼2-fold induction 6 hours after heat stress, which then returned to baseline levels after 24 hours and was unaffected by exposure to ELF-MF before or after heat stress. The results of independent experiments are combined in Figure 4. Experiments with high background levels, which did not show at least a 1.5-fold or 2-fold induction of Hsp60 in Western and Northern blots, respectively, in response to the heat shock positive control, were not used for further analysis. Exposure of the cells to heat stress after MF even tended toward a reduced induction of hsp60, compared with heat stress alone, although this was not a consistent result and was not significant (t-test, P = 0.075). The reverse of this regimen, heat stress (42°C, 30 minutes) followed by 700-μT ELF-MF for 24 hours, also failed to reveal a protective or synergistic effect on hsp60 expression levels compared with heat stress alone (42°C, 30 minutes, recovery for 24 hours at 37°C).
Fig 3.
Representative Northern hybridization analysis of the expression of hsp60 messenger ribonucleic acid (mRNA) in human umbilical vein endothelial cells after treatment with heat or magnetic field (MF) stress. (A) Lanes 1 and 2: untreated control cells; lanes 3 and 4: 700μT MF at 50 Hz for 24 hours; lane 5: heat stress (42°C, 30 minutes) followed by 6 hours at 37°C; lane 6: 700 μT MF at 50 Hz for 24 hours followed by heat stress and recovery as in lane 2; lane 7: heat stress (42°C, 30 minutes) followed by 700 μT MF at 50 Hz for 24 hours; lane 8: heat stress (42°C, 30 minutes) followed by 6 hours at 37°C; lane 9: 700 μT MF at 50 Hz for 6 hours only. (B) Ribosomal RNA stained on the membrane with ethidium bromide, which was used to control loading and integrity of the RNA.
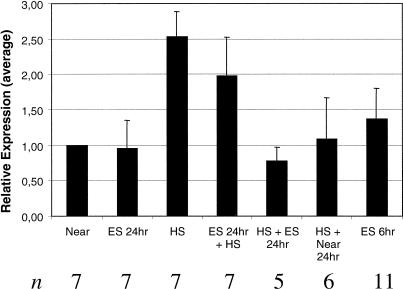
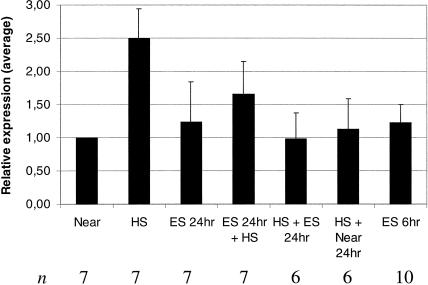
Fig 4.
Response of hsp60 mRNA expression to heat stress, magnetic field (MF) exposure, or combinations from independent experiments. Northern blot analysis was quantified by densitometric analysis, with the negative control being set to a relative value of 1.0, whereas loading was normalized by ethidium bromide staining of ribosomal ribonucleic acid (RNA) on the membrane. Results of independent experiments were combined, with the average response shown graphically. The error bars represent the standard deviation from the mean, and n represents the number of samples that was combined for the analysis. Heat stress alone induced approximately 2-fold induction of hsp60 messenger RNA compared with control cells, whereas pretreatment with 700 μT MF tended to reduce the strength of the induction. Treatment with 700 μT alone for 6 or 24 hours, or heat shock followed by 24 hours recovery in or out of the 700 μT field failed to show an influence on hsp60 expression
Expression levels of Hsp60 protein
The expression of Hsp60 determined by Western blotting generally mirrored that observed for messenger RNA (mRNA) expression (Fig 5). We found no evidence of a consistent effect of exposure to 700 μT ELF-MF for either 6 or 24 hours. Induction of Hsp60 protein after heat shock was inconsistent, and, in general, less than that observed on the RNA level. Because heat shock was a positive control, experiments failing to show at least a 1.5-fold increase in Hsp60 expression in Western blots were not used for further analysis of protein expression levels (Fig 6).
Fig 5.
Expression of Hsp60 protein after subjecting human umbilical venous endothelial cells to heat, magnetic field (MF) stress, or combinations. Typical results observed using Western blot of untreated control cells (lane 1), cells treated by heat stress (42°C, 30 minutes) followed by 6 hours recovery at 37°C (lane 2), MF exposed for 24 hours (700 μT, 50 Hz; lane 3), MF exposed for 24 hours followed by heat stress (and recovery as in lane 2; lane 4), heat stress (42°C, 30 minutes) followed by 700 μT MF at 50 Hz for 24 hours (lane 5), heat stress (42°C, 30 minutes) followed by 6 hours at 37°C (lane 6), 700 μT MF at 50 Hz for 6 hours only (lane 7).
Fig 6.
Average response of Hsp60 protein expression to heat stress and magnetic field (MF) exposure. Western blots were quantified by densitometry, setting the negative control to a relative value of 1.0 to allow comparison and combination of independent experiments. The average expression is shown graphically, whereas the standard deviation from the mean is shown by the error bars, and n is the number of experimental samples used in the analysis. The numbering on the x-axis is identical to the lane numbering in Fig 3. Exposure of the cells to a 700 μT MF for 24 hours or 6 hours, or to heat stress (42°C, 30 minutes) followed by exposure for 24 hours at 37°C either inside or outside a 700 μT field had no effect on the expression level of Hsp60 in comparison with a nonexposed control. Treatment of the cells with heat stress (42°C, 30 minutes) followed by 6 hours of recovery at 37°C, with or without pre-exposure to a 700 μT field, resulted in increased expression of Hsp60. Induction of Hsp60 expression was reduced if cells were pre-exposed to the field, although this was not significant (P = 0.060).
As with the results observed at the transcript level, a slight difference was observed between heat shock alone and pre-exposure of the cells to 700 μT ELF-MFs for 24 hours in the expression of Hsp60. Pre-exposure of the cells to 700 μT led to a reduction of the expression levels of Hsp60 after heat stress, compared with heat stress alone. However, this was not found to be significant (t-test, P = 0.060). Subjecting the cells to heat shock first followed by 700 μT ELF-MF for 24 hours or 24 hours NC revealed no difference in the expressed Hsp60 level.
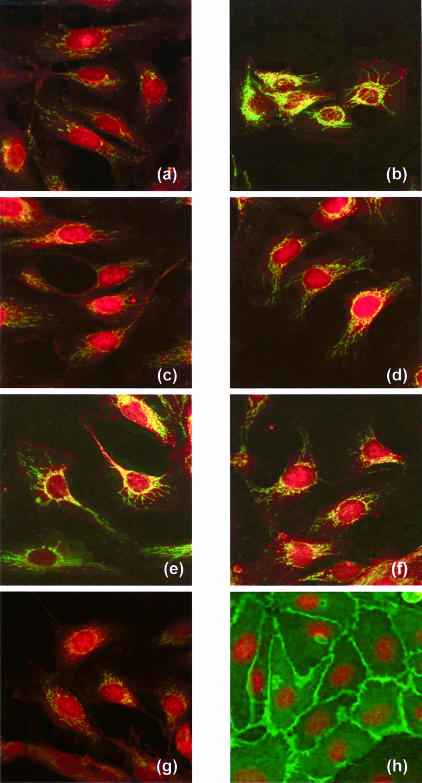
Confocal imaging of Hsp60 expression by immunofluorescence in fixed cells was also performed (Fig 7a–g). Results were in good agreement with those observed for Western and Northern blots, with no change in expression of either Hsps after 700 μT ELF-MF for 6 or 24 hours. Heat-stressed (42°C, 30 minutes) HUVECs displayed a more consistent and higher response in the confocal studies than in Western blot analysis, similar to those observed in Northern blot analysis. No significant effect of pretreatment with 700 μT ELF-MF for 24 hours before heat stress (42°C, 30 minutes) or with heat stress (42°C, 30 minutes) before 24 hours ELF-MF exposure was evident in the confocal microscopy data. Immunofluorescence labeling of the endothelial cell–specific marker CD-31 (PECAM-1) demonstrated the high purity of the HUVEC cell lines used (Fig 7h).
Fig 7.
Confocal microscopic images of human umbilical venous endothelial cells (HUVECs) after their exposure to different potential stress conditions. Cells were fixed and stained for α-actin (red stain by tetramethyl rhodamine iso-thiocyanate [TRITC]-phalloidin, Sigma) and Hsp60 (green stain by fluorescein isothiocyanate [FITC]–labeled Ab II-13) in (a–g). Only a weak expression of Hsp60 was observed in the mitochondria of HUVEC from the “near” control [NC] (a), after 24 hours (c) and 6 hours (g) of exposure to the extremely low–frequency magnetic fields (ELF-MF). Highest expression of Hsp60 was observed after heat stress (b). Moderate expression of Hsp60 was seen after heat stress followed by 24 hours of exposure to ELF-MF (e) or as NC (f), as well as 24 hours of exposure to ELF-MF followed by heat exposure (d). The expression of the FITC-labeled endothelial cell–specific adhesion molecule PECAM-1 (CD31) is shown in green in (h), counterstained by the red nuclei after a DNA stain by TOPRO-3 (Molecular Probes)
DISCUSSION
Increased levels of Hsp60 expressed by endothelial cells have been hypothesized to play an important role in the development of atherosclerotic lesions by the induction of an autoimmune reaction against Hsp60 (Wick et al 1995, 2001). Indeed, there have been reports showing a positive correlation between the levels of antibodies against Hsp60 from various microbial sources in peripheral blood and the incidence of atherosclerosis (Xu et al 1994, 1999, 2000; Mayr et al 2000; Kiechl et al 2001). Among the various stressors that have been reported to influence Hsp expression, several publications have shown that in particular, Hsp70 expression can be increased by exposure of cells to 50-Hz MFs (Goodman and Henderson 1988; Lin et al 1997, 1998; Goodman and Blank 1998; Pipkin et al 1999). Similar data on the influence of MF exposure on Hsp60 levels were not present in the literature. An increase in the expression levels of Hsp60 after exposure of endothelial cells to 50-Hz MF could lead to an increased risk of the development of atherosclerotic lesions according to the autoimmune hypothesis of atherogenesis (Wick et al 1995).
We saw no influence of MF exposure at 700 μT for up to 24 hours on the expression levels of Hsp60 in HUVECs either at the mRNA or at the protein level. Additional studies investigating a combination of MF exposure either before or after heat shock also failed to reveal an effect on transcript levels of hsp60. At the protein level, however, a trend of reduction in Hsp60 expression was found when cells were prestressed with 700 μT for 24 hours followed by heat stress compared with cells treated with heat stress alone, although this was not significant. Hsp 60 expression was not affected if cells were treated with heat stress first followed by MF exposure (700 μT, 24 hours) compared with heat stress alone. In addition, we failed to see any change of expression in Hsp70 protein levels or HSF-1 activation after MF exposure (data not shown).
Because of our own special interest and lack of information on the literature, we concentrated on Hsp60 expression. One of the main differences between this study and earlier reports that have found an effect on Hsp levels is the use of HUVECs, a primary (nontransformed) cell line, and not HL60 (leukemia cell line) cells. It is possible that ELF-MF effects may be cell line specific, and there have been reports that the activation state of the cell could play an important role in any response (Liburdy et al 1993b). The exposure system and conditions were also quite different among the experiments. The intensity of the ELF-MFs used in our system (700 μT) was much greater than had been found previously to have an effect (8 μT). Initial experiments at lower intensities failed to show any effect (data not shown); thus, the intensity was increased to a higher, occupational level of exposure. Interestingly, there have been reports of possible window effects of ELF-MFs, where an increase in Hsp70 expression was observed at 8 μT but not at 100 μT (Goodman and Blank 1998). Also, the timescale of the analysis was different from that used by Goodman and Blank (1998). They claim to have found an increase in expression of Hsp70 levels after a 20-minute exposure to ELF-MF or heat (42°C) followed by only a 20-minute recovery. To ensure that exposure to ELF-MFs did not alter the kinetics of the heat shock response, recovery times from 20 minutes to 6 hours were surveyed (data not shown). At no time did we observe an increase in the heat shock response after exposure of the HUVECs to 700 μT ELF-MF. Other publications have enclosed the entire coil system in a metal container to prevent stray fields from affecting other cells (Goodman and Blank 1998). Attempts to house the negative control in a perforated metal container simply led to extremely high levels of both Hsp60 and Hsp70 being expressed, possibly due to insufficient atmospheric exchange (data not shown). This led to a background exposure of 7 μT to the negative control sample. The dimensions of a metal container sufficient to house the coil, but not to interfere with the field produced, were too large to be practically housed in a standard laboratory incubator. A further difference was that our exposure system had the field running vertical to the plane of the cells and not horizontally (Goodman and Blank 1998), which may be more important with adherent cells such as HUVECs than with cells that grow in suspension.
The high constitutive expression of Hsp60 in cultured HUVECs makes interpretation of results difficult. We were obliged to rule out a number of experiments in the combined analysis, in which the heat-stressed positive control was not sufficiently induced (the cutoff was 2-fold induction at the mRNA level and 1.5-fold at the protein level). We believe this to be due to the inherent stresses of in vitro cell culture, although even the best of conditions are stressful to cells. From our experience, the expression level of Hsp60 in HUVECs is a very sensitive measure of the stress experienced by cultured cells compared with, for example, smooth muscle cells or fibroblasts. Other groups have also reported that heat stress does not always induce Hsp60 expression in HUVECs (Wagner et al 1999). In an attempt to allow the HUVECs to adapt to cell culture conditions, cells were not used for experiments until the fifth passage and were not used past the 10th passage, when they may have lost their endothelial morphology and characteristics (personal communication).
Although 700 μT is a very intense field, it is relevant in an occupational setting and only slightly outside the recommended guideline (ICNIRP 1998). The HUVECs were grown to confluence before exposure to the ELF-MF, so this study cannot rule out the effects on actively proliferating cells.
Our experiments have shown that under the strictly standardized conditions used, endothelial cells do not respond with an altered expression of Hsp60 to MF exposure at 700 μT in any of the visualization techniques (Northern and Western blottings, confocal immunofluorescence) used. The HUVECs did not respond with significantly altered Hsp60 expression if the cells were stressed by heat either before or after exposure to MF. Therefore, exposure to MF is not considered to be a risk factor for atherogenesis.
Acknowledgments
We would like to thank Drs Bauhofer and Wörle for their advice on appropriate exposure conditions. Dr R. Gupta (Hamilton, Ontario, Canada) graciously provided the hybridoma for the monoclonal antibody II-13. This work was supported by a grant from the Organisation of Austrian Electricity Suppliers (Verband der Elektrizitätsunternehmen Österreichs [VEÖ]) and the Austrian Research Fund (project number 14741).
REFERENCES
- Ahlbom IC, Cardis E, Green A, Linet M, Savitz D, and Swerdlow A 2001 Review of the epidemiologic literature on EMF and health. Environ Health Perspect. 109(Suppl 6). 911–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberger A, Maczek C, and Jurgens G. et al. 1997 Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones. 2:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Goodman R. Do electromagnetic fields interact directly with DNA? Bioelectromagnetics. 1997;18:111–115. doi: 10.1002/(sici)1521-186x(1997)18:2<111::aid-bem3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Blank M, Goodman R. Electromagnetic fields may act directly on DNA. J Cell Biochem. 1999;75:369–374. doi: 10.1002/(sici)1097-4644(19991201)75:3<369::aid-jcb2>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- Byus CV, Pieper SE, Adey WR. The effects of low-energy 60-Hz environmental electromagnetic fields upon the growth-related enzyme ornithine decarboxylase. Carcinogenesis. 1987;8:1385–1389. doi: 10.1093/carcin/8.10.1385. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Chow K, Tung WL. Magnetic field exposure enhances DNA repair through the induction of DnaK/J synthesis. FEBS Lett. 2000a;478:133–136. doi: 10.1016/s0014-5793(00)01822-6. [DOI] [PubMed] [Google Scholar]
- Chow KC, Tung WL. Magnetic field exposure stimulates transposition through the induction of DnaK/J synthesis. Biochem Biophys Res Commun. 2000b;270:745–748. doi: 10.1006/bbrc.2000.2496. [DOI] [PubMed] [Google Scholar]
- Deguchi Y, Negoro S, Kishimoto S. Heat-shock protein synthesis by human peripheral mononuclear cells from SLE patients. Biochem Biophys Res Commun. 1987;148:1063–1068. doi: 10.1016/s0006-291x(87)80239-5. [DOI] [PubMed] [Google Scholar]
- DiCarlo AL, Farrell JM, Litovitz TA. A simple experiment to study electromagnetic field effects: protection induced by short-term exposures to 60 Hz magnetic fields. Bioelectromagnetics. 1998;19:498–500. doi: 10.1002/(sici)1521-186x(1998)19:8<498::aid-bem8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, White N, Guo F, Garrett P, Litovitz T. Chronic electromagnetic field exposure decreases Hsp70 levels and lowers cytoprotection. J Cell Biochem. 2002;84:447–454. doi: 10.1002/jcb.10036.abs. [DOI] [PubMed] [Google Scholar]
- Elias D, Markovits D, Reshef T, van der Zee R, Cohen IR. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990;87:1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaghier A, Prantera C, Bothamley G, Wilkins E, Jindal S, Ivanyi J. Disease association of antibodies to human and mycobacterial hsp70 and hsp60 stress proteins. Clin Exp Immunol. 1992;89:305–309. doi: 10.1111/j.1365-2249.1992.tb06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam WZ, Mikhail EL. Lymphoma induced in mice chronically exposed to very strong low-frequency electromagnetic field. Cancer Lett. 1996;105:257–269. doi: 10.1016/0304-3835(96)04324-8. [DOI] [PubMed] [Google Scholar]
- Fernie KJ, Bird DM. Evidence of oxidative stress in American kestrels exposed to electromagnetic fields. Environ Res. 2001;86:198–207. doi: 10.1006/enrs.2001.4263. [DOI] [PubMed] [Google Scholar]
- Feychting M, Ahlbom A. Magnetic fields and cancer in children residing near Swedish high-voltage power lines. Am J Epidemiol. 1993;138:467–481. doi: 10.1093/oxfordjournals.aje.a116881. [DOI] [PubMed] [Google Scholar]
- Fuller KJ, Issels RD, Slosman DO, Guillet JG, Soussi T, Polla BS. Cancer and the heat shock response. Eur J Cancer. 1994;12:1884–1891. doi: 10.1016/0959-8049(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Goodman R, Blank M. Magnetic field stress induces expression of hsp70. Cell Stress Chaperones. 1998;3:79–88. doi: 10.1379/1466-1268(1998)003<0079:mfsieo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Blank M, Lin H, Khorkova O, Soo L, Weisbrot D, Henderson A. Increased levels of Hsp70 transcripts are induced when cells are exposed to low frequency electromagnetic fields. Bioelectrochem Bioenerg. 1994;33:115–120. [Google Scholar]
- Goodman R, Henderson AS. Exposure of salivary gland cells to low-frequency electromagnetic fields alters polypeptide synthesis. Proc Natl Acad Sci U S A. 1988;85:3928–3932. doi: 10.1073/pnas.85.11.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Wei LX, Xu JC, Henderson A. Exposure of human cells to low-frequency electromagnetic fields results in quantitative changes in transcripts. Biochim Biophys Acta. 1989;1009:216–220. doi: 10.1016/0167-4781(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Graham C, Cook MR, Riffle DW. Human melatonin during continuous magnetic field exposure. Bioelectromagnetics. 1997;18:166–171. [PubMed] [Google Scholar]
- Graham C, Cook MR, Riffle DW, Gerkovich MM, Cohen HD. Nocturnal melatonin levels in human volunteers exposed to intermittent 60 Hz magnetic fields. Bioelectromagnetics. 1996;17:263–273. doi: 10.1002/(SICI)1521-186X(1996)17:4<263::AID-BEM2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Han L, Lin H, Head M, Jin M, Blank M, Goodman R. Application of magnetic field-induced heat shock protein 70 for presurgical cytoprotection. J Cell Biochem. 1998;71:577–583. doi: 10.1002/(sici)1097-4644(19981215)71:4<577::aid-jcb12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hochleitner BW, Hochleitner EO, and Obrist P. et al. 2000 Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 20:617–623. [DOI] [PubMed] [Google Scholar]
- ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Nonionizing Radiation Protection. Health Phys. 1998;74:494–522. [PubMed] [Google Scholar]
- Jin M, Blank M, Goodman R. ERK1/2 phosphorylation, induced by electromagnetic fields, diminishes during neoplastic transformation. J Cell Biochem. 2000;78:371–379. doi: 10.1002/1097-4644(20000901)78:3<371::aid-jcb3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Junkersdorf B, Bauer H, Gutzeit HO. Electromagnetic fields enhance the stress response at elevated temperatures in the nematode Caenorhabditis elegans. Bioelectromagnetics. 2000;21:100–106. doi: 10.1002/(sici)1521-186x(200002)21:2<100::aid-bem4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Juutilainen J, Huuskonen H, Komulainen H. Increased resorptions in CBA mice exposed to low-frequency magnetic fields: an attempt to replicate earlier observations. Bioelectromagnetics. 1997;18:410–417. doi: 10.1002/(sici)1521-186x(1997)18:6<410::aid-bem2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Heat shock proteins in health and disease. Int J Clin Lab Res. 1992;21:221–226. doi: 10.1007/BF02591650. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Egger G, and Mayr M. et al. 2001 Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 103:1064–1070. [DOI] [PubMed] [Google Scholar]
- Liburdy RP. Calcium signaling in lymphocytes and ELF fields. Evidence for an electric field metric and a site of interaction involving the calcium ion channel. FEBS Lett. 1992;301:53–59. doi: 10.1016/0014-5793(92)80209-y. [DOI] [PubMed] [Google Scholar]
- Liburdy RP, Callahan DE, Sloma TR, and Yaswen P 1993a Intracellular calcium, calcium transport and cMYC mRNA induction in lymphocytes exposed to 60 Hz magnetic fields: the cell membrane and the signal transduction pathway. In: The Electricity and Magnetism in Biology and Medicine, ed Blank M. San Francisco Press, San Francisco, CA, 311–314. [Google Scholar]
- Liburdy RP, Sloma TR, Sokolic R, Yaswen P. ELF magnetic fields, breast cancer, and melatonin: 60 Hz fields block melatonin's oncostatic action on ER+ breast cancer cell proliferation. J Pineal Res. 1993b;14:89–97. doi: 10.1111/j.1600-079x.1993.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Lin H, Head M, Blank M, Han L, Jin M, Goodman R. Myc-mediated transactivation of Hsp70 expression following exposure to magnetic fields. J Cell Biochem. 1998;69:181–188. doi: 10.1002/(sici)1097-4644(19980501)69:2<181::aid-jcb8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lin H, Opler M, Head M, Blank M, Goodman R. Electromagnetic field exposure induces rapid, transitory heat shock factor activation in human cells. J Cell Biochem. 1997;66:482–488. doi: 10.1002/(sici)1097-4644(19970915)66:4<482::aid-jcb7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Linet MS, Hatch EE, and Kleinerman RA. et al. 1997 Residential exposure to magnetic fields and acute lymphoblastic leukemia in children. N Engl J Med. 337:1–7. [DOI] [PubMed] [Google Scholar]
- Litovitz TA, Krause D, Mullins JM. Effect of coherence time of the applied magnetic field on ornithine decarboxylase activity. Biochem Biophys Res Commun. 1991;178:862–865. doi: 10.1016/0006-291x(91)90970-i. [DOI] [PubMed] [Google Scholar]
- Loscher W, Liburdy RP. Animal and cellular studies on carcinogenic effects of low frequency (50/60-Hz) magnetic fields. Mutat Res. 1998;410:185–220. doi: 10.1016/s1383-5742(97)00039-2. [DOI] [PubMed] [Google Scholar]
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–1566. doi: 10.1161/01.cir.99.12.1560. [DOI] [PubMed] [Google Scholar]
- Mayr M, Keichl S, Willeit J, Wick G, Xu Q. Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobactor pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 adn carotid or femoral atherosclerosis. Circulation. 2000;102:833–839. doi: 10.1161/01.cir.102.8.833. [DOI] [PubMed] [Google Scholar]
- McBride ML, Gallagher RP, and Theriault G. et al. 1999 Power-frequency electric and magnetic fields and risk of childhood leukemia in Canada. Am J Epidemiol. 149:831–842. [DOI] [PubMed] [Google Scholar]
- Mevissen M, Lerchl A, Szamel M, Loscher W. Exposure of DMBA-treated female rats in a 50-Hz, 50 microTesla magnetic field: effects on mammary tumor growth, melatonin levels, and T lymphocyte activation. Carcinogenesis. 1996;17:903–910. doi: 10.1093/carcin/17.5.903. [DOI] [PubMed] [Google Scholar]
- Minota S, Cameron B, Welch WJ, Winfield JB. Autoantibodies to the constitutive 73-kD member of the hsp70 family of heat shock proteins in systemic lupus erythematosus. J Exp Med. 1988a;168:1475–1480. doi: 10.1084/jem.168.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minota S, Koyasu S, Yahara I, Winfield J. Autoantibodies to the heat-shock protein hsp90 in systemic lupus erythematosus. J Clin Invest. 1988b;81:106–109. doi: 10.1172/JCI113280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Heat shock: the role of transient inducible responses in cell damage, transformation, and differentiation. Cancer Cells. 1991;3:295–301. [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Nakasono S, Saiki H. Effect of ELF magnetic fields on protein synthesis in Escherichia coli K12. Radiat Res. 2000;154:208–216. doi: 10.1667/0033-7587(2000)154[0208:eoemfo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Olsen JH, Nielsen A, Schulgen G. Residence near high voltage facilities and risk of cancer in children. BMJ. 1993;307:891–895. doi: 10.1136/bmj.307.6909.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, Haggren W, Thomas WJ, Ishida-Jones T, Adey WR. Magnetic field-induced changes in specific gene transcription. Biochim Biophys Acta. 1992;1132:140–144. doi: 10.1016/0167-4781(92)90004-j. [DOI] [PubMed] [Google Scholar]
- Pipkin JL, Hinson WG, and Young JF. et al. 1999 Induction of stress proteins by electromagnetic fields in cultured HL- 60 cells. Bioelectromagnetics. 20:347–357. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- Reiter RJ 1992 Changes in circadian melatonin synthesis in the pineal gland of animals exposed to extremely low frequency electromagnetic radiation: a summary of observations and speculation on their implications. In: Electromagnetic Fields and Circadian Rhythmicity, ed Moore-Ede MC, Campbell SS, Reiter RJ. Birkhauser, Boston, 13–17. [Google Scholar]
- Res PC, Schaar CG, Breedveld FC, van Eden W, van Embden JD, Cohen IR, de Vries RR. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988;2:478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Salvetti M, Buttinelli C, and Ristori G. et al. 1992 T-lymphocyte reactivity to the recombinant mycobacterial 65- and 70-kDa heat shock proteins in multiple sclerosis. J Autoimmun. 5:691–702. [DOI] [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaoui B, Lambrozo J, Touitou Y. Magnetic fields and pineal function in humans: evaluation of nocturnal acute exposure to extremely low frequency magnetic fields on serum melatonin and urinary 6-sulfatoxymelatonin circadian rhythms. Life Sci. 1996;58:1539–1549. doi: 10.1016/0024-3205(96)00128-2. [DOI] [PubMed] [Google Scholar]
- Selmaoui B, Lambrozo J, Touitou Y. Endocrine functions in young men exposed for one night to a 50-Hz magnetic field. A circadian study of pituitary, thyroid and adrenocortical hormones. Life Sci. 1997;61:473–486. doi: 10.1016/s0024-3205(97)00407-4. [DOI] [PubMed] [Google Scholar]
- Singh B, Gupta RS. Expression of human 60-kD heat shock protein (Hsp60 or P1) in Escherichia coli and the development and characterization of corresponding monoclonal antibodies. DNA Cell Biol. 1992;11:489–496. doi: 10.1089/dna.1992.11.489. [DOI] [PubMed] [Google Scholar]
- Sobel E, Dunn M, Davanipour Z, Qian Z, Chui HC. Elevated risk of Alzheimer's disease among workers with likely electromagnetic field exposure. Neurology. 1996;47:1477–1481. doi: 10.1212/wnl.47.6.1477. [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21:315–320. doi: 10.1006/cbir.1997.0144. [DOI] [PubMed] [Google Scholar]
- Stevens TR, Winrow VR, Blake DR, Rampton DS. Circulating antibodies to heat-shock protein 60 in Crohn's disease and ulcerative colitis. Clin Exp Immunol. 1992;90:271–274. doi: 10.1111/j.1365-2249.1992.tb07941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W, Hogervorst EJ, van der Zee R, van Embden JD, Hensen EJ, Cohen IR. The mycobacterial 65 kD heat-shock protein and autoimmune arthritis. Rheumatol Int. 1989;9:187–191. doi: 10.1007/BF00271878. [DOI] [PubMed] [Google Scholar]
- Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- Walleczek J, Liburdy RP. Nonthermal 60 Hz sinusoidal magnetic-field exposure enhances 45Ca2+ uptake in rat thymocytes: dependence on mitogen activation. FEBS Lett. 1990;271:157–160. doi: 10.1016/0014-5793(90)80396-z. [DOI] [PubMed] [Google Scholar]
- Wei LX, Goodman R, Henderson A. Changes in levels of c-myc and histone H2B following exposure of cells to low-frequency sinusoidal electromagnetic fields: evidence for a window effect. Bioelectromagnetics. 1990;11:269–272. doi: 10.1002/bem.2250110403. [DOI] [PubMed] [Google Scholar]
- Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979;109:273–284. doi: 10.1093/oxfordjournals.aje.a112681. [DOI] [PubMed] [Google Scholar]
- Wick G, Perschinka H, Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 2001;22:665–669. doi: 10.1016/s1471-4906(01)02089-0. [DOI] [PubMed] [Google Scholar]
- Wick G, Schett G, Amberger A, Kleindienst R, Xu Q. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995;16:27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, and Perschinka H. et al. 2000 Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 102:14–20. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, Seitz CS, Hu Y, Gupta RS, Wick G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994;75:1078–1085. doi: 10.1161/01.res.75.6.1078. [DOI] [PubMed] [Google Scholar]
- Yasui M, Kikuchi T, Ogawa M, Otaka Y, Tsuchitani M, Iwata H. Carcinogenicity test of 50 Hz sinusoidal magnetic fields in rats. Bioelectromagnetics. 1997;18:531–540. doi: 10.1002/(sici)1521-186x(1997)18:8<531::aid-bem1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Rott D, Csako G, Wu H, Halcox J, Epstein SE. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation. 2001;103:1071–1075. doi: 10.1161/01.cir.103.8.1071. [DOI] [PubMed] [Google Scholar]