Abstract
The influence of prolonged ischemic time on outcomes after lung transplant is controversial, but no research has investigated ischemic time in the context of center volume. We used data from the United Network for Organ Sharing to estimate the influence of ischemic time on patient survival conditional on center volume in the post–lung allocation score era (2005–2015). The analytic sample included 14 877 adult lung transplant recipients, of whom 12 447 were included in multivariable survival analysis. Patient survival was improved in high-volume centers compared with low-volume centers (log-rank test p = 0.001), although mean ischemic times were longer at high-volume centers (5.16 ± 1.70 h vs. 4.83 ± 1.63 h, p < 0.001). Multivariable Cox proportional hazards regression stratified by transplant center found an adverse influence of longer ischemic time at low-volume centers but not at high-volume centers. At centers performing 50 transplants in the period 2005–2015, for example, 8 versus 6 h of ischemia were associated with an 18.9% (95% confidence interval 6.5–32.7%; p < 0.001) greater mortality hazard, whereas at centers performing 350 transplants in this period, no differences in survival by ischemic time were predicted. Despite longer mean ischemic time at high-volume transplant centers, these centers had favorable patient outcomes and no adverse survival implications of prolonged ischemia.
Introduction
Lung transplantation (LTx) is a common treatment option for patients afflicted with certain advanced lung diseases, but limited donor organ availability continues to be the major obstacle in providing this option to all potential candidates (1,2). The implementation of the lung allocation score (LAS) prioritized candidates with a higher risk of death, regardless of time accrued on the waitlist (3), but allocation of organs to candidates with the highest priority remains limited because of efforts to minimize graft ischemia time. Extending the acceptable donor organ ischemic time could potentially remedy this constraint; however, reluctance about universally prolonging acceptable graft ischemia times has persisted because the medical literature has not clearly demonstrated acceptable outcomes for transplantation of grafts with prolonged ischemic times.
Adverse effects of prolonged ischemic time have been used to justify upper limits on ischemia time in LTx, although recent reports of no such adverse influences of prolonged ischemia have contributed to an emerging controversy on this point. Longer graft ischemia has been associated with posttransplant ischemia–reperfusion injury, primary graft failure and increased risk of bronchiolitis obliterans syndrome, while adversely influencing survival after LTx (4–7). In contrast, other studies have failed to find adverse outcomes associated with prolonged ischemic time, including both single-center experiences and retrospective analyses of the United Network for Organ Sharing (UNOS) registry (8–10). The evident lack of adverse influences of prolonged ischemia in an analysis of a large national registry has been cited to support the recommendation that acceptable ischemic times for LTx be prolonged (8).
With conflicting findings regarding prolonged ischemic time after LTx, it is vital to consider whether center experience influences potential risks of transplanting grafts with longer ischemic time. Experienced centers may have accumulation of expertise or access to resources that allows performance of LTx with prolonged ischemic times that are comparable to LTx performed with ischemic times in a conventionally acceptable range. The volume of lung transplant procedures is a well-established indicator of experience and proficiency, so we investigated whether greater transplant volume at a center would ameliorate negative survival effects of prolonged ischemia in LTx. With no previous research investigating this important question, we tested this hypothesis in a contemporary cohort of lung transplant recipients.
Methods
Data
Nationwide Children’s Hospital’s institutional review board approved the study with a waiver of individual consent (IRB14-00716). Lung transplant recipients in the United States were identified using the UNOS registry (11) during the contemporary LAS era (May 2005 to June 2015). Whereas all lung transplants were used to classify centers according to volume during the LAS era, inclusion of individual transplant records in subsequent analysis was contingent on adult age (≥18 years); no prior history of transplantation; known graft ischemic time; and, for survival analysis, known and nonzero days at risk after LTx. Multivariable models of patient survival were further limited to cases with complete covariate data.
Statistical methods
Descriptive statistics were presented as means and standard deviations for continuous variables and as counts and percentages for categorical variables. Center volume was calculated as the total number of lung transplants performed between 2005 and 2015. For descriptive comparisons and univariate analysis, a threshold of 150 total lung transplants over this period (i.e. an average of 15 lung transplants per year) was used to distinguish low- and high-volume centers. The median transplant center performed ≈150 lung transplants over the study period. Comparisons between centers above and below this threshold were performed using independent t-tests and chi-square tests for continuous and categorical variables, respectively. Patient survival in days was the end point in univariate and multivariable survival analyses. Kaplan–Meier curves with log-rank tests were used to test survival differences by dichotomized center volume (<150 vs. >150 lung transplants over a 10-year period) and dichotomized ischemic time (<6 vs. ≥6 h).
Continuous ischemic time (in hours) was included in multivariable analyses. Multivariable models compared conventional Cox proportional hazards analysis to a Cox model with the baseline hazard stratified on transplant center (12). In a conventional Cox model, the baseline hazard is shared across all patients, and hazard ratio (HR) assumes proportional hazards between patients with, for example, higher and lower ischemic times. When baseline hazards are stratified on the transplant center, the HR between high and low ischemic times would still be constant across centers but would imply a greater difference in absolute survival at centers for which the baseline hazard is greater. Consequently, stratification by the center-specific baseline hazard reveals the influence of ischemic time (and other covariates) on within-center variation in survival (13). Further multivariable analysis added interactions between continuous ischemic time and continuous center volume (the number of lung transplants performed over the 2005–2015 period). In the final model, ischemic time was treated as a quadratic polynomial to account for a nonlinear effect on mortality hazard. This model allowed the HR of ischemic time to vary between centers according to their volume of LTx, in addition to accounting for center differences in the baseline hazard.
To clarify the interpretation of the final multivariable model, the volume of LTx was centered at 150 total lung transplants performed in the period 2005–2015. With this centering, the HR of ischemic time represented the estimated influence of this variable in a center performing 150 lung transplants in the 2005–2015 period (comparable to the volume of the median transplant center). Based on this model, estimated HRs of ischemic time (relative to 6 h) were plotted for a center performing 50 lung transplants (with ≈25% of centers at or below this volume threshold) and for a center performing 350 lung transplants (with ≈25% of centers at or above this volume threshold) over the study period. Model diagnostics included Grambsch–Therneau tests of the proportional hazards assumption and a plot of Martingale residuals against ischemic time to verify the adequacy of modeling this variable as a quadratic function. This model was refitted with distance (in kilometers) between donor and transplant centers as the main independent variable to examine whether geographic distance and ischemic time similarly influenced patient survival. Analyses were performed in Stata/IC 13.1 (StataCorp LP, College Station, TX), and p < 0.05 was considered statistically significant.
Results
Study cohort
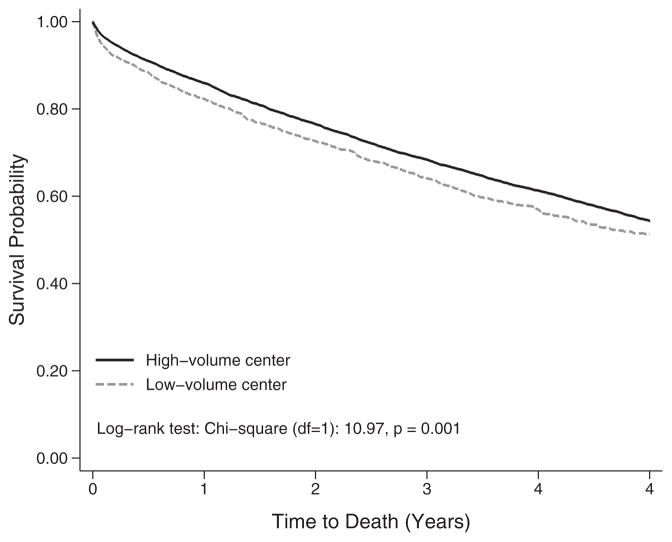
There were 14 877 adult lung transplant recipients included in the descriptive analysis, with 14 842 available for survival analysis and 12 447 included in multivariable survival models. The sample included lung transplants performed at 74 centers, of which 54 (73%) performed >50, 37 (50%) performed >150 and 15 (20%) performed >350. Figure 1 demonstrates the survival advantage of LTx performed in high-volume centers compared with low-volume centers (log-rank test p = 0.001). Descriptive statistics presented in Table 1 indicate that lung transplants performed at high-volume centers (top 50% of centers; >150 lung transplants in 2005–2015) had significantly longer ischemic time (5.16 ± 1.70 h vs. 4.83 ± 1.63 h, p < 0.001). Lung transplants performed at high-volume centers were also more likely to be single lung transplants, more likely to involve older recipients and more likely to involve recipients with greater expected transplant benefit, as indicated by higher LAS.
Figure 1.
Kaplan–Meier survival functions of adult lung transplants by center volume among adult patients transplanted between May 2005 and June 2015 (N = 14 842), log-rank test: chi-square (df = 1): 10.97, p = 0.001.
Table 1.
Characteristics of adults receiving lung transplants between May 2005 and June 2015, stratified by total center volume of adult lung transplants (n = 14 877)
| Variable | Valid patients, n | Center
|
p-value1 | |
|---|---|---|---|---|
| Low volume (n = 1851) | High volume (n = 13 026) | |||
| Ischemic time (h) | 14 877 | 4.83 (1.63) | 5.16 (1.70) | <0.001 |
| Male recipient | 14 877 | 1083 (59) | 7793 (60) | 0.280 |
| Male donor | 14 877 | 1109 (60) | 7842 (60) | 0.812 |
| Recipient race | 14 877 | 0.710 | ||
| White | 1540 (83) | 10 924 (84) | ||
| Black | 166 (9) | 1097 (8) | ||
| Other | 145 (8) | 1005 (8) | ||
| Donor race | 14 877 | <0.001 | ||
| White | 1180 (64) | 8000 (61) | ||
| Black | 379 (20) | 2463 (19) | ||
| Other | 292 (16) | 2563 (20) | ||
| Bilateral LTx | 14 877 | 1308 (71) | 8595 (66) | <0.001 |
| Diagnosis | 14 877 | <0.001 | ||
| PPH | 34 (2) | 226 (2) | ||
| CF | 314 (17) | 1522 (12) | ||
| IPF | 667 (36) | 5601 (43) | ||
| COPD | 540 (29) | 3363 (26) | ||
| Sarcoidosis | 55 (3) | 413 (3) | ||
| A1AD | 69 (4) | 335 (3) | ||
| Other | 172 (9) | 1566 (12) | ||
| ECMO | 14 877 | 35 (2) | 303 (2) | 0.240 |
| Mechanical ventilation | 14 877 | 92 (5) | 891 (7) | 0.002 |
| Donor infection | 14 661 | 1015 (56) | 7447 (58) | 0.064 |
| CMV matching | 13 977 | 0.004 | ||
| R−D− | 260 (15) | 2078 (17) | ||
| R+D− | 398 (22) | 2423 (20) | ||
| R−D+ | 435 (24) | 3180 (26) | ||
| R+D+ | 690 (39) | 4513 (37) | ||
| Donor cause of death | 14 877 | 0.005 | ||
| Head trauma | 928 (50) | 6043 (46) | ||
| Cerebrovascular | 628 (34) | 4613 (35) | ||
| Other | 295 (16) | 2370 (18) | ||
| Age (years) | 14 877 | 52.71 (13.95) | 55.43 (12.86) | <0.001 |
| Year of transplant | 14 877 | 2010.39 (2.85) | 2010.18 (2.82) | 0.003 |
| Creatinine (mg/dL) | 14 852 | 0.86 (0.67) | 0.85 (0.42) | 0.266 |
| BMI (kg/m2) | 14 871 | 24.83 (4.88) | 25.10 (4.57) | 0.019 |
| Final LAS | 14 871 | 45.66 (16.87) | 46.71 (17.28) | 0.014 |
| FEV1 (% predicted) | 14 531 | 35.77 (20.51) | 38.83 (20.82) | <0.001 |
| FVC (% predicted) | 14 610 | 47.24 (17.44) | 48.69 (17.50) | <0.001 |
| O2 requirement (L/min) | 14 706 | 5.08 (4.87) | 5.25 (4.94) | 0.155 |
| Six-minute walk distance (m) | 14 698 | 230.49 (132.43) | 236.20 (134.42) | 0.088 |
| Mean PAP (mmHg) | 14 017 | 27.87 (10.64) | 27.27 (10.60) | 0.026 |
| Donor age (years) | 14 877 | 32.68 (13.32) | 34.70 (14.35) | <0.001 |
| Donor PaO2 (mmHg) | 14 782 | 359.23 (152.37) | 380.48 (148.83) | <0.001 |
Data are shown as n (%) and mean (standard deviation) for continuous and categorical variables, respectively.
A1AD, α1-antitrypsin deficiency; CF, cystic fibrosis; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; D, donor; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; LAS, lung allocation score; LTx, lung transplantation; PAP, pulmonary artery pressure; PaO2, partial pressure of oxygen; PPH, primary pulmonary hypertension; R, recipient.
The p-value was assessed by chi-square test for categorical variables and by t-test for continuous variables.
Survival implications of ischemic time
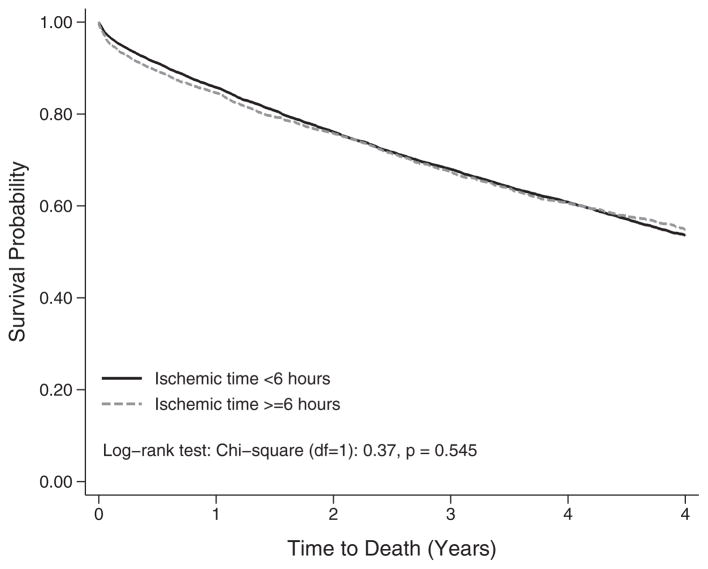
A Kaplan–Meier plot stratified on ischemic time ≥6 h identified no differences in survival in the overall cohort (Figure 2) (p = 0.545). Multivariable Cox proportional hazards regressions were used to estimate the survival implications of continuous ischemic time with and without stratification of the baseline hazard by transplant center. In model 1 of Table 2, no stratification of the baseline hazard was performed, and the adjusted HR of ischemic time failed to reach statistical significance (HR 1.017, 95% confidence interval [CI] 0.998–1.036; p = 0.082). This result suggested that, comparing outcomes among all lung transplant recipients, longer ischemic time did not influence survival after adjusting for recipient, donor and transplant characteristics. In contrast, stratifying the baseline hazard by transplant center, as shown in model 2 of Table 2, identified a significant adverse influence of longer ischemic time (HR 1.035; 95% CI 1.013–1.058; p = 0.001) when comparing lung transplant recipients within each center and adjusting for the same covariates as model 1.
Figure 2.
Kaplan–Meier survival functions by allograft ischemic time among adult patients transplanted between May 2005 and June 2015 (N = 14 842), log-rank test: chi-square (df = 1): 0.37, p = 0.545.
Table 2.
Multivariable Cox proportional hazards models of patient survival among adult lung transplant recipients (n = 12 447)
| Variable | Model 1: Conventional Cox regression
|
Model 2: Cox regression with baseline hazard stratified by transplant center
|
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Ischemic time (h) | 1.017 (0.998–1.036) | 0.082 | 1.035 (1.013–1.058) | 0.002 |
| Male recipient | 1.119 (1.047–1.196) | 0.001 | 1.119 (1.046–1.198) | 0.001 |
| Male donor | 0.951 (0.889–1.019) | 0.153 | 0.944 (0.881–1.012) | 0.106 |
| Race of recipient | ||||
| White | Ref | Ref | ||
| Black | 1.068 (0.956–1.194) | 0.244 | 1.014 (0.905–1.138) | 0.806 |
| Other | 0.900 (0.800–1.013) | 0.082 | 0.890 (0.788–1.006) | 0.062 |
| Race of donor | ||||
| White | Ref | Ref | ||
| Black | 1.170 (1.087–1.260) | <0.001 | 1.160 (1.076–1.252) | <0.001 |
| Other | 1.075 (0.995–1.163) | 0.068 | 1.055 (0.972–1.145) | 0.200 |
| Bilateral LTx | 0.778 (0.726–0.834) | <0.001 | 0.737 (0.683–0.796) | <0.001 |
| Diagnosis | ||||
| PPH | 1.349 (1.059–1.717) | 0.015 | 1.377 (1.079–1.758) | 0.010 |
| CF | 1.284 (1.101–1.498) | 0.001 | 1.273 (1.086–1.492) | 0.003 |
| IPF | Ref | Ref | ||
| COPD | 1.004 (0.899–1.122) | 0.939 | 1.004 (0.895–1.126) | 0.944 |
| Sarcoidosis | 0.920 (0.765–1.106) | 0.373 | 0.933 (0.774–1.124) | 0.467 |
| A1AD | 0.969 (0.790–1.188) | 0.760 | 0.999 (0.811–1.229) | 0.990 |
| Other | 1.124 (1.013–1.247) | 0.027 | 1.125 (1.010–1.253) | 0.033 |
| ECMO | 1.219 (0.929–1.600) | 0.153 | 1.169 (0.888–1.540) | 0.265 |
| Mechanical ventilation | 1.251 (1.082–1.447) | 0.002 | 1.355 (1.164–1.576) | <0.001 |
| Donor infection | 0.999 (0.942–1.061) | 0.986 | 0.992 (0.933–1.055) | 0.795 |
| CMV matching | ||||
| R−D− | Ref | Ref | ||
| R+D− | 1.033 (0.936–1.140) | 0.522 | 1.019 (0.922–1.126) | 0.712 |
| R−D+ | 1.286 (1.173–1.410) | <0.001 | 1.282 (1.168–1.408) | <0.001 |
| R+D+ | 1.128 (1.032–1.233) | 0.008 | 1.091 (0.996–1.194) | 0.061 |
| Donor cause of death | ||||
| Head trauma | Ref | Ref | ||
| Cerebrovascular | 1.002 (0.926–1.083) | 0.968 | 0.997 (0.921–1.080) | 0.949 |
| Other | 1.023 (0.940–1.114) | 0.595 | 1.022 (0.938–1.114) | 0.612 |
| Age (years) | 1.014 (1.010–1.018) | <0.001 | 1.014 (1.010–1.017) | <0.001 |
| Year of transplant | 0.999 (0.985–1.012) | 0.859 | 0.998 (0.984–1.012) | 0.766 |
| Creatinine (mg/dL) | 1.103 (1.055–1.154) | <0.001 | 1.092 (1.040–1.147) | <0.001 |
| BMI (kg/m2) | 1.003 (0.995–1.010) | 0.488 | 1.002 (0.994–1.010) | 0.619 |
| Final LAS | 1.000 (0.997–1.003) | 0.839 | 1.000 (0.997–1.004) | 0.782 |
| FEV1 (% predicted) | 1.001 (0.998–1.003) | 0.493 | 1.001 (0.999–1.004) | 0.352 |
| FVC (% predicted) | 0.999 (0.996–1.001) | 0.296 | 0.998 (0.995–1.000) | 0.102 |
| O2 requirement (L/min) | 1.011 (1.002–1.021) | 0.015 | 1.009 (0.999–1.018) | 0.078 |
| Six-minute walk distance (m) | 0.999 (0.999–1.000) | <0.001 | 0.999 (0.999–1.000) | <0.001 |
| Mean PAP (mmHg) | 1.001 (0.998–1.004) | 0.537 | 1.001 (0.998–1.005) | 0.388 |
| Donor age (years) | 1.003 (1.001–1.006) | 0.008 | 1.004 (1.001–1.006) | 0.003 |
| Donor PaO2 (mmHg) | 1.000 (1.000–1.000) | 0.797 | 1.000 (1.000–1.000) | 0.252 |
A1AD, α1-antitrypsin deficiency; CF, cystic fibrosis; CI, confidence interval; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; D, donor; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HR, hazard ratio; IPF, idiopathic pulmonary fibrosis; LAS, lung allocation score; LTx, lung transplantation; PAP, pulmonary artery pressure; PaO2, partial pressure of oxygen; PPH, primary pulmonary hypertension; R, recipient; Ref, reference.
Center variation in influence of ischemic time
Patient survival varied by ischemic time when comparing patients within the same center; therefore, further analysis tested whether continuous center volume (number of lung transplants performed in the period 2005–2015) moderated the survival implications (i.e. the HR) of longer ischemic time. Linear and quadratic terms for ischemic time were interacted with continuous center volume, as shown in Table 3. The baseline hazard remained stratified on transplant center. The statistically significant linear term of ischemic time (HR 1.058; 95% CI 1.028–1.089; p < 0.001) indicated that at a center performing 150 lung transplants in 2005–2015, ischemic time >6 h was associated with increased mortality hazard. Furthermore, the statistically significant (HR 1.009; 95% CI 1.000–1.018; p < 0.001) quadratic term of ischemic time indicated that the association between prolonged ischemia and increased mortality hazard grew stronger at ischemic times >6 h.
Table 3.
Multivariable Cox proportional hazards models of patient survival among adult lung transplant recipients showing ischemic time interacting with center volume and stratifying the baseline hazard by transplant center (n = 12 447)
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Ischemic time (h)1 | ||
| Linear term | 1.058 (1.028–1.089) | <0.001 |
| Quadratic term | 1.009 (1.000–1.018) | 0.048 |
| Center LTx volume Hundreds of transplants2 | ||
| Interaction with linear ischemic time | 0.990 (0.980–1.000) | 0.047 |
| Interaction with quadratic ischemic time | 0.999 (0.996–1.002) | 0.410 |
| Male recipient | 1.122 (1.049–1.200) | 0.001 |
| Male donor | 0.945 (0.882–1.013) | 0.110 |
| Race of recipient | ||
| White | Ref | |
| Black | 1.016 (0.906–1.139) | 0.790 |
| Other | 0.893 (0.790–1.009) | 0.069 |
| Race of donor | ||
| White | Ref | |
| Black | 1.159 (1.074–1.250) | <0.001 |
| Other | 1.052 (0.969–1.142) | 0.228 |
| Bilateral LTx | 0.746 (0.691–0.807) | <0.001 |
| Diagnosis | ||
| PPH | 1.377 (1.078–1.757) | 0.010 |
| CF | 1.267 (1.081–1.486) | 0.004 |
| IPF | Ref | |
| COPD | 1.004 (0.895–1.126) | 0.943 |
| Sarcoidosis | 0.932 (0.773–1.123) | 0.457 |
| A1AD | 0.997 (0.810–1.227) | 0.979 |
| Other | 1.124 (1.009–1.252) | 0.034 |
| ECMO | 1.174 (0.891–1.546) | 0.254 |
| Mechanical ventilation | 1.355 (1.165–1.577) | <0.001 |
| Donor infection | 0.991 (0.932–1.054) | 0.770 |
| CMV matching | ||
| R−D− | Ref | |
| R+D− | 1.018 (0.922–1.125) | 0.723 |
| R−D+ | 1.281 (1.167–1.406) | <0.001 |
| R+D+ | 1.090 (0.995–1.193) | 0.064 |
| Donor cause of death | ||
| Head trauma | Ref | |
| Cerebrovascular | 0.998 (0.922–1.080) | 0.952 |
| Other | 1.024 (0.940–1.116) | 0.581 |
| Age (years) | 1.014 (1.010–1.017) | <0.001 |
| Year of transplant | 0.998 (0.984–1.012) | 0.767 |
| Creatinine (mg/dL) | 1.092 (1.040–1.147) | <0.001 |
| BMI (kg/m2) | 1.002 (0.994–1.010) | 0.614 |
| Final LAS | 1.000 (0.997–1.004) | 0.819 |
| FEV1 (% predicted) | 1.001 (0.999–1.004) | 0.333 |
| FVC (% predicted) | 0.998 (0.995–1.000) | 0.093 |
| O2 requirement (L/min) | 1.009 (0.999–1.018) | 0.072 |
| Six-minute walk distance (m) | 0.999 (0.999–1.000) | <0.001 |
| Mean PAP (mmHg) | 1.002 (0.998–1.005) | 0.370 |
| Donor age (years) | 1.004 (1.001–1.006) | 0.003 |
| Donor PaO2 (mmHg) | 1.000 (1.000–1.000) | 0.235 |
A1AD, α1-antitrypsin deficiency; CF, cystic fibrosis; CI, confidence interval; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; D, donor; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HR, hazard ratio; IPF, idiopathic pulmonary fibrosis; LAS, lung allocation score; LTx, lung transplant; PAP, pulmonary artery pressure; PaO2, partial pressure of oxygen; PPH, primary pulmonary hypertension; R, recipient; Ref, reference.
Centered at 6 h.
Total lung transplants performed in 2005–2015, centered at 150.
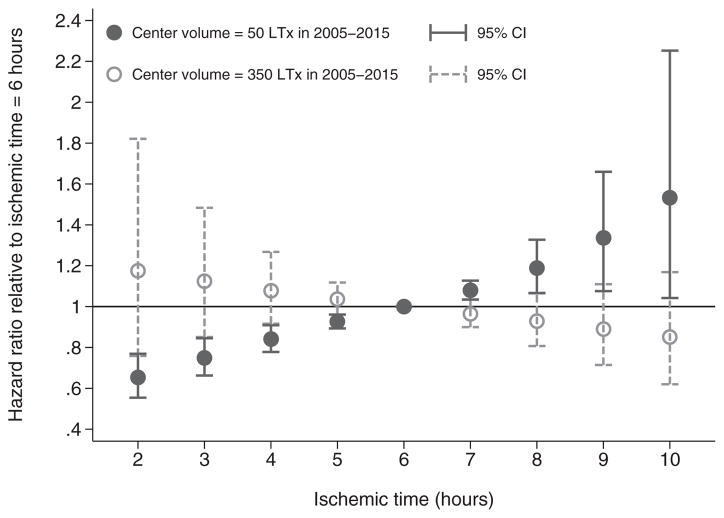
Whereas these findings reflect the association between prolonged ischemia and diminished survival, given a total transplant center volume of 150 lung transplants over the 2005–2015 period, the interaction between the linear ischemic time term and continuous center lung transplant volume (p = 0.047) suggested that the HR of ischemic time declined at higher volume centers, representing a weaker association with survival. To illustrate this point, Figure 3 plots predicted HRs of ischemic time relative to 6 h for centers performing 50 and 350 lung transplants, respectively. Given a total center volume of 50 lung transplants in the 2005–2015 period, there is a clear gradient toward increased mortality hazard with higher ischemic times; for example, the HR of 8 versus 6 h of ischemia is 1.189 (95% CI 1.065–1.327; p < 0.001); however, for a center performing 350 lung transplants in 2005–2015, the predictions illustrated in Figure 3 implied that ischemic time was not associated with survival.
Figure 3. Predicted hazard ratios of continuous ischemic time relative to 6 h, according to center volume of lung transplants, based on multivariable Cox proportional hazards regression.
CI, confidence interval; LTx, lung transplantation.
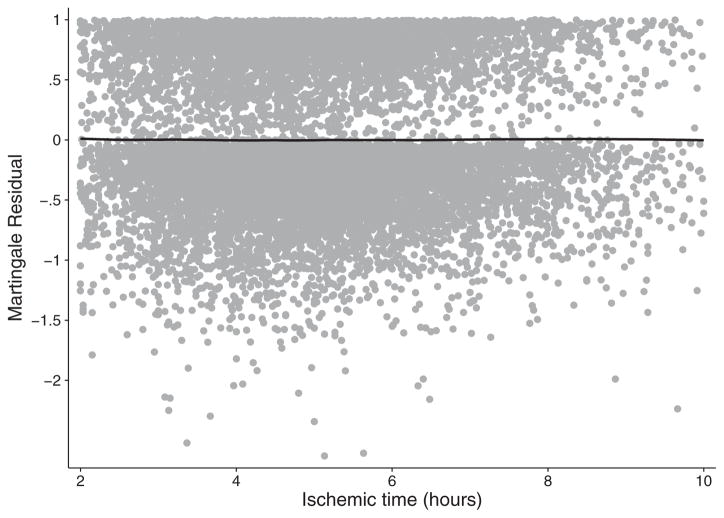
Model diagnostics indicated that none of the variables for ischemic time and center volume violated the proportional hazards assumption and that the quadratic specification of ischemic time achieved excellent fit to the data (Figure 4). The global Grambsch–Therneau test (p < 0.001) found five covariates with nonproportional hazards (donor race, bilateral LTx, indication for LTx, year of transplant, and mean pulmonary artery pressure). Interacting these covariates with the log of analysis time did not change the main results reported. Furthermore, analyzing geographic distance rather than ischemic time revealed no association between distance and survival in multivariable regression (Table 4).
Figure 4.
Martingale residuals from multivariable Cox proportional hazards regression plotted against ischemic time and smoothed by locally weighted regression.
Table 4.
Multivariable Cox proportional hazards models of patient survival among adult lung transplant recipients showing geographic distance to donor center interacting with center volume and stratifying the baseline hazard by transplant center (n = 12 447)
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Distance to donor center (Hundreds of kilometers)1 | 0.995 (0.984–1.006) | 0.380 |
| Center LTx volume (Hundreds of transplants)2 | ||
| Interaction with distance to donor center | 1.002 (0.998–1.006) | 0.322 |
| Male recipient | 1.129 (1.055–1.207) | <0.001 |
| Male donor | 0.947 (0.884–1.015) | 0.123 |
| Race of recipient | ||
| White | Ref | |
| Black | 1.015 (0.905–1.138) | 0.802 |
| Other | 0.892 (0.789–1.008) | 0.066 |
| Race of donor | ||
| White | Ref | |
| Black | 1.162 (1.077–1.254) | <0.001 |
| Other | 1.053 (0.970–1.144) | 0.215 |
| Bilateral LTx | 0.768 (0.715–0.825) | <0.001 |
| Diagnosis | ||
| PPH | 1.379 (1.080–1.760) | 0.010 |
| CF | 1.277 (1.089–1.497) | 0.003 |
| IPF | Ref | |
| COPD | 1.002 (0.893–1.124) | 0.974 |
| Sarcoidosis | 0.939 (0.779–1.131) | 0.506 |
| A1AD | 0.988 (0.803–1.216) | 0.911 |
| Other | 1.126 (1.010–1.254) | 0.032 |
| ECMO | 1.175 (0.892–1.547) | 0.252 |
| Mechanical ventilation | 1.345 (1.156–1.564) | <0.001 |
| Donor infection | 0.991 (0.932–1.054) | 0.781 |
| CMV matching | ||
| R−D− | Ref | |
| R+D− | 1.019 (0.923–1.126) | 0.706 |
| R−D+ | 1.281 (1.167–1.407) | <0.001 |
| R+D+ | 1.090 (0.995–1.193) | 0.064 |
| Donor cause of death | ||
| Head trauma | Ref | |
| Cerebrovascular | 0.997 (0.921–1.079) | 0.931 |
| Other | 1.023 (0.939–1.115) | 0.603 |
| Age (years) | 1.013 (1.010–1.017) | <0.001 |
| Year of transplant | 0.998 (0.984–1.012) | 0.807 |
| Creatinine (mg/dL) | 1.092 (1.040–1.146) | <0.001 |
| BMI (kg/m2) | 1.002 (0.995–1.010) | 0.588 |
| Final LAS | 1.001 (0.998–1.004) | 0.646 |
| FEV1 (% predicted) | 1.001 (0.999–1.004) | 0.366 |
| FVC (% predicted) | 0.998 (0.995–1.000) | 0.094 |
| O2 requirement (L/min) | 1.008 (0.999–1.018) | 0.087 |
| Six-minute walk distance (m) | 0.999 (0.999–1.000) | <0.001 |
| Mean PAP (mmHg) | 1.002 (0.998–1.005) | 0.348 |
| Donor age (years) | 1.004 (1.001–1.006) | 0.003 |
| Donor PaO2 (mmHg) | 1.000 (1.000–1.000) | 0.219 |
A1AD, α1-antitrypsin deficiency; CI, confidence interval; CF, cystic fibrosis; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; D, donor; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HR, hazard ratio; IPF, idiopathic pulmonary fibrosis; LAS, lung allocation score; LTx, lung transplant; PAP, pulmonary artery pressure; PaO2, partial pressure of oxygen; PPH, primary pulmonary hypertension; R, recipient; Ref, reference.
Quadratic term not included because the quadratic specification did not improve model fit.
Total lung transplants performed in 2005–2015, centered at 150.
Discussion
The most important finding from the current study is that high-volume centers tend to perform lung transplants with longer graft ischemic time, but in these high-volume centers, there are no evident adverse influences of prolonged ischemic time compared with patients transplanted at the same center with shorter graft ischemic times. In contrast, a survival disadvantage associated with prolonged graft ischemic times persists in low-volume lung transplant centers, including centers performing the median number of lung transplants over the study period (150 total lung transplants between 2005 and 2015). Because of differing implications of prolonged ischemia between high- and low-volume transplant centers, previous estimates of ischemic time effects on LTx outcomes in national registry data have likely been biased toward the null by inclusion of lung transplants from high-volume centers. Our finding that ischemic time remains associated with worse patient survival in smaller centers counters recent research that has argued for relaxing ischemic time limits on the acceptability of lung allografts.
The debate about the influence of ischemic time on patient outcomes in LTx has evolved from early findings of adverse influences to more recent results demonstrating favorable outcomes with ischemic times of ≥8 h. A recent analysis of the UNOS registry by Grimm et al (8) found no difference in survival or primary graft failure at 1 and 5 years after LTx between patients who received grafts that were exposed to ischemia that lasted ≥6 h or more and patients who received grafts with shorter ischemic times (8). The authors of that study recommended extending the acceptable ischemia time in certain patient populations to expand organ availability (8). Bharat (14) urged caution in moving forward with this recommendation because of limited data on potential confounding factors in the UNOS registry and potential risk due to prolonged ischemia in certain cases, such as bilateral LTx. We have argued that differences in the case mix between small and large transplant programs strongly confound the association between ischemic time and survival, as shown by the fact that large programs tend to perform LTx involving longer ischemia but achieve better patient outcomes. After demonstrating that adverse influences of prolonged ischemia become apparent with stratification of the baseline hazard in proportional hazards regression, we further examined the assumption that the effect of ischemia time on lung transplant outcomes is truly shared across centers regardless of their volume and expertise.
We analyzed center volume as moderating the survival implications of prolonged ischemic time. Interaction analysis demonstrated that the within-center effect of ischemic time was adverse and statistically significant in low-volume but not high-volume centers; for example, the predicted difference in mortality hazard between 6 and 8 h of ischemic time at a center performing 50 total lung transplants in 2005–2015 (HR 1.189) was intermediate between the effect sizes of recipient sex and procedure type (single vs. bilateral). In contrast, there were no differences in survival by geographic distance, even after taking into account center differences in the baseline hazard and interaction of distance with center volume. Consequently, earlier judgments about prolonged allograft ischemia as a risk factor for patient survival (4–7) remain applicable to contemporary LTx performed in small centers.
The effect of ischemia on the donor lung is incompletely understood, but ischemic preconditioning is thought to be an important component of organ transplantation, as described in animal models (15–18). The lung is a low metabolic organ (19,20), so ischemia may not be as detrimental during organ preservation in the setting of hypothermia for LTx. At the time of procurement, the lung is also filled with 100% oxygen, so the presence of oxygen may cause less ischemic injury compared with other organs. In an animal model of donation after cardiac death (DCD), hypoventilation was required with hypoxia before significantly impaired DCD lung graft function was seen (21). Based on these mechanisms, it is credible that in some settings (e.g. LTx performed at experienced centers), prolonged ischemia time will not adversely affect recipient outcomes.
Although our study design prevents us from identifying specific factors related to high-volume centers that mitigate the risk of prolonged ischemic time, we speculate that the influences are multifactorial and provide some insight into factors meriting further study. First, it is unlikely that our findings are related to biological or physiological differences between patients undergoing LTx at low- versus high-volume centers because effects of center volume are generally thought to reflect institutional differences in practice, expertise, and available resources.
Second, we note that high-volume centers commonly use a dedicated surgeon and team for procurement, so that particular surgeon or team may perform 50–100 procurements annually. With that type of experience, clinical and surgical skills are enhanced in the setting of a superior process for donor assessment and technical performance of the procurement and storage. Third, the surgical management of the recipient at time of LTx by the high-volume centers may mitigate risk associated with ischemia; for example, the roles played by variations in reperfusion techniques or warm ischemic times during implantation may be as important as the duration of cold ischemia. A fourth consideration is a potential rescue phenomenon in which short-term effects or complications of prolonged ischemic times are better managed at more experienced centers, leading to no adverse impact on long-term survival. In addition to these elements of LTx, a potential explanation of our findings could include technical issues not related to the procurement that contribute to ischemia. We provided these speculations to suggest that these factors should be considered in future prospective research. We assume, however, that outcomes related to ischemic times are not related to the distance traveled, as our analysis found no association between geographic distance and survival.
The approach to studying outcomes of prolonged allograft ischemia in the UNOS registry is limited by some aspects of this database. Most important, details of warm and cold ischemia are not available, the length of time on cardiopulmonary bypass could not be determined, and the use of ex vivo lung perfusion (EVLP) was not tracked during the study period, so the roles of these factors in explaining the moderating influence of center volume could not be assessed. With EVLP being considered for inclusion in the UNOS data collection form, future studies should examine whether this factor explains the improved outcomes at high-volume centers. Furthermore, the extent of variation in allograft ischemia observed in this retrospective study is biased by the fact that all donor organs reflected in these data were considered acceptable for transplantation. Finally, our study focused on survival implications of allograft ischemic time apart from other transplant, recipient and donor characteristics. In practice, the composite risk arising from the combination of these factors likely drives decisions to accept specific organs for transplantation. Generalizing from our findings, we would expect that more experienced centers are more likely to undertake LTx in higher risk candidates or involving higher risk donors, but would have better success in ensuring good patient outcomes despite these risk factors (22).
In conclusion, the current study presents timely results on the role of ischemic time in LTx, a treatment growing rapidly as an option for patients with advanced lung disease. Reanalysis of the national registry data used in recent studies demonstrates the importance of considering center differences in survival and interactions between center expertise and individual-level risk factors before moving toward uniform acceptance of LTx involving >6 h of ischemic time. With center volume evaluated over the entire study period, it is unclear how gaining experience in perioperative management by performing more lung transplants might remedy the post-LTx survival disadvantage associated with longer ischemic times in a given center currently performing few lung transplants. Nevertheless, center volume and expertise in LTx should be taken into account when considering extension of acceptable ischemic times for LTx, as recommended in recent reports.
Abbreviations
- A1AD
α1-antitrypsin deficiency
- CF
cystic fibrosis
- CI
confidence interval
- CMV
cytomegalovirus
- COPD
chronic obstructive pulmonary disease
- DCD
donation after cardiac death
- D
donor
- ECMO
extracorporeal membrane oxygenation
- EVLP
ex vivo lung perfusion
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- HR
hazard ratio
- IPF
idiopathic pulmonary fibrosis
- LAS
lung allocation score
- LTx
lung transplantation
- PaO2
partial pressure of oxygen
- PAP
pulmonary artery pressure
- PPH
primary pulmonary hypertension
- Ref
reference
- R
recipient
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Valapour M, Skeans MA, Heubner BM, et al. OPTN/SRTR 2013 Annual Data Report: Lung. Am J Transplant. 2015;15(Suppl 2):1–28. doi: 10.1111/ajt.13200. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: Implications for transplant researchers, 2005. Am J Transplant. 2006;6(5 Pt 2):1228–1242. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 4.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: A multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 5.Snell GI, Rabinov M, Griffiths A, et al. Pulmonary allograft ischemic time: An important predictor of survival after lung transplantation. J Heart Lung Transplant. 1996;15:160–168. [PubMed] [Google Scholar]
- 6.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 7.Fiser SM, Kron IL, Long SM, et al. Influence of graft ischemic time on outcomes following lung transplantation. J Heart Lung Transplant. 2001;20:1291–1296. doi: 10.1016/s1053-2498(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 8.Grimm JC, Valero V, 3rd, Kilic A, et al. Association between prolonged graft ischemia and primary graft failure or survival following lung transplantation. JAMA Surg. 2015;150:547–553. doi: 10.1001/jamasurg.2015.12. [DOI] [PubMed] [Google Scholar]
- 9.Gammie JS, Stukus DR, Pham SM, et al. Effect of ischemic time on survival in clinical lung transplantation. Ann Thorac Surg. 1999;68:2015–2019. doi: 10.1016/s0003-4975(99)00903-0. discussion 2019–2020. [DOI] [PubMed] [Google Scholar]
- 10.Hennessy SA, Hranjec T, Emaminia A, et al. Geographic distance between donor and recipient does not influence outcomes after lung transplantation. Ann Thorac Surg. 2011;92:1847–1853. doi: 10.1016/j.athoracsur.2011.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United Network for Organ Sharing/Organ Procurement and Transplantation Network Standard Transplant Analysis and Research Database. [cited 2016 Jun 26]. Available from: http://optn.transplant.hrsa.gov/data/about/OPTNDatabase.asp.
- 12.Thabut G, Christie JD, Mal H, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med. 2013;187:1335–1340. doi: 10.1164/rccm.201303-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asrani SK, Lim YS, Therneau TM, Pedersen RA, Heimbach J, Kim WR. Donor race does not predict graft failure after liver transplantation. Gastroenterology. 2010;138:2341–2347. doi: 10.1053/j.gastro.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharat A. Prolonged lung allograft ischemia and posttransplant outcome. JAMA Surg. 2015;150:554. doi: 10.1001/jamasurg.2015.0416. [DOI] [PubMed] [Google Scholar]
- 15.Du ZY, Hicks M, Winlaw D, Spratt P, Macdonald P. Ischemic preconditioning enhances donor lung preservation in the rat. J Heart Lung Transplant. 1996;15:1258–1267. [PubMed] [Google Scholar]
- 16.Friedrich I, Spillner J, Lu EX, et al. Ischemic pre-conditioning of 5 minutes but not of 10 minutes improves lung function after warm ischemia in a canine model. J Heart Lung Transplant. 2001;20:985–995. doi: 10.1016/s1053-2498(01)00290-x. [DOI] [PubMed] [Google Scholar]
- 17.Markart P, Schmidt R, Ruppert C, et al. Ischemic and endotoxin pre-conditioning reduce lung reperfusion injury-induced surfactant alterations. J Heart Lung Transplant. 2005;24:1680–1689. doi: 10.1016/j.healun.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Merry HE, Wolf PS, Fitzsullivan E, Keech JC, Mulligan MS. Lipopolysaccharide pre-conditioning is protective in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2010;29:471–478. doi: 10.1016/j.healun.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tierney DF. Lung metabolism and biochemistry. Annu Rev Physiol. 1974;36:209–231. doi: 10.1146/annurev.ph.36.030174.001233. [DOI] [PubMed] [Google Scholar]
- 20.Touya JJ, Rahimian J, Corbus HF, et al. The lung as a metabolic organ. Semin Nucl Med. 1986;16:296–305. doi: 10.1016/s0001-2998(86)80016-2. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi K, Oto T, Otani S, et al. Effect of donor pre-mortem hypoxia and hypotension on graft function and start of warm ischemia in donation after cardiac death lung transplantation. J Heart Lung Transplant. 2011;30:445–451. doi: 10.1016/j.healun.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Hayes D, Jr, Tobias JD, Tumin D. Center volume and extracorporeal membrane oxygenation support at lung transplantation in the lung allocation score era. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201511-2222OC. Epub ahead of print. [DOI] [PubMed] [Google Scholar]