Abstract
The neuroactive steroid (NAS) tetrahydrodeoxycorticosterone (THDOC) increases protein kinase C (PKC) mediated phosphorylation of extrasynaptic GABAA receptor (GABAAR) subunits leading to increased surface expression of α4/β3 subunit-containing extrasynaptic GABAARs, leading to a sustained increase in GABAAR tonic current density. Whether other naturally occurring and synthetic NASs share both an allosteric and metabotropic action on GABAARs is unknown. Here, we examine the allosteric and metabotropic properties of allopregnanolone (ALLO), and synthetic NASs SGE-516 and ganaxolone. ALLO, SGE-516, and ganaxolone all allosterically enhanced prototypical synaptic and extrasynaptic recombinant GABAARs. In dentate gyrus granule cells (DGGCs) all three NASs, when applied acutely, allosterically enhanced tonic and phasic GABAergic currents. In separate experiments, slices were exposed to NASs for 15 min, and then transferred to a steroid naïve recording chamber followed by ≥ 30 min wash before tonic currents were measured. A sustained increase in tonic current was observed following exposure to ALLO, or SGE-516 and was prevented by inhibiting PKC with GF 109203X. No increase in tonic current was observed with exposure to ganaxolone. In agreement with the observations of an increased tonic current, the NASs ALLO and SGE-516 increased the phosphorylation and surface expression of the β3 subunit-containing GABAARs. Our studies demonstrate that neuroactive steroids have differential abilities to induce sustained increases in the efficacy of tonic inhibition by promoting GABAAR phosphorylation and membrane trafficking dependent on PKC activity.
Keywords: GABA receptor, protein kinase C (PKC), Neuroactive steroid, tonic inhibition, dentate gyrus
1. Introduction
γ-Aminobutyric acid type A receptors (GABAARs) mediate both phasic and tonic inhibitory neurotransmission in the CNS and are the sites of action of benzodiazepines, barbiturates, general anesthetics and neuroactive steroids (NAS). GABAARs containing the α4 subunit are located primarily extrasynaptically in the dentate gyrus of the hippocampus, neocortex, striatum and the thalamus where they are persistently activated by low concentrations of GABA. They have distinct pharmacological properties that distinguish them from synaptic GABAARs (Glykys and Mody, 2007). Extrasynaptic GABAARs are responsible for mediating the tonic inhibition that determines the gain and offset of the neuronal output, thus regulating the excitability of neurons and the activity of neuronal circuits (Belelli et al., 2009; Semyanov et al., 2004). In addition to regulating neuronal activity, α4/δ subunit-containing GABAARs are implicated in a multitude of neurological disorders including epilepsy, neurodevelopment disorders and anxiety disorders (Belelli et al., 2009; Brickley and Mody, 2012; D’Hulst and Kooy, 2007).
The endogenous NASs ALLO, and THDOC, and the synthetic NAS ganaxolone, are potent positive allosteric modulators (PAMs) of GABAARs. Consistent with their role as GABAAR PAMs, NASs have dose dependent anxiolytic, anti-convulsant, hypnotic and sedative actions (Mitchell et al., 2008; Paul and Purdy, 1992). The ability of NASs to allosterically potentiate both phasic and tonic inhibition makes them attractive anticonvulsants however, despite these favorable properties the therapeutic potential of NASs is very limited because of their low bioavailability, and rapid clearance preventing the concentration in the brain to reach therapeutic levels (Rupprecht, 2014). Recently, Botella et al. (Botella et al., 2015) developed a series of synthetic NASs with modification that highly improves their pharmacokinetic properties yet retain similar pharmacological profile as ALLO. Among those compounds, SGE-516 was developed with improved bioavailability, and pharmacokinetic properties.
In addition to their actions as PAMs, recent studies have demonstrated that THDOC has a metabotropic mechanism of action on GABAARs. THDOC increase the PKC-dependent phosphorylation of S443 in α4 subunits and S408/9 in β3 subunits. Upon THDOC exposure, enhanced phosphorylation at S443 and S408/9 leads to a sustained increase in GABAAR tonic current density due to increased receptor insertion into the plasma membrane and decreased receptor endocytosis (Abramian et al., 2014; Abramian et al., 2010; Adams et al., 2015). In this study we sought to examine if the PKC-dependent metabotropic pathway previously identified with THDOC exposure is stimulated by another endogenous NAS, ALLO, the first generation synthetic NAS, ganaxolone, and a more recently developed synthetic NAS, SGE-516.
2. Methods
2.1. Recombinant GABAA Pharmacology
Cellular electrophysiology was used to measure the pharmacological properties of ALLO, ganaxolone and SGE-516 in heterologous cell systems. Compounds were tested for their ability to affect GABA mediated currents at a submaximal agonist dose (GABA EC20 = 2μM).
LTK cells were stably transfected with the α1β2γ2 subunits of the GABA receptor and CHO cells are transiently transfected with the α4β3δ subunits via the Lipofecatamine method. Cells were passaged at a confluence of about 50–80% and then seeded onto 35mm sterile culture dishes containing 2 ml culture complete medium without antibiotics or antimycotics. Cells were cultivated at a density that enabled the recording of single cells without visible connections to other cells.
Whole-cell currents were measured with HEKA EPC-10 amplifiers using PatchMaster software. Bath solution for all experiments contained (in mM): NaCl 137, KCl 4, CaCl2 1.8, MgCl2 1, HEPES 10, D-Glucose 10, pH 7.4 with NaOH. Intracellular (pipette) solution contained (in mM): KCl 130, MgCl2 1, Mg-ATP 5, HEPES 10, EGTA 5, pH 7.2 with KOH. During experiments, cells and solutions were maintained at room temperature (19°C–30°C). For manual patch-clamp recordings, cell culture dishes were placed on the dish holder of the microscope and continuously perfused (1 ml/min) with bath solution. After formation of a Gigaohm seal between the patch electrode and the cell (pipette resistance range: 2.5 MΩ–6.0 MΩ; seal resistance range: >1 GΩ) the cell membrane across the pipette tip was ruptured to assure electrical access to the cell interior (whole-cell patch configuration).
Cells were voltage-clamped at a holding potential of −80 mV. GABAA receptors were activated by 2 μM GABA and compounds were sequentially applied at increasing concentrations for 30s prior to a 2s application of GABA. GABA and compounds were applied to cells via the Dynaflow perfusion system (Cellectricon, Sweden). Test compounds were dissolved in DMSO to form 10 mM stock solution and serially diluted to 0.01, 0.1, 1, and 10 μM in bath solution. There was no effect on GABA currents when DMSO was applied to cells at its maximal concentration in solution (0.1%). All concentrations of test compound were tested on each cell. The relative percentage potentiation was defined as the peak amplitude in response to GABA EC20 in the presence of test compound divided by the peak amplitude in response to GABA EC20 alone, multiplied by 100.
2.2. Hippocampal Slice preparation
Brain slices were prepared from 3- to 5-week-old male C57 mice. Mice were anesthetized with isoflurane, decapitated, and brains were rapidly removed and submerged in ice-cold cutting solution containing (mM): 126 NaCl, 2.5 KCl, 0.5 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 1.5 sodium pyruvate, and 3 kynurenic acid. Coronal 310 μm thick slices were cut with the vibratome VT1000S (Leica Microsystems, St Louis, MO, USA). The slices were then transferred into incubation chamber filled with prewarmed (31–32°C) oxygenated artificial cerebro-spinal fluid (ACSF) of the following composition (in mM): 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 1.5 sodium pyruvate, 1 glutamine, 3 kynurenic acid and 0.005 GABA bubbled with 95% O2–5% CO2. Slices were allowed to recover at 32°C for at least 1hr before recording. Exogenous GABA was added in an attempt to standardize ambient GABA in the slice and provide an agonist source for newly inserted extrasynaptic GABAARs.
2.3. Electrophysiology Recordings
After recovery, a single slice was transferred to a submerged, dual perfusion recording chamber (Warner Instruments, Hamden, CT, USA) on the stage of an upright microscope (Nikon FN-1) with a 40× water immersion objective equipped with DIC/IR optics. Slices were maintained at 32°C and gravity-superfused with ACSF solution throughout experimentation and perfused at rate of 2 ml/min with oxygenated (O2/CO2 95/5%) ACSF.
Whole-cell currents were recorded from the dentate gyrus granule cells (DGGCs) in 310-μm-thick coronal hippocampal slices. Patch pipettes (5–7 MΩ) were pulled from borosilicate glass (World Precision Instruments) and filled with intracellular solution of the composition (in mM) as follows: 140 CsCl, 1 MgCl2, 0.1 EGTA, 10 HEPES, 2 Mg-ATP, 4 NaCl and 0.3 Na-GTP (pH = 7.2 with CsOH). A 5 min period for stabilization after obtaining the whole-cell recording conformation (holding potential of −60 mV) was allowed before currents were recorded using an Axopatch 200B amplifier (Molecular Devices), low-pass filtered at 2 kHz, digitized at 20 kHz (Digidata 1440A; Molecular Devices), and stored for off-line analysis.
2.4. Electrophysiology Analysis
For tonic current measurements, an all-points histogram was plotted for a 10 s period before and during 100 μM picrotoxin application, once the response reached a plateau level. Recordings with unstable baselines were discarded. Fitting the histogram with a Gaussian distribution gave the mean baseline current amplitude and the difference between the amplitudes before and during picrotoxin was considered to be the tonic current. The negative section of the all-points histogram which corresponds to the inward IPSCs was not fitted with a Gaussian distribution (Kretschmannova et al., 2013; Nusser and Mody, 2002). Series resistance and whole-cell capacitance were continually monitored and compensated throughout the course of the experiment. Recordings were eliminated from data analysis if series resistance increased by >20%.
Spontaneous inhibitory post-synaptic currents (sIPSCs) were analyzed using the mini-analysis software (version 5.6.4; Synaptosoft, Decatur, GA). Minimum threshold detection was set to 3 times the value of baseline noise signal. To assess sIPSC kinetics, the recording trace was visually inspected and only events with a stable baseline, sharp rising phase, and single peak were used to negate artifacts due to event summation. Only recordings with a minimum of 200 events fitting these criteria were analyzed. sIPSCs amplitude, and frequency from each experimental condition was pooled and expressed as mean ± SEM. To measure sIPSC decay we averaged 100 consecutive events and fitted the decay to a double exponential and took the weighted decay constant (τw). Statistical analysis was performed by using Student t-test (paired and unpaired where appropriate), where p<0.05 is considered significant.
2.5. Metabolic Labeling and Biotinylation
Slices were labeled with 0.5 mCi/mL [32P]orthophosphoric acid for 1–4 hr in phosphate-free DMEM before lysis. The β3 subunit was isolated using immunoprecipitation with β3 antibodies, after correction for protein content and the specific activity of labeling. Results were attained by SDS/PAGE followed by autoradiography (Abramian et al. 2010). For biotinylation experiments hippocampi were dissected out of acute slices from 8–12 week old C57/Bl6 mice and incubated in artificial cerebrospinal fluid (ACSF) described above at 30°C for 1 hr for recovery befor e experimentation. Slices were then placed on ice and incubated for 30 min with 1 mg/mL NHS-SS-biotin (Pierce). Excess biotin was removed by a 50 mM glycine quenching buffer, followed by washing of slices three times in ice-cold ACSF. The tissue was snap frozen on dry ice for 5 minutes, thawed at 4°C and lysed. The lysates were solubilized with 2% Triton at 4°C on a rotating wheel for 1 hr. The insoluble material was removed by centrifugation, and 350–500 μg of protein lysate were incubated with NeutrAvidin beads (Pierce) for 18–24 h at 4°C. Bound material was eluted with sample buffer and subjected to SDS/PAGE and then immunoblotted with the indicated antibodies. Blots were then quantified using the CCD-based ChemiDoc XRS+ system. Antibodies against the β3 subunit and the phospho- β3 antibody were generated and verified by the laboratory of S.J.M. (Vien et al., 2015).
3. Results
3.1. Comparing the effects of endogenous and synthetic neurosteriods on the activity of recombinant GABAARs
ALLO and ganaxolone are known PAMs of both synaptic and extrasynaptic GABAAR-mediated currents. The ability of SGE-516 to act as a PAM was compared to ALLO and ganaxolone using the whole-cell recordings of recombinant human GABAA receptors expressed in mammalian cells. The α1β2γ2 or α4β3δ subunit combinations were chosen as representatives of typical synaptic and extrasynaptic GABAA receptors respectively. Similar to previous reports (Botella et al., 2015), ALLO, ganaxolone and SGE-516 allosterically potentiated currents induced by EC20 concentration of GABA in a concentration-dependent manner in both synaptic- and extrasynaptic-type GABAARs. ALLO potentiated α1β2γ2 receptors with an EC50 of 115 nM and Emax of 229% and potentiated α4β3δ receptors with an EC50 of 57 nM and Emax of 426%. SGE-516 potentiated α1β2γ2 receptors with an EC50 of 61 nM and Emax of 219%. Likewise, SGE-516 potentiated α4β3δ receptors with an EC50 of 193 nM and Emax of 400%. Ganaxolone potentiated α1β2γ2 receptors with an EC50 of 256 nM and Emax of 307% and potentiated α4β3δ receptors with an EC50 of 94 nM and Emax of 225% (Table 1). To confirm the presence of the γ2 subunit in α1β2γ2 receptors, we also checked the effects of diazepam, a well-known benzodiazepine PAM specifically for synaptic GABAARs (data not shown). It was observed that diazepam potentiates the GABA evoked currents in α1β2γ2 receptors with an EC50 of 69 nM and Emax of 184%. In contrast, it did not alter GABA evoked currents in α4β3δ receptors. The results indicate that like ALLO, and ganaxolone, SGE-516 is a potent and efficacious PAM for both synaptic and extrasynaptic type GABAARs.
Table 1.
Properties of neuroactive steroids on recombinant GABAARs.
| Compound | α1β2γ2 | α4β3δ | ||
|---|---|---|---|---|
| EC50 (pEC50 ± S.E.M.) |
Emax ± S.E.M. (%) |
EC50 (pEC50 ± S.E.M.) |
Emax ± S.E.M. (%) |
|
| ALLO | 115 (6.9 ± 0.2) |
229 ± 19 | 57 (7.2 ± 0.3) |
426 ± 42 |
| SGE-516 | 61 (7.2 ± 0.3) |
219± 21 | 193 (6.7 ± 0.1) |
505± 19 |
| Ganaxolone | 256 (6.6 ± 0.2) |
400± 27 | 94 (7.0 ± 0.1) |
225± 8 |
EC50 values are given in nM with −pEC50 ± S.E.M., n=3 for all.
3.2. Acute exposure to NASs allosterically potentiates tonic current in the dentate gyrus granule cells
The results from the above experiments with synaptic-, and extrasynaptic-like GABAARs expressed in HEK293 cells demonstrated the ability of ALLO, SGE-516, and ganaxolone to potentiate sub-maximal GABA-mediated currents. We next examined the ability of acute application of these NASs to allosterically modulate phasic and tonic currents in dentate gyrus granule cells (DGGCs) in hippocampal slices from 3–5 weeks old male C57/Bl6 mice. As would be expected, both ALLO, and SGE-516 modulated the tonic holding current in DGGCs in hippocampal slices (Fig. 1). At 100nM, ALLO modulated the tonic current from 31.8 ± 5.9 pA, to 58.3 ± 13.0 pA, (n=5) and 100nM SGE-516 modulated the tonic current from 34.2 ± 11.1 pA, to 49.2 ± 8.9 (n=7). The only significant modulation was observed with 100 nM ganaxolone which modulated DGGC tonic current from 42.1 ± 17.5 to 78.8 ± 23.2 pA (n=6; p=0.004 paired t-test).
Figure 1. NASs allosterically modulate DGGC tonic currents.
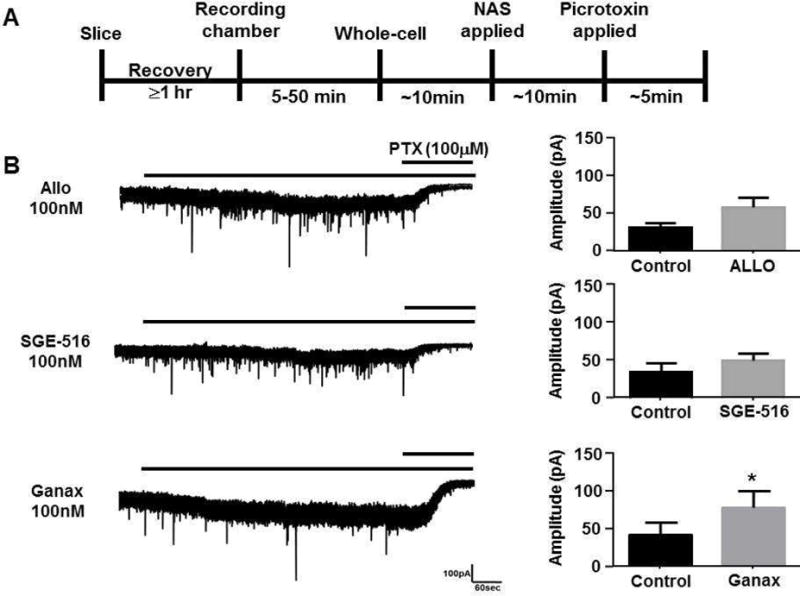
A. Scheme demonstrating experimental protocol. Hippocampal slices from p21–35 (C57/Bl6) mice were allowed to recover for at least 1 hr following slicing. Slices were transferred to the recording chamber of the microscope. After achieving the whole-cell configuration approximately 10 minutes was allowed for membrane currents to stabilize. Hippocampal slices were acutely exposed to 100nM ALLO, SGE-516, or ganaxolone (Ganax) for 10 min followed by 100 μM picrotoxin (PTX). B. Example of current recordings from DGGCs showing modulation of tonic currents by acutely applied NASs (left panel). Bars above current recordings represent application of NAS and picrotoxin. Bar graphs summarizing the average tonic current (mean ± SEM) before and during acute exposure with ALLO, SGE-516, or ganaxolone (right panel). * = significantly different to control (p=0.004; t-test), n=4–12 cells.
3.3. Comparing the acute effects of NASs on phasic currents in DGGCs
We also compared the properties of inhibitory synaptic currents in DGGCs before and during exposure to NASs (Fig. 2). It was observed that there was no significant difference in the mean sIPSC amplitude before and during exposure with 100 nM ALLO (p=0.85, n=5), 100 nM SGE-516 (p=0.46, n=7) and 100 nM ganaxolone (p=0.07, n=6) (Table 2). However, the mean sIPSC decay time significantly increased in the presence of ALLO, SGE-516, and ganaxolone (p=0.03, p=0.01, and p=0.04 respectively, Table 2).
Figure 2. Allosteric modulation of phasic currents by acutely applied NASs.
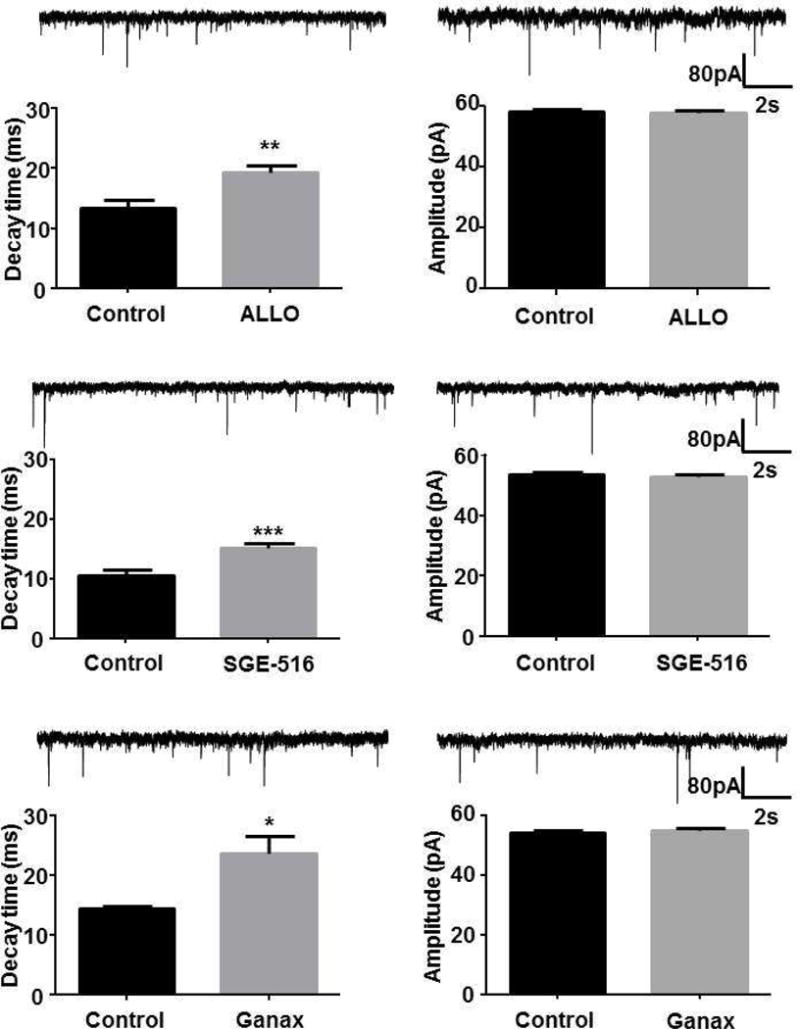
Recordings of sIPSCs from DGGCs before and during acute exposure to 100nM ALLO, 100nM SGE-516, or 100 nM ganaxolone for 10 min. Example of current recordings from DGGCs showing phasic currents before and during acutely applied NASs (left panel). Bar graphs summarizing the effects of acute exposure of ALLO, SGE-516, or ganaxolone on the amplitude and decay of sIPSCs (right panel). *** p=0.01, ** p=0.03, * p=0.04, paired t-test, n=5–7 cells.
Table 2.
Allosteric modulation of DGGC sIPSCs evoked by 100 nM ALLO, SGE-516, and ganaxolone
| sIPSC | Control | ALLO | Control | SGE-516 | Control | Ganax |
|---|---|---|---|---|---|---|
|
Amplitude (pA) |
59.2±0.7 | 59.1±0.6 | 53.7±0.7 | 52.9±0.7 | 54.2±0.6 | 55.1±0.7 |
|
Frequency (Hz) |
4.5±0.9 | 3.4±1.0 | 4.1±0.4 | 4.6±0.6 | 6.4±1.3 | 5.6±1.7 |
|
Decay (ms) |
13.4±1.3 | 19.2±1.3** | 10.5±1.1 | 15.1±0.8*** | 14.4±0.5 | 23.6±2.9* |
Data is mean SEM, n=5 to 7 neurons.
p=0.01
p=0.03
p=0.04
3.4. Exposure to NASs metabotropically enhances tonic current in DGGCs
Recently we have shown that, in addition to the allosteric modulation of GABAA receptors, THDOC, exert sustained effects on GABAergic tonic current by enhancing the PKC-dependent phosphorylation of the α4 and β3 subunits, leading to enhanced insertion and stability of GABAARs into the membrane and a long lasting increase in tonic current (Abramian et al., 2014; Abramian et al., 2010).
Here, we analyzed the sustained effects of ALLO, or the new synthetic NAS SGE-516 on the tonic current in DGGCs in hippocampal slices from 3–5 week old C57 male mice. Hippocampal slices were incubated for 15 min in a chamber containing NASs dissolved in ACSF. Slices were then transferred to the recording chamber of the microscope followed by a wash period between 30 to 60 mins of continuous perfusion of NAS-free ACSF before recordings were started (Fig. 3A).
Figure 3. NAS-mediated metabotropic enhancement of tonic inhibitory current in DGGC neurons.
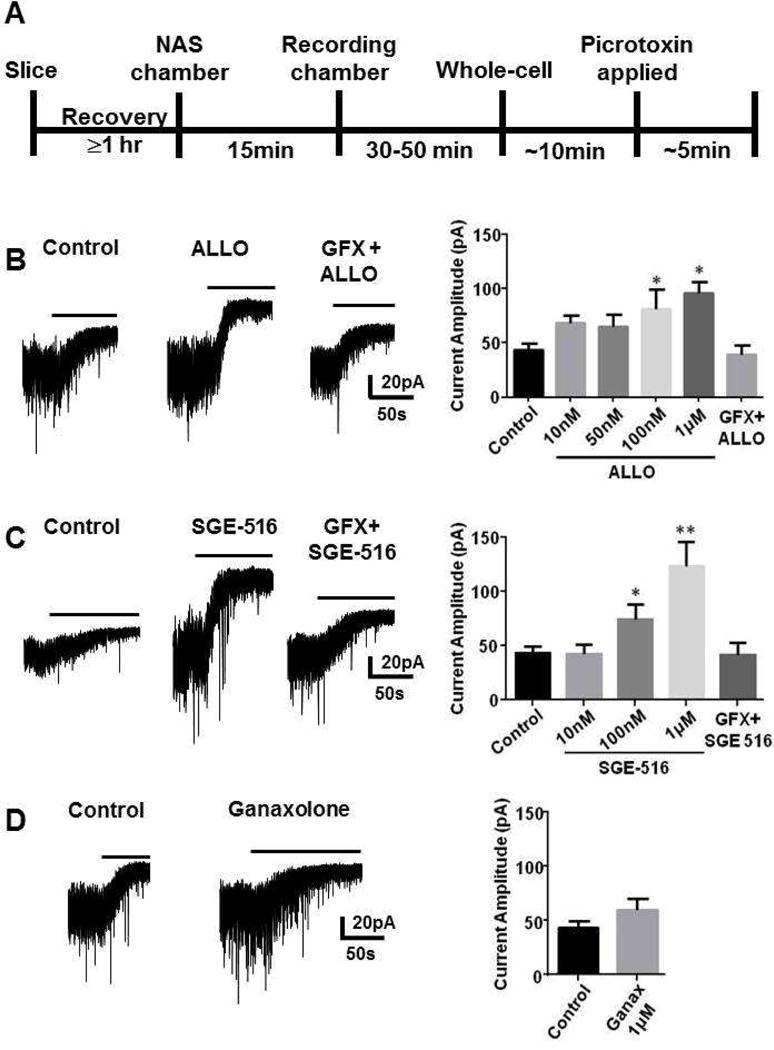
A. Scheme demonstrating experimental protocol. Hippocampal slices were allowed to recover for at least 1 hr following slicing. Slices were then incubated for 15 min in a chamber containing NASs dissolved in ACSF. Slices were then transferred to the recording chamber of the microscope followed by a wash period between 30 to 60 min of continuous perfusion of NAS-free ACSF before recordings were started. Recordings were made from DGGCS in hippocampal slices from p21–35 C57 mice in the presence of 5 μM GABA followed by 100 μM picrotoxin and the difference in holding current was then determined. Left panel B, C, D. Example tonic currents from slices following exposures to vehicle (control) or 100 nM ALLO (B), 100 nM SGE-516 (C), or 1 μM ganaxolone (D) for 15 min. No change in tonic current was observed in slices pre-incubated for 15 min with GFX followed by ALLO, or SGE-516. Bar above current represents application of picrotoxin (100 μM). Right panel B, C, D. Bar graph shows average tonic current was significantly enhanced following exposure to different concentrations of ALLO and SGE-516. No significant change in tonic current was observed following exposure to 1 μM ganaxolone for 15 min. In all panels * = significantly different to control (p<0.05; un-paired t-test, n=4–12 cells).
Slices exposed to ALLO, or SGE-516 demonstrated a concentration-dependent increase in the tonic current measured by the addition of picrotoxin with the maximal effects at 1 μM. Control, vehicle-treated slices had a tonic current of 43.9 ± 5.7 pA (n=12), whereas the tonic currents for slices treated with SGE-516 (1 μM) was 123.0 ± 22.2 pA, (n=6, p= 0.0003), or ALLO (1 μM) was 95.8 ± 10.8 pA (n=4, p= 0.0005, Fig. 3B&C).
In addition, we also examined if the synthetic NAS, ganaxolone, also had a metabotropic effect following 15 min incubation. In contrast to the naturally occurring NASs THDOC, and ALLO, and the synthetic NAS, SGE-516, ganaxolone (1 μM) did not significantly alter the magnitude of tonic current in DGGCs (57.4 ± 6.3 pA, n=7, p=0.14, Fig. 3D).
To assess if the effects of NASs are dependent upon PKC, hippocampal slices were treated with the established PKC inhibitor GF 109203X (GFX 50 μM) for 15 min followed by co-exposure of to ALLO, or SGE-516 and GFX for 15 mins. When tonic current was measured following ≥30 min washout, there was no significant difference to the tonic current measured in ALLO/GFX, or SGE-516/GFX treated slices with vehicle treated slices (Fig. 3).
We measured the tonic current by blocking extrasynaptic GABAARs with picrotoxin. Because picrotoxin also inhibits glycine receptors we examined if glycine receptors contributed to tonic current by using the specific glycine receptor inhibitor, strychnine. There was no difference in tonic current measured under control conditions in the absence or presence of strychnine (100nM). Similarly, there was no difference in the increase in tonic current following a 15 minute exposure to 100 nM ALLO in the absence or presence of strychnine (Fig. 4). These results suggest that glycine receptors have an undetected contribution to tonic current in DGGCs and that the metabotropic increase in tonic current by NAS exposure do not involve glycine receptors.
Figure 4. Glycine receptors do not contribute to tonic current in DGGCs.
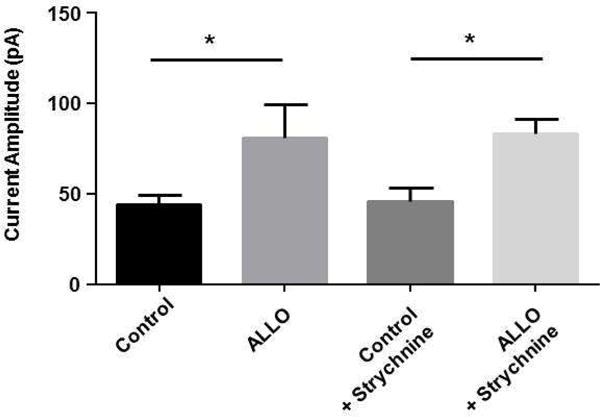
Hippocampal slices were incubated for 15 min with 100 nM ALLO or vehicle dissolved in ACSF then transferred to the recording chamber and washed for 30 to 60 min with NAS-free ACSF before recordings were started. Tonic current was measured by applying 100 μM picrotoxin in the absence or presence of the glycine receptor, strychnine (100 nM). Exposure to ALLO caused a significant increase in tonic current. Addition of strychnine did not alter the tonic current measured with picrotoxin. * p=0.01, unpaired t-test, n=4–12 neurons).
Collectively, these results suggest that the exposure of hippocampal slices to SGE-516 and ALLO has a strong sustained metabotropic effects on tonic current. In contrast, ganaxolone produce major effects via allosteric mechanism as compared to metabotropic effects.
3.5. The metabotropic effects of NASs on phasic current in DGGCs
In parallel with our measurements on tonic current we assessed the effects of prior exposure of NASs on sIPSC properties. The amplitude of sIPSCs was significantly increased after incubation with 100 nM SGE-516 compared to vehicle control (control 39 ± 0.9 pA, n=12; SGE-516 58 ± 1.9 pA, n=6, p=0.0001). This increase in sIPSC amplitude following exposure to SGE-516 was significantly reduced following co-exposure with SGE-516 and GFX (Fig. 5C). No change in sIPSC amplitude was observed following exposure with 100 nM ALLO or 1 μM ganaxolone (Fig. 5B&D). In contrast to the changes observed with the allosteric modulation of IPSC decay time, no metabotropic-mediated changes in sIPSC decay time was observed following exposure to ALLO, SGE-516, or ganaxolone (Fig. 5).
Figure 5. sIPSC amplitude and decay was largely uncharged following exposure to NASs.
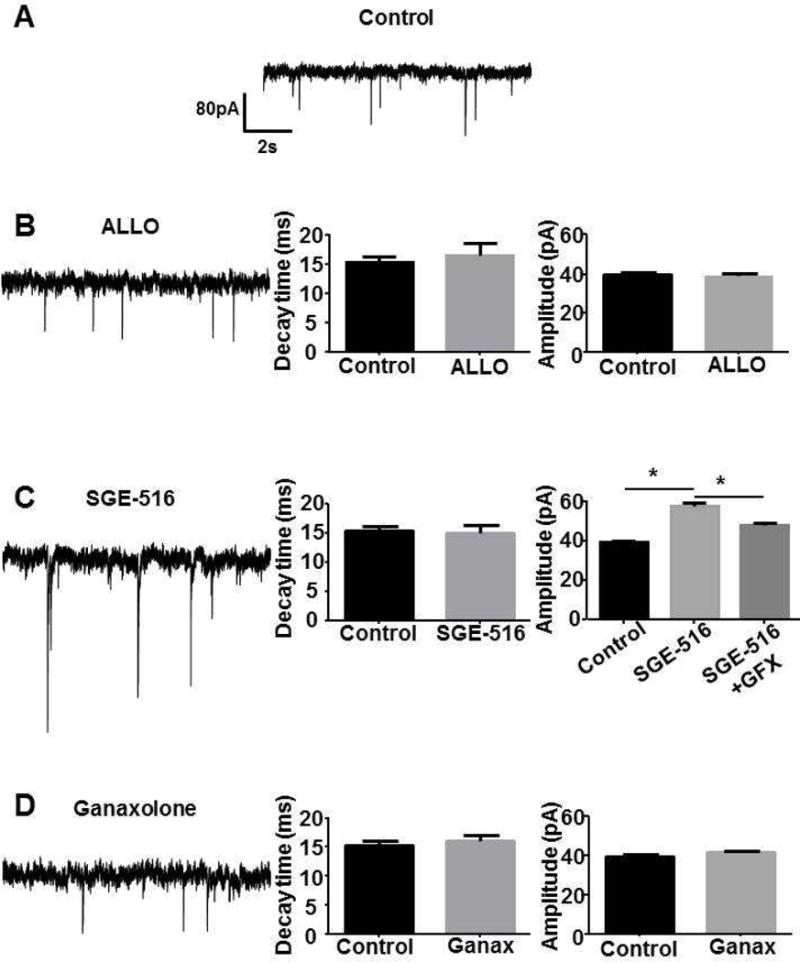
A. IPSC recordings were made from DGGCS in hippocampal slices from p21–35 C57 mice. The slices were exposed to vehicle (control, n=6 neurons from 3 mice), B. 100 nM ALLO (n=4 neurons from, 2 mice), C. 100 nM SGE-516 (n=5 neurons from 2 mice), or D. 1 μM ganaxolone (n=5 neurons from 2 mice) for 15 min and then washed for >30 min prior to measurement of sIPSCs. Bar graphs shows average sIPSC decay and amplitude. Only sIPSC amplitude was significantly enhanced following exposure to 100nM SGE-516 but GFX (n=5 neurons from 2 mice) significantly reduced SGE-516 enhancement. * = significantly different to control (p<0.05; unpaired t-test).
3.6. SGE-516 and ALLO increase the phosphorylation and cell surface stability of GABAARs
We have previously shown that the naturally occurring NAS, THDOC, increased tonic current in part through phosphorylation of S408/409 of the β3 subunit (Abramian et al., 2014; Vien et al., 2015). To further our understanding of the NAS-mediated increase in tonic current we measured the effects of ALLO, SGE-516, on the phosphorylation and membrane expression of β3 subunits in hippocampal slices. Twenty min exposure to 100nM ALLO, or SGE-516 increased phosphorylation of β3 subunits at S408/409 to 141 ± 10% of control (n=10, p<0.01), and 143 ± 14% of control (n=4, p<0.05) respectively (Fig. 6A&B).
Figure 6. NAS exposure increases phosphorylation and surface expression of β3 subunits.
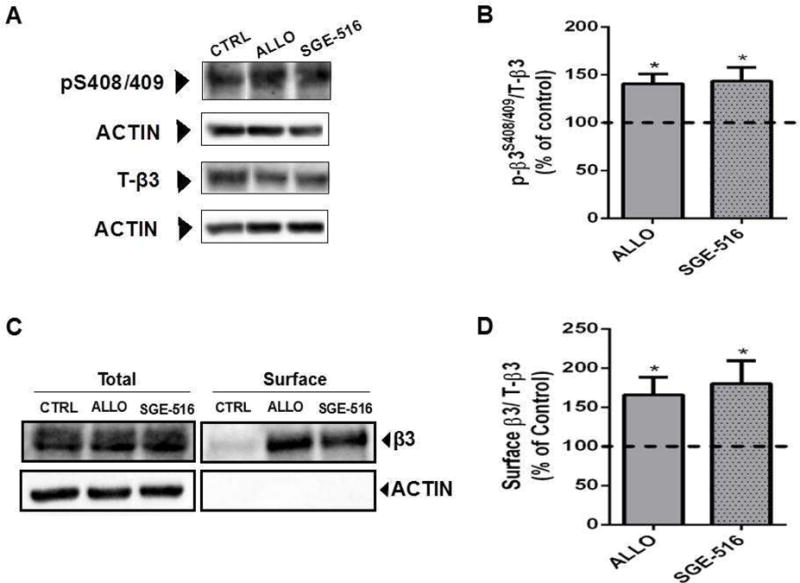
A. Exposure to 100 nM of the NASs, ALLO or SGE-516, for 20 min increases β3 S408/409 phosphorylation in acute hippocampal slices. B. The ratio of p-β3/T-β3 was measured and values were normalized to those in control (100%). Asterisks represent a significant difference from control (ALLO: p <0.01, Student’s t test, n=10 slices, from 10 mice; SGE-516: p<0.05, Student’s t test, n=4 slices, from 4 mice). C. Exposure to 100 nM ALLO or SGE-516 for 20 min increases GABAA-β3–containing receptors at the plasma membrane in acute hippocampal slices. D. The ratio of surface β3/T-β3 was measured and values were normalized to cell surface levels in control treated slices (100%). Asterisks represent a significant difference from control (ALLO: p <0.05, Student’s t test, n=8 slices; SGE-516: p<0.05, Student’s t test, n=4 slices).
In parallel with modulating phosphorylation, exposure to 100 nM ALLO, or SGE-516 increased the cell surface expression levels of receptors containing β3 subunits to 166 ± 22% of control (n=8, p<0.05), and 180 ± 29% of control (n=4, p<0.05) respectively (Fig. 6C&D). Therefore, in parallel with inducing sustained effects on GABAergic inhibition ALLO and SGE-516 enhance the cell surface levels and phosphorylation of the GABAARs containing β3 subunits dependent upon PKC activity.
4. Discussion
We have previously shown that the endogenous NAS, THDOC, exerts a sustained increase on GABAergic tonic current in cultured neurons. The THDOC-mediated increase in α4/β3 subunit-mediated current is independent of the allosteric potentiation typically described of NASs. Rather, we described a metabotropic signaling mechanism by which THDOC induced a PKC-mediated phosphorylation of α4 and β3 subunits with a subsequent increase in the insertion of GABAARs, and a reduction in their endocytosis (Abramian et al., 2014; Abramian et al., 2010; Adams et al., 2015). Here we have assessed this putative regulatory mechanism for other endogenous and synthetic NASs in the dentate gyrus.
To do so, hippocampal slices were exposed to NAS for 15 min followed by ≥30 min wash in a separate recording chamber before the start of the experiment. Both ALLO and SGE-516 significantly enhanced the tonic current compared to vehicle treated slices via a metabotropic mechanism. This is in contrast with the non-significant allosteric enhancement of tonic current we observed with ALLO and SGE-516. However, we also examined the first generation synthetic NAS, ganaxolone, and although it significantly produced an allosteric potentiated tonic current it failed to metabotropically modulate tonic current. This result demonstrates that the metabotropic pathway that increases the PKC-mediated phosphorylation of α4 and β3 subunits can be stimulated by both endogenous and synthetic NASs but is not universally stimulated by all steroids.
Ganaxolone, ALLO, and SGE-516 all allosterically prolonged the decay of sIPSCs, and allosterically potentiated recombinant GABAARs demonstrating their recognized role as GABAARs PAMs. However, in our metabotropic studies only SGE-516 significantly altered sIPSC properties, with an increase in sIPSC amplitude. Our previous data has demonstrated the link between PKC-mediated phosphorylation of β3 subunits with a subsequent reduction in GABAAR endocytosis and an increase in IPSC amplitude (Jovanovic et al., 2004; Kittler et al., 2005; Vien et al., 2015).
In DGGCs, from mice that are 3 weeks and older the predominant extrasynaptic combination is thought to be α4βnδ or even just α4βn (Bencsits et al., 1999; Chandra et al., 2006; Mortensen and Smart, 2006). Extrasynaptic GABAARs in hippocampal CA1 pyramidal neurons have the α5 subunit whereas, extrasynaptic GABAARs in hippocampal interneurons are comprised of α1βnδ subunits (Caraiscos et al., 2004; Glykys et al., 2007). We have previously shown that with NAS exposure, α5 and α1 subunits are not phosphorylated nor is there an increase in α5 and α1 subunit trafficking to the membrane surface (Abramian et al., 2014). We have only observed the NAS-evoked PKC-dependent increase in tonic current through the phosphorylation of α4 and β3 subunits and this phosphorylation occurs independently of the presence of δ subunits (Abramian et al., 2014), suggesting a brain region selective change in extrasynaptic GABAAR trafficking. Extrasynaptic GABAARs in dentate gyrus granule cells have been suggested to be a mix of α4β2δ and α4β3δ subunits (Herd et al., 2008). GABAARs comprised of α4β2δ have been shown to undergo increased endocytosis upon PKC phosphorylation of β2 S410 (Bright and Smart, 2013). A hypothetical consequence of NAS-mediated increase in PKC phosphorylation of GABAA subunits would be the predominance of extrasynaptic GABAARs comprised of α4β3δ subunits.
Reduced expression of extrasynaptic GABAARs has been demonstrated in human and rodent models of postpartum depression, schizophrenia, epilepsies, Angelman, Rett, and Fragile X syndromes (Curia et al., 2009; D’Hulst and Kooy, 2007; Gantois et al., 2006; Maguire and Mody, 2008; Maldonado-Aviles et al., 2009). We have previously described a decrease in phosphorylation of residues S408/409 of the β3 subunit following status epilepticus leading to an increase in GABAAR endocytosis and increased excitation (Terunuma et al., 2008). Thus, by increasing the phosphorylation of the β3 subunit by exposure to NAS the expression and trafficking of these extrasynaptic GABAAR subunits could be manipulated and may provide novel therapeutic areas for these difficult to treat disorders.
The NAS-mediated increase in phosphorylation and surface levels of extrasynaptic GABAARs leading to an increase in tonic current is a further mechanism by which NASs can increase the inhibitory tone in the brain in addition to the more commonly described allosteric effect. While the allosteric modulation only exists while the NAS is present, the PKC-mediated metabotropic enhancement can cause a prolonged increase in inhibitory tone. We have noted before that other positive allosteric modulators such as the intravenous general anesthetic, propofol, do not have the ability to metabotropically enhance extrasynaptic GABAARs (Abramian et al., 2014). Here, we have demonstrated that not all steroidal modulators (ganaxolone) have the ability to increase tonic current through this metabotropic mechanism either. However, the metabotropic pathway seems to be activated by various NAS including endogenous THDOC, and ALLO and the synthetic NAS, SGE-516. The activation of this pathway increases phosphorylation of the β3 subunit, leading to increased surface expression of the receptor and an increase in tonic current. All of which leads to an interesting area of potential therapeutic targets aimed at modulating the trafficking of a particularly important subset of GABAARs.
Highlights.
Short exposure to ALLO or SGE-516 produces long lasting enhancement of tonic current.
Exposure to ganaxolone failed to produce long term enhancement of tonic current.
Enhancement was dependent upon PKC.
Exposure to ALLO or SGE-516 increased phosphorylation of β3 subunits.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH)-National Institute of Alcoholism and Alcohol Abuse grant AA017938 (P.A.D), NIH-National Institute of Mental Health grant, MH097446, and DOD, AR140209 (PAD & SJM), NIH-National Institute of Neurological Disorders and Stroke grant NS051195, NS056359, NS081735, NS080064, NS087662 (SJM), the Simons Foundation #206026 (S.J.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SJM serves as a consultant for SAGE therapeutics and AstraZeneca, relationships that are regulated by Tufts University and do not impact on this study. JJD and MAA are employees of SAGE therapeutics.
Author Contributions
AM performed and analysed the patch clamp experiments and drafted the paper, MLP performed phosphorylation and biotinylation experiments and drafted the paper, MAA conceived and coordinated the study, JJD conceived and coordinated the study, SJM conceived and coordinated the study, and wrote the paper PAD conceived and coordinated the study and wrote the paper.
References
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Thomas P, Smart TG. Modulation of neurosteroid potentiation by protein kinases at synaptic- and extrasynaptic-type GABAA receptors. Neuropharmacology. 2015;88:63–73. doi: 10.1016/j.neuropharm.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native gamma-aminobutyric AcidA receptors containing alpha4 subunits do not contain gamma or delta subunits. J Biol Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- Botella GM, Salituro FG, Harrison BL, Beresis RT, Bai Z, Shen KS, Belfort GM, Loya CM, Ackley MA, Grossman SJ, Hoffmann E, Jia SL, Wang JM, Doherty JJ, Robichaud AJ. Neuroactive Steroids. 1. Positive Allosteric Modulators of the (gamma-Aminobutyric Acid)(A) Receptor: Structure-Activity Relationships of Heterocyclic Substitution at C-21. J Med Chem. 2015;58:3500–3511. doi: 10.1021/acs.jmedchem.5b00032. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Smart TG. Protein kinase C regulates tonic GABA(A) receptor-mediated inhibition in the hippocampus and thalamus. Eur J Neurosci. 2013;38:3408–3423. doi: 10.1111/ejn.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30:425–431. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D’Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABA(A) receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis. 2006;21:346–357. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmannova K, Hines RM, Revilla-Sanchez R, Terunuma M, Tretter V, Jurd R, Kelz MB, Moss SJ, Davies PA. Enhanced tonic inhibition influences the hypnotic and amnestic actions of the intravenous anesthetics etomidate and propofol. J Neurosci. 2013;33:7264–7273. doi: 10.1523/JNEUROSCI.5475-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O’Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Rupprecht R. New perspectives in neurosteroid action: open questions for future research. Front Cell Neurosci. 2014;8:268. doi: 10.3389/fncel.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vien TN, Modgil A, Abramian AM, Jurd R, Walker J, Brandon NJ, Terunuma M, Rudolph U, Maguire J, Davies PA, Moss SJ. Compromising the phosphodependent regulation of the GABAAR beta3 subunit reproduces the core phenotypes of autism spectrum disorders. Proc Natl Acad Sci U S A. 2015;112:14805–14810. doi: 10.1073/pnas.1514657112. [DOI] [PMC free article] [PubMed] [Google Scholar]


