Abstract
Irreversible acetylcholinesterase (AChE) inhibition accumulates to high levels in the central nervous system (CNS) because AChE turnover in the brain is much slower than in peripheral tissues. As expected from this CNS selectivity, the irreversible AChE inhibitor methanesulfonyl fluoride (MSF) produces significant cognitive improvement in Alzheimer’s patients without the gastrointestinal toxicity that plagues other AChE inhibitors. However, without dose-limiting gastrointestinal toxicity, one shortcoming of the prior human studies of MSF is that the upper limits of CNS AChE inhibition that might be tolerated could not be tested. Therefore, in this study, monkeys were treated with escalating intramuscular doses of MSF that culminated with several weeks of 1.5 mg/kg dosing, more than eight times the prior human clinical dose, still without signs of toxicity. Brain biopsies showed that ~ 80% AChE inhibition had been produced and that the new synthesis of cortical AChE had a half-time (t1/2) of ~ 12 days. A single IM dose of 1.5 mg/kg MSF produced ~ 59% inhibition in cerebrospinal fluid (CSF) AChE as measured one day later. This corresponds to a peak of ~ 80% inhibition in CSF AChE at the time of the injection, recovering with a t1/2 of 2.4 days. Computational analyses suggest that MSF at clinically relevant doses could theoretically produce a steady-state AChE inhibition between 65% and 85% in the CNS. These data suggest that the full therapeutic advantage of AChE inhibition therapy can be realized without interference from dose-limiting gastrointestinal toxicity if an irreversible inhibitor is employed.
Keywords: Alzheimer’s, acetylcholinesterase, EC 3.1.1.7, butyrylcholinesterase, EC 3.1.1.8, methanesulfonyl fluoride, CAS 558-25-8, Lewy body, Parkinson’s, central nervous system
INTRODUCTION
Inhibition of cholinesterase, primarily acetylcholinesterase (AChE; EC 3.1.1.7), is a mainstay strategy for treating dementing disorders that involve a critical loss of central nervous system (CNS) acetylcholine [1]. This approach is well grounded in the findings that dementia in Alzheimer’s is due, at least in part, to a severe loss of acetylcholine in the nucleus basalis of Meynert, as well as in other midbrain nuclei, cortex and hippocampus [2 – 4]. Recent evidence also suggests that cholinesterase inhibitors may be useful in treating vascular cognitive impairment [5 – 7], Parkinson’s disease dementia and Lewy body dementia [8, 9], and for age-related memory impairment as suggested by animal experiments [10]. In addition, a wide variety of preclinical and clinical studies show that AChE inhibitors may have neuroprotective effects that can delay or modify the course of the disease [11 – 17]. In view of the key role that cholinergic dysfunction plays in Alzheimer’s and related diseases, it is disappointing that the development of AChE inhibitors has focused on only those with competitive or pseudo-irreversible mechanisms of action as, for example, with donepezil and rivastigmine). These have produced only marginal clinical improvement, far below what might be expected from treating such a well validated therapeutic target.
For the purposes of this paper, a “competitive” AChE inhibitor occupies the catalytic site temporarily (without a covalent bond) and is readily established and reversed according to concentration-driven kinetics. “Pseudo-irreversible” inhibitors such as the carbamates (e.g., rivastigmine) form a covalent enzyme-inhibitor bond at the catalytic site which then undergoes spontaneous hydrolysis according to pseudo-first-order kinetics [18], eventually allowing the recovery of enzyme activity. Neither competitive nor pseudo-irreversible inhibitors require the new synthesis of enzyme for the recovery of activity. An “irreversible” AChE inhibitor, on the other hand, forms a permanent covalent enzyme-inhibitor bond at the catalytic site that does not undergo spontaneous hydrolysis to restore enzyme activity. Thus, recovery from irreversible inhibition requires the synthesis of new, uninhibited enzyme.
The main problem with AChE inhibitors that rely on competitive or pseudo-irreversible mechanisms of action is that they do not deliver the high level of CNS selectivity required for successful therapy. Besides being necessary in the CNS, acetylcholine is also the major neurotransmitter in all autonomic ganglia, in the nerve/muscle junction on somatic striated muscle, and in the parasympathetic control of smooth muscle, cardiac muscle, and glands. When pharmacological levels of AChE inhibition begin to be achieved in the CNS, drugs without sufficient CNS selectivity wreak havoc in these other critical peripheral tissues, especially in the gastrointestinal system. This is not a trivial problem. Gastrointestinal toxicity (nausea, vomiting, and diarrhea) has been an impenetrable barrier to using the high doses of conventional AChE inhibitors that are required to correct severe acetylcholine deficits in the brain [19]. To realize the full, as yet untested therapeutic benefit of AChE inhibition for CNS disorders, exceptionally high CNS selectivity must be obtained.
One strategy for establishing and maintaining the necessary high levels of AChE inhibition in the brain, but without dose-limiting nausea, vomiting, and diarrhea, is to use an AChE inhibitor with an irreversible mechanism of action. Importantly, irreversible AChE inhibitors are inherently CNS-selective because the new synthesis of AChE in the brain occurs very slowly, with a half-time (t½) of ~ 12 days [20, 21]. In contrast to the CNS, new synthesis of AChE in peripheral tissues is, by comparison, very rapid, with a t½ of only 1 day in intestines [20]. Slow new synthesis of AChE in the brain thus allows irreversible inhibition to carry over, dose after dose, stacking inhibition on top of accumulating inhibition, until high levels of enzyme blockade are produced and maintained. In peripheral tissues, on the other hand, AChE is replaced by 10+ times more rapid new synthesis. When AChE in peripheral tissues is blocked by an irreversible inhibitor, most of the inhibited enzyme is immediately replaced with newly synthesized active enzyme during the time between doses. The rapid replacement of AChE in peripheral tissues prevents the accumulation of inhibition such that these tissues remain unaffected.
The idea of using an irreversible AChE inhibitor for Alzheimer’s disease is not new. Metrifonate, an organophosphate tested for the treatment of Alzheimer’s, is often described as being “irreversible” [19, 22]. However, metrifonate is a pro-drug, a slow release formulator, that is converted non-enzymatically to DDVP (O,O-dimethyl O-(2,2,-dichlorovinyl) phosphate), its active AChE inhibitor intermediate [23, 24]. However, in vivo administration of metrifonate, or DDVP itself, results in mainly pseudo-irreversible AChE inhibition which then undergoes rapid spontaneous reactivation within a few hours, like that seen with rivastigmine, a classic pseudo-irreversible inhibitor [18]. Only the lesser residual part of metrifonate and DDVP-induced AChE inhibition is thus truly irreversible [21, 24]. For example, after a single injection of 10 mg/kg DDVP in mice, a dose which markedly suppressed locomotor activity, rotarod performance, and rectal temperature, produced a peak of 88% CNS AChE inhibition at 2 hours which then underwent spontaneous reversal to only 20% inhibition within 24 hours, showing a t1/2 of ~12 hours like that found with a pseudo-irreversible inhibitor [21]. However, in spite of the rapid spontaneous reversal of most of the daily DDVP-induced AChE inhibition, ten daily injections in that study accumulated ~ 65% inhibition of cortical AChE which did not undergo spontaneous reactivation. In the absence of further DDVP treatment, cortical enzyme activity recovered with a t1/2 of several days as is characteristic of irreversible inhibition [21]. This outcome would occur if as little as 15% of the DDVP-induced inhibition produced by each daily dose “ages” into the more stable, irreversible organophosphate bond that resists reactivation [25]. Thus, because of its predominantly pseudo-irreversible mechanism of action, metrifonate was not an adequate test of the real therapeutic potential of an irreversible AChE inhibitor for treating Alzheimer’s dementia.
Methanesulfonyl fluoride (MSF), in contrast to metrifonate, forms an extremely stable covalent bond at the catalytic site that is totally refractory to reactivation [20, 21, 26]. MSF-induced AChE inhibition can only be overcome by the new synthesis of uninhibited enzyme. Moreover, it is important to note that MSF is a sulfonyl fluoride and does not interact with the neuropathy target enzyme associated with organophosphate-induced delayed neuropathy [27]. MSF is, therefore, free of the risk of life-threatening respiratory paralysis and muscle weakness which contributed to the termination of metrifonate development [28]. Moreover, the organophosphates have the disadvantage of producing a wide range of serious non-cholinergic side effects [29]. Because of its truly irreversible mechanism of action, and its freedom from organophosphate-induced toxic effects, MSF was selected to determine the uppermost practical and theoretical limits of AChE inhibition that can be tolerated in primates, the purpose of this study.
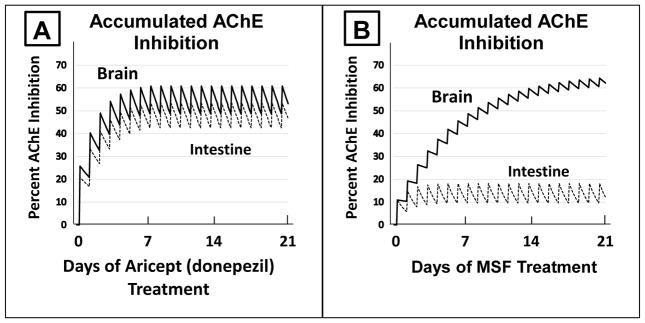
The critical importance of an irreversible mechanism of AChE inhibition to the eventual success in treating Alzheimer’s dementia cannot be overemphasized. Figure One presents a side-by-side comparison of the results that can be expected with an AChE inhibitor with either a competitive or pseudo-irreversible mechanism of action as compared to an AChE inhibitor with an irreversible mechanism of action. The left side of Figure One [A] is modeled after a competitive inhibitor with a clearance t1/2 of 70 hours and strong CNS selectivity, producing 1.25 times more inhibition in the brain than in intestines (e.g., Aricept/donepezil). As shown in Figure One A, there is considerable overlap between AChE inhibition in the CNS and intestines. When brain inhibition is maintained above the 50% level required for a therapeutic effect, inhibition in the intestines is also at or near 50%, a level that causes gastrointestinal toxicity [19]. The unavoidable overlap between brain and intestinal AChE inhibition by inhibitors without sufficient CNS selectivity (e.g., competitive or pseudo-irreversible inhibitors) is the cause of the unbearable nausea, vomiting, and diarrhea that has limited such AChE inhibitors to ineffective doses. In sharp contrast to side A, Figure One B shows the separation of brain versus intestinal AChE inhibition that can be obtained with an irreversible inhibitor. MSF-induced AChE inhibition is expected to accumulate to a high level in the CNS (>60%, estimated t1/2; for AChE replacement = 12 days) without producing clinically significant inhibition of AChE in the periphery (<25%, estimated t1/2 for AChE replacement = 1 day). The clear separation between brain and peripheral AChE inhibition in response to MSF is based solely on the powerful effect of the 10x slower replacement of AChE that occurs in the brain.
FIGURE ONE. Side-by-Side Computational Comparison of Expected Competitive versus Irreversible AChE Inhibition Through 21 Days of Treatment.
A computational/theoretical model of the expected accumulated AChE inhibition in the brain (upper solid line) and intestine (lower dotted line) after three weeks of daily doses of a competitive inhibitor (A, e.g. donepezil, producing 25% inhibition in the CNS versus 20% inhibition in the intestine, a ratio of 1.25 more inhibition in the CNS) or an irreversible inhibitor (B, e.g. MSF, producing an equal 10% inhibition in both CNS and intestine). The saw-tooth appearance of the lines shows the increase in inhibition added with each dose. MSF is particularly well suited for these computations because it reaches a peak concentration within minutes after oral administration and then disappears rapidly from blood, within a few hours, producing the pulsatile inhibition shown in B [30]. The downward slope between doses is the decrease of inhibition during the dose-to-dose interval. These pharmacological calculations (repeated dosing with enzyme recovery between doses) predict the estimated accumulated effects occurring over 21 days as shown.
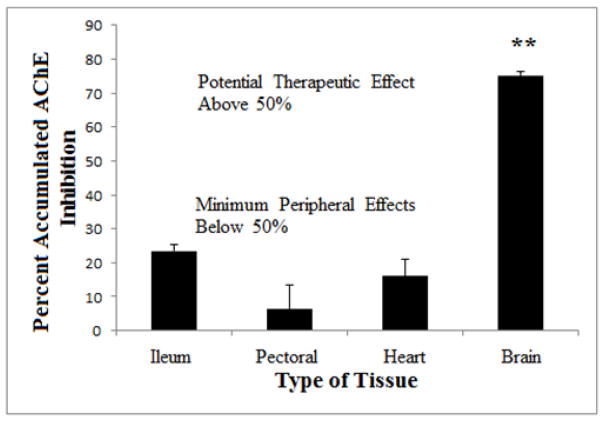
A prior study has empirically validated the theoretical advantage of an irreversible inhibitor, the clear separation of AChE inhibition in the brain and peripheral tissues, in an experiment in which young rats were treated with MSF over a period of three weeks [30]. According to a priori computations like those shown in Figure One, the dose of MSF and the dosing schedule used during the three weeks were expected to produce an average of 70% AChE inhibition in the brain (using t1/2 = 12 days) and 25% inhibition in the intestines (using t1/2 = 1 day). The ex vivo results shown in Figure Two show excellent agreement with the a priori estimates. The 75% CNS AChE inhibition (Figure Two) was achieved without toxicity and is more than the 50% minimum required for a therapeutic effect [19]. Less than 30% AChE inhibition is sufficient to avoid gastrointestinal as well as cardiac and skeletal muscle toxicity [19]. As is expected from such powerful AChE inhibition, MSF also produces larger and longer lasting increases in brain acetylcholine than donepezil [31].
FIGURE TWO. Actual Accumulated AChE Inhibition in Rat Tissues after Three Weeks of Repeated Doses of MSF.
Accumulated AChE inhibition in the brain, pectoral muscle (skeletal muscle), heart (cardiac muscle), and ileum (smooth muscle) after 0.3 mg/kg MSF (IM) given three times per week for three weeks. The animals were sacrificed and tissues assayed for AChE inhibition, compared to untreated controls, 24 hours after the last injection. AChE inhibition in the brain was significantly greater than in the other tissues (** p<0.01) (From Moss et al, [30]).
The absence of drug-induced nausea, vomiting, and diarrhea presents a unique challenge to estimating the dose of MSF that should be tested in Alzheimer’s patients. The dose cannot be established by simple dose escalation, going up until higher doses cannot be tolerated, as with competitive and pseudo-irreversible inhibitors (e.g., donepezil, rivastigmine, etc.).
The CNS selectivity shown in Figure Two has real-world significance. In a recent study of MSF in normal humans, reports of nausea were, as expected, uncommon with only 5 reports of mild, transient nausea out of 56 administrations of the highest dose. Diarrhea and vomiting were even more infrequent and inconsequential [30]. Without dose-limiting gastrointestinal toxicity, however, the starting point for what might be an effective clinical dose of MSF in humans had to be estimated a priori based on animal experiments [32]. The absence of dose-limiting nausea, vomiting, or diarrhea in the humans suggests that the doses of MSF tested in the clinical trials conducted thus far may not have, in fact, been anywhere close to the limits of tolerability or clinical effectiveness that might be possible [32].
In view of the untested bounds of MSF tolerability or potential clinical efficacy, the purpose of the present study was, therefore, to determine the upper practical and theoretical limits of CNS AChE inhibition and tolerability in primates.
MATERIALS AND METHODS
Four three-year old male Macaca fascicularis (Crab Eating Cynomolgus) monkeys (Charles River Primate Breeding Facility, Port Washington, NY) served as subjects in all of the followed protocols which were approved by the U.T. El Paso Institutional Animal Care and Use Committee and following guidelines recommended by NIH. Methanesulfonyl fluoride was purchased from Aldrich Chemical Company (Milwaukee, WI).
Experiment One – Effects of Long-term MSF on Cortical and RBC AChE
The first experiment was conducted to assess the toxicity/tolerability of escalating doses of MSF followed by cortical biopsies to validate the expected level of accumulated CNS AChE inhibition. Two monkeys were randomly selected to receive 33 IM injections of MSF in peanut oil over 93 days. The other two monkeys received matching injections of pure peanut oil as a vehicle control. The monkeys were monitored daily for the appearance of vomit, diarrhea, loss of appetite, loss of body weight, or changes in general behavior. RBC AChE activity was measured at various times to monitor the effects of dosing. The schedule of escalated doses of MSF as well as the corresponding MSF-treated monkeys’ RBC AChE inhibition, expressed as percent of control monkey RBC AChE activity, are shown in Table One.
TABLE ONE.
Doses and Schedule of MSF Administration
| Dose of MSF (IM) | # of doses | Day of Experiment in which the dose was administered | RBC AChE Inhibition in MSF Monkeys* |
|---|---|---|---|
| 0.05 mg/kg | 3 | Days 1, 3, and 5 | Day 8: 48% INH |
| 0.15 mg/kg | 4 | Days 10, 15, 18, and 20 | No data |
| 0.5 mg/kg | 3 | Days 22, 24, and 26 | Day 29: 59% INH |
| 1.0 mg/kg | 11 | Days 30, 32, 34, 36, 37, 38, 39, 40, 45, 47, and 50 | Days 36, 43, 51: 62%, 83%, 88% INH |
| 1.5 mg/kg | 12 | Days 53, 57, 59, 61, 64, 71, 75, 78, 82, 85, 87, and 93 | Days 53, 65, 71, 87: 80%, 98%, 94%, 81% INH |
The days listed are those during which RBC AChE was assayed (500 μM acetylthiocholine iodide substrate). The percent inhibition on an assay day is shown, in corresponding order, in bold following the listing of days.
All cholinesterase assays were conducted in 0.1 M (Na) PO4 buffer, pH 7.0, and assayed in triplicate according to the procedure of Ellman et al. [33] using acetylthiocholine and butyrylthiocholine as substrates for AChE and butyrylcholinesterase (BChE), respectively. Assays of RBC AChE activity on the days of MSF treatment shown in Table One were conducted on a few drops of blood drawn up into heparinized capillary tubes which were then sealed and centrifuged at low speed at 4° C for 5 min. The volume of the packed erythrocytes was measured and then added directly into prepared cholinesterase assays containing 500 μM acetylthiocholine substrate. Cortical biopsies were taken under ketamine anesthesia 2.5 days after the last MSF injection on day 93. The cortical samples (mean of 55 mg, SEM=8.8 mg) were homogenized as a 2% w/v (1:50) wet weight in 0.1 M (Na) PO4 buffer, pH 7.0, and assayed in triplicate using acetylthiocholine and butyrylthiocholine as substrates for AChE and BChE, respectively.
Experiment Two – The Effects of MSF on CSF
The purpose of this experiment was to determine if cerebrospinal fluid (CSF) sampling might be a better and less invasive method for estimating brain AChE inhibition than taking cortical biopsies. Therefore, inhibition of AChE in primate CSF and the rate at which CSF AChE activity is replaced after a single injection of 1.5 mg/kg MSF (IM) were determined. Experiment Two was initiated seven months after the conclusion of Experiment One, using the same four monkeys.
CSF was taken by lumbar tap under ketamine anesthesia as necessary to maintain adequate restraint. The taps were conducted under sterile conditions by inserting a 21 gauge sterile needle (1 inch) into an intervertebral space in the lumbar region and allowing about 0.5 ml CSF to drip spontaneously into a collection tube. If blood appeared in the CSF, the first few drops were discarded and the remaining CSF was centrifuged at low speed at 4° C for five minutes. The clear supernatant was then used for the AChE assays which were conducted according to the method of Ellman et al. [33] described earlier. CSF was sampled twice before the beginning of MSF treatment to establish a pre-drug baseline for each individual monkey. All four monkeys then received one IM injection of 1.5 mg/kg MSF in peanut oil. CSF was then sampled and assayed for AChE activity at 1.0, 4.25, 7.25, and 10.25 days after that single injection. The first CSF sample was delayed for one day to distinguish toxicity from the MSF injection from adverse events related to CSF sampling. The monkeys showed mild weight loss (~ 5%) over the duration of the 10 day experiment, quite probably because of the stress of repeated anesthesia and CSF sampling.
No attempt was made in either Experiment One or Two to measure the concentration of MSF or its metabolites in brain, blood, or CSF because MSF undergoes rapid spontaneous hydrolysis to methanesulfonic acid and disappears from blood with a t1/2 of ~ 2 hours [30, 34]. There appears to be no conventional metabolic processing of MSF.
RESULTS
Experiment One – Effects of Long-term MSF on Cortical AChE
During the escalating doses of MSF shown in Table One, there were no instances of vomiting or diarrhea in either control or MSF-treated monkeys and similarly, there were no differences in eating behavior or body weights (further suggesting that the animals experienced no nausea or anorexia). There were also no adverse events of any type observed and no changes in the comprehensive clinical blood profiles that measured, among other things, kidney and liver function.
The average Km for RBC AChE was 80.4 μM (SEM = 9.75 μM) acetylthiocholine and the Vmax for the packed erythrocytes was 3.61 × 10−6 (SEM = 4.77 × 10−7) moles of substrate hydrolyzed/ml packed RBC/minute. The times at which the RBC AChE assays were conducted and the corresponding levels of inhibition present during the escalation of MSF dosing are shown in Table One.
Analyses of the cortical biopsies showed that the average Km for cortical AChE was 87 μM (SEM = 41 μM) acetylthiocholine with a Vmax of 2.55 × 10−6 moles/gram/min (SEM = 6.2 × 10−7 moles/gram/min). The average Km for cortical BChE was 161 μM (SEM = 2.5 μM) butyrylthiocholine with a Vmax of 1.22 × 10−6 moles/gram/min (SEM = 1.44 × 10−7 moles/gram/min). Table Two shows the results of the cortical biopsy enzyme assays run using 500 μM acetylthiocholine and 2 mM butyrylthiocholine, the substrate concentrations used to estimate total accumulated cortical AChE and BChE activity remaining at the time of the biopsies.
TABLE TWO.
Cholinesterase Activity Determined from Monkey Cortex Biopsies. AChE and BChE activities were assayed at 500 μM acetylthiocholine iodide and 2 mM butyrylthiocholine iodide substrate concentrations, respectively. Activity is expressed as moles of substrate hydrolyzed/gram of tissue/minute.
| Mean AChE Activity of Control Cortex | Mean AChE Activity of MSF Treated Cortex | % AChE Inhibition |
|---|---|---|
| 2.07 × 10−6 (SEM 2.2 × 10−7) | 4.31 × 10−7 (SEM 5.4 × 10−8) | 79.2% |
|
| ||
| Mean BChE Activity of Control Cortex | Mean BChE Activity of MSF Treated Cortex | % BChE Inhibition |
| 1.17 × 10−6 (SEM 1.6 × 10−7) | 6.34 × 10−7 (SEM 8.2 × 10−8) | 45.8 % |
As expected from earlier experiments in rodents [30], Table Two shows that MSF-treated monkeys showed a mean of ~80% inhibition of cortical AChE activity relative to the controls. Surprisingly, the MSF-treated monkeys showed a mean of 46% inhibition of cortical BChE, more than was expected in view of in vitro experiments showing that MSF is 16 times more reactive against human cortical AChE than BChE [35].
Experiment Two – The Effects of MSF on CSF
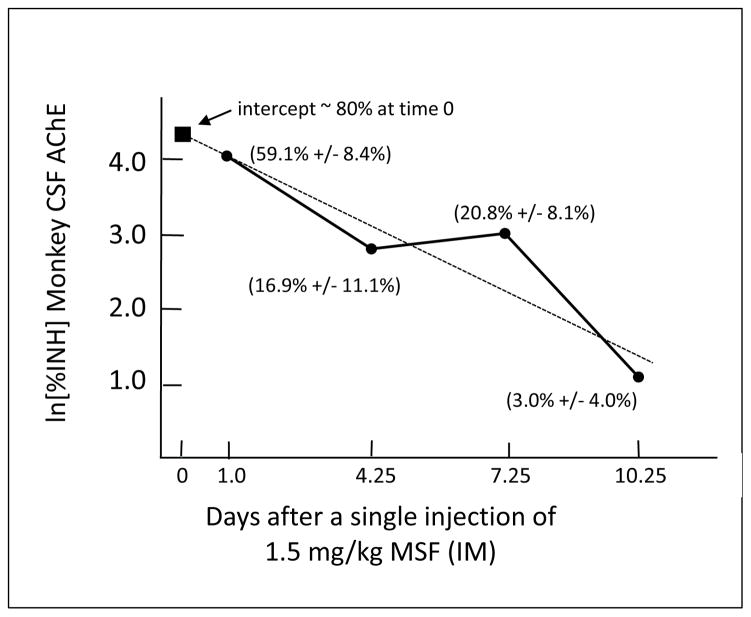
The single injections of 1.5 mg/kg (IM) were tolerated without any signs of toxicity (e.g., vomiting, diarrhea, or behavioral changes). Least squares linear regression analysis of the CSF AChE recovery (Figure Three) shows that monkey CSF AChE recovers with a t1/2 of 2.4 days. The Y-intercept of the least squares linear regression applied to the data shown in Figure Three indicates that a peak of ~ 80% CSF AChE inhibition was produced by the 1.5 mg/kg dose at the time it was administered (Day 0).
FIGURE THREE. Recovery of Monkey CSF AChE.
Recovery of monkey CSF AChE after a single injection of 1.5 mg/kg MSF on Day 0. The dotted line shows the least squares linear regression. Percent AChE inhibition is shown in parentheses on the semi-log chart (Mean +/− SEM). The filled square at Day 0 shows the computed intercept which is an estimate of the peak inhibition at the time of the injection.
DISCUSSION
To provide a human clinical reference for the escalating MSF doses shown in Table One, the clinically effective dose of MSF in Alzheimer’s patients was 180 μg/kg given three times per week [30, 32]. Using these human data as a reference point, the MSF-treated monkeys in Experiment One were receiving more than 5 times, and then more than 8 times, the human clinical dose of MSF when they received 1.0 mg/kg and 1.5 mg/kg, respectively. The ability of the monkeys to tolerate these high doses may be due, at least in part, to the prior escalation from lower doses [19].
As shown in Table One, the repeated escalating doses produced significant accumulated inhibition of RBC AChE with an asymptote at ~ 90% inhibition during the final weeks of repeated 1.5 mg/kg doses of MSF. The dose/response equation for the effect of single doses of MSF on human RBC AChE derived from Moss et al. [30] is:
Using the above equation to estimate the effects of MSF on monkey RBC AChE, it is expected that 1.5 mg/kg MSF would produce 43% inhibition of the remaining active enzyme at the time of each new dose. Given that M. fascicularis RBC’s, like those in humans, have a 70 – 90 day lifespan, [36], it is expected that the dosing schedule used in this experiment would produce a mean of ~ 90% RBC AChE inhibition, in good agreement with the results obtained (Table One). The accumulated monkey RBC AChE inhibition in this experiment was limited by a ceiling effect and it is not expected to be sensitive to dose-dependent differences like those that are found at more clinically relevant doses [30, 32].
Besides showing that ~ 80% cortical AChE inhibition can be produced and maintained by repeated doses of 1.5 mg/kg MSF, the data in Table Two can also be used to estimate the rate at which monkey brain AChE is replaced by new synthesis, a factor that is critical for predicting the cumulative effect of MSF in the brain when it is administered repeatedly during clinical use (e.g., computations as shown in Figure One).
Even though the level of brain AChE inhibition was determined by biopsies at only one time point (2.5 days after the last MSF injection), the lower limit of the t1/2 for de novo AChE synthesis in monkey cortex can be estimated by proposing, for purposes of calculations only, that the last injection of 1.5 mg/kg MSF produced a peak of 100% inhibition. If the brain showed 100% AChE inhibition at the time of the final injection (time 0 for calculations of enzyme recovery), and it still retained 80% inhibition 2.5 days later, the t1/2 for new synthesis of cortical AChE could not be less than 8 days. It is, of course, highly unlikely that the brain had 100% inhibition at the end of MSF treatment. With anything less than 100% inhibition, the t1/2 will be longer, very likely similar to the 12 days that is observed in mice and rats [20, 21]. Using a proposed t1/2 of 12 days for AChE recovery in monkey cortex and estimating the effect of the repeated doses of 1.5 mg/kg at 43% inhibition with each dose (from human RBC AChE dose-response function shown above [30]), retrospective calculations indicate that a range of 84% (peak) declining to 73% AChE (trough) inhibition between doses would be expected from the series of injections shown in Table One. The actual finding of ~ 80% cortical inhibition 2.5 days after the last injection is within the expected range, confirming that a t1/2 of 12 days is a useful estimate for primate brain AChE turnover.
Whether or not the estimated t1/2 of 12 days or the calculated maximum AChE inhibition of 92% are accurate is of only theoretical interest. The empirically established level of 80% cortical AChE inhibition actually achieved and confirmed by enzyme assays far exceeds the minimum of 50% inhibition estimated to be required for clinically significant therapeutic effects [19].
Experiment Two showed that monkey CSF AChE recovered with a t1/2 of 2.4 days, which is faster than the minimum possible t1/2 of 8 days for cortical AChE recovery, and especially the more likely t1/2 of 12 days. Monkey CSF AChE recovery with a t1/2 of 2.4 days (Figure Three) compares favorably to the 2.2 day t1/2 of CSF AChE reported in humans [37]. The high turnover rate of CSF AChE, as compared to brain tissue, suggests that monitoring CSF AChE is not a useful indicator of the accumulated effects of repeated doses of an irreversible AChE inhibitor. It is important to note that two monkeys in this experiment had never received MSF, the controls in the previous experiment, and the other two had not received MSF for seven months. In spite of this, the abrupt administration of 1.5 mg/kg, without prior escalating doses, did not produce any discernable side effects.
The above monkey experiments using MSF demonstrate that dose-limiting side-effects need not be major barriers to obtaining the clinical doses required to effectively treat Alzheimer’s dementia or other CNS disorders. Indeed, an irreversible AChE inhibitor can produce and maintain substantially more inhibition than is probably needed for effective treatment of CNS disorders. An irreversible inhibitor circumvents the major barrier of unbearable dose-limiting nausea, vomiting, and diarrhea that is experienced by patients given AChE inhibitors that have competitive or pseudo-irreversible mechanisms of action. While the use of an irreversible inhibitor appears to effectively reduce the risk of peripheral toxicity, there is an as yet inadequately tested possibility of limiting centrally-mediated hypercholinergic side effects.
MSF was used in these experiments because it is the only irreversible inhibitor for which a comprehensive series of experiments, from mice to monkeys and humans, is available. Similar results would be expected from any truly irreversible AChE inhibitor that crosses the blood-brain barrier and has a suitable partitioning coefficient into mainly lipid tissues.
If toxic side-effects do not determine the upper limit of doses of an irreversible AChE inhibitor that might be used to treat dementia, what then can be the basis for a guideline for optimal dosing?
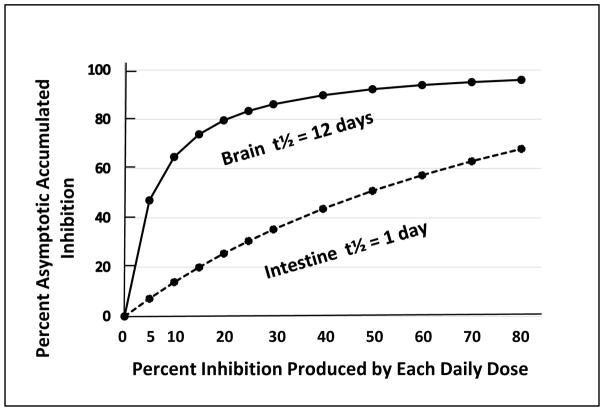
One of the problematic pharmacological realities of irreversible AChE inhibitors is that they produce decreasing returns with increasing doses. This is especially evident at the higher end of the dosing scale. Accumulated AChE inhibition produced by an irreversible inhibitor eventually approaches an asymptotic level that is a function of the percent inhibition produced by each dose, the frequency with which the doses are administered, and the t1/2 for the synthesis of new enzyme. An example of this is shown in Figure One (B) wherein the asymptotic accumulated AChE inhibition in the intestines was achieved in 4 days, yet even after 21 days of dosing the brain was still approaching, but had not yet achieved, the final asymptotic accumulated AChE inhibition even at 65%.
Figure Four shows the estimated asymptotic level of accumulated AChE inhibition that can be expected from increasing doses of an irreversible inhibitor in brain and intestine. These computations assume a t1/2 of 12 days for brain and 24 hour dose-to-dose intervals. As shown in Figure Four, doses of an irreversible inhibitor that produce as little as 10% and 20% inhibition of the remaining active AChE with each dose, will eventually produce an expected asymptotic inhibition of 65% or 80%, respectively. Figure Four also shows that higher doses produce the sharply decreasing returns as discussed above. Even though the monkey experiments show that very high doses are well tolerated, it seems unlikely that the clinical use of a daily dose that produces more than 20% inhibition with each dose would be useful.
FIGURE FOUR. Hypothetical Relationship Between Dose Size and Accumulated Asymptotic Brain AChE Inhibition.
The expected (computed) relationship between % inhibition produced by each dose of an irreversible inhibitor and the eventual accumulated AChE inhibition expected after extended dosing (asymptotic level, equilibrium between drug-induced inhibition and ongoing new synthesis). Computations assume daily dosing and half-times of 12 and 1 day for brain and intestine, respectively.
As a basis for estimating the effect of an irreversible inhibitor in a clinical trial, the asymptotic inhibition of brain AChE that can be expected with extended daily administration of any particular dose of an irreversible inhibitor (at equilibrium between drug-induced inhibition and new enzyme synthesis) can be estimated from a double reciprocal plot which gives a straight line with the equation:
In this equation, Asym% is the expected asymptotic accumulated AChE inhibition in the brain expected after extended daily administrations of an irreversible inhibitor; and, %INH is the percent inhibition of the remaining active enzyme at the time of each dose. The slope of this equation is unique to a t1/2 of 12 days (brain) and will not predict asymptotic accumulated AChE inhibition in other tissues. It is not surprising that with a t1/2 of 1 day (intestine) and a schedule of 1 day between doses, that there is an almost linear increase in asymptotic AChE inhibition in the intestines with increasing dose size (Figure Four).
As mentioned above, a cursory examination of Figure Four shows that there is little benefit to be gained by daily doses of an irreversible inhibitor that produce more than 20% inhibition. Extended use of a 20% dose will eventually produce and maintain ~ 80% and 25% AChE inhibition in the brain and intestines, respectively. The maximum practical dose of an irreversible inhibitor is not only limited by decreasing returns in brain AChE inhibition. It is also subject to limitations imposed by the accumulated asymptotic inhibition in the intestines. For example, Figure Four shows that a daily dose which produces 30% inhibition with each administration will approach ~ 40% accumulated AChE inhibition in the intestines, a level that will possibly allow the reappearance of gastrointestinal toxicity [19]. Satisfactory results may even be obtained by a daily dose of drug that produces as little as 10% inhibition. This dosing would result in an estimated 65% inhibition in the brain, within the therapeutic range, with as little as 15% inhibition in the intestines (Figure Four), well below levels expected to be toxic [19]. In the specific case of MSF, the percent inhibition produced by single doses can be determined from the human RBC AChE dose-response equation from Moss et al. [30] shown above.
The above computations are designed to estimate a practical schedule of daily dosing that will produce an adequate clinical effect in the brain with minimum peak levels of inhibition and risk of gastrointestinal side-effects. Other dosing schedules such as twice- or three-times per week, as used in the present monkey experiments, can be satisfactory, but will produce a wider range of peaks and valleys of AChE inhibition. Higher peak levels of inhibition may increase the risk of gastrointestinal toxicity. New estimated effects would need to be computed for different dosing schedules.
In summary, a myriad of in vitro and in vivo experiments in mice, rats and monkeys clearly demonstrate, as a proof-of-concept, that irreversible AChE inhibition, like that produced by MSF, can produce and maintain high levels of CNS inhibition without interference associated with AChE inhibition in peripheral tissues. The high CNS selectivity of an irreversible inhibitor can be understood within a simple biochemical model based on pseudo first-order kinetics. Irreversible inhibitors are unique in that they alone exploit the slow de novo synthesis of AChE in the brain to increase therapeutic benefit and decrease the risk of peripheral toxicity. Indeed, the promise of strong efficacy and excellent tolerability of an irreversible AChE inhibitor has already been realized in human clinical trials [30, 32].
Irreversible AChE inhibitors thus open the door to the full potential of CNS cholinesterase inhibitor therapy for the treatment of memory loss. Because of the important clinical implications for effective CNS cholinesterase inhibitor therapy, irreversible AChE inhibitors, especially MSF, deserve serious evaluation in humans experiencing cognitive loss, especially when the aging population around the world is growing exponentially.
Acknowledgments
The assistance of Jon Esposito, DVM, in the collection of CSF samples and the cortical biopsies, and Dr. Isabel Sumaya, PhD, and Jim Summerton, PhD, for valuable criticisms during the preparation of the manuscript, are gratefully acknowledged. This work was supported in part by Grant 2G12MD007592 from the National Institutes of Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH), MIRDP Grant MH47167, NIMH Grant RR08012, the El Paso Chapter of the Alzheimer’s Association, and the Coldwell Foundation of El Paso, Texas. RGP and HK report no conflict of interest. Dr. Moss served as a paid consultant to SeneXta Therapeutics (Switzerland) for the clinical trial conducted in Germany in 2010–2011. All rights to Dr. Moss’ now-expired patents for the use of MSF for the treatment of Alzheimer’s were licensed by the University of Texas at El Paso to SeneXta Therapeutics. Dr. Moss has no ownership or any other financial interest in SeneXta Therapeutics. Dr. Moss is Co-Manager of Brain-Tools, LLC.
References
- 1.Ashford JW. Treatment of Alzheimer’s disease: the legacy of the cholinergic hypothesis, neuroplasticity, and future directions. J Alzheimers Dis. 2015;47:149–156. doi: 10.3233/JAD-150381. [DOI] [PubMed] [Google Scholar]
- 2.Davies P, Maloney AJR. Selective loss of cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 3.Whitehouse PJ, Price DL, Clarke AW, Coyle JT, DeLong MR. Alzheimer’s disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 4.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 5.Schneck M. Vascular dementia. Top Stroke Rehab. 2008;15:22–26. doi: 10.1310/tsr1501-22. [DOI] [PubMed] [Google Scholar]
- 6.Birks J, McGuinness B, Craig D. Rivastigmine for vascular cognitive impairment. Cochrane Database of Systemic Reviews. 2013;(5) doi: 10.1002/14651858.CD004744.pub3. Art. No. CD004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlongan CV, Sumaya IC, Moss DE. Methanesulfonyl fluoride, an acetylcholinesterase inhibitor, attenuates simple learning and memory deficits in ischemic rats. Brain Res. 2005;1038:50–58. doi: 10.1016/j.brainres.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Rolinski M, Fox C, Maidment I, McShane R. Cholinsterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database of Systematic Reviews. 2012;(3) doi: 10.1002/14651858.CD006504.pub2. Art. No.: CD006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang HF, Yu JT, Tang SW, Jiang T, Tan CC, Meng XF, Wang C, Tan MS, Tan L. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychia. 2015;86:135–143. doi: 10.1136/jnnp-2014-307659. [DOI] [PubMed] [Google Scholar]
- 10.Malin DH, Plotner RE, Radulescu SJ, Perebee RN, Lake JR, Negrete PG, Schaefer PJ, Crothers MK, Moss DE. Chronic methanesulfonyl fluoride enhances one-trial per day reward learning in aged rats. Neurobiol Aging. 1993;14:393–395. doi: 10.1016/0197-4580(93)90127-w. [DOI] [PubMed] [Google Scholar]
- 11.Castro A, Martinez A. Targeting beta-amyloid pathogenesis through acetylcholinesterase inhibitors. Curr Pharmaceutical Des. 2006;12:4377–4387. doi: 10.2174/138161206778792985. [DOI] [PubMed] [Google Scholar]
- 12.Akaike A. Preclinical evidence of neuroprotection by cholinesterase inhibitors. Alzheimer Dis Assoc Disord. 2006;20(Suppl 1):S8–11. doi: 10.1097/01.wad.0000213802.74434.d6. [DOI] [PubMed] [Google Scholar]
- 13.Nordberg A. Mechanisms behind the neuroprotective actions of cholinesterase inhibitors in Alzheimer disease. Alz Dis Assoc Dis. 2006;20(Suppl 1):S12–18. doi: 10.1097/01.wad.0000213804.59187.2d. [DOI] [PubMed] [Google Scholar]
- 14.Beach TG, Walker DG, Sue S, Scott KJ, Layne KJ, Newell AJ, Potter PE, Durham RA, Emmerling MR, Webster SD, Honer WG, Fisher A, Roher AE. Immunotoxin lesion of the cholinergic nucleus basalis causes Aβ deposition: towards a physiologic animal model of Alzheimer’s disease. Curr Med Chem – Immun Endo Metab Agents. 2003;3:57–75. [Google Scholar]
- 15.Winblad B, Jelic V. Long-term treatment of Alzheimer disease: efficacy and safety of acetylcholinesterase inhibitors. Alz Dis Assoc Dis. 2004;18(Suppl 1):S2–S8. doi: 10.1097/01.wad.0000127495.10774.a4. [DOI] [PubMed] [Google Scholar]
- 16.Atri A, Shaughnessy LW, Locascio JJ, Growden JH. Long-term course and effectiveness of combination therapy in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2008;22:209–221. doi: 10.1097/WAD.0b013e31816653bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behl P, Edwards JD, Kiss A, Lanctot KL, Streiner DL, Black SE, Stuss DT. Treatment effects in multiple cognitive domains in Alzheimer’s disease: a two-year cohort study. Alzheimers Res Ther. 2014;18:6. doi: 10.1186/alzrt280. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groner E, Ashani Y, Schorer-Apelbaum D, Sterling J, Herzig Y, Weinstock M. The kinetics of inhibition of human acetylcholinesterase and butyrylcholinesterase by two series of novel carbamates. Mol Pharmacol. 2007;71:1610–1617. doi: 10.1124/mol.107.033928. [DOI] [PubMed] [Google Scholar]
- 19.Imbimbo BP. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs. 2001;15:375–390. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 20.Moss DE, Kobayashi H, Pacheco G, Palacios R, Perez R. Methanesulfonyl fluoride: a CNS selective cholinesterase inhibitor. In: Giacobini E, Becker R, editors. Current Research in Alzheimer Therapy. Taylor and Francis; New York: 1988. pp. 305–314. [Google Scholar]
- 21.Kobayashi H, Nakano T, Moss DE, Suzuki T. Effects of a central anticholinesterase, methanesulfonyl fluoride on the cerebral cholinergic system and behavior in mice: comparison with an organophosphate DDVP. J Health Sci. 1999;45:191–202. [Google Scholar]
- 22.Becker RE, Colliver JA, Markwell SJ, Moriearty PL, Unni LK, Vicari S. Double-blind, placebo-controlled study of metrifonate, an acetylcholinesterase inhibitor, for Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:124–131. doi: 10.1097/00002093-199601030-00003. [DOI] [PubMed] [Google Scholar]
- 23.Nordgren I, Bergstrom M, Holmstedt B, Sandoz M. Transformation and action of metrifonate. Arch Toxicol. 1978;41:31–41. doi: 10.1007/BF00351767. [DOI] [PubMed] [Google Scholar]
- 24.Hallak M, Giacobini E. A comparison of two inhibitors on brain cholinesterase. Neuropharmacol. 1987;26:521–530. doi: 10.1016/0028-3908(87)90143-2. [DOI] [PubMed] [Google Scholar]
- 25.Cochran R, Kalisiak J, Küçükkilinç T, Radić Z, Garcia E, Zhang L, Ho K-Y, Amitai G, Kovarik A, Fokin VV, Sharpless B, Taylor P. Oxime-assisted acetylcholinesterase catalytic scavengers of organophosphates that resist aging. J Biol Chem. 2011;286(34):29718–29724. doi: 10.1074/jbc.M111.264739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitz R, Wilson IB. Esters of methanesulfonic acid as irreversible inhibitors of acetycholinesterase. J Biol Chem. 1962;237:3245–3249. [PubMed] [Google Scholar]
- 27.Osman KA, Moretto A, Lotti M. Sulfonyl fluorides and the promotion of diisopropyl fluorophosphates neuropathy. Fund Appl Toxicol. 1996;33:294–297. doi: 10.1006/faat.1996.0167. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Arrieta J, Schneider L. Metrifonate for Alzheimer’s disease (Review) The Cochrane Collection. 2006;(2) doi: 10.1002/14651858.CD003155.pub3. Art. No.: CD003155. [DOI] [PubMed] [Google Scholar]
- 29.Terry AV., Jr Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Thera. 2012;134:355–365. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss DE, Fariello RG, Sahlmann J, Sumaya I, Pericle F, Braglia E. A randomized Phase I study of methanesulfonyl fluoride, an irreversible acetylcholinesterse inhibitor, for the treatment of Alzheimer’s disease. Brit J Clin Pharmacol. 2013;75:1231–1239. doi: 10.1111/bcp.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imanishi T, Hossain MM, Suzuki T, Xu P, Sato I, Kobayashi H. Effects of a CNS sensitive anticholinesterase methane sulfonyl fluoride on hippocampal acetylcholine release in freely moving rats. Adv Pharmacol Sci. 2012;2012:708108. doi: 10.1155/2012/708178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moss DE, Berlanga P, Hagan MM, Sandoval H, Ishida C. Methanesulfonyl fluoride (MSF): a double-blind, placebo-controlled study of the safety and efficacy in the treatment of senile dementia of the Alzheimer type. Alz Dis Assoc Dis. 1999;13:20–25. doi: 10.1097/00002093-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 34.Snow AW, Barger WR. A chemical comparison of methanesulfonyl fluoride with organofluorophosphorus ester anticholinesterase compounds. Chem Res Toxicol. 1988;1:379–384. doi: 10.1021/tx00006a009. [DOI] [PubMed] [Google Scholar]
- 35.Pacheco G, Palacios-Esquivel R, Moss DE. Cholinesterase inhibitors proposed for the treatment of Alzheimer’s Disease: selectivity toward human brain acetylcholinesterase compared to butyrylcholinesterase. J Pharmacol Exp Thera. 1995;274:767–770. [PubMed] [Google Scholar]
- 36.Landaw SA. Factors that accelerate or retard red blood cell senescense. Blood Cells. 1988;14:47–67. [PubMed] [Google Scholar]
- 37.Unni L, Vicari S, Moriearty P, Schaefer F, Becker R. The recovery of cerebrospinal fluid acetylcholinesterase activity in Alzheimer’s disease patients after treatment with metrifonate. Meth Find Exp Clin Pharmacol. 2000;22:57–61. doi: 10.1358/mf.2000.22.1.795849. [DOI] [PubMed] [Google Scholar]