Abstract
Aims
To describe the baseline characteristics of participants in the Kerala Diabetes Prevention Programme.
Methods
The Kerala Diabetes Prevention Programme is a cluster randomized controlled trial of lifestyle intervention for prevention of Type 2 diabetes mellitus in India. Participants in the study were those aged 30–60 years who had an Indian Diabetes Risk Score ≥60 and who were without Type 2 diabetes on oral glucose tolerance test. Data on demographic, lifestyle, clinical and biochemical characteristics were collected using standardized tools.
Results
A total of 2586 individuals were screened with the Indian Diabetes Risk Score, of these 1529 people (59.1%) had a score ≥60, of whom 1209 (79.1%) underwent an oral glucose tolerance test. A total of 202 individuals (16.7%) had undiagnosed Type 2 diabetes and were excluded, and the remaining 1007 individuals were enrolled in the trial (control arm, n = 507; intervention arm, n = 500). The mean participant age was 46.0 ± 7.5 years, and 47.0% were women. The mean Indian Diabetes Risk Score was 67.1 ± 8.4. More than two-thirds (69.0%) had prediabetes and 31.0% had normal glucose tolerance. The prevalence of cardiometabolic risk factors was high, including current tobacco use (34.4% in men), current alcohol use (39.3% in men), no leisure time exercise (98.0%), no daily intake of fruit and vegetables (78.8%), family history of diabetes (47.9%), overweight or obesity (68.5%), hypertension (22.3%) and dyslipidemia (85.3%).
Conclusions
The Kerala Diabetes Prevention Programme recruited participants using a diabetes risk score. A large proportion of the participants had prediabetes and there were high rates of cardiometabolic risk factors. The trial will evaluate the effectiveness of lifestyle intervention in a population selected on the basis of a diabetes risk score.
(Clinical Trial registration: Australia and New Zealand Clinical Trials Registry: ACTRN12611000262909.)
Introduction
Globally, 415 million adults are estimated by the International Diabetes Federation to have Type 2 diabetes mellitus and this figure is projected to rise to 642 million by 2040 [1]. Approximately 75% of adults with Type 2 diabetes live in low- and middle-income countries, with an estimated 69.2 million of these living in India [1]. Type 2 diabetes confers an economic burden on the affected individuals and their families in India [2], therefore, there is an urgent need for the development and widespread implementation of cost-effective approaches for the prevention of Type 2 diabetes in the country.
Randomized controlled trials have consistently shown that lifestyle interventions are effective in preventing or delaying the onset of Type 2 diabetes among high-risk groups [3]. To date, the majority of diabetes prevention trials have primarily targeted those with impaired glucose tolerance or impaired fasting glucose, identified based on an oral glucose tolerance test (OGTT) [3]; however, in resource-constrained settings like India, it is important to explore simple and low-cost methods, such as non-invasive diabetes risk scores, to identify high-risk individuals who may benefit from lifestyle intervention. Similarly, it is important to explore low-cost methods of delivering a lifestyle intervention. Most previous trials of lifestyle intervention have involved individual counselling from health professionals in primary care settings [3]. In India, where there are a substantial number of people at high risk of developing Type 2 diabetes [1], one-to-one counselling is not feasible. Further, more than two-thirds of India’s population still resides in rural areas [4], where there is a severe shortage of qualified health professionals; therefore, approaches to Type 2 diabetes prevention in this setting should be less dependent on healthcare providers and services. Peer support interventions have been shown to be effective in disease prevention and management in various countries [5]. Indeed, members of our research group showed that community-based approaches with a strong emphasis on peer support could improve health behaviours and metabolic risk factors in Finland and Australia [6,7]. A comprehensive needs assessment conducted by our research group in the Indian state of Kerala has shown peer support delivered in community groups to be a feasible intervention for Type 2 diabetes prevention [8].
Kerala state in India is in the late stage of epidemiological transition and is likely to be an example of what will happen to the rest of India with regard to the increasing burden of non-communicable diseases over the coming years [9]; therefore, Kerala provides an appropriate setting in which to implement and evaluate a community-based diabetes prevention programme in India. The Kerala Diabetes Prevention Programme (K-DPP) is a cluster randomized controlled trial, designed to evaluate the effectiveness of a culturally adapted, group-based and peer-led lifestyle intervention programme for the prevention of Type 2 diabetes in Asian Indians [10]. The present paper describes the baseline demographic, lifestyle, clinical and biochemical characteristics of participants in the K-DPP.
Methods
Study design
The K-DPP study design has been described in detail elsewhere [10]. Briefly, the trial was undertaken in 60 'polling booths' (electoral divisions with ~900–1500 people aged ≥18 years per polling booth) of the Neyyattinkara taluk (sub-district) in the district of Thiruvananthapuram, Kerala. Neyyattinkara is located only ~20 km from the city of Thiruvananthapuram, with a total population of 880 986, according to the 2011 census [4]. The geographical terrain of Neyyattinkara encompasses the three natural divisions of Kerala, the highlands, midlands and coastal areas. The trial statistician used a computer-generated randomization sequence, with constant block size and stratification by polling booth size, to randomly assign the polling booths (1:1) to the intervention arm (received lifestyle intervention programme for 12 months) or the control arm (received a health education booklet on lifestyle modification). The study protocol was approved by the ethics committees of the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, India and Monash University and the University of Melbourne in Australia. All study participants provided written informed consent.
Screening and recruitment
Individuals aged 30–60 years were selected randomly from the electoral roll of polling booths and contacted in their homes by trained staff ('home screening'). Eligibility criteria were: literacy in the local language (Malayalam); no history of diabetes, heart disease, stroke, cancer, epilepsy, arthritis or dementia; not pregnant; and not currently taking medications known to influence glucose tolerance. Individuals satisfying the eligibility criteria were screened using the Indian Diabetes Risk Score (IDRS) [11]. The IDRS is a non-invasive diabetes risk score, comprising questions about age, physical activity and family history of diabetes, and waist circumference. The total score ranges between 0 and 100. Individuals with IDRS score ≥60 were identified as being at high risk of Type 2 diabetes and invited to clinics conducted in the community using locally based buildings ('mobile clinic'). Among those who attended mobile clinics, a 2-h 75-g OGTT was performed and those with undiagnosed Type 2 diabetes [fasting plasma glucose (FPG) ≥7.0 mmol/l and/or 2-h plasma glucose (2-h PG) ≥11.1 mmol/l] [12] were excluded, while those with prediabetes (impaired fasting glucose: FPG 5.6 and 6.9 mmol/l and 2-h PG <7.8 mmol/l or impaired glucose tolerance: FPG <7.0 mmol/l and 2-h PG 7.8 and 11.0 mmol/l) and normal glucose tolerance (FPG <5.6 mmol/l and 2-h PG <7.8 mmol/l), based on the American Diabetes Association criteria, were enrolled in the trial. Participants were blinded to their study arm allocation until they had completed their baseline assessment.
Measurements
Because the measurements have been previously described in detail [10], they are briefly summarized here. Data on demographic characteristics, lifestyle habits and medical history were collected using standardized questionnaires. Anthropometric measurements such as height, weight, body fat percent, waist circumference and hip circumference and blood pressure (mean of the second and third readings was used in the analysis) were obtained using standardized tools and protocols. In addition to the OGTT, other biochemical measurements included HbA1c and lipids. The assays used for analysis of biochemical samples and the quality control measures are given in supplementary file. Data collection staff and laboratory technicians were masked to the participants’ study arm allocation.
Statistical analysis
Data analysis was performed using STATA version 14.0 (StataCorp LP, College Station, TX, USA). Mean ± SD values are presented for approximately normally distributed variables, and medians (interquartile range) are presented for skewed variables. Categorical variables are summarized with frequencies and percentages. Baseline characteristics of participants were compared between study arms using linear regression for continuous variables and logistic regression (including multinomial for unordered categories and ordinal for ordered categories) for categorical variables, with P values based on Huber–White standard errors that were adjusted for clustering by polling booths. Skewed variables were log-transformed before analysis. Two-sided P values <0.05 were taken to indicate statistical significance.
Results
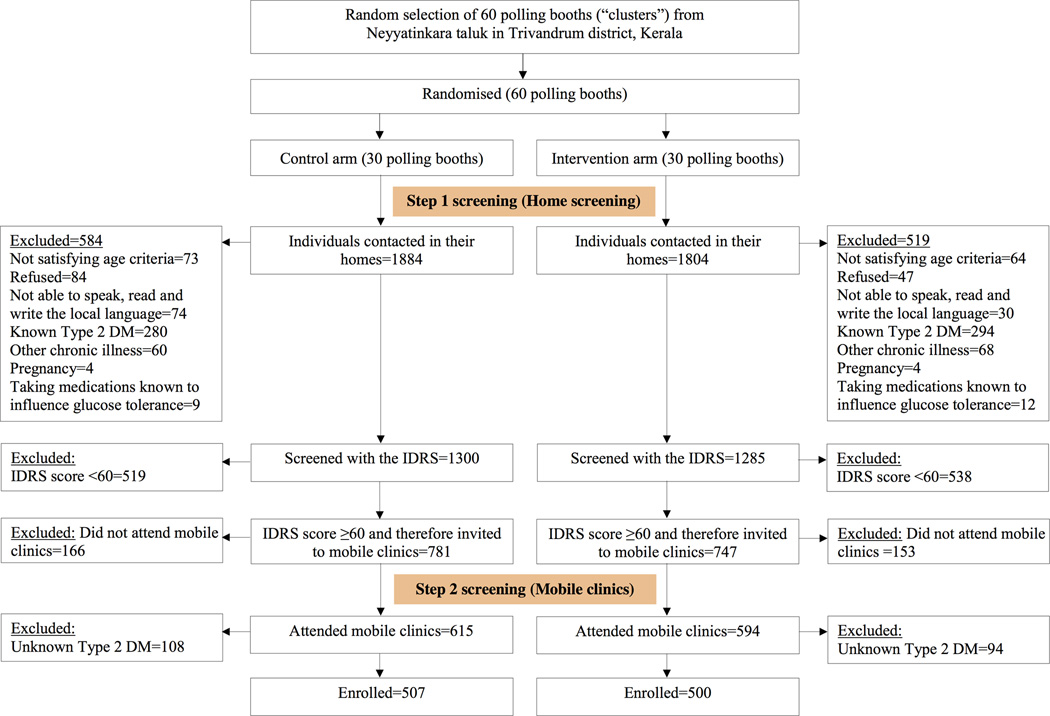
Screening and recruitment was carried out from January to October 2013. Figure 1 shows the K-DPP screening and enrolment flowchart. A total of 3552 individuals, aged 30–60 years, were contacted during home visits and of these, 3421 (96.3%) consented to participate. After excluding those not satisfying the eligibility criteria (n=835), 2586 individuals were screened using the IDRS. Of these, 1529 (59.1%) had a score ≥60 and were invited to mobile clinics to undergo an OGTT. Among those who underwent the OGTT (n=1209, 79.1%), 202 (16.7%) had undiagnosed Type 2 diabetes and were excluded from the study. Of the remaining 1007 individuals, 695 (69.0%) had prediabetes [579 (57.5%) had impaired fasting glucose and 116 (11.5%) had impaired glucose tolerance] and 312 (31.0%) had normal glucose tolerance. These 1007 individuals were enrolled in the trial. The control arm had 507 participants and the intervention arm had 500 participants. The mean IDRS among the study participants was 67.1 ± 8.4 (range 60–100), and the mean FPG and 2-h PG levels were 5.8 ± 0.5 mmol/l and 5.9 ± 1.6 mmol/l, respectively.
FIGURE 1.
Flow chart showing the screening and enrolment of participants in the Kerala Diabetes Prevention Programme. IDRS, Indian Diabetes Risk Score.
Table 1 shows the demographic characteristics of the study participants. The mean age was 46.0 ± 7.5 years, and 47.0% were women. The majority were educated up to secondary school (75.6%), were involved in skilled or unskilled labour (72.0%), were married (95.1%) and were Hindus (59.2%). The median (range) monthly household expenditure was 7000 (5000–10000) Indian Rupees (~US$105) and the mean household size was 4.3 ± 1.4 people.
Table 1.
Demographic characteristics of participants in the Kerala Diabetes Prevention Programme
| Total (N=1007) | Control arm (n=507) | Intervention arm (n=500) | P* | |
|---|---|---|---|---|
| Age, years | 46.0 ± 7.5 | 45.7 ± 7.4 | 46.2 ± 7.6 | 0.45 |
| Men | 532 (52.8) | 272 (53.7) | 260 (52.0) | 0.53 |
| Education | ||||
| Up to primary | 253 (25.1) | 117 (23.1) | 136 (27.2) | 0.54 |
| Middle | 272 (27.0) | 143 (28.2) | 129 (25.8) | |
| Secondary | 237 (23.5) | 123 (24.3) | 114 (22.8) | |
| Higher secondary | 85 (8.4) | 42 (8.3) | 43 (8.6) | |
| Vocational education | 59 (5.9) | 31 (6.1) | 28 (5.6) | |
| College or above | 101 (10.0) | 51 (10.1) | 50 (10.0) | |
| Occupation | ||||
| Skilled/unskilled | 728 (72.3) | 361 (71.2) | 367 (73.4) | 0.58 |
| Homemaker | 268 (26.6) | 139 (27.4) | 129 (25.8) | |
| Unemployed/retired | 11 (1.1) | 7 (1.4) | 4 (0.8) | |
| Monthly household expenditure†, Indian Rupees | 7000 (5000–10000) | 6000 (5000–10000) | 7000 (5000–10000) | 0.88 |
| Marital status | ||||
| Single | 11 (1.1) | 3 (0.6) | 8 (1.6) | 0.10 |
| Married | 958 (95.1) | 482 (95.1) | 476 (95.2) | |
| Widowed | 28 (2.8) | 19 (3.8) | 9 (1.8) | |
| Divorced/separated | 10 (1.0) | 3 (0.6) | 7 (1.4) | |
| Religion | ||||
| Hindu | 596 (59.2) | 276 (54.4) | 320 (64.0) | 0.31 |
| Christian | 365 (36.3) | 210 (41.4) | 155 (31.0) | |
| Muslim | 46 (4.6) | 21 (4.1) | 25 (5.0) |
INR, Indian Rupees.
Data are mean ± sd or median (interquartile range) or n (%). Percentages may not add up to 100% because of rounding. No missing data for any of the variables.
Based on Huber–White standard errors that were adjusted for clustering by polling booths in regression analysis.
Log-transformed data were used in regression analysis.
Table 2 shows the lifestyle, clinical and biochemical characteristics of participants. Among men, 26.9% reported current smoking, 15.0% reported current smokeless tobacco use, 34.4% reported current tobacco use and 39.3% reported current alcohol use. The corresponding figures for women were 0, 2.3, 2.3 and 0.4%, respectively. A large proportion reported no leisure time exercise (98.0%) and only less than a quarter (21.3%) consumed fruit and vegetables daily. Nearly half (47.9%) had a family history of diabetes (one or both parents), and slightly more than two-thirds were overweight or obese (68.5%) and centrally obese (69.6%). More than half (54.7%) had prehypertension or hypertension. Among those with hypertension, 44.0% were aware of their hypertensive status, 33.3% were treated with blood pressure-lowering medication and 24.4% had their blood pressure under control (systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg associated with the use of blood pressure-lowering medications). More than four-fifths (85.3%) had dyslipidemia, with high total cholesterol and high LDL cholesterol being more common than low HDL cholesterol and high triglycerides. Only 2.4% of those with dyslipidemia were taking lipid-lowering medications and 90.9% had more than three cardiometabolic risk factors (Appendix S1).
Table 2.
Lifestyle, clinical and biochemical characteristics of participants in the Kerala Diabetes Prevention Programme
| Total (N=1007) | Control arm (n=507) | Intervention arm (n=500) | Pa | |
|---|---|---|---|---|
| Current smokingb | 143 (14.2) | 68 (13.4) | 75 (15.0) | 0.53 |
| Current smokeless tobacco usec | 91 (9.0) | 43 (8.5) | 48 (9.6) | 0.64 |
| Current tobacco used | 194 (19.3) | 92 (18.2) | 102 (20.4) | 0.47 |
| Current alcohol usee | 211 (21.0) | 97 (19.1) | 114 (22.8) | 0.27 |
| Leisure time exercise | 20 (2.0) | 10 (2.0) | 10 (2.0) | 0.98 |
| Daily intake of fruits and vegetables | 214 (21.3) | 108 (21.3) | 106 (21.2) | 0.97 |
| Family history of diabetes (one or both parents) | 482 (47.9) | 260 (51.3) | 222 (44.4) | 0.05 |
| Anthropometry | ||||
| Weight, kg | 63.6 ± 11.9 | 64.5 ± 12.1 | 62.6 ± 11.6 | 0.06 |
| BMI, kg/m2 | 24.9 ± 4.0 | 25.1 ± 4.1 | 24.6 ± 3.9 | 0.15 |
| Overweight or obesityf | 690 (68.5) | 357 (70.4) | 333 (66.6) | 0.35 |
| Waist circumference, cmg | 88.3 ± 9.7 | 88.7 ± 9.7 | 87.9 ± 9.7 | 0.28 |
| Central obesityh | 699 (69.6) | 359 (71.1) | 340 (68.0) | 0.38 |
| Hip circumference, cmi | 94.8 ± 8.7 | 95.4 ± 9.0 | 94.2 ± 8.4 | 0.10 |
| Waist-to-hip ratioj | 0.93 ± 0.07 | 0.93 ± 0.06 | 0.93 ± 0.07 | 0.74 |
| Body fat percent, %k | 29.8 ± 8.4 | 30.0 ± 8.7 | 29.7 ± 8.2 | 0.55 |
| Blood pressure | ||||
| Systolic, mmHg | 123.2 ± 17.7 | 123.4 ± 17.9 | 123.0 ± 17.6 | 0.81 |
| Diastolic, mmHg | 74.9 ± 11.8 | 74.8 ± 12.1 | 75.0 ± 11.5 | 0.81 |
| Prehypertensionl | 326 (32.4) | 169 (33.3) | 157 (31.4) | 0.50 |
| Hypertensionm | 225 (22.3) | 118 (23.3) | 107 (21.4) | 0.55 |
| Blood pressure-lowering drugs | 75 (7.5) | 40 (7.9) | 35 (7.0) | 0.59 |
| Plasma glucose, mmol/l | ||||
| Fasting | 5.8 ± 0.5 | 5.8 ± 0.5 | 5.8 ± 0.5 | 0.21 |
| 2-h | 5.9 ± 1.6 | 6.0 ± 1.5 | 5.9 ± 1.6 | 0.48 |
| HbA1c, mmol/mol (%)n | 38 ± 6 (5.6 ± 0.5) | 38 ± 6 (5.6 ± 0.5) | 38 ± 6 (5.6 ± 0.5) | 0.93 |
| Serum lipids, mmol/l | ||||
| Total cholesterol | 5.7 ± 1.0 | 5.7 ± 1.1 | 5.7 ± 1.0 | 0.90 |
| High total cholesterolo | 704 (69.9) | 355 (70.0) | 349 (69.8) | 0.95 |
| LDL cholesterol | 3.8 ± 0.9 | 3.8 ± 1.0 | 3.8 ± 0.9 | 0.99 |
| High LDL cholesterolp | 678 (67.3) | 343 (67.7) | 335 (67.0) | 0.82 |
| HDL cholesterol | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.65 |
| Low HDL cholesterolq | 344 (34.2) | 172 (33.9) | 172 (34.4) | 0.89 |
| Triglyceridesr | 1.1 (0.9–1.6) | 1.1 (0.9–1.6) | 1.2 (0.9–1.6) | 0.40 |
| High triglyceridess | 210 (20.9) | 106 (20.9) | 104 (20.8) | 0.97 |
| Dyslipidemiat | 859 (85.3) | 437 (86.2) | 422 (84.4) | 0.43 |
| Lipid-lowering drugs | 21 (2.1) | 12 (2.4) | 9 (1.8) | 0.50 |
| IDRS score | 67.1 ± 8.4 | 67.5 ± 8.4 | 66.8 ± 8.3 | 0.28 |
IDRS, Indian Diabetes Risk Score.
Data are mean ± sd or median (interquartile range) or n (%).
Based on Huber–White standard errors that were adjusted for clustering by polling booths in regression analysis.
Smoked cigarettes, bidis (hand-rolled cigarettes), cigars or pipes in the last 30 days [21].
Use of smokeless tobacco (snuff or chewing tobacco) in the last 30 days [21].
Current smoking or current smokeless tobacco use [21].
Consumption of spirits, beer, wine or toddy (locally made alcoholic drink) in the last 30 days [21].
Overweight was defined as BMI ≥23 kg/m2 but <25 kg/m2 and obesity as BMI ≥25 kg/m2 [22].
Missing data for two participants in the control arm.
Waist circumference ≥90 cm for men and ≥80 cm for women [22].
Missing data for five participants in the control arm.
Missing data for five participants in the control arm.
Missing data for one participant in the intervention arm.
Systolic blood pressure between 120 and 139 mmHg and/or diastolic blood pressure between 80 and 89 mmHg and not taking blood pressure lowering medications [23].
Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or currently taking blood pressure lowering medications [23].
Missing data for one participant in the intervention arm.
Total cholesterol ≥5.2 mmol/l [24].
LDL cholesterol ≥3.4 mmol/l [24].
HDL cholesterol <1.04 mmol/l for men and <1.3 mmol/l for women [24].
Median (interquartile range). Log-transformed data was used in regression analysis.
Triglycerides ≥1.7 mmol/l [24].
Taking lipid-lowering medications and/or high total cholesterol and/or high LDL cholesterol and/or low HDL cholesterol and/or high triglycerides [24].
Reflecting the randomization of the study design, there were no statistically significant differences in the baseline characteristics of participants between the study arms (Tables 1 and 2).
Discussion
The K-DPP trial is the first diabetes prevention programme from a low- and middle-income country to use a simple and low-cost diabetes risk score as a screening tool to identify high-risk individuals. A large proportion of study participants had prediabetes and high rates of cardiometabolic risk factors. Even among those with normal glucose tolerance, more than three-quarters (84.6%) had FPG levels in the range of 5.1–5.5 mmol/l, levels that confer an independent risk for the development of Type 2 diabetes, as shown by previous studies [13], and a similar proportion (87.5%) had more than three cardiometabolic risk factors.
The prevalence of cardiometabolic risk factors in the K-DPP cohort was higher than in the general population of rural Kerala [9]. For example, the prevalence rates of generalized obesity and central obesity (defined using waist circumference) in the general population (aged 30–60 years) were 29 and 56.5%, respectively [9]; the corresponding figures in the K-DPP cohort were 47 and 69.6%, respectively. When the K-DPP cohort was compared with participants in previous diabetes prevention trials conducted in Asian Indians, a lower proportion of those enrolled in the Indian Diabetes Prevention Programme-1 [23] had hypertension (≥135/80 mmHg; 34 vs 31%) and high total cholesterol (68 vs 42%). Similarly, compared with participants in the baseline survey of the Diabetes Community Lifestyle Improvement Program trial from urban Chennai in India [14], a greater proportion of K-DPP participants were current smokers (27 vs 13%), had hypertension (22 vs 18%) and had high total cholesterol (68 vs 35%). The K-DPP cohort also differed from those enrolled in diabetes prevention trials conducted in white populations. For example, the K-DPP participants were younger and leaner than those enrolled in the US Diabetes Prevention Program (mean age 51 years and mean BMI 34 kg/m2) [15] or in the Finnish Diabetes Prevention Study (mean age 55 years and mean BMI 31 kg/m2) [16]. These differences are primarily attributable to the selection criteria used in the K-DPP trial.
Large-scale use of the OGTT as a prerequisite for entering a diabetes prevention programme is a major financial and practical barrier, particularly in low- and middle-income countries; therefore, one of the key aims of the K-DPP was to determine whether a diabetes prevention programme could recruit individuals at high risk of developing Type 2 diabetes using a diabetes risk score. The baseline characteristics of the K-DPP confirmed that the study population does indeed differ from those who have been enrolled in other diabetes prevention trials; a large proportion of participants had prediabetes and there were high rates of cardiometabolic risk factors. Sensitivity analyses will be able to investigate to what extent using higher IDRS thresholds would have produced a population with a higher incidence of diabetes and whether that would alter the findings.
The K-DPP trial has a number of strengths. Firstly, recruiting participants for community-based randomized controlled trials, particularly in a country like India, is complex and challenging [28]; however, we were able to screen and recruit the targeted sample size from the community within the expected timeframe, with high response rates at each step of the screening and recruitment process. Secondly, almost 50% of participants in the K-DPP were women compared with 0–36% women in other diabetes prevention trials conducted in Asian Indians [17–20]. Thus, successful completion of the K-DPP trial will provide valuable data on the effects of lifestyle intervention among high-risk women in Asian Indians. Thirdly, standardized questionnaires, tools and protocols were used for data collection. Fourthly, completeness of data for key baseline variables was very high (missing data for key variables ranged between 0.1 and 0.4%). Finally, despite collecting blood samples in a community setting, we were able to achieve very-high-quality glucose measures (intra-class correlation coefficient almost 1.0), using standardized protocols, laboratories with national and international accreditation and stringent quality control measures.
The study also has some limitations. Glucose tolerance status was based on a single OGTT. This might have led to some misclassification because of high intra-individual variability in glucose levels. Although, repeated testing is recommended for clinical diagnosis [12], a single OGTT result is commonly accepted in epidemiological studies. Demographic measures of K-DPP participants, including age structure, education, occupation, marital status and household size were broadly similar to the general population of rural Kerala (aged 30–59 years) [4]; however, the gender ratio was lower than the state’s average and there was an under-representation of Muslims in the study. Although, Muslims are the second largest religious group in rural Kerala, they are the minority group in the study area [4].
Using a diabetes risk score, the K-DPP trial recruited participants among whom a large proportion had prediabetes and there were high rates of cardiometabolic risk factors. The trial will evaluate the effectiveness of lifestyle intervention in a population selected on the basis of a diabetes risk score.
Supplementary Material
What's new?
Large-scale use of an oral glucose tolerance test (OGTT) as a prerequisite for entering a diabetes prevention programme is a major financial and practical barrier.
The Kerala Diabetes Prevention Programme is the first diabetes prevention trial from a low- and middle-income country to use a simple and low-cost diabetes risk score as a screening tool to identify high-risk individuals.
Of 1209 screen positives, 202 (16.7%) had undiagnosed Type 2 diabetes mellitus on OGTT.
The trial will evaluate the effectiveness of lifestyle intervention in a population selected on the basis of a risk score, a large proportion of whom had prediabetes and among whom there were high rates of cardiometabolic risk factors.
Acknowledgments
T.S. was supported by the Victoria India Doctoral Scholarship (VIDS) for his PhD at the University of Melbourne, Australia. In addition, T.S. and E.M. were supported by the ASCEND Program, funded by the Fogarty International Centre of the National Institutes of Health (NIH) under Award Number: D43TW008332. We also acknowledge Peers for Progress, a programme of the American Academy of Family Physicians Foundation supported by the Eli Lily and Company Foundation. The contents of this paper are solely the responsibility of the authors and do not reflect the views of NHMRC, NIH, Peers for Progress or the ASCEND Program.
Funding sources
The Kerala Diabetes Prevention Programme is funded by the National Health and Medical Research Council (NHMRC), Australia (Project Grant ID 1005324). The NHMRC had no role in study design, data collection, data analysis, manuscript preparation or publication decision.
Footnotes
Competing interests
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article: Appendix S1.
References
- 1.International Diabetes Federation. [Last accessed 10 March 2016];Diabetes Atlas. (7th). Available at: http://www.diabetesatlas.org/ [Google Scholar]
- 2.Yesudian CAK, Grepstad M, Visintin E, Ferrario A. The economic burden of diabetes in India: a review of the literature. Globalization Health. 2014;10:80. doi: 10.1186/s12992-014-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Woods HB, et al. Preventing the progression to Type 2 diabetes mellitus in adults at high risk: A systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pr. 2015;107:320–331. doi: 10.1016/j.diabres.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Office of the Registrar General and Census Commissioner of India. [Last accessed 30 October 2015];Census. 2011 Available at: http://censusindia.gov.in.
- 5.Fisher EB, Coufal MM, Parada H, Robinette JB, Tang PY, Urlaub DM, et al. Peer support in health care and prevention: cultural, organizational, and dissemination issues. Annu Rev Public Health. 2014;35:363–383. doi: 10.1146/annurev-publhealth-032013-182450. [DOI] [PubMed] [Google Scholar]
- 6.Laatikainen T, Dunbar JA, Chapman A, Kilkkinen A, Vartiainen E, Heistaro S, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health. 2007;7:249. doi: 10.1186/1471-2458-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care. 2009;32:1418–1420. doi: 10.2337/dc09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daivadanam M, Absetz P, Sathish T, Thankappan KR, Fisher EB, Philip NE, et al. Lifestyle change in Kerala, India: needs assessment and planning for a community-based diabetes prevention trial. BMC Public Health. 2013;13:95. doi: 10.1186/1471-2458-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thankappan KR, Shah B, Mathur P, Sarma PS, Srinivas G, Mini GK, et al. Risk factor profile for chronic non-communicable diseases: Results of a community-based study in Kerala, India. Indian J Med Res. 2010;131:53–63. [PubMed] [Google Scholar]
- 10.Sathish T, Williams ED, Pasricha N, Absetz P, Lorgelly P, Wolfe R, et al. Cluster randomised controlled trial of a peer-led lifestyle intervention program: study protocol for the Kerala diabetes prevention program. BMC Public Health. 2013;13:1035. doi: 10.1186/1471-2458-13-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan V, Deepa R, Deepa M, Somannavar S, Datta M. A simplified Indian Diabetes Risk Score for screening for undiagnosed diabetic subjects. J Assoc Physicians India. 2005;53:759–763. [PubMed] [Google Scholar]
- 12.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl.):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla P, La Valle E, Falbo R, Limonta G, Signorini S, Cappellini F, et al. Normal fasting plasma glucose and risk of type 2 diabetes. Diabetes Care. 2011;34:1372–1374. doi: 10.2337/dc10-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anjana RM, Ranjani H, Unnikrishnan R, Weber MB, Mohan V, Narayan KMV. Exercise patterns and behaviour in Asian Indians: Data from the baseline survey of the Diabetes Community Lifestyle Improvement Program (D-CLIP) Diabetes Res Clin Pract. 2015;107:77–84. doi: 10.1016/j.diabres.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran A, Snehalatha C, Mary S, Selvam S, Kumar CK, Seeli AC, Shetty AS. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2) Diabetologia. 2009;52:1019–1026. doi: 10.1007/s00125-009-1315-x. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1:191–198. doi: 10.1016/S2213-8587(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 20.Ranjani H, Weber MB, Anjana RM, Lakshmi N, Venkat Narayan KM, Mohan V. Recruitment challenges in a diabetes prevention trial in a low- and middle-income setting. Diabetes Res Clin Pract. 2015;110:51–59. doi: 10.1016/j.diabres.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. [Last accessed 30 October 2015];STEPwise approach to surveillance (STEPS) Available at: http://www.who.int/chp/steps/en/
- 22.World Health Organization. [Last accessed 30 October 2015];The Asia-Pacific Perspective: Redefining Obesity and its Treatment. 2000 Available at: http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity.pdf.
- 23.US Department of Health and Human Services. Seventh Report of the Joint National Committee On Prevention, Detection, Evaluation and Treatment of High Blood Pressure. [Last accessed 30 October 2015]; Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf.
- 24.National Cholesterol Education Program Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.