Abstract
Small heat shock/α-crystallin proteins function as molecular chaperones, protecting other proteins from irreversible denaturation by an energy-independent process. The brine shrimp, Artemia franciscana, produces a small heat shock/α-crystallin protein termed p26, found in embryos undergoing encystment, diapause, and metabolic arrest. These embryos withstand long-term anoxia and other stresses normally expected to cause death, a property likely dependent on molecular chaperone activity. The association of p26 with tubulin in unfractionated cell-free extracts of Artemia embryos was established by affinity chromatography, suggesting that p26 chaperones tubulin during encystment. To test this possibility, both proteins were purified by modifying published protocols, thereby simplifying the procedures, enhancing p26 yield about 2-fold, and recovering less tubulin than before. The denaturation of purified tubulin as it “aged” and exposed hydrophobic sites during incubation at 35°C was greatly reduced when p26 was present; however, tubulin polymerization into microtubules was reduced. On incubation at 35°C, centrifugation in sucrose density gradients demonstrated the association of purified p26 with tubulin. This is the first study where the relationship between a small heat shock/α-crystallin protein and tubulin from the same physiologically stressed organism was examined. The results support the proposal that p26 binds tubulin and prevents its denaturation, thereby increasing the resistance of encysted Artemia embryos to stress. Additional factors are apparently required for release of tubulin from p26 and restoration of efficient assembly, events that would occur as embryos resume development and the need for microtubules is established.
INTRODUCTION
The small heat shock/α-crystallin proteins, discovered as heat-inducible proteins in Drosophila larvae and now known to be widely distributed phylogenetically, share a conserved sequence called the α-crystallin domain (MacRae 2000; Scharf et al 2001; Narberhaus 2002). The small heat shock/α-crystallin proteins function as molecular chaperones, preventing heat-induced aggregation of substrates in vitro by an adenosine triphosphate (ATP)–independent mechanism that involves recognition of proteins exhibiting aggregation-prone molten globule structure (Ehrnsperger et al 1997; Wang and Spector 2000; Goenka et al 2001; Carver et al 2002). Although substrate denaturation is prevented in the absence of ATP, the nucleotide may influence chaperone activity, and additional molecular chaperones are usually required in concert with an energy source for regeneration of protein activity (Lee and Vierling 2000; Wang and Spector 2001; Valdez et al 2002). This is true for citrate synthase and luciferase when inactivated in the company of α-crystallin (Ehrnsperger et al 1997; Wang and Spector 2000, 2001) and prevails for remobilization of luciferase in the presence of Pisum sativum Hsp18.1 (Lee et al 1997). Thus, the primary function of small heat shock/α-crystallin proteins in the context of molecular chaperoning is to bind denaturing proteins at hydrophobic sites and to maintain them in a folding competent state until other chaperones act (Ehrnsperger et al 1997; Lee and Vierling 2000; Shroff et al 2001; Van Montfort et al 2001), although Synechocystis HSP17 also stabilizes heat-stressed membranes (Török et al 2001). The small heat shock/α-crystallin proteins confer thermotolerance on cells (Liang and MacRae 1999; Crack et al 2002; Valdez et al 2002), inhibit apoptotic death (Kamradt et al 2001; Mao et al 2001; Samali et al 2001; Andley et al 2002; Merendino et al 2002; Paul et al 2002), and generally protect organisms from physiological stress (MacRae 2000; Narberhaus 2002).
Oviparously developing embryos of Artemia franciscana cease development and are released from females as encysted gastrulae, or cysts, enclosed in protective shells (Clegg et al 1999; Liang and MacRae 1999). Cysts enter a reversible state of dormancy termed diapause (MacRae 2001), wherein metabolic activity is extremely low (Clegg and Jackson 1998; Warner and Clegg 2001). The tolerance of encysted Artemia embryos to stress is remarkable, including resistance to complete desiccation (Drinkwater and Clegg 1991) and survival under anoxia for several years, even when fully hydrated at physiological temperature (Clegg and Jackson 1998; Clegg et al 1999, 2000). Encysted embryos contain abundant quantities of p26, a small heat shock/α-crystallin protein not produced in embryos that develop directly into nauplii (Clegg et al 1995, 1999; Liang et al 1997a, 1997b; Liang and MacRae 1999). This protein has a molecular mass of 20.7 kDa and possesses an α-crystallin domain but differs from other small heat shock/α-crystallin proteins in its amino- and carboxy-terminal sequences. p26 forms oligomers of approximately 700 kDa and acts as a molecular chaperone, entering nuclei during stress (Willsie and Clegg 2002), endowing transformed bacteria with thermotolerance (Liang and MacRae 1999; Crack et al 2002), and preventing aggregation of citrate synthase on heating in vitro (Liang et al 1997a).
As a small heat shock/α-crystallin protein, p26 may associate with denaturing proteins in encysted embryos during diapause and anoxia. To test this possibility, the relationship between p26 and tubulin, a major structural protein of eukaryotic cells, was examined. Tubulin most commonly exists as a heterodimer of α- and β-tubulin, although there are at least 7 different isotypes within the superfamily, producing a multitude of isotubulins as a consequence of differential gene expression and posttranslational processing (MacRae 1997; Nogales 2000; Dutcher 2001). Tubulin depends on molecular chaperones such as prefoldin and chaperonin (CCT/Tric) and postchaperonin proteins (cofactors) for proper folding and dimerization during synthesis (Llorca et al 2001; Lopez-Fanarraga et al 2001), and it interacts with Hsp70 and Hsp90 (Liang and MacRae 1997; Williams and Nelsen 1997; Garnier et al 1998; Agueli et al 2001).
Tubulin dimers polymerize into microtubules, dynamic cytoskeletal elements with a role in chromosome segregation and cytoplasmic organization, activities dependent on microtubule-associated proteins (Wittmann et al 2001; Cassimeris 2002). It is noteworthy that tubulin, a protein reported to possess chaperone-like activity (Manna et al 2001; Sarkar et al 2001), undergoes biphasic unfolding and exposes hydrophobic regions as it “ages” (Sarkar et al 1995; Guha and Bhattacharyya 1997; Roychowdhury et al 2000), providing reactive sites recognizable by small heat shock/α-crystallin proteins. An association between p26 and tubulin that limits denaturation on heating in vitro was demonstrated in this study, but assembly competence was nonetheless reduced. It is therefore possible that p26 binds partially denatured tubulin in vivo, perhaps in a molten globule state, thereby contributing to the ability of Artemia embryos to withstand stress during encystment and diapause. The resumption of assembly would depend on tubulin liberation from p26 and regeneration of native structure, subjects for future study.
MATERIALS AND METHODS
Preparation of cell-free extract from Artemia embryos
Cell-free extract was prepared from encysted Artemia embryos (Sanders Brine Shrimp Co., Ogden, UT, USA) hydrated overnight at 4°C in distilled water, collected under suction on a Buchner funnel, washed twice with cold distilled water followed by 2 washes with Pipes buffer (100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) [Pipes], 1 mM MgCl2, 1 mM ethylene glycol-bis(aminoethylether)-tetraacetic acid, pH 6.5), and then homogenized in Pipes buffer with a Retsch motorized mortar and pestle (Brinkman Instruments Canada, Rexdale, Ontario, Canada). The homogenate was stirred at 4°C for 15 minutes, centrifuged at 16 000 × g for 10 minutes at 4°C, and the resulting supernatant filtered through 2 layers of Calbiochem Miracloth (Cedarlane Laboratories Ltd, Hornby, Ontario, Canada) before centrifugation at 40 000 × g for 30 minutes at 4°C. The upper 80% of the supernatant was recovered and centrifuged for 20 minutes under the same conditions. The upper 80% of this supernatant, termed the cell-free extract, was removed and either used immediately or stored at −70°C. Protein concentrations for this and other preparations were determined using bovine serum albumin (BSA) as a standard (Lowry et al 1951).
Association of p26 and tubulin in cell-free extract from encysted Artemia embryos
Interaction between p26 and tubulin in cell-free extract from encysted Artemia embryos was examined by affinity chromatography. Specifically, immunoglobulin (IgG) was purified by individually mixing preimmune and immune (anti-p26) rabbit serum with Protein A Sepharose (Amersham Pharmacia Biotech, Baie d'Urfé, Quebec, Canada), washing with 20 mM Na2HPO4, pH 7.4, and eluting with 0.1 M sodium citrate, pH 2.5, into tubes containing 0.1 volume of 1.0 M Tris-HC1, pH 8.0 (Harlow and Lane 1999). The eluate was dialyzed overnight against 40 mM 3-(N-morpholino)propane-sulfonic acid (MOPS), 10 mM C2H3O2Na, 2 mM ethylenediamine-tetraacetic acid, pH 7.5 (MOPS buffer), at 4°C. IgG fractions were then attached covalently to either Protein A Sepharose (Harlow and Lane 1999) or Affi-Gel 10 (manufacturer's instructions). In the former case, 8 mg of purified IgG was mixed with 100 mg of Protein A Sepharose for 1 hour at room temperature, centrifuged, and the supernatant discarded. The affinity matrix was washed once with 0.2 M NaB2O7, pH 8.0, incubated in 2 volumes of 0.2 M NaB2O7 containing 20 mM di-methylpimelimidate for 30 minutes at room temperature, washed once with NaB2O7, and then incubated for 2 hours at room temperature in 2 volumes of 0.2 M NaB2O7 containing 0.2 M ethanolamine. The affinity matrix was washed exhaustively with phosphate-buffered saline (PBS), 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4. A sample from the final wash was electrophoresed in a sodium dodecyl sulfate (SDS)–polyacrylamide gel, followed either by staining with Coomassie blue or by blotting to nitrocellulose and reacting with anti-rabbit IgG antibody (described later) to ensure that all free IgG was gone. Affi-Gel 10 affinity columns were prepared by incubating IgG and Affi-Gel 10, in amounts equivalent to those used for preparation of the IgG-Protein A Sepharose affinity matrix, in MOPS buffer at 4°C, with gentle agitation for 4 hours, and then draining and washing with MOPS buffer followed by Pipes buffer. The affinity matrix was washed with 0.1 M glycine, pH 2.5, until no IgG was released (see above), equilibrated in MOPS buffer, and stored at 4°C.
To absorb p26 and associated proteins, 100 mg of each affinity matrix, containing either immune or preimmune IgG, was incubated with 0.5 mL of cell-free extract containing 16 mg of protein for 30 minutes at 4°C and then washed with 20 mL of PBS. One hundred microliters of gel sample buffer was added to half of the affinity matrix; the mixture was heated in a boiling water bath for 3 minutes and then centrifuged before 20 μL was removed for electrophoresis in 7–14% SDS polyacrylamide gradient gels. As an additional control, each affinity matrix was incubated with Pipes buffer rather than with cell-free extract, followed by heating in treatment buffer and electrophoresis in SDS polyacrylamide gradient gels. The gels were either stained with Coomassie blue or blotted to nitrocellulose and probed, as described later, using the antibodies indicated in the figure legends.
Preparation of p26 and tubulin from Artemia
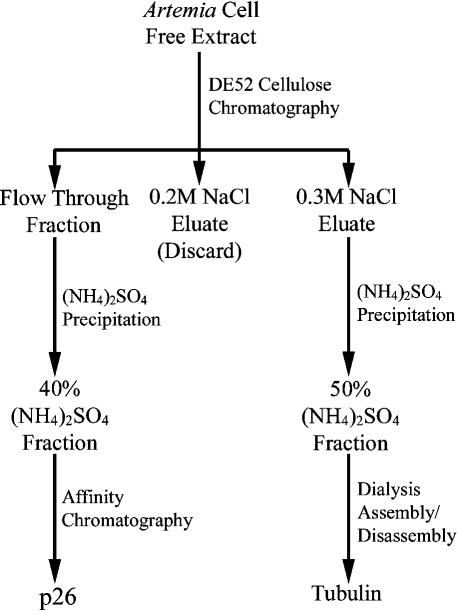
p26 and tubulin were prepared from Artemia embryos by initially applying 40 mL of cell-free extract to diethylaminoethyl (DE52) cellulose (Whatman, Mandel Scientific Co. Ltd, Guelph, Ontario, Canada) columns (2.5 × 18 cm) previously washed with 0.5× Pipes buffer containing 1 M NaCl and equilibrated with Pipes buffer (Fig 1). Columns were washed with Pipes buffer after application of cell-free extract, and adhering proteins were removed by sequential washes with Pipes buffer containing 0.2 M and 0.3 M NaCl. Flow-through and 0.3 M NaCl protein fractions from DE52 columns were retained, whereas the 0.2 M NaCl fraction was discarded.
Fig 1.
Flow chart representing the simplified purification procedure for p26 and tubulin from encysted Artemia embryos
For purification of p26, powdered (NH4)2SO4 was added with stirring to the DE52 flow-through fraction to a final concentration of 40% (22 g/100 mL at 4°C); the mixture was stirred for 15 minutes and then centrifuged at 12 000 × g for 10 minutes at 4°C. The precipitate was resuspended in 8 mL of Pipes buffer and stored at −70°C in 1-mL aliquots. Approximately 70 mg of an IgG fraction obtained by the use of Protein A Sepharose from anti-p26 rabbit serum (Liang and MacRae 1999) was incubated in MOPS buffer with 2 mL of Affi-Gel 10 to prepare an affinity column, which was washed with 0.1 M glycine, pH 2.5, equilibrated in PBS, and stored at 4°C. Two milliliters of 40% (NH4)2SO4 fraction and 2 mL of PBS were added to affinity matrices before incubation, with gentle agitation for 1 hour at 4°C. Matrices were drained and washed twice with PBS, followed by PBS containing 0.25 M NaCl and 0.1 M Tris-glycine, pH 7.4. p26 was eluted from the matrix with 0.1 M glycine, pH 2.5, into tubes containing 0.1 volume of 1.0 M Tris, pH 8.0.
Discontinuous 9-mL sucrose gradients for analysis of p26 oligomerization were generated by layering equal volumes of 50%, 30%, and 10% sucrose dissolved in 0.1 M Tris-glycine buffer, pH 7.4, and centrifuging at 200 000 × g for 3 hours at 4°C. Affinity-purified p26 was applied to gradients and centrifuged at 200 000 × g for 21 hours at 4°C in a Beckman SW41TI rotor. Fractions of 0.8 mL were collected through tube bottoms punctured with an 18-gauge needle, after which p26 was detected by electrophoresis in SDS polyacrylamide gels, blotting to nitrocellulose, and immunoprobing.
Tubulin was prepared from 0.3 M NaCl DE52 cellulose fractions by addition of (NH4)2SO4 to 50% saturation (29.1 g/100 mL at 4°C), by overnight dialysis against Pipes buffer containing 1.8 mM guanosine triphosphate (GTP) and 8 M glycerol, and by assembly/disassembly, all as described previously, with the exception that chromatography on phosphocellulose was omitted (MacRae and Ludueña 1984). Purified Artemia tubulin at a final concentration of 1.0 mg/mL was assembled by incubation at 37°C for 30 minutes in Pipes buffer containing 1.8 mM GTP, after which 5 μL of the sample was placed on carbon-stabilized, formvar-coated copper grids, negatively stained with 1% uranyl acetate, and examined under a Philips 201 electron microscope.
Chaperoning of tubulin by p26 in vitro
Tubulin at a final concentration of 1.1 mg/mL was incubated either in the absence or in the presence of p26 for 8 hours at 35–37°C in covered 96-well microplates (Castor, Fisher Scientific Ltd, Whitby, Ontario, Canada), using a SPECTRA max PLUS384 Microplate Spectrophotometer. BSA was added as a negative control to demonstrate that p26 effects on turbidity development were specific and not merely due to the presence of a protein other than tubulin in the assay mixture. Solution turbidity was measured at 350 nm every 5 minutes after agitation for 3 seconds. Five-microliter samples were removed after 6 hours of incubation and examined under an electron microscope to ensure that turbidity increase was not due to tubulin assembly. Reaction mixtures containing tubulin and p26 were also examined for association of these proteins, wherein entire reaction mixtures of 250 μL were applied to 10 mL of 17–40% continuous sucrose gradients in 0.1 M Tris-glycine buffer, pH 7.4, and centrifuged at 200 000 × g for either 21 or 3 hours at 4°C in a Beckman SWTi40 rotor. Gradients were fractionated by collecting samples of approximately 0.7 mL after tube bottoms were punctured with an 18-gauge needle. Tubulin and p26 were localized by electrophoresis of samples from each gradient fraction in 12% SDS polyacrylamide gels, blotting to nitrocellulose, and probing with antibodies. Molecular mass markers, 29 kDa (carbonic anhydrase), 66 kDa (BSA), 150 kDa (alcohol dehydrogenase), 200 kDa (β-amylase), 443 kDa (apoferritin), and 669 kDa (thyroglobulin) (Sigma, St Louis, MO, USA), were centrifuged in separate gradients and their positions determined by reading the absorbance at 280 nm for each sample obtained on gradient fractionation.
Electrophoresis, Western blotting, and protein immunodetection
Electrophoresis was done in 12.5%, 10%, or 7–14% gradient SDS polyacrylamide gels, followed by staining with Coomassie blue. Molecular weight standards were from BioRad Laboratories Ltd. Proteins were also transferred to Protram™ nitrocellulose membranes (Mandel Scientific Co. Ltd) after electrophoresis (Towbin et al 1979). Blots were stained with Ponceau S (Sigma) to ensure efficient protein transfer, destained, and probed. Primary antibodies to p26 (Liang and MacRae 1999) and α/β-tubulin (Day et al 2000) were raised in rabbit, whereas the monoclonal anti–α-tubulin antibody, DM 1A (Sigma), was from mouse. All antibodies were diluted in 10 mM Tris-HCl, 140 mM NaCl, 0.1% (v/v) Tween 20, pH 7.4 (TBS-Tween), and incubated with blots for 15 minutes at room temperature with vigorous agitation. Membranes were washed and then incubated for 15 minutes at room temperature, with peroxidase-conjugated secondary antibodies diluted in 10 mM Tris-HCl, 1 M NaCl, 0.5% (v/v) Tween 20, pH 7.4 (HST), including goat anti-rabbit IgG (Bio/Can Scientific, Mississauga, Ontario, Canada), goat anti-mouse IgG (Sigma) and mouse anti-rabbit IgG, and γ-chain–specific clone RG-96 (Sigma), which recognizes only the heavy chain of IgG. Blots were washed after secondary antibody exposure, and immunoreactive proteins were revealed by use of the enhanced chemiluminescence technique (Renaissance®, NEM™, Life Science Products Inc, Boston, MA, USA) and exposure to autoradiography film (Labscientific, Livingston, NJ, USA).
RESULTS
Association of tubulin and p26 in cell-free extracts of encysted Artemia embryos
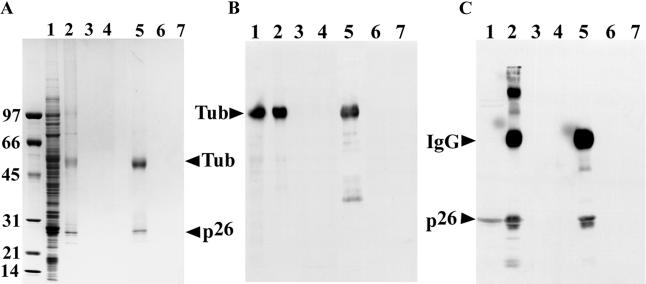
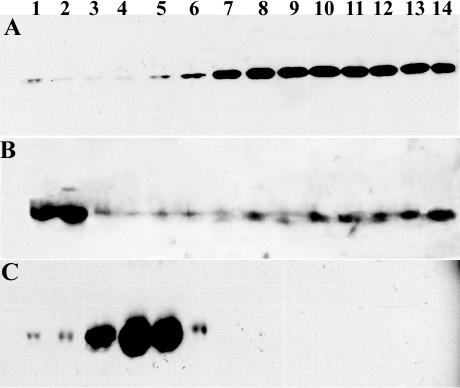
Incubation of cell-free extracts from Artemia embryos with anti-p26 antibody covalently linked to Protein A Sepharose, followed by electrophoresis in SDS polyacrylamide gels and Coomassie blue staining yielded protein bands of the size expected for tubulin and p26 (Fig 2A, lane 2), in addition to other lighter-staining bands. In contrast, when preimmune IgG was used to construct affinity matrices, no proteins were observed in Commassie blue–stained gels (Fig 2A, lane 3), as was true when affinity matrices were incubated with Pipes buffer rather than with cell-free extract (Fig 2A, lane 4). Similar results were obtained when Affi-Gel 10 was substituted for Protein A Sepharose (Fig 2A, lanes 5–7). To determine whether the major gel bands at approximately 55 kDa and 26 kDa represented tubulin and p26, respectively, samples were blotted to nitrocellulose and probed with polyclonal antibodies to p26 raised in rabbit (Liang and MacRae 1999) and tubulin (Day et al 2000), followed by peroxidase-conjugated goat anti-rabbit IgG secondary antibody. Although reactive polypeptides of the appropriate size were obtained, Western blots incubated with PBS lacking primary antibody also produced bands of the same molecular mass, indicating release of IgG from affinity matrices (not shown). In light of this observation, DM 1A, a monoclonal antibody raised in mouse, was used with goat anti-mouse IgG secondary antibody, shown in preliminary studies not to recognize rabbit IgG, to determine the location of tubulin on blots. Additionally, anti-rabbit IgG high–molecular weight chain (γ-chain specific, clone RG-96) secondary antibody was used with polyclonal anti-p26 antibody to detect p26. The anti-rabbit IgG high–molecular weight γ-chain–specific secondary antibody reacted with high– but not low–molecular weight polypeptides of the anti-p26 antibody used in the affinity columns. By using the antibodies just described, both types of affinity matrices constructed with anti-p26 IgG were shown to capture p26 and tubulin, whereas neither matrix containing preimmune IgG yielded these proteins (Figs 2 B,C). These results indicate that tubulin and p26 were associated with one another in cell-free extracts of encysted Artemia embryos.
Fig 2.
Association of tubulin and p26 in cell-free extracts of encysted Artemia embryos. (A) Affinity matrices were constructed by coupling Protein A Sepharose (lanes 2–4) and Affi-Gel 10 (lanes 5–7) with anti-p26 immunoglobulin (IgG) (lanes 2, 4, 5, 7) and preimmune IgG (lanes 3, 6). The affinity matrices were incubated with either Artemia cell-free extract (lanes 2, 3, 5, 6) or Pipes buffer (lanes 4, 7), harvested, heated in treatment buffer, electrophoresed in sodium dodecyl sulfate (SDS) polyacrylamide gels, and stained with Coomassie blue. Lane 1: 60 μg of Artemia cell-free extract protein. The positions of tubulin and p26 are indicated by labeled arrowheads. Molecular weight markers (×10−3) are on the left side of the figure. (B) Affinity matrices were constructed with Protein A Sepharose (lanes 2–4) and Affi-Gel 10 (lanes 5–7) coupled to anti-p26 IgG (lanes 2, 5), preimmune IgG (lanes 3, 6), and no IgG (lanes 4, 7). These affinity matrices were incubated with Artemia cell-free extracts, harvested, heated in treatment buffer, electrophoresed in SDS polyacrylamide gels, blotted to nitrocellulose, and probed with DM 1A followed by peroxidase-conjugated goat anti-mouse IgG secondary antibody. Lane 1: 30 μg of Artemia cell-free extract. (C) The same as panel B except that the membrane was probed with anti-p26 antibody followed by peroxidase-conjugated anti-rabbit IgG heavy-chain (α-chain specific, clone RG-96) secondary antibody
Preparation of tubulin and p26
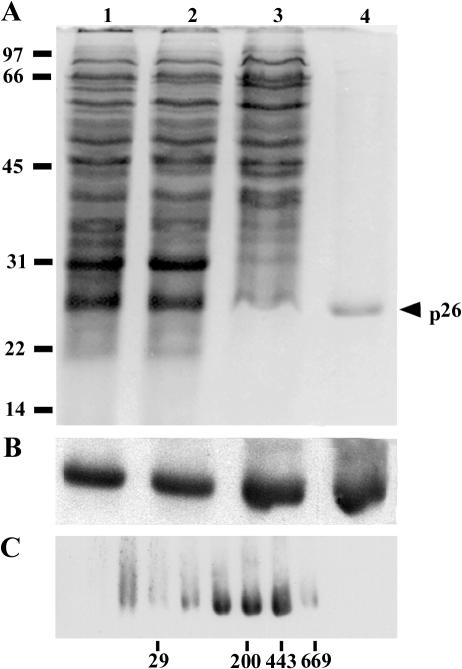
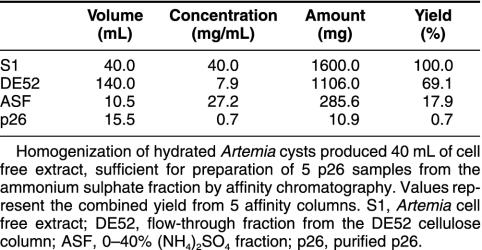
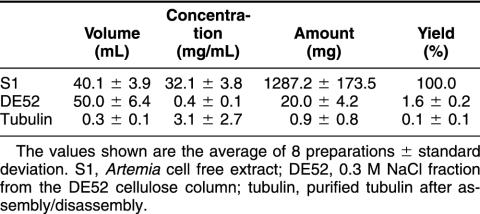
Established procedures for purification of p26 (Liang et al 1997a) and tubulin (MacRae and Ludueña 1984) from Artemia were modified such that the proteins were obtained from the same cell-free extract (Fig 1). Specifically, 40% (NH4)2SO4 samples prepared from DE52 cellulose column flow-through fractions were applied to p26 affinity columns, and adhering proteins were eluted with low-pH citrate buffer, yielding a major low–molecular weight protein with minor amounts of high–molecular weight contaminants in some preparations (Fig 3A). The low–molecular weight protein reacted strongly with antibody to p26, confirming its identity (Fig 3B), and approximately 11 mg of p26 representing 0.7% of starting protein was recovered (Table 1). Purified p26 consisted of a heterogeneous oligomer population that varied in composition between preparations (Fig 3C). Maximum oligomer mass was 669 kDa, but most of the p26 mass was in the range of 150–443 kDa. For a monomer mass of 20.7 kDa (Liang et al 1997a), oligomers were composed of 7–21 subunits, although monomers and larger assemblages were present.
Fig 3.
Purification and oligomerization of p26. Protein fractions obtained during p26 purification from encysted Artemia embryos were electrophoresed in sodium dodecyl sulfate (SDS) polyacrylamide gels and either stained with Coomassie blue (A) or transferred to nitrocellulose and probed with antibody to p26 (B). Lane 1: cell-free extract, 100 μg in A and 25 μg in B; lane 2: flow-through fraction from diethylaminoethyl cellulose, 80 μg in A and 20 μg in B; lane 3: ammonium sulfate fraction, 80 μg in A and 20 μg in B; lane 4: purified p26, 3.5 μg in A and 0.9 μg in B. (C) Purified p26 was centrifuged in discontinuous 10–50% sucrose gradients, after which samples from gradient fractions were electrophoresed in SDS polyacrylamide gels, blotted to nitrocellulose, and probed with anti-p26 antibody. The top of the gradient is to the left, and positions of molecular weight markers (×10−3) are indicated at the bottom and left of the figure. Labeled arrowhead, p26
Table 1.
Purification of p26
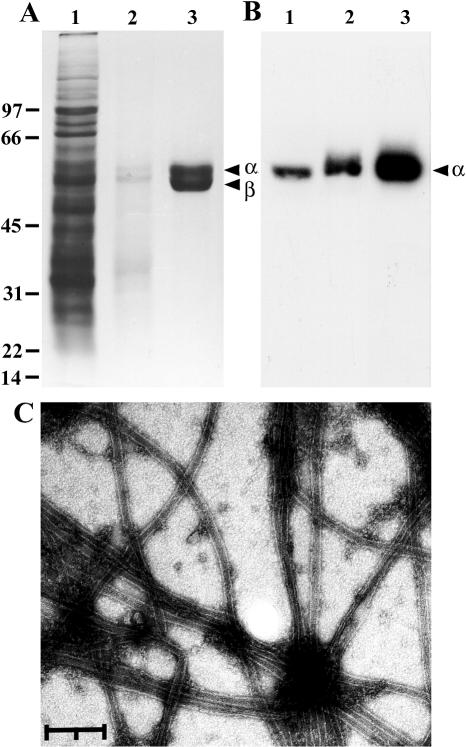
Much of the protein applied to DE52 cellulose columns either eluted in the loading buffer, there being remarkably little difference between the protein composition of cell-free extract and DE52 cellulose flow-through fractions, or was removed with Pipes buffer containing 0.2 M NaCl, after which tubulin was recovered in Pipes buffer supplemented with 0.3 M NaCl (Fig 4A). Tubulin was precipitated with 50% (NH4)2SO4, dialyzed overnight at 4°C against Pipes buffer containing 8 M glycerol, diluted 1:1 with Pipes buffer, and assembled/disassembled to remove contaminants. Purified tubulin consisted of 2 polypeptides, and reaction with DM 1A identified α-tubulin (Fig 4 A,B). Approximately 1300 mg of cell-free extract protein gave 0.9 mg of tubulin, equivalent to about 0.1% of starting protein (Table 2). Purified tubulin assembled into morphologically normal microtubules, as shown by uranyl acetate staining and examination by transmission electron microscopy (Fig 4C).
Fig 4.
Purification of Artemia tubulin. Protein fractions obtained during purification of Artemia tubulin were electrophoresed in sodium dodecyl sulfate polyacrylamide gels and either stained with Coomassie blue (A) or blotted to nitrocellulose and reacted with DM 1A (B). Lane 1: cell-free extract, 65 μg in A and 30 μg in B; lane 2: 0.3 M NaCl fraction from DE52 cellulose, 5 μg in A and 2.5 μg in B; lane 3: purified tubulin, 9 μg in A and 2 μg in B. Labeled arrowheads, α- and β-tubulins; molecular weight markers (×10−3), left side of diagram. (C) Purified Artemia tubulin at 1.0 μg/μL was incubated at 37°C for 30 minutes in the presence of guanosine triphosphate, placed on carbon-coated, formvar-covered grids, negatively stained with uranyl acetate, and examined by transmission electron microscopy. The bar represents 0.2 μm
Table 2.
Purification of tubulin
Chaperoning of Artemia tubulin by p26
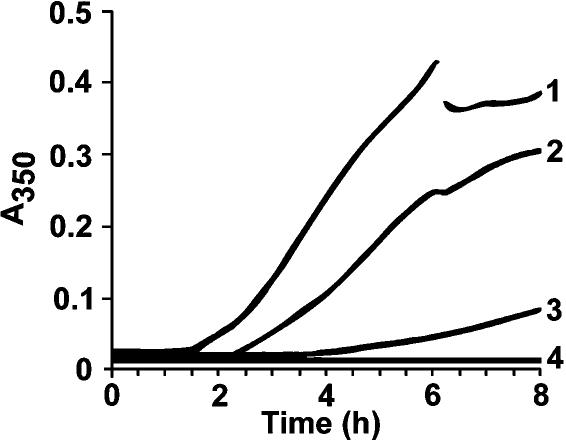
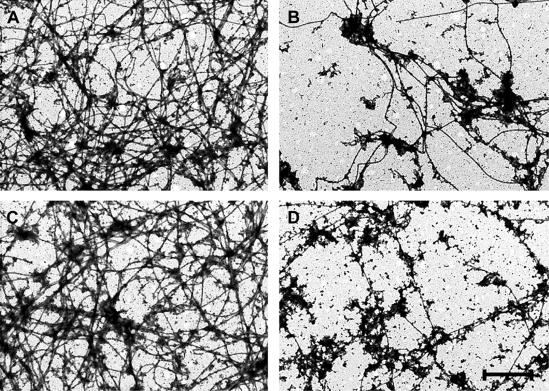
The turbidity of purified Artemia tubulin solutions remained low for approximately 2 hours when incubated at 35°C and then increased rapidly for 3 hours before reaching an A350 of approximately 0.4 after 8 hours (Fig 5, curve 1). The inflections in this and other curves at 6 hours occurred on removal of 5-μL samples to check for tubulin assembly. Addition of p26 to reaction mixtures delayed turbidity development, and final absorbance was reduced substantially in the presence of p26 (Fig 5, curve 3). BSA lowered final turbidity slightly on heating but to a much lesser extent than p26 (Fig 5, curve 2). The turbidity of solutions containing only p26 (Fig 5, curve 4) or BSA (not shown) changed little on heating. Doubling the concentration of p26 yielded an even greater reduction in turbidity, an effect that persisted for as long as 22 hours (not shown), although this phenomenon has not been studied in detail. Tubulin incubated at 35°C for several hours assembled poorly in comparison with unheated tubulin (Fig 6 A,B), but microtubules were morphologically normal in both cases. Unheated tubulin mixed with p26 polymerized readily, but after exposure to 35°C for 6 hours, assembly was reduced (Fig 6 C,D), even though turbidity measurements indicated only limited tubulin denaturation. Tubulin heated in the presence of p26 advanced further down sucrose density gradients on centrifugation than did tubulin heated in Pipes buffer in the absence of p26 (Fig 7). Tubulin was also incubated in the absence of p26 in 0.1 M Tris-glycine buffer, pH 7.4, before centrifugation on sucrose gradients, with the same result (not shown). p26 comigrated with tubulin and moved a greater distance down sucrose gradients than was expected on the basis of oligomer size of the purified protein. The results indicate complex formation between tubulin and p26, perhaps causing the reduction in tubulin assembly observed after heating at 35°C.
Fig 5.
Chaperoning of Artemia tubulin by p26. Purified Artemia tubulin at 1.1 μg/μL was incubated at 35°C for 8 hours in the presence or absence of p26, with solution turbidity measured every 5 minutes at 350 nm. Reaction mixtures contained 1, tubulin only; 2, tubulin and 0.25 μg/μL bovine serum albumin; 3, tubulin and purified p26 at 0.25 μg/μL; 4, p26 only at 0.25 μg/μL. Five-microliter samples were removed at 6 hours, resulting in inflection of the curves
Fig 6.
Assembly of Artemia tubulin after incubation at 35°C. Artemia tubulin was assembled before (A, C) and after (B, D) incubation for 6 hours at 35°C in the absence (A, B) or presence (C, D) of p26. Magnification is the same for all panels, and the bar in D represents 2 μm
Fig 7.
Association of purified tubulin and p26. Purified Artemia tubulin at 1.2 μg/μL was incubated at 35°C for 7 hours in the presence (A, B) or absence (C) of p26 in Pipes buffer. Solutions were centrifuged at 200 000 × g for 3 (A, B) or 22 hours (C) in 17–40% continuous sucrose gradients and fractionated, and samples from each fraction were electrophoresed in sodium dodecyl sulfate polyacylamide gels before blotting to nitrocellulose and immunostaining. The membranes were probed with DM 1A (A), anti-p26 (B), and α/β-tubulin (C). The top of each gradient is to the left
DISCUSSION
The adsorption of cell-free extracts from encysted Artemia embryos to affinity matrices containing anti-p26 IgG revealed association of tubulin with p26, presumably to protect tubulin in vivo from irreversible denaturation during encystment and diapause. During these studies it was noted that incubation of cyst cell–free extract with affinity matrices containing anti-p26 IgG caused release of antibody even though the matrices were washed thoroughly before extract addition. In contrast, IgG was not released when cyst cell–free extract was mixed with matrices constructed with preimmune IgG. Apart from representing technical difficulties overcome with the use of appropriate primary and secondary antibodies, the observations may be instructive of p26 function. For example, it is intriguing that antibody was released from matrices constructed with immune IgG each time p26 was captured from cell-free extract, but matrices containing preimmune IgG did not lose antibodies under the same conditions. Seemingly, the interaction of p26 with IgG caused a change in the latter that permitted release, and resolution of this process may reveal mechanisms related to the function of p26 and other small heat shock/α-crystallin proteins.
Published protocols for purification of tubulin (MacRae and Ludueña 1984) and p26 (Liang et al 1997a) were combined by eliminating Phosphocellulose P-11 chromatography. As before, purified tubulin appeared homogeneous, but lower returns pertained, even though the procedure was quicker. Reduced yields may either reflect the use of different cysts or result from lingering contaminants normally removed by Phosphocellulose P-11 and that affect tubulin polymerization after dialysis. On the other hand, elimination of Phosphocellulose P-11 chromatography doubled p26 recoveries compared with those reported previously from this laboratory (Liang et al 1997a), facilitated purification of the protein (Liang et al 1997a; Viner and Clegg 2001), and generated adequate p26 and tubulin for testing of chaperone activity in vitro.
This report represents the first time small heat shock/α-crystallin protein chaperoning of tubulin has been studied in a homogeneous system subject to physiological stress and where potentially important interactions between these proteins were known to occur. In other work, drug-induced microtubule disassembly in C6 glioma cells enhanced accumulation of αB crystallin but not Hsp27 (Kato et al 1996). Additionally, the overexpression of αB crystallin, but not Hsp27, improved microtubule preservation in rat cardiac myocytes exposed to simulated ischemia (Bluhm et al 1998). However, in neither case was recognition of tubulin by small heat shock/α-crystallin proteins demonstrated. Arai and Atomi (1997) reported association of αB-crystallin with tubulin in extracts of nonstressed L6 cells and that bovine αB-crystallin complexed with porcine brain tubulin when heated at 37°C. Unexpectedly, αB-crystallin was more effective at 0.05 mg/mL than at higher concentrations in preventing tubulin aggregation in vitro. Hsp27 colocalized with mitotic but not interphase microtubules in immunofluorescently stained HeLa cells (Hino et al 2000). Reaction between Hsp27 and tubulin or microtubules was shown by antibody “pull down” experiments in the same study and by cosedimentation of Hsp27 with taxol-induced microtubules from mitotic and interphase HeLa cells. Stressed cells and purified proteins were not examined in these experiments.
The turbidity of Artemia tubulin solutions increased when incubated at 35°C, but this was almost completely suppressed for 8 hours by approximately equimolar amounts of p26 monomers, a chaperone : substrate ratio of 1:4 (w/w). If the average molecular mass of oligomers is taken as 200 kDa (Fig 3), then the molar ratio of p26 to tubulin when almost complete inhibition of turbidity increase is achieved was 1:4. Monomers and small oligomers of bovine αB-crystallin suppressed porcine tubulin aggregation for 6 hours at 37°C; however, an αB-crystallin : tubulin ratio of 1:30 (w/w) was more effective than ratios of 1:15, 1:7.5, and 1:5 (Arai and Atomi 1997). Although p26 suppressed tubulin aggregation, the chaperone : substrate molar ratio and hence the role of oligomerization in chaperoning can only be estimated, given the heterogeneity of p26 quaternary structure. It has been proposed that proteins associate with the exteriors of small heat shock/α-crystallin protein oligomers and because of steric hindrance the numbers that are bound depend on substrate size (Leroux et al 1997; Kumar and Rao 2000), but several reports run counter to this proposal (Marini et al 2000; Feil et al 2001; Sreelakshmi and Sharma 2001; Crack et al 2002). In other models, oligomers are storage forms of small heat shock/α-crystallin proteins with chaperone function dependent on multimer disassembly and subunit exchange (Van Montfort et al 2001; Bera and Abraham 2002; Cobb and Petrash 2002; Gu et al 2002), but again not all reports support this idea (Borrelli et al 2002).
The defining activity of α/β-tubulin is the ability to assemble into microtubules. Qualitative analysis of negatively stained samples by transmission electron microscopy demonstrated that tubulin incubated for 6 hours at 35°C assembled poorly as compared with nonincubated samples, regardless of reduced protein denaturation in the presence of p26. It would be instructive, pending availability of sufficient tubulin and p26, to quantitate tubulin polymerization, but this may add little to the key observation that assembly is reduced by heating whether or not p26 is present. In comparison, α-crystallin, murine Hsp25, and human Hsp27 fail to alter inactivation kinetics of denaturing enzymes, although turbidity development is prevented in stressed solutions (Rao et al 1993; Ehrnsperger et al 1997; Wang and Spector 2000). Exceptions to this observation include protection of lens sorbitol dehydrogenase and glucose-6-phosphate activity by α-crystallin (Marini et al 2000; Reddy et al 2001). Rajaraman et al (2001) also showed that transient recognition of citrate synthase by α-crystallin reduces inactivation of the former, but this mechanism is not applicable to all enzymes. The interplay between suppression of denaturation and protein functional inactivation may depend on the nature of the substrate, as suggested by Kitagawa et al (2002) in their study of IbpA and IbpB from Escherichia coli. Moreover, ATP and other molecular chaperones are usually required to regenerate enzyme activity after stress but do so only when denaturation occurs in the presence of small heat shock/α-crystallin proteins (Ehrnsperger et al 1997; Lee and Vierling 2000; Török et al 2001; Wang and Spector 2001). p26 could therefore function as a protein scaffold, binding tubulin and preventing irreversible aggregation. Demonstration of complex formation between tubulin and p26 supports the “scaffold hypothesis,” a possibility even more plausible given that tubulin exposes hydrophobic regions on denaturation (Sarkar et al 1995; Guha and Bhattacharyya 1997; Roychowdhury et al 2000).
To conclude, a small heat shock/α-crystallin protein from encysted Artemia embryos was shown to chaperone tubulin, a substrate protein from the same physiologically stressed organism. Although purified p26 prevented heat-induced denaturation of tubulin for several hours, assembly competence was reduced, possibly because of complex formation between the molecules. This may mimic the function of p26 in vivo, wherein proteins of encysting or diapause embryos bind p26 and are protected until resumption of metabolism and development. At this time, p26 releases substrate proteins, native conformations are restored either spontaneously or with the help of other chaperones, and proteins regain their functions, which in the case of tubulin is polymerization into microtubules.
Acknowledgments
The work was supported by a Natural Sciences and Engineering Research Council of Canada Research Grant and a Nova Scotia Health Research Foundation New Opportunity Grant to T.H.M.
REFERENCES
- Agueli C, Geraci F, Giudice G, Chimenti L, Cascino D, Sconzo G. A constitutive 70 kDa heat shock protein is localized on the fibres of spindles and asters at metaphase in an ATP-dependent manner: a new chaperone role is proposed. Biochem J. 2001;360:413–419. doi: 10.1042/0264-6021:3600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP, Patel HC, Xi J-H. The R116C mutation in αA-crystallin diminishes its protective activity against stress-induced lens epithelial cell apoptosis. J Biol Chem. 2002;277:10178–10186. doi: 10.1074/jbc.M109211200. [DOI] [PubMed] [Google Scholar]
- Arai H, Atomi Y. Chaperone activity of αB-crystallin suppresses tubulin aggregation through complex formation. Cell Struct Funct. 1997;22:539–544. doi: 10.1247/csf.22.539. [DOI] [PubMed] [Google Scholar]
- Bera S, Abraham EC. The αA-crystallin R116C mutant has a higher affinity for forming heteroaggregates with αB-crystallin. Biochemistry. 2002;41:297–305. doi: 10.1021/bi011010v. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, Martin JL, Mestril R, Dillmann WH. Specific heat shock proteins protect microtubules during simulated ischemia in cardiac myocytes. Am J Physiol. 1998;275:H2243–H2249. doi: 10.1152/ajpheart.1998.275.6.H2243. [DOI] [PubMed] [Google Scholar]
- Borrelli MJ, Bernock LJ, Landry J, Spitz DR, Weber LA, Hickey E, Freeman ML, Corry PM. Stress protection by a fluorescent Hsp27 chimera that is independent of nuclear translocation or multimeric dissociation. Cell Stress Chaperones. 2002;7:281–296. doi: 10.1379/1466-1268(2002)007<0281:spbafh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver JA, Lindner RA, Lyon C, Canet D, Hernandez H, Dobson CM, Redfield C. The interaction of the molecular chaperone α-crystallin with unfolding α-lactalbumin: a structural and kinetic spectroscopic study. J Mol Biol. 2002;318:815–827. doi: 10.1016/S0022-2836(02)00144-4. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA. The metabolic status of quiescent and diapause embryos of Artemia franciscana (Kellogg) Arch Hydrobiol. 1998;52:425–439. [Google Scholar]
- Clegg JS, Jackson SA, Liang P, MacRae TH. Nuclear-cytoplasmic translocations of protein p26 during aerobic-anoxic transitions in embryos of Artemia franciscana. Exp Cell Res. 1995;219:1–7. doi: 10.1006/excr.1995.1197. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA, Popov VI. Long-term anoxia in encysted embryos of the crustacean, Artemia franciscana: viability, ultrastructure, and stress proteins. Cell Tissue Res. 2000;301:433–446. doi: 10.1007/s004410000249. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Willsie JK, Jackson SA. Adaptive significance of a small heat shock/α-crystallin protein (p26) in encysted embryos of the brine shrimp, Artemia franciscana. Am Zool. 1999;39:836–847. [Google Scholar]
- Cobb BA, Petrash JM. α-Crystallin chaperone-like activity and membrane binding in age-related cataracts. Biochemistry. 2002;41:483–490. doi: 10.1021/bi0112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack JA, Mansour M, Sun Y, MacRae TH. Functional analysis of a small heat shock/α-crystallin protein from Artemia franciscana: oligomerization and thermotolerance. Eur J Biochem. 2002;269:933–942. doi: 10.1046/j.0014-2956.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- Day R, Criel GRJ, Walling MA, MacRae TH. Posttranslationally modified tubulins and microtubule organization in hemocytes of the brine shrimp, Artemia franciscana. J Morphol. 2000;244:153–166. doi: 10.1002/(SICI)1097-4687(200006)244:3<153::AID-JMOR1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Drinkwater LE, Clegg JS 1991 Experimental biology of cyst diapause. In: Artemia Biology, ed Browne RA, Sorgeloos P, Trotman CNA. CRC Press, Boca Raton, FL, 93–117. [Google Scholar]
- Dutcher SK. The tubulin fraternity: alpha to eta. Curr Opin Cell Biol. 2001;13:49–54. doi: 10.1016/s0955-0674(00)00173-3. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil IK, Malfois M, Hendle J, van der Zandt H, Svergun DI. A novel quaternary structure of the dimeric α-crystallin domain with chaperone-like activity. J Biol Chem. 2001;276:12024–12029. doi: 10.1074/jbc.M010856200. [DOI] [PubMed] [Google Scholar]
- Garnier C, Barbier P, Gilli R, Lopez C, Peyrot V, Briand C. Heat-shock protein 90 (hsp90) binds in vitro to tubulin dimer and inhibits microtubule formation. Biochem Biophys Res Commun. 1998;250:414–419. doi: 10.1006/bbrc.1998.9319. [DOI] [PubMed] [Google Scholar]
- Goenka S, Raman B, Ramakrishma T, Rao ChM. Unfolding and refolding of a quinone oxidoreductase: α-crystallin, a molecular chaperone assists its reactivation. Biochem J. 2001;359:547–556. doi: 10.1042/0264-6021:3590547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Abulimiti A, Li W, Chang Z. Monodisperse Hsp16.3 nonamer exhibits dynamic dissociation and reassociation, with the nonamer dissociation prerequisite for chaperone-like activity. J Mol Biol. 2002;319:517–526. doi: 10.1016/S0022-2836(02)00311-X. [DOI] [PubMed] [Google Scholar]
- Guha S, Bhattacharyya B. Refolding of urea-denatured tubulin: recovery of nativelike structure and colchicine binding activity from partly unfolded states. Biochemistry. 1997;36:13208–13213. doi: 10.1021/bi970993m. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D 1999 Using Antibodies, A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Hino M, Kurogi K, Okubo M-A, Murata-Hori M, Hosoya H. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun. 2000;271:164–169. doi: 10.1006/bbrc.2000.2553. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Inaguma Y, Okamoto K, Saga S. Synthesis and accumulation of αB crystallin in C6 glioma cells is induced by agents that promote the disassembly of microtubules. J Biol Chem. 1996;271:26989–26994. doi: 10.1074/jbc.271.43.26989. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Miyakawa M, Matsumura Y, Tsuchido T. Escherichia coli small heat shock proteins, IbpA and IbpB, protect enzymes from inactivation by heat and oxidants. Eur J Biochem. 2002;269:2907–2917. doi: 10.1046/j.1432-1033.2002.02958.x. [DOI] [PubMed] [Google Scholar]
- Kumar LVS, Rao ChM. Domain swapping in human αA and αB crystallins affects oligomerization and enhances chaperone-like activity. J Biol Chem. 2000;275:22009–22013. doi: 10.1074/jbc.M003307200. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Vierling E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000;122:189–198. doi: 10.1104/pp.122.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux MR, Melki R, Gordon B, Batelier G, Candido EPM. Structure-function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides. J Biol Chem. 1997;272:24646–24656. doi: 10.1074/jbc.272.39.24646. [DOI] [PubMed] [Google Scholar]
- Liang P, Amons R, MacRae TH, Clegg JS. Purification, structure and in vitro molecular-chaperone activity of Artemia p26, a small heat shock/α-crystallin protein. Eur J Biochem. 1997a;243:225–232. doi: 10.1111/j.1432-1033.1997.0225a.x. [DOI] [PubMed] [Google Scholar]
- Liang P, Amons R, Clegg JS, MacRae TH. Molecular characterization of a small heat shock/α-crystallin protein in encysted Artemia embryos. J Biol Chem. 1997b;272:19051–19058. doi: 10.1074/jbc.272.30.19051. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110:1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of a small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138. [DOI] [PubMed] [Google Scholar]
- Llorca O, Martin-Benito J, Gómez-Puertas P, Ritco-Vonsovici M, Willison KR, Carrascosa JL, Valpuesta JM. Analysis of the interaction between the eukaryotic chaperonin CCT and its substrates actin and tubulin. J Struct Biol. 2001;135:205–218. doi: 10.1006/jsbi.2001.4359. [DOI] [PubMed] [Google Scholar]
- Lopez-Fanarraga M, Avila J, Guasch A, Coll M, Zabala JC. Review: postchaperonin tubulin folding cofactors and their role in microtubule dynamics. J Struct Biol. 2001;135:219–229. doi: 10.1006/jsbi.2001.4386. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MacRae TH. Tubulin post-translational modifications: enzymes and their mechanisms of action. Eur J Biochem. 1997;244:265–278. doi: 10.1111/j.1432-1033.1997.00265.x. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH 2001 Do stress proteins protect embryos during metabolic arrest and dormancy? In: Molecular Mechanisms of Metabolic Arrest: Life in Limbo, ed Storey KB. BIOS Scientific Publishers, Oxford, 169–186. [Google Scholar]
- MacRae TH, Ludueña RF. Developmental and comparative aspects of brine shrimp tubulin. Biochem J. 1984;219:137–148. doi: 10.1042/bj2190137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna T, Sarkar T, Poddar A, Roychowdhury M, Das KP, Bhattacharyya B. Chaperone-like activity of tubulin: binding and reactivation of unfolded substrate enzymes. J Biol Chem. 2001;276:39742–39747. doi: 10.1074/jbc.M104061200. [DOI] [PubMed] [Google Scholar]
- Mao Y-W, Xiang H, Wang J, Korsmeyer S, Reddan J, Li DW-C. Human bcl-2 gene attenuates the ability of rabbit lens epithelial cells against H2O2-induced apoptosis through down-regulation of αB-crystallin gene. J Biol Chem. 2001;276:43435–43445. doi: 10.1074/jbc.M102195200. [DOI] [PubMed] [Google Scholar]
- Marini I, Moschini R, Corso AD, Mura U. Complete protection by α-crystallin of lens sorbitol dehydrogenase undergoing thermal stress. J Biol Chem. 2000;275:32559–32565. doi: 10.1074/jbc.M006133200. [DOI] [PubMed] [Google Scholar]
- Merendino AM, Paul C, and Vignola AM. et al. 2002 Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: possible implication in asthma. Cell Stress Chaperones. 7:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F. α-Crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo A-P. Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman K, Raman B, Ramakrishna T, Rao ChM. Interaction of human recombinant αA- and αB-crystallins with early and late unfolding intermediates of citrate synthase on its thermal denaturation. FEBS Lett. 2001;497:118–123. doi: 10.1016/s0014-5793(01)02451-6. [DOI] [PubMed] [Google Scholar]
- Rao PV, Horwitz J, Zigler JS Jr.. Alpha-crystallin, a molecular chaperone, forms a stable complex with carbonic anhydrase upon heat denaturation. Biochem Biophys Res Commun. 1993;282:786–793. doi: 10.1006/bbrc.1993.1118. [DOI] [PubMed] [Google Scholar]
- Reddy GB, Reddy PY, Suryanarayana P. αA- and αB-crystallins protect glucose-6-phosphate dehydrogenase against UVB irradiation-induced inactivation. Biochem Biophys Res Commun. 2001;282:712–716. doi: 10.1006/bbrc.2001.4642. [DOI] [PubMed] [Google Scholar]
- Roychowdhury M, Sarkar N, Manna T, Bhattacharyya S, Sarkar T, BasuSarkar P, Roy S, Bhattacharyya B. Sulfhydryls of tubulin: a probe to detect conformational changes of tubulin. Eur J Biochem. 2000;267:3469–3476. doi: 10.1046/j.1432-1327.2000.01369.x. [DOI] [PubMed] [Google Scholar]
- Samali A, Robertson JD, and Peterson E. et al. 2001 Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 6:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar T, Manna T, and Bhattacharyya S. et al. 2001 Role of the carboxy-termini of tubulin on its chaperone-like activity. Proteins: Struct Funct Genet. 44:262–269. [DOI] [PubMed] [Google Scholar]
- Sarkar N, Mukhopadhyay K, Parrack PK, Bhattacharyya B. Aging of tubulin monomers using 5,5′-bis (8-anilino-1-naphthalenesulfonate) as a probe. Biochemistry. 1995;34:13367–13373. doi: 10.1021/bi00041a013. [DOI] [PubMed] [Google Scholar]
- Scharf K-D, Siddique M, Vierling E. The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing α-crystallin domains (Acd proteins) Cell Stress Chaperones. 2001;6:225–237. doi: 10.1379/1466-1268(2001)006<0225:tefoat>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff NP, Bera S, Cherian-Shaw M, Abraham EC. Substituted hydrophobic and hydrophilic residues at methionine-68 influence the chaperone-like function of αB-crystallin. Mol Cell Biochem. 2001;220:127–133. doi: 10.1023/a:1010834107809. [DOI] [PubMed] [Google Scholar]
- Sreelakshmi Y, Sharma K. Interaction of α-lactalbumin with mini-αA-crystallin. J Prot Chem. 2001;20:123–130. doi: 10.1023/a:1011077307262. [DOI] [PubMed] [Google Scholar]
- Török Z, Goloubinoff P, and Horwáth I. et al. 2001 Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc Natl Acad Sci U S A. 98:3098–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electorphoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez MM, Clark JI, Wu GJS, Muchowski PJ. Functional similarities between the small heat shock proteins Mycobacterium tuberculosis HSP 16.3 and human αB-crystallin. Eur J Biochem. 2002;269:1806–1813. doi: 10.1046/j.1432-1033.2002.02812.x. [DOI] [PubMed] [Google Scholar]
- Van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Viner RI, Clegg JS. Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/α-crystallin protein. Cell Stress Chaperones. 2001;6:126–135. doi: 10.1379/1466-1268(2001)006<0126:iototm>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Spector A. α-Crystallin prevents irreversible protein denaturation and acts cooperatively with other heat-shock proteins to renature the stabilized partially denatured protein in an ATP-dependent manner. Eur J Biochem. 2000;267:4705–4712. doi: 10.1046/j.1432-1327.2000.01521.x. [DOI] [PubMed] [Google Scholar]
- Wang K, Spector A. ATP causes small heat shock proteins to release denatured protein. Eur J Biochem. 2001;268:6335–6345. doi: 10.1046/j.0014-2956.2001.02580.x. [DOI] [PubMed] [Google Scholar]
- Warner AH, Clegg JS. Diguanosine nucleotide metabolism and the survival of artemia embryos during years of continuous anoxia. Eur J Biochem. 2001;268:1568–1576. [PubMed] [Google Scholar]
- Williams NE, Nelsen EM. HSP70 and HSP90 homologs are associated with tubulin in hetero-oligomeric complexes, cilia and the cortex of Tetrahymena. J Cell Sci. 1997;110:1665–1672. doi: 10.1242/jcs.110.14.1665. [DOI] [PubMed] [Google Scholar]
- Willsie JK, Clegg JS. Small heat shock protein p26 associates with nuclear lamins and HSP70 in nuclei and nuclear matrix fractions from stressed cells. J Cell Biochem. 2002;84:601–614. [PubMed] [Google Scholar]
- Wittmann T, Hyman A, Desai A. The spindle: a dynamic assembly of microtubules and motors. Nat Cell Biol. 2001;3:E28–E34. doi: 10.1038/35050669. [DOI] [PubMed] [Google Scholar]