Abstract
INTRODUCTION
Tau protein levels in plasma may be a marker of neuronal damage. We examined associations between plasma tau levels and Alzheimer's Disease (AD)-related MRI and PET neuroimaging measures among non-demented individuals.
METHODS
Participants included 378 cognitively normal (CN) and 161 Mild Cognitive Impairment (MCI) individuals enrolled in the Mayo Clinic Study of Aging with concurrent neuropsychological measures and amyloid PET, FDG-PET, and MRI imaging. Baseline plasma tau levels were measured using the Quanterix Simoa-HD1 tau assay.
RESULTS
Plasma tau levels were higher in MCI compared to CN (4.34 vs. 4.14 pg/mL, P=0.078). In regression models adjusted for age, sex, education, and APOE, higher plasma tau was associated with worse memory performance (b=−0.30, P=0.02) and abnormal cortical thickness in an AD signature region (OR=1.80, P=0.018).
DISCUSSION
Plasma tau is associated with cortical thickness and memory performance. Longitudinal studies will better elucidate the associations between plasma tau, neurodegeneration, and cognition.
Keywords: plasma tau, amyloid, cortical thickness, MCI, cognition, memory, MRI
1. Introduction
Several recent large Alzheimer's disease (AD) clinical trials targeting amyloid in mild or moderate AD have failed to show efficacy. These results have led to the hypothesis that treating individuals earlier in the disease course may be more effective at slowing or stopping disease progression. However, identifying asymptomatic individuals in the pre-clinical or early mild cognitive impairment (MCI) stages of AD dementia for inclusion in clinical trials is difficult. Current non-invasive and inexpensive biomarkers have not been sufficiently sensitive to identify which patients are at greatest risk of progressing to MCI and AD dementia in the shortest period of time. For example, in the asymptomatic AD population imaging and cerebral spinal fluid (CSF) biomarkers have shown the most promise, but are yet insufficient to identify who will progress to MCI over a time frame amenable to an affordable clinical trial.
CSF levels of tau and phosphorylated (p)-tau are associated with the pathophysiology of AD and have been extensively shown to have utility in identifying patients at risk for progression to AD. However, CSF markers have limited application in large studies involving elderly patients due to associated costs, invasiveness of the procedure, and the common side effect of positional headache. For these reasons, blood-based biomarkers would be more amenable to large-scale screening to identify which subjects are at highest risk of progressing from cognitively normal to MCI or AD dementia.
Recent advances in the development of sensitive immunoassays have made it possible to detect tau in plasma. These methods have been used to investigate neurological damage from sports related injury and hypoxic brain injury after cardiac arrest [1,2]. Through these and other studies, the Zetterberg lab has demonstrated that acute brain injury can result in an increase in plasma tau levels, which do not always return back to baseline levels and may be associated with long term neurological damage [1,2]. In another study exploring the utility of plasma tau as diagnostic markers for MCI and AD, Zetterberg et al., reported that plasma tau levels were significantly elevated in AD, but not in MCI, compared to cognitively normal subjects [3]. Since there was significant overlap between the diagnostic groups, it was concluded that cross-sectional measurement of plasma tau would not be useful for AD diagnosis [3]. In an effort to replicate the initial findings by Zetterberg et al., and extend the assessment of plasma tau as a potential biomarker for preclinical and prodromal AD, we investigated plasma tau levels in cognitively normal and MCI subjects enrolled in the population-based Mayo Clinic Study of Aging (MCSA) [4,5]. We also examined the associations between plasma tau and neuroimaging measures of amyloid and neurodegeneration in AD-specific regions.
2. Methods
2.1. Participants
The present study was conducted with data from the MCSA, a prospective population-based study that began in 2004 with the primary goal of determining the incidence and prevalence of MCI in Olmsted County, Minnesota [4]. The study initially recruited Olmsted County residents between the ages of 70 and 89 using an age- and sex-stratified random sampling design to ensure that men and women were equally represented in each 5-year age strata. Since 2004, the population has been re-enumerated several times and has been extended to cover the ages of 50–90+ following the same sampling strategy. Subjects randomly chosen for recruitment were invited to participate in the MCSA and those without a medical contraindication (e.g., pacemaker) were invited to participate in imaging studies. The present analyses included 539 participants, aged 56–95, who had plasma tau measures, neuroimaging (amyloid positron emission tomography (PET), fluorodeoxyglucose-PET (FDG-PET), and magnetic resonance imaging (MRI)) and/or cognitive testing at the same study visit.
The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent.
2.2. Participant assessment
MCSA visits included a physician examination, an interview by a study coordinator, and neuropsychological testing administered by a psychometrist [4]. Physician examinations included a review of the participant's medical history, a complete neurological examination, and administration of the Short Test of Mental Status [6]. Study coordinator interviews reviewed participant demographic information, medical history, and completion of the participant and informant Clinical Dementia Rating scale [7].
The neuropsychological battery included nine tests covering four domains: 1) memory (Auditory Verbal Learning Test Delayed Recall Trial [8]; Wechsler Memory Scale-Revised Logical Memory II & Visual Reproduction II [9]); 2) language (Boston Naming Test [10] and Category Fluency [11]); 3) executive function (Trail Making Test B [12] and WAIS-R Digit Symbol subtest [13]); and 4) visuospatial skills (WAIS-R Picture Completion and Block Design subtests) [13]. Using the mean and standard deviation (SD) from the MCSA 2004 enrollment cohort, which excluded subjects with dementia, participant test scores were converted to z-scores. Global cognition was calculated using the z-transformed averages of the four other domains.
2.3. MCI diagnostic determination
For each participant, performance in a cognitive domain was compared with the age-adjusted scores of cognitively normal individuals previously obtained using Mayo's Older American Normative Studies [14]. This approach relies on prior normative work and extensive experience with the measurement of cognitive abilities in an independent sample of subjects from the same population. Subjects with scores more than 1.5 SD below the age-specific mean in the general population were considered for possible cognitive impairment. A final decision about impairment in a cognitive domain was made after taking into account education, prior occupation, visual or hearing deficits, and reviewing all other participant information. The diagnosis of MCI was made by a consensus agreement between the study coordinator, examining physician, and neuropsychologist using published criteria [4]. Individuals who performed in the normal range and did not meet criteria for MCI or dementia were deemed cognitively normal.
2.4. Plasma tau enzyme-linked immunosorbent assay (ELISA) of MCSA samples
Participants' blood was collected in-clinic after an overnight fast. The blood was centrifuged, aliquoted, and stored at −80°C. Plasma tau was meas ured on the Quanterix Simoa-HD1 Platform. hTau kits (101444) were purchased from Quanterix and used according to the kit protocol with minor modifications. The capture antibody recognizes amino acid 16–24 and the detector antibody recognizes amino acid 218–222. Briefly, plasma samples were analyzed at a 1/8 dilution and in triplicate across three identically prepared sample plates. Standard curves and individual sample measurements were calculated using Excel 2010 and Graph Pad Prism (version 6). Each batch was analyzed using six standard replicate curves and applying a four parameter logistic fit with 1/Y2 weighting. The reported sample values represented the mean of three replicate measures with an overall CV of 10.5% across all batches. During data analysis, a small number (<2 %) of individual measurements did not result in experimental values or were deemed outliers due to incomplete washing, and were excluded from calculation of mean results. Both of these reasons for excluding one of three replicates were due to known instrument malfunctions and never resulted in failure to report a subject's mean value. In all cases at least two replicate values were available to calculate a mean result for each subject.
2.5. Imaging methods
Amyloid PET imaging was performed with Pittsburgh Compound B (PiB) [15], and FDG-PET was obtained on the same day. Participants also completed computed tomography (CT) at that time for attenuation correction. Amyloid PET images were acquired from 40–60 minutes and FDG from 30–50 minutes after injection. Amyloid PET and FDG-PET were analyzed with our in-house fully automated image processing pipeline [16], where image voxel values are extracted from automatically labeled regions of interest (ROIs) propagated from an MRI template. An amyloid PET standardized uptake value ratio (SUVR) was formed from the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus ROIs normalized to uptake in cerebellar grey matter. The data was partial volume corrected for voxel CSF content using segmented coregistered MRI. An AD-characteristic FDG-PET SUVR was formed from the angular gyrus, posterior cingulate, and inferior temporal cortical ROIs normalized to pons and vermis [17]. FDG-PET data were not partial volume corrected (in our experience doing so eliminates a substantial portion of the apparent biological signal) [18–20].
All MRI scans were completed on one of three 3T machines from the same vendor, and cortical surface was parcellated using FreeSurfer version 5.3.0 (https://surfer.nmr.mgh.harvard.edu/). Hippocampal volume (HVa) was adjusted for total intracranial volume (TIV), using our in-house fully automated imaging processing pipeline [16]. An AD-signature cortical thickness measure was composed of the following individual cortical thickness ROIs: entorhinal, inferior temporal, middle temporal, and fusiform.
2.6. Definition of elevated amyloid PET and abnormal HVa
We set the cut point for elevated PiB PET and MRI measures using the 90th percentile from a sample of 75 AD dementia subjects from the Mayo Clinic, as described by Jack and colleagues [21]. Elevated amyloid PET was defined as SUVR >1.40, a value validated with autopsy correlation [22]. Abnormal HVa was defined as departure of −2.39 cm3 or more from expected HVa, adjusted for TIV. Abnormal AD-signature region cortical thickness was defined at <2.74 mm. Finally, abnormal glucose uptake was demarcated at SUVR<1.32.
2.7. Assessment of covariates
Participant demographics (e.g., sex, age, years of education) were ascertained during the in-person interview at the in-clinic exam. Participants' height (cm) and weight (kg) were also measured during the in-clinic exam to calculate body mass index (BMI). A history of diabetes, hypertension, atrial fibrillation, and myocardial infarction were abstracted from the medical records. Participants were asked to bring all of their medication into the visit and were asked if they were currently taking each medication. Apolipoprotein E (APOE) ε4 allele genotyping was performed from a blood draw taken at the in-clinic exam.
2.8 Statistical analyses
The relationships between plasma tau levels and dichotomous variables were examined using Mann-Whitney rank sum tests. The distribution of plasma tau was right-skewed so the variable was natural log-transformed prior to subsequent regression analyses. We used logistic regression to determine the cross-sectional association between log-transformed plasma tau levels (as a continuous measure or in quartiles) and odds of having abnormal neuroimaging. Linear regression models were used to determine the association between plasma tau and cognitive test z-scores. Both linear and logistic regression models were adjusted for multiple covariates based on the literature and their association with plasma tau. Model 1 was unadjusted. Model 2 adjusted for age, sex, education, and APOE ε4. Model 3 adjusted for the variables in Model 2 and BMI, medical conditions (hypertension, diabetes, atrial fibrillation, myocardial infarction), and medications (statins, diabetes, medication, Coumadin). All analyses were completed using Stata Version 12.0 (StataCorp, College Station, TX).
3. Results
3.1. Participants characteristics and description of plasma tau data
Of the 539 participants, the median age (Interquartile range [IQR]) was 80 (77, 84) and 331 (61.4%) were male. The median (IQR) education was 14 (12, 16) and BMI was 27.1 (24.4, 30.3). There were 159 (29.5%) with an APOE ε4 allele and 161 (29.9%) had a diagnosis of MCI. The raw plasma tau levels ranged from 0.88 to 16.38 pg/mL with median and mean values of 4.18 (interquartile range, (IQR) = 3.33, 5.12) and 4.39 (SD = 1.72) pg/mL, respectively.
The associations between plasma tau and dichotomous participant characteristics are shown in Tables 1, stratified by cognitive status. In general, the associations with plasma tau were similar in the cognitive normal and MCI groups but the results with the cognitively normal individuals were more frequently statistically significant. Among the cognitively normal participants, plasma tau levels were significantly higher in those who were older than 80 years, had a diagnosis of diabetes, hypertension, atrial fibrillation, myocardial infarction and those who took non-steroidal anti-inflammatory medications, statins, diabetic medications, and statins. Among the MCI participants, plasma tau levels were significantly higher in those with a diagnosis of diabetes, hypertension and atrial fibrillation, and among those who were taking Coumadin. Examining neuroimaging measures, cognitively normal individuals (but not MCI) with abnormal amyloid-PET or abnormal cortical thickness had higher plasma tau levels.
Table 1.
Plasma tau levels by dichotomous participant characteristics among individuals who are cognitively normal or have mild cognitive impairment.
| Cognitively normal | Mild Cognitive Impairment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||||||
| Characteristic | N | Median (IQR) | N | Median (IQR) | z | p-value | N | Median (IQR) | N | Median (IQR) | z | p-value |
| Age ≥ 80 years | 218 | 4.33 (3.62, 5.19) | 160 | 3.76 (3.05, 4.61) | −3.97 | <0.001 | 89 | 4.57 (3.39, 6.02) | 72 | 4.09 (3.29, 5.23) | −1.54 | 0.124 |
| Men | 232 | 4.06 (3.31, 4.93) | 146 | 4.29 (3.41, 4.96) | 0.96 | 0.339 | 99 | 4.09 (3.34, 5.34) | 62 | 4.63 (3.39, 6.02) | 1.39 | 0.166 |
| Education >12 years | 250 | 4.03 (3.23, 4.89) | 128 | 4.29 (3.63, 5.06) | 1.74 | 0.082 | 92 | 4.33 (3.37, 5.49) | 69 | 4.34 (3.35, 5.71) | −0.24 | 0.808 |
| ≥1 APOE E4 allele | 101 | 4.26 (3.62, 5.00) | 277 | 4.12 (3.23, 4.93) | −1.32 | 0.187 | 58 | 4.58 (3.47, 6.12) | 103 | 4.18 (3.32, 5.33) | −1.67 | 0.096 |
| BMI>25 | 261 | 4.21 (3.48, 4.99) | 117 | 4.01 (3.23, 4.73) | −1.84 | 0.065 | 115 | 4.38 (3.39, 5.70) | 46 | 3.91 (3.24, 5.34) | −1.14 | 0.254 |
| Diabetes | 85 | 4.77 (3.82, 5.73) | 293 | 3.99 (3.20, 4.78) | −4.47 | <0.0001 | 43 | 4.95 (3.88, 6.55) | 110 | 4.13 (3.24, 5.33) | −2.58 | 0.010 |
| Hypertension | 296 | 4.19 (3.49, 5.08) | 82 | 3.81 (2.96, 4.60) | −2.69 | 0.007 | 125 | 4.56 (3.54, 5.78) | 28 | 3.57 (3.04, 4.34) | −2.76 | 0.006 |
| Atrial Fibrillation | 64 | 4.80 (3.66, 5.61) | 314 | 4.03 (3.27, 4.81) | −3.37 | <0.001 | 27 | 5.33 (4.08, 6.32) | 126 | 4.13 (3.31, 5.46) | −2.56 | 0.011 |
| Myocardial Infarction | 70 | 4.36 (3.69, 5.45) | 308 | 4.12 (3.27, 4.85) | −1.99 | 0.047 | 32 | 4.70 (3.35, 6.35) | 121 | 4.33 (3.39, 5.48) | −1.06 | 0.290 |
| Angina | 133 | 4.22 (3.42, 5.19) | 245 | 4.08 (3.23, 4.90) | −1.51 | 0.131 | 57 | 4.37 (3.40, 5.50) | 96 | 4.34 (3.29, 5.67) | −0.46 | 0.643 |
| Current NSAID Use | 270 | 4.22 (3.35, 5.12) | 108 | 3.92 (3.25, 4.58) | −2.53 | 0.011 | 112 | 4.34 (3.29, 5.71) | 49 | 4.33 (3.65, 5.48) | 0.80 | 0.423 |
| Current Statin Use | 225 | 4.30 (3.60, 5.19) | 153 | 3.90 (3.02, 4.60) | −3.84 | <0.001 | 88 | 4.37 (3.35, 5.71) | 73 | 4.18 (3.38, 5.46) | −0.44 | 0.664 |
| Current Diabetes Treatment (oral or insulin) | 59 | 4.58 (3.71, 5.80) | 319 | 4.13 (3.24, 4.84) | −2.78 | 0.005 | 31 | 4.91 (3.88, 5.85) | 122 | 4.23 (3.27, 5.50) | −1.50 | 0.134 |
| Coumadin | 29 | 4.66 (3.51, 5.51) | 349 | 4.12 (3.31, 4.93) | −1.56 | 0.119 | 18 | 5.33 (4.33, 6.34) | 143 | 4.13 (3.30, 5.48) | −2.60 | 0.009 |
| Abnormal Pib-PET (SUVR>1.40) | 224 | 4.20 (3.59, 5.01) | 152 | 3.97 (3.05, 4.78) | −2.17 | 0.030 | 102 | 4.43 (3.34, 5.82) | 58 | 4.11 (3.38, 5.07) | −0.97 | 0.334 |
| Abnormal Hva (<2.40 cm3) | 81 | 4.18 (3.35, 4.89) | 294 | 4.14 (3.29, 4.96) | −0.19 | 0.851 | 57 | 4.61 (3.39, 5.48) | 102 | 4.11 (3.32, 5.50) | −1.00 | 0.320 |
| Abnormal cortical thickness (<2.74 mm) | 141 | 4.46 (3.69, 5.19) | 234 | 3.97 (3.17, 4.77) | −3.32 | 0.001 | 85 | 4.56 (3.44, 5.85) | 73 | 4.08 (3.24, 5.34) | −1.76 | 0.079 |
| Abnormal FDG PET (SUVR<1.32) | 153 | 4.02 (3.14, 5.14) | 225 | 4.18 (3.35, 4.82) | −0.40 | 0.690 | 84 | 4.25 (3.42, 5.63) | 77 | 4.37 (3.26, 5.65) | −0.23 | 0.818 |
Abbreviations: BMI, Body Mass Index; HVa, hippocampal volume; FDG-PET, fluorodeoxyglucose-positron emission tomography; SUVR, standardized uptake value ratio; PiB-PET, Pittsburgh Compound B-positron emission tomography; IQR, interquartile range; APOE, Apolipoprotein E.
Hippocampal volume was adjusted for total intracranial volume. P values were determined using Mann-Whitney rank sum tests.
3.2 Plasma tau levels and imaging measures of amyloid and neurodegeneration
We evaluated the associations of plasma tau and abnormal neuroimaging biomarkers using logistic regression models. In unadjusted models, continuous levels of plasma tau were associated with higher odds of elevated PIB SUVR (odds ratio (OR) = 1.73; 95% confidence interval (CI), 1.10–2.72) (Table 2). Similarly, those in the highest quartile of plasma tau had almost a 2-fold greater odds of elevated PiB SUVR compared to those in the lowest quartile (OR = 1.80; 95% CI, 1.10–2.96). However, these associations were no longer significant in multivariable adjusted models. Among neuroimaging measures of neurodegeneration, we did not observe an association between plasma tau and abnormal HVa or FDG-PET. However higher plasma tau log levels were associated with higher odds of lower cortical thickness in the AD signature meta-ROI, including in the univariate and fully adjusted models (Table 2).
Table 2.
Association between log plasma tau and abnormal amyloid and neurodegeneration imaging biomarkers
| Log Plasma tau (pg/mL) | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | OR (95% CI) | P value | N | OR (95% CI) | P value | N | OR (95% CI) | P value | ||
| Abnormal PiB-PET (SUVR>1.40) | ||||||||||
| Continuous Quartiles | 536 | 1.73 (1.10–2.72) | .017 | 536 | 1.38 (0.85–2.24) | .188 | 524 | 1.54 (0.93–2.55) | .093 | |
| 1 | 536 | Reference | 536 | Reference | 524 | Reference | ||||
| 2 | 1.42 (0.87–2.31) | .159 | 1.30 (0.77–2.17) | .324 | 1.33 (0.78–2.28) | .295 | ||||
| 3 | 1.28 (0.79–2.07) | .324 | 1.04 (0.62–1.75) | .882 | 1.04 (0.61–1.78) | .875 | ||||
| 4 | 1.80 (1.10–2.96) | .020 | 1.38 (0.81–2.36) | .234 | 1.37 (0.76–2.46) | .289 | ||||
| Abnormal Hippocampal volume (<2.40 cm3) | ||||||||||
| Continuous Quartiles | 534 | 1.25 (0.76–2.07) | .379 | 534 | 1.00 (0.58–1.71) | .992 | 522 | 1.15 (0.66–2.00) | .632 | |
| 1 | 534 | Reference | 534 | Reference | 522 | Reference | ||||
| 2 | 1.00 (0.57–1.75) | 1.000 | 0.88 (0.49–1.61) | .689 | 0.95 (0.51–1.77) | .860 | ||||
| 3 | 1.16 (0.67–2.01) | .599 | 1.02 (0.56–1.84) | .948 | 1.06 (0.57–1.96) | .850 | ||||
| 4 | 1.30 (0.75–2.25) | .344 | 1.05 (0.58–1.91) | .868 | 0.86 (0.45–1.66) | .655 | ||||
| Abnormal cortical thickness (<2.74 mm) | ||||||||||
| Continuous Quartiles | 533 | 2.30 (1.44–3.68) | .001 | 533 | 1.80 (1.11–2.94) | .018 | 521 | 1.93 (1.17–3.19) | .010 | |
| 1 | 533 | Reference | 533 | Reference | 521 | Reference | ||||
| 2 | 1.40 (0.85–2.32) | .187 | 1.29 (0.76–2.18) | .348 | 1.11 (0.64–1.93) | .705 | ||||
| 3 | 1.74 (1.06–2.87) | .028 | 1.45 (0.86–2.44) | .166 | 1.36 (0.80–2.34) | .260 | ||||
| 4 | 2.36 (1.43–3.88) | .001 | 1.80 (1.06–3.05) | .029 | 1.43 (0.81–2.54) | .218 | ||||
| Abnormal FDG–PET (SUVR<1.32) | ||||||||||
| Continuous Quartiles | 539 | 1.28 (0.83–1.99) | .266 | 539 | 1.10 (0.70–1.73) | .683 | 527 | 1.05 (0.65–1.69) | .843 | |
| 1 | 539 | Reference | 539 | Reference | 527 | Reference | ||||
| 2 | 1.16 (0.72–1.88) | .541 | 1.10 (0.67–1.80) | .710 | 1.00 (0.60–1.67) | .994 | ||||
| 3 | 0.76 (0.46–1.23) | .263 | 0.66 (0.40–1.09) | .107 | 0.62 (0.37–1.04) | .071 | ||||
| 4 | 1.33 (0.82–2.15) | .248 | 1.12 (0.68–1.84) | .669 | 0.89 (0.51–1.53) | .672 | ||||
Abbreviations: OR, odds ratio; CI, confidence interval; PiB-PET, Pittsburgh Compound B-positron emission tomography; SUVR, standardized uptake value ratio; FDG-PET, fluorodeoxyglucose-positron emission tomography.
Model 1 is unadjusted. Model 2 adjusted for sex, age, education, and APOE ε4. Model 3 adjusted for the variables in Model 2 and BMI, medical conditions (hypertension, diabetes, atrial fibrillation, myocardial infarction), and medications (statins, diabetes medications, Coumadin).
3.3 Plasma tau levels by MCI status
In logistic regression models adjusting for age, sex, education, and APOE genotype, each log unit increase in plasma tau was associated with increased odds of MCI (OR: 1.45; 95% CI, 0.88–2.37), but the results did not reach significance at the P < .05 level (Table 3). Adjusting for additional factors in Model 3 did not change the association. Examining plasma tau in quartiles, the highest quartile versus the lowest was also not significantly associated with increased odds in all three models (Table 3).
Table 3.
Association between plasma log tau and odds of MCI
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log plasma tau (pg/mL) | N | OR (95% CI) | P value | N | OR (95% CI) | P value | N | OR (95% CI) | P value | |
| Continuous Quartiles | 539 | 1.45 (0.90–2.36) | .130 | 539 | 1.45 (0.88–2.37) | .145 | 527 | 1.44 (0.85–2.46) | .177 | |
| 1 | 539 | Reference | 539 | Reference | 527 | Reference | ||||
| 2 | 1.04 (0.61–1.76) | .893 | 0.99 (0.58–1.69) | .957 | 0.93 (0.52–1.64) | .803 | ||||
| 3 | 0.79 (0.46–1.37) | .405 | 0.76 (0.44–1.33) | .334 | 0.82 (0.46–1.45) | .494 | ||||
| 4 | 1.62 (0.97–2.70) | .065 | 1.59 (0.94–2.70) | .086 | 1.65 (0.93–2.95) | .090 | ||||
Abbreviations: MCI, mild cognitive impairment; OR, odds ratio; CI, confidence interval
Model 1 is unadjusted. Model 2 adjusted for sex, age, education, and APOE ε4. Model 3 adjusted for the variables in Model 2 and BMI, medical conditions (hypertension, diabetes, atrial fibrillation, myocardial infarction), and medications (statins, diabetes medications, Coumadin).
3.4 Association between plasma tau and cognitive test performance
We next examined the association between plasma tau and cognitive test performance (Table 4). Higher log plasma tau levels were significantly associated with worse performance on tests of global cognition, memory, and attention/executive function, but not on tests of visuospatial or language ability (Table 4). After adjusting for covariates in Models 2 and 3, the association between higher plasma tau and worse memory performance remained. The analysis of plasma tau by quartiles did not substantially alter the associations between plasma tau and cognition.
Table 4.
Association between log plasma tau and cognitive z-scores
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log Plasma tau (pg/mL) | N | B (95% CI) | P value | N | B (95% CI) | P value | N | B (95% CI) | P value | |
| Global z-score | ||||||||||
| Continuous Quartiles | 502 | −0.29 (−0.54 – −0.04) | .023 | 502 | −0.20 (−0.44 – 0.03) | .087 | 494 | −0.19 (−0.44 – 0.06) | .139 | |
| 1 | 502 | Reference | 502 | Reference | 494 | Reference | ||||
| 2 | −0.03 (−0.31 – 0.25) | .846 | 0.05 (−0.21 – 0.30) | .728 | 0.06 (−0.20 – 0.33) | .628 | ||||
| 3 | −0.22 (−0.50 – 0.05) | .113 | −0.07 (−0.33 – 0.19) | .589 | −0.08 (−0.34 – 0.18) | .539 | ||||
| 4 | −0.54 (−0.74– −0.34) | .018 | −0.21 (−0.47 – 0.05) | .117 | −0.18 (−0.47 – 0.10) | .204 | ||||
| Memory z-score | ||||||||||
| Continuous Quartiles | 533 | −0.38 (−0.64 – −0.12) | .005 | 533 | −0.30 (−0.56 – −0.05) | .020 | 522 | −0.28 (−0.54, −0.01) | .044 | |
| 1 | 533 | Reference | 533 | Reference | 522 | Reference | ||||
| 2 | −0.18 (−0.47 – 0.11) | .218 | −0.09 (−0.37 – 0.19) | .516 | −0.05 (−0.34 – 0.23) | .703 | ||||
| 3 | −0.31 (−0.60 – −0.02) | .036 | −0.22 (−0.50 – 0.06) | .125 | −0.21 (−0.50 – 0.07) | .137 | ||||
| 4 | −0.41 (−0.70 – −0.11) | .007 | −0.31 (−0.59 – −0.02) | .034 | −0.26 (−0.57 – 0.04) | .088 | ||||
| Attention/Executive Function z-score | ||||||||||
| Continuous Quartiles | 511 | −0.32 (−0.58 – −0.06) | .017 | 511 | −0.20 (−0.45 – 0.05) | .116 | 502 | −0.14 (−0.40 – 0.12) | .288 | |
| 1 | 511 | Reference | 511 | Reference | 502 | Reference | ||||
| 2 | 0.03 (−0.27 – 0.32) | .863 | 0.10 (−0.17 – 0.38) | .451 | 0.11 (−0.16 – 0.39) | .420 | ||||
| 3 | −0.11 (−0.40 – 0.18) | .460 | 0.05 (−0.22 – 0.33) | .698 | 0.03 (−0.25 – 0.30) | .852 | ||||
| 4 | −0.35 (−0.64 – −0.06) | .019 | −0.19 (−0.47 – 0.09) | .184 | −0.14 (−0.44 – 0.16) | .351 | ||||
| Visuospatial z-score | ||||||||||
| Continuous Quartiles | 512 | −0.10 (−0.34 – 0.14) | .404 | 512 | −0.05 (−0.28 – 0.17) | .632 | 502 | −0.05 (−0.29 – 0.19) | .67 | |
| 1 | 512 | Reference | 512 | Reference | 502 | Reference | ||||
| 2 | 0.002 (−0.26 – 0.27) | .987 | 0.03 (−0.21 – 0.28) | .785 | 0.10 (−0.15 – 0.35) | .431 | ||||
| 3 | −0.16 (−0.42 – 0.10) | .223 | −0.06 (−0.31 – 0.18) | .613 | −0.04 (−0.29 – 0.21) | .735 | ||||
| 4 | −0.14 (−0.40 – 0.13) | .304 | −0.03 (−0.28 – 0.21) | .785 | −0.01 (−0.28 – 0.25) | .919 | ||||
| Language z-score | ||||||||||
| Continuous Quartiles | 520 | −0.22 (−0.48 – 0.03) | .083 | 520 | −0.15 (−0.39 – 0.09) | .227 | 510 | −0.16 (−0.41 – 0.10) | .222 | |
| 1 | 520 | Reference | 520 | Reference | 510 | Reference | ||||
| 2 | −0.07 (−0.35 – 0.21) | .632 | 0.02 (−0.24 – 0.29) | .871 | 0.04 (−0.23 – 0.30) | .787 | ||||
| 3 | −0.19 (−0.47 – 0.10) | .197 | −0.05 (−0.31 – 0.22) | .733 | −0.07 (−0.34 – 0.20) | .603 | ||||
| 4 | −0.29 (−0.57 – −0.01) | .046 | −0.16 (−0.43 – 0.11) | .234 | −0.16 (−0.45 – 0.13) | .281 | ||||
Abbreviation: CI, confidence interval.
Model 1 is unadjusted. Model 2 adjusted for sex, age, education, and APOE ε4. Model 3 adjusted for the variables in Model 2 and BMI, medical conditions (hypertension, diabetes, atrial fibrillation, myocardial infarction), and medications (statins, diabetes medications, Coumadin).
3.4 Plasma tau levels, MCI, and imaging measures of amyloid and neurodegeneration
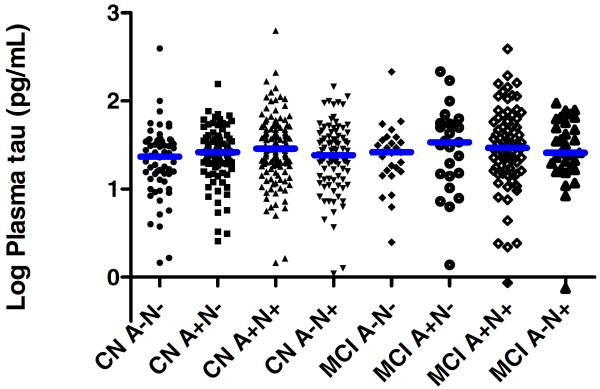
Lastly, we evaluated the median plasma levels by cognitive status (CN or MCI) and amyloid (A) and neurodegeneration (N) imaging status (A−N−, A+N−, A+N+, A−N+) using Kruskal-Wallis tests (Figure 1). We did not observe an overall difference across the groups (chi2, 7 df = 10.864, P = .145).
Fig. 1. Median log plasma tau levels by cognitive and neuroimaging amyloid and neurodegeneration biomarker status.
Amyloid positive (A+) was defined as amyloid PET SUVR > 1.40. Neurodegeneration positive (N+) was defined as either an AD-signature region cortical thickness <2.74 mm or FDG-PET SUVR<1.32 in AD-signature regions. CN = cognitively normal; MCI = Mild cognitive impairment. The number of subjects in each group are as follows: 62 CN A−N−; 90 CN A+N−; 133 CN A+N+; 89 CN A−N+; 27 MCI A−N−; 25 MCI A+N−; 76 MCI A+N+; 31 MCI A−N+.
4. Discussion
This study evaluated the utility of plasma tau levels as a peripheral biomarker in the preclinical and prodromal stages of AD in a well-characterized population-based study with neuroimaging and cognitive measures. Higher levels of plasma tau were cross-sectionally associated with worse memory performance and lower cortical thickness in an AD-signature region, with a trend toward higher values in MCI than CN. In analyses comparing eight groups defined by cognitive status and amyloid and neurodegeneration neuroimaging markers, there was high overlap in plasma tau levels. These results suggest that plasma tau will not be useful as a stand-alone diagnostic biomarker in preclinical or prodromal AD. However, when p-tau or other neuronal degeneration related assays are available, plasma tau should be considered as a key member in a peripheral biomarker panel for AD. Future research will also be needed to determine the prognostic value of plasma tau or a biomarker panel.
Zetterberg et al. (2013) examined plasma tau among individuals clinically diagnosed as CN, MCI, or AD. They reported that plasma tau levels were significantly elevated in AD, but not in MCI, compared to CN subjects. Additionally, there was substantial overlap in plasma tau levels between the groups. Notably, the Zetterberg study had a small group of CN individuals (n = 25) and used a different assay, but the same highly sensitive Simoa-HD1 platform. Despite the differences in the assays and sample size, the plasma tau levels among MCI subjects were similar between the two studies (mean = 4.68 pg/mL in the Zetterberg study and mean = 4.64 pg/mL in the present study). Our results are highly consistent with their results in that there was much overlap between groups and no difference between individuals who were CN or MCI. When we stratified by cognitive status (CN versus MCI) in table 1, plasma tau levels were also similarly elevated with age, the presence of vascular risk factors, and cortical thinning in both groups; although, the MCI group still tended to have higher, non-significant, levels in the presence of each factor. Additional longitudinal studies are needed to determine whether plasma tau could be useful as a pathological or prognostic biomarker or risk factor for AD. Our data suggest that plasma tau is cross-sectionally associated with both cortical thinning and memory performance, even after controlling for potentially confounding factors such as age and diabetes. For example, it has been reported that type II diabetes patients exhibit cortical thinning patterns parallel to those observed in AD patients [23], but our observed association between plasma tau and cortical thickness remained significant after accounting for diabetes diagnosis and diabetes medications. Thus, our results support the hypothesis that plasma tau may be a peripheral biomarker of brain tissue injury [1,2] and further extends these findings to neurodegeneration associated with preclinical and prodromal AD.
We found associations between higher plasma tau levels and worse memory performance, which remained significant after adjusting for multiple covariates. Although we are unaware of other reports of plasma tau correlating with cognition in any stage of the AD continuum, there are many studies showing that greater postmortem tau pathology is associated with worse pre-mortem cognition (reviewed in Nelson et al. [24]). Recent evidence using tau-PET also demonstrates increasing neurofibrillary tangle pathology with advancing AD stages [25]. The relationship between the measures of tau pathology in the brain, CSF tau, or plasma tau has yet to be fully explored, and we are aware only of one study, Zetterberg et al. (2013), that reported on the lack of relationship between CSF and plasma tau. CSF tau levels were not available to report for this study, so we were unable to compare plasma and CSF tau levels between groups. Future studies carefully investigating the relationship between tau-PET, CSF tau and plasma tau in the same cohort of subjects are needed to longitudinally ascertain their relationship with each other and in relation to disease progression. The reported disconnect between CSF tau and plasma tau will need further exploration, and it will be critical to measure CSF and plasma with similar assays and under similar conditions. It is reported that CSF tau is actually a compilation of many sized polypeptides originating from full length tau and similar to other proteins. Therefore the population and specific polypeptides formed in CSF and plasma may be different and, could have different utilities as biomarkers for AD [26].
Despite its strengths, limitations of the study warrant consideration. First, the plasma sample set was used for two separate studies to conserve samples and, thus, underwent at least one freeze-thaw cycle prior to analysis. Second, our findings may not be directly generalizable to other populations. Third, the study did not include subjects with AD dementia. The MCSA was designed to determine the incidence and prevalence of MCI in the population, and therefore initially excluded dementia patients at baseline. However, as these participants are followed, some will convert to dementia, so we will have the opportunity to investigate plasma tau levels across the AD spectrum. Fourth, plasma assays of p-tau are being developed but are not currently available. Future research is needed to determine whether plasma p-tau, in combination with plasma amyloid and total tau, could be a more useful diagnostic marker. Lastly, this study was cross-sectional. Due to the overlap across cognitive and neuroimaging groups, we cannot make a meaningful clinical decision at an individual subject level based on plasma tau levels. Future studies to evaluate the longitudinal changes of plasma tau over the preclinical and clinical AD time frame, and in relation to changes in amyloid and neurodegenerative neuroimaging measures, will provide additional insight into the utility of plasma tau in clinical trials or for patient management.
A peripheral measure associated with acute neuronal damage or chronic neurodegeneration in elderly subjects would be a major breakthrough in the field of neurodegeneration research, especially if the peripheral marker is directly associated with one of the defining pathologies of AD. Existing models and genetics of AD suggest amyloid deposition is necessary, but insufficient, to predict the development of AD dementia in the short term. A peripheral measure, plasma tau, could allow for routine, repeat testing of amyloid-positive subjects to determine their rate of disease progression and the effectiveness of potential disease-modifying future therapies. However, the longitudinal collection of plasma tau data over the next decades in controlled clinical trials of AD, traumatic brain injury, sports-related concussion and in aging studies is necessary to enhance our understanding of the utility of plasma tau as a biomarker or a risk factor of neuronal injury and AD-associated neurodegeneration. These studies will need to determine the appropriate sampling frequency and normal variation in an individual's plasma tau level in order to understand normal and disease related changes in a subjects tau levels.
Highlights.
Higher plasma tau levels were cross-sectionally associated with worse memory performance and lower cortical thickness in an AD-signature region.
In analyses comparing eight groups defined by cognitive status and neuroimaging measures, there was high overlap in plasma tau levels. These results suggest that plasma tau will not be useful as a stand-alone diagnostic biomarker in preclinical or prodromal AD.
Future studies are needed to evaluate the longitudinal associations between plasma tau and neuroimaging measures of amyloid and neurodegeneration in the preclinical and clinical AD stages. This research is critical to assess the utility of plasma tau as a prognostic marker, for use in clinical trials, or for patient management.
Research in context.
Systematic review: We reviewed the literature using traditional (e.g., PubMED) resources. While CSF tau has utility as an Alzheimer's disease (AD) biomarker, few studies have examined the utility of plasma tau.
Interpretation: Higher plasma tau levels were cross-sectionally associated with worse memory performance and lower cortical thickness in an AD-signature region. In analyses comparing eight groups defined by cognitive status and neuroimaging measures, there was high overlap in plasma tau levels. These results suggest that plasma tau will not be useful as a stand-alone diagnostic biomarker in preclinical or prodromal AD.
Future Directions: Future studies are needed to evaluate the longitudinal associations between plasma tau and neuroimaging measures of amyloid and neurodegeneration in the preclinical and clinical AD stages. This research is critical to assess the utility of plasma tau as a prognostic marker, for use in clinical trials, or for patient management.
Acknowledgments
This study would not be possible without the participants enrolled in the Mayo Clinic Study of Aging. This study was supported by funding from the National Institutes of Health/National Institute on Aging grants U01 AG006786, R01 AG011378, R01 AG041851, and R01 AG049704, and was made possible by the Rochester Epidemiology Project (R01 AG034676). The sample preparation and analysis was carried out by Andrew Hiday of Advanced Testing Laboratories.
Conflict of interest Drs. Dage and Airey are employees of Eli Lilly. Drs. Wennberg, Machulda, and Roberts have no disclosures. Mr. Hagen has no disclosures. Dr. Knopman serveed as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRx Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer's Disease Cooperative Study; and receives research support from the NIH. Dr. Jack has provided consulting services for Eli Lilly. He receives research funding from the National Institutes of Health, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. Dr. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Roche, Inc., Merck, Inc., and Genentech, Inc.; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA. Dr. Mielke served as a consultant to AbbVie and Lysosomal Therapeutics, Inc., and receives research support from the NIH/NIA and the Michael J. Fox Foundation.
Abbreviations
- AD
Alzheimer's disease
- APOE
Apolipoprotein E
- BMI
body mass index
- CI
confidence interval
- CN
cognitively normal
- CSF
cerebral spinal fluid
- CT
computed tomography
- ELISA
enzyme-linked immunosorbent assay
- FDG-PET
fluorodeoxyglucose-positron emission tomography
- HVa
hippocampal volume
- IQR
interquartile range
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- MRI
magnetic resonance imaging
- OR
odds ratio
- PET
positron emission tomography
- PiB
Pittsburgh Compound B
- ROIs
regions of interest
- SD
standard deviation
- SUVR
standardized uptake value ratio
- TIV
total intracranial volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Randall J, Mortberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84:351–6. doi: 10.1016/j.resuscitation.2012.07.027. [DOI] [PubMed] [Google Scholar]
- [2].Shahim P, Tegner Y, Wilson DH, Randall J, Skillback T, Pazooki D, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71:684–92. doi: 10.1001/jamaneurol.2014.367. [DOI] [PubMed] [Google Scholar]
- [3].Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer's disease. Alz Research & Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Negash S, Smith GE, Pankratz S, Aakre J, Geda YE, Roberts RO, et al. Successful aging: definitions and prediction of longevity and conversion to mild cognitive impairment. Am J Geriatric Psychiatry. 2011;19:581–8. doi: 10.1097/JGP.0b013e3181f17ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–8. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- [7].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- [8].Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Archives de Psychologie. 1964;28:286–340. [Google Scholar]
- [9].Wechsler D. Wechsler Memory Scale - Revised Manual. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- [10].Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Lee & Febiger; Philadelphia: 1983. [Google Scholar]
- [11].Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3rd ed. Oxford University Press; New York: 2006. [Google Scholar]
- [12].Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- [13].Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual. The Psychological Corporation; New York: 1981. [Google Scholar]
- [14].Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo's older americans normative studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- [15].Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- [16].Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–8. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Curiati PK, Tamashiro-Duran JH, Duran FL, Buchpiguel CA, Squarzoni P, Romano DC, et al. Age-related metabolic profiles in cognitively healthy elders: results from a voxel-based [18F]fluorodeoxyglucose-positron-emission tomography study with partial volume effects correction. AJNR Am J Neuroradiol. 2011;32:560–5. doi: 10.3174/ajnr.A2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lowe VJ, Kemp BJ, Jack CR, Jr, Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–86. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Su Y, Blazey TM, Snyder AZ, Raichle ME, Marcus DS, Ances BM, et al. Partial volume correction in quantitative amyloid imaging. Neuroimage. 2015;107:55–64. doi: 10.1016/j.neuroimage.2014.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain. 2015;138:1370–81. doi: 10.1093/brain/awv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–42. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Experiment Neurol. 2012;71:362–81. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau PET imaging in aging and early Alzheimer's disease. Ann Neurol (in press) 2015 doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Meredith JE, Jr, Sankaranarayanan S, Guss V, Lanzetti AJ, Berisha F, Neely RJ, et al. Characterization of novel CSF Tau and ptau biomarkers for Alzheimer's disease. PloS One. 2013;8:e76523. doi: 10.1371/journal.pone.0076523. [DOI] [PMC free article] [PubMed] [Google Scholar]