Abstract
Background
Phenotypic presentations in young children with asthma are varied and may contribute to differential responses to asthma controller medications.
Methods
The Individualized Therapy for Asthma in Toddlers (INFANT) study was a multicenter, randomized, double-blind, double-dummy, clinical trial in children age 12-59 months (n=300) with asthma necessitating treatment with daily controller (Step 2) therapy. Participants completed a 2-8 week run-in period followed by three crossover periods with daily inhaled corticosteroid (ICS), daily leukotriene receptor antagonist (LTRA), and as-needed ICS treatment co-administered with albuterol. The primary outcome was differential response to asthma medication based on a composite measure of asthma control. The primary analysis involved two stages: determination of differential response, and assessment of whether three pre-specified features (aeroallergen sensitization, previous exacerbations, sex) predicted differential response.
Results
74% (170 of 230) of children with analyzable data had a differential response to the three treatment strategies. Within differential responders, the probability of best response was highest for daily ICS and was predicted by aeroallergen sensitization, but not exacerbation history or sex. The probability of best response to daily ICS was further increased in children with both aeroallergen sensitization and blood eosinophils ≥300/μL. In these children, daily ICS was associated with more asthma control days and fewer exacerbations compared to the other treatments.
Conclusions
In young children with asthma necessitating Step 2 treatment, phenotyping with aeroallergen sensitization and blood eosinophils is useful for guiding treatment selection and identifies children with a high exacerbation probability for whom treatment with daily ICS is beneficial despite possible risks of growth suppression.
Keywords: Asthma, Asthma treatment, Asthma biomarkers, Asthma phenotype, Inhaled corticosteroid, Leukotriene receptor antagonist, Personalized medicine, Treatment response
Graphical abstract
INTRODUCTION
Although asthma treatment guidelines1,2 have proven useful in care standardization and reduction of adverse outcomes,3 there is phenotypic heterogeneity within the disorder and growing appreciation for “personalized” medicine as opposed to a “one-size-fits-all” treatment approach.4,5 Young children are particularly diverse with numerous and variable phenotypic presentations in early life that correspond to different outcomes,6-9 yet they are incompletely studied and significant treatment gaps remain.10,11 Even among young children who warrant treatment with daily inhaled corticosteroids (ICS), the response to ICS is inconsistent,12 perhaps due to differences in symptom presentation and/or persistence13 or other underlying inflammatory features.14 Indeed, many young children have asymptomatic periods between respiratory viral illnesses,15 raising the question whether daily therapy with ICS is warranted in all children since ICS administration does not significantly alter the long-term disease course16 and may contribute to dose-dependent and sustained reductions in linear growth in selected subpopulations.17,18
Given these challenges and the mandate for personalized and more efficient medicine,19 the Individualized Therapy for Asthma in Toddlers (INFANT) trial characterized phenotypic heterogeneity in young children with asthma necessitating treatment with daily controller medications (i.e., Step 2 therapy2) and examined the relationship of phenotypic features and biomarkers to asthma medication response profiles. This study demonstrates for the first time differential responses to asthma medications in young children that can be predicted with clinical biomarkers. The results support personalization of asthma therapy and highlight a phenotype of children with aeroallergen sensitization and elevated blood eosinophils at risk for exacerbation for whom daily ICS treatment is beneficial despite the possible risk of growth suppression.
METHODS
Study design and oversight
The INFANT study was a multicenter, randomized, double-blind, double-dummy, clinical trial conducted from March, 2013 through April, 2015. A run-in period of 2-8 weeks was followed by a randomized cross-over of three 16-week treatment periods with daily ICS (fluticasone propionate, two inhalations, 44 μg each, twice daily, GlaxoSmithKline, Evreux, France), daily leukotriene receptor antagonist (LTRA) (montelukast, 4 mg, once daily at bedtime, Merck and Co., Inc., Whitehouse Station, NJ), and as-needed ICS co-administered with an open-label short-acting bronchodilator for symptom relief (fluticasone propionate, two inhalations, 44 μg each; albuterol sulfate, two inhalations, 90 μg each, GlaxoSmithKline, Research Triangle Park, NC) (Figure 1A). Antipyretic/analgesic therapy was blinded and controlled in a linked protocol (NCT01606319) through a factorial design. Details of that study were previously published.20 Children were randomized in two processes: the first determined the crossover sequence of asthma therapy and the second determined the blinded antipyretic/analgesic medication to be used as needed for fever or pain throughout the 48 week duration of the crossover study, with stratification by clinical center.
Figure 1.
Study diagram and procedures. ICS = inhaled corticosteroid; LTRA = leukotriene receptor antagonist; Diary = electronic diary distribution and data review; Height = height measurement, Blood = blood collection; Urine = urine collection. The “+” sign indicates that the procedure was performed.
The National Heart, Lung and Blood Institute's asthma network (AsthmaNet) funded the study, which was managed by a Data Coordinating Center (Hershey, PA). The protocol was developed by the AsthmaNet Steering Committee (NCT01606306) and was approved by an external Protocol Review committee, a Data Safety Monitoring Board, and each site's Institutional Review Board. Caregivers provided written informed consent.
Sites and patients
The study was conducted in children 12-59 months of age at 18 sites in the United States. Children were recruited for the study through a variety of methods including advertisements, primary care and specialty care clinic referrals, and screenings of urgent care facility visits and after-hours telephone logs. Children were eligible for study entry if they met guideline-based criteria for daily asthma controller medication (i.e., Step 2 treatment).2 To encourage recruitment and generalization of results, this protocol enrolled ICS and LTRA-naïve children treated only with intermittent bronchodilators who required step-up therapy, as well as children currently treated with low-dose ICS or LTRA for whom daily controller therapy was warranted. Children symptomatic on current ICS or LTRA were enrolled with the rationale that 1) they may require treatment with LTRA and not ICS or vice versa, 2) they may benefit from the ICS formulation (i.e., directly inhaled versus nebulized), and 3) medication delivery may be improved with educational intervention and adherence monitoring.
Children were eligible for the study irrespective of current medication use if their caregivers reported daytime asthma symptoms >2 days per week (averaged over the preceding 4 weeks), nighttime awakening from asthma at least once over the previous 4 weeks, or ≥4 wheezing episodes, each lasting ≥24 hours, in the preceding 12 months. Children not receiving current ICS or LTRA treatment were also eligible if they reported ≥2 exacerbations requiring systemic corticosteroids in the preceding 6 months. Children receiving current ICS or LTRA treatment were also eligible if they reported ICS or LTRA receipt for >90 days during the preceding 6 months or ≥2 exacerbations requiring systemic corticosteroids in the preceding 12 months.
Run-in period
Eligible children received one oral medication and one inhaled medication for daily use, open-label albuterol sulfate and open-label prednisolone. The run-in duration was variable and based upon whether or not the child was currently receiving step 2 therapy (i.e., daily low-dose ICS or LTRA) and whether or not the child qualified based on exacerbation history. Children not on step 2 therapy during the 6 months prior to enrollment (including children who received ICS or LTRA intermittently) received placebo oral and inhaled therapy during the run-in. The run-in was completed in 2 weeks if the participant had a previous exacerbation requiring systemic corticosteroids. If the participant did not have an exacerbation, the run-in period could be extended up to 8 weeks to elicit symptoms. Children who were currently receiving step 2 therapy received active ICS or active LTRA during the run-in period. If the participant had a previous exacerbation requiring systemic corticosteroids, the run-in was completed in two weeks. If not, the run-in lasted 4 weeks in total. Caregivers recorded symptoms, healthcare utilization and medication use in electronic diaries each day at bedtime (Spirotel®, Medical International Research, Rome, Italy). Children were ineligible for randomization if the following were observed during the run-in period: 1) completion of <75% of daily electronic diaries, 2) an exacerbation requiring systemic corticosteroids, 3) daily asthma symptoms if not receiving active therapy, or 4) asthma symptoms >2 days per week if receiving active therapy. Further details are provided in Figure 1 and the online supplement.
Biomarker determination
Peripheral blood eosinophil counts were determined from one aliquot of whole blood by an automated assay at each clinical site. ECP,21 total serum immunoglobulin E (IgE) and specific IgE concentrations were quantified by a commercial laboratory (Advanced Diagnostic Laboratories, National Jewish Health, Denver, CO). Specific IgE (ImmunoCAP®) was performed for a nationally representative panel of inhalant aeroallergens (details provided in the online supplement). Aeroallergen test results were considered positive if values were ≥ 0.35 kU/L. Urinary leukotriene E4 (LTE4) concentrations were measured by mass spectrometry as previously described22,23 and were expressed per mg of creatinine.
Outcome measures
The primary outcome was the differential response to three therapies on the basis of fixed threshold criteria for the following asthma control measures, which encompassed domains of risk and impairment:1,2 the time from the start of the treatment period to an asthma exacerbation treated with systemic corticosteroids, and the annualized number of asthma control days (ACDs) from within that period. ACDs were defined as full calendar days without symptoms, rescue medication use, or unscheduled healthcare visits. Children were defined as differential responders if, first, the time to an asthma exacerbation was at least four weeks longer, or second, if the number of annualized ACDs was at least 31 days more for one treatment than another, in that order. If neither threshold was met, the participant was considered a non-differential responder. Four weeks between the onset of treatment and an asthma exacerbation was selected as a clinically meaningful outcome based on the results of a previous study in school-age children that noted differences in asthma exacerbation prevalence in children treated with fluticasone (16%) versus montelukast (32%) over a 16-week period.24 A difference of 31 days of more with regard to ACDs was also thought to be clinically meaningful based on the results of a prior study in school-age children24 and preschool children at high risk for asthma development.16
Differential response was determined in children completing at least two treatment periods and at least 50% of the daily diary entries for each period. Because placebo washouts were not performed, the data collected during the first two weeks of each period were not included in the analysis of ACDs. Days with missing diary data were also excluded from ACD determination. Secondary outcomes included exacerbations, ACDs, albuterol use, unscheduled healthcare for asthma, and protocol-defined treatment failures.
Criteria for treatment period failure and study failure
Treatment period failure was achieved if a child experienced two exacerbations, separated by at least one week, in a single 16-week treatment period. When two exacerbations occurred, the child was advanced to the next treatment period. Criteria for study failure were met if the participant: 1) received four courses of prednisolone after randomization, 2) was hospitalized >24 hours for an acute asthma exacerbation, or 3) was moved forward to the next treatment period two times during the course of the study.
Statistical Analysis
The primary analysis involved two stages: first testing the null hypothesis of all three treatments having equal probability to yield the best response as defined by the criteria described above, and second to determine whether any of three pre-specified phenotypic characteristics (sensitization to at least one aeroallergen, previous exacerbations requiring systemic corticosteroids, and sex) predict different patterns of treatment response. The overall type I error rate for the primary analysis was 0.05 using a significance level of 0.0125 for the first stage test and for each of the three pre-specified predictors. Rank-ordered logistic regression25 was used to model the probability of yielding best response for each treatment and bootstrapping was used to calculate confidence intervals. Secondary analyses employed the generalized linear model framework to compare treatments with respect to secondary outcomes, using the Generalized Estimating Equations approach to incorporate the longitudinal aspect of the cross over design, and including period and treatment-by-period interaction effects to examine potential carryover effects. Exploratory analyses utilized rank-ordered logistic regression to examine other phenotypic characteristics that might predict patterns of treatment response. Pre-specified exploratory analyses focused on serum eosinophil cationic protein (ECP) levels21 and urinary leukotriene E4 (LTE4) concentrations26 as predictors of treatment response. Blood eosinophils, specific aeroallergen tests results, serum IgE and modified Asthma Predictive Index27 (mAPI) status, defined by a history of 4 or more wheezing episodes plus one major criteria (parental history of asthma, physician-diagnosed atopic dermatitis, or allergic sensitization to ≥1 aeroallergen) or two minor criteria (allergic sensitization to milk, egg, or peanut, wheezing unrelated to colds, or blood eosinophils ≥4%),28 were examined post-hoc as potential predictors. Secondary and exploratory analyses utilized a 0.05 significance level without adjustment for multiple testing. Exploratory models also included allergic sensitization, history of exacerbations and sex as covariates.
A sample size of 294 participants was selected to test the primary null hypothesis of all three treatments having equal probability (one-third) to yield the best response with statistical power of at least 0.90 if any one of the three treatments actually has probability of at least one-half to yield the best response. This study was also powered to detect differences in patterns of treatment response for the prespecified predictors. The sample size allowed for up to 25% of participants to drop-out and up to 45% of participants to not demonstrate differential response.29 These sample size assumptions were met. SAS® statistical software (version 9.4, SAS Inc., Cary, NC) was used for all analyses.
RESULTS
Study patients
443 children were enrolled and 300 were randomized (Figure 2). Of these, 42% were sensitized to at least one aeroallergen and 60% had a positive mAPI28 (Table 1). Specific aeroallergen testing results are shown in Table E1. 226 children completed all three periods while 230 children completed at least two periods with adequate diary completion, permitting assessment of differential response.
Figure 2.
Flow chart depicting the number of participants who enrolled in the study, underwent randomization, and completed the study, and provided analyzable data for analysis.
Table 1.
Features of the study participants. Data represent the mean ± SD, the median (IQR) or the number of participants (%).
| All participants N = 300 | Not Evaluable N = 70 | Evaluable1 N = 230 | Non-Differential Response N = 60 | Differential Response N = 170 | |
|---|---|---|---|---|---|
| Age at enrollment (months) | 39.9 +/− 13.2 | 40.3 +/− 14.1 | 39.7 +/− 13.0 | 40.8 +/− 12.3 | 39.4 +/− 13.2 |
| Males | 179 (59.7%) | 36 (51.4%) | 143 (62.2%) | 37 (61.7%) | 106 (62.4%) |
| Race/Ethnicity | |||||
| African American race | 97 (32.3%) | 31 (44.3%) | 66 (28.7%) | 23 (38.3%) | 43 (25.3%) |
| White race | 148 (49.3%) | 28 (40.0%) | 120 (52.2%) | 23 (38.3%) | 97 (57.1%) |
| Hispanic | 72 (24.0%) | 16 (22.9%) | 56 (24.3%) | 14 (23.3%) | 42 (24.7%) |
| Parental asthma | 178 (59.3%) | 36 (51.4%) | 142 (61.7%) | 36 (60.0%) | 106 (62.4%) |
| Positive mAPI | 181 (60.3%) | 39 (55.7%) | 142 (61.7%) | 39 (65.0%) | 103 (60.6%) |
| Exacerbation history | |||||
| Systemic corticosteroid use (previous 12 months) | 224 (74.7%) | 51 (72.9%) | 173 (75.2%) | 39 (65.0%) | 134 (78.8%) |
| Systemic corticosteroid courses (previous 6 months) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 1 (0, 1) | 1 (0, 2) |
| Urgent/ED visits past year | 3 (1, 4) | 2 (1, 4) | 3 (1, 4) | 2.5 (1, 4) | 3 (2, 5) |
| Wheezing episodes past year | 5 (3, 7) | 4.5 (3, 7) | 5 (3, 7) | 5 (3.5, 7.5) | 5 (3, 7) |
| Hospitalized in past year | 65 (21.7%) | 18 (25.7%) | 47 (20.4%) | 9 (15%) | 38 (22.4%) |
| Allergic/inflammatory features | |||||
| Positive Aeroallergen Test | 126 (42.0%) | 26 (37.1%) | 100 (43.5%) | 21 (35.0%) | 79 (46.5%) |
| Eczema | 160 (53.3%) | 34 (48.6%) | 126 (54.8%) | 35 (58.3%) | 91 (53.5%) |
| Blood eosinophils >4% | 123 (41.0%) | 27 (38.6%) | 96 (41.7%) | 21 (35.0%) | 75 (44.1%) |
| Blood eosinophils (per μL) | 257.6 (158.4, 492.0) | 246.6 (175.6, 463.5) | 257.6 (153.4, 495.0) | 232.2 (133.2, 487.0) | 259.6 (162.0, 495.6) |
| Serum IgE (kU/L) | 70.0 (22.0, 208.0) | 64.0 (19.0, 313.0) | 70.0 (24.0, 206.0) | 64.0 (26.5, 195.0) | 77.5 (21.0, 208.0) |
| Serum ECP (μg/L) | 11.1 (5.8, 21.4) | 11.1 (5.8, 18.0) | 11.1 (5.9, 22.2) | 10.2 (4.7, 22.4) | 11.3 (6.3, 21.5) |
| Urinary LTE4 (pg/mg creatinine) | 117.3 (72.0, 182.1) | 124.2 (74.8, 193.7) | 115.6 (70.3, 178.5) | 123.5 (79.1, 189.3) | 112.4 (64.6, 176.3) |
| Environmental exposures | |||||
| Tobacco smoke exposure | 110 (36.7%) | 33 (47.1%) | 77 (33.5%) | 20 (33.3%) | 57 (33.5%) |
| Pets in Home | 139 (46.3%) | 27 (38.6%) | 112 (48.7%) | 25 (41.7%) | 87 (51.2%) |
| Run-in characteristics | |||||
| Run-in ICS | 189 (63.0%) | 40 (57.1%) | 149 (64.8%) | 38 (63.3%) | 111 (65.3%) |
| Run-in LTRA | 18 (6.0%) | 3 (4.3%) | 15 (6.5%) | 4 (6.7%) | 11 (6.5%) |
| Run-in percent ACDs | 85.5 +/− 17.6 | 86.6 +/− 17.4 | 85.2 +/− 17.7 | 89.0 +/− 14.4 | 83.8 +/− 18.6 |
mAPI = modified Asthma Predictive Index; ED = Emergency Department; IgE = immunoglobulin E; ECP = eosinophil cationic protein; LTE4 = leukotriene E4; ICS = inhaled corticosteroid; LTRA = leukotriene receptor antagonist; ACD = asthma control day
Includes participants who completed at least two study periods with adequate diary completion
Differential response to the treatment strategies
A differential response to the three treatments occurred in 170 (74%) of the 230 children with evaluable data. Among the differential responders, the probability of best response was highest for daily ICS (Figure 3A). Sixty children (26%) did not demonstrate a differential response and had indicators of less disease activity, including more ACDs and lower exacerbation probability (Figure 2, B-C). Seasonal adjustment did not impact results. No interactions with antipyretic/analgesic use (NCT01606319) were noted. Sensitivity analyses also indicated no interactions based upon ICS treatment during the run-in (Figure E1A) or based upon the order in which the study treatments were received (Figure E1, B-C).
Figure 3.
(A) Probability of each asthma treatment being the best of the three. Gray shading depicts participants who did not have a differential response. (B) The percentage of Asthma Control Days (ACDs) and (C) the probability of an exacerbation.. Boxplots represent the median value, 25th to 75th percentile (shading) and 5th to 95th percentile (whiskers). Outliers are shown as triangles.
Primary analysis of pre-specified predictors of differential response
The second stage of the primary analysis focused on sensitization to at least one aeroallergen, previous exacerbations requiring systemic corticosteroids, and sex as predictors of differential response. Aeroallergen sensitization, but not exacerbation history or sex, was associated with a differential response favoring daily ICS (Figure 3, A-C).
Exploratory analyses of predictors of differential response
Blood eosinophils ≥300/μL were also associated with a higher probability of responding best to daily ICS (Figure 3D) and predictive ability was significantly enhanced when both elevated eosinophils and aeroallergen sensitization were included in the model (Figure 3E). Further analyses demonstrated that serum ECP levels ≥10 μg/L and dog and/or cat sensitization also predicted better response to daily ICS, while mAPI status, serum IgE and urinary LTE4 concentrations did not predict differential response pattern (Figure E2, A-E). Cut-points for quantitative biomarker predictors were identified based on analyses in which they were treated as continuous predictors. No predictor identified a group in which LTRA or as-needed ICS were more likely than daily ICS to yield best response.
Secondary Outcomes
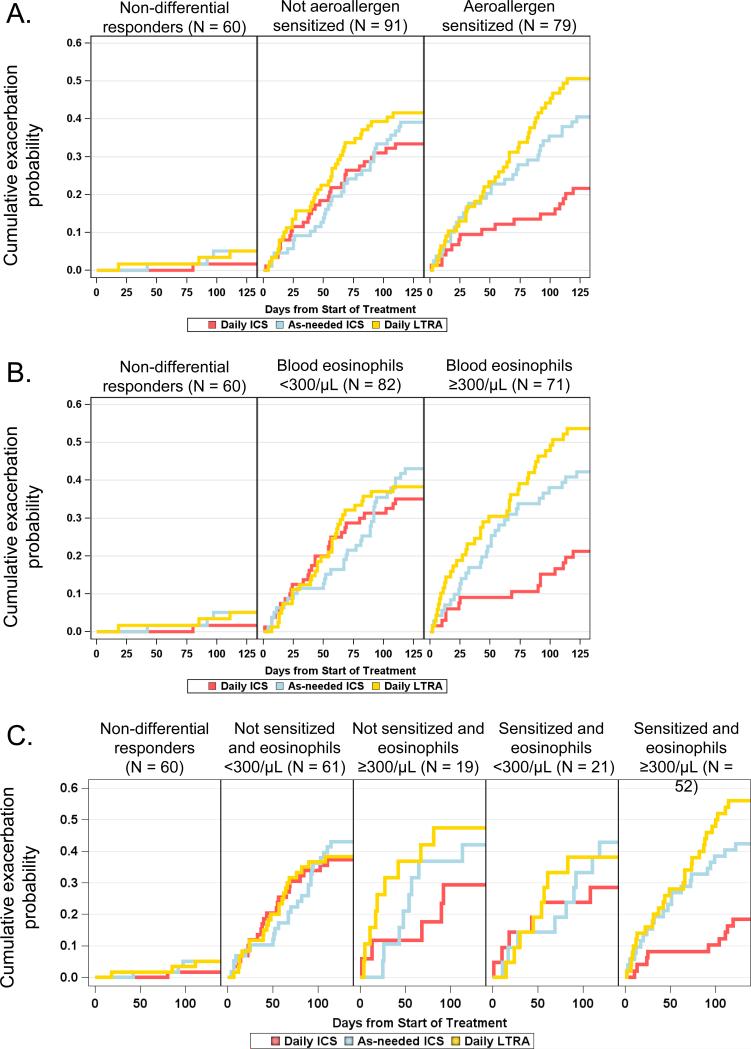
Daily ICS treatment was associated with more ACDs, fewer rescue albuterol inhalations, and fewer exacerbations (Table E2). The average weekly ICS dose was approximately 1200 μg fluticasone in the daily ICS group versus 270 μg fluticasone in the as-needed ICS group. Descriptive analyses further indicated greater improvement in ACDs (Figure E3) and a prolonged time to exacerbation (Figure 4) with daily ICS treatment in children with aeroallergen sensitization, children with blood eosinophils ≥300/μL and children with both aeroallergen sensitization and blood eosinophils ≥300/μL.
Figure 4.
The probability of best response based on (A) aeroallergen sensitization, (B) previous exacerbation, (C) sex, (D) eosinophils ≥300/μL, and (E) combinations of sensitization and eosinophils. P-values correspond to the test of interaction between the predictor and treatment and indicate whether the pattern of treatment response differs according to subgroup. Sample sizes correspond to participants with evaluable data (N = 230).
Adherence to the study therapies
75% of daily diaries were completed throughout the study. Self-reported adherence to daily medication was ≥96% for all treatments. As-needed ICS was used concomitantly with albuterol on 99% of occasions per electronic diary report. Albuterol was administered on approximately 70% and 90% of days with mild and moderate-severe symptoms reported, respectively.
Adverse events
There were no marked differences in adverse events between treatments (Table E3). There was a non-significant trend toward decreased height velocity in children treated with daily ICS (Table E4).
DISCUSSION
Young children with asthma are a heterogeneous group of patients with significant morbidity and healthcare utilization who are challenging to treat.30,31 Initial medication selection and timing of delivery is controversial32,33 given a limited number of studies and overall low quality of evidence in this age group.34,35 Moreover, while differential responses to asthma medications have been observed in older children,36,37 and argue against a universal treatment approach, no study has assessed how treatment decisions should be made in young children using phenotypic characteristics and biomarkers to estimate the likelihood of improvement. Utilizing a composite measure of asthma control, we found that 74% of young children demonstrated clinically relevant improvements in response to one treatment versus others, most often daily ICS, and that clinically accessible biomarkers can be used to predict the medication strategy associated with the best response in these children. We further noted a phenotype of children with type-2 inflammation evidenced by aeroallergen sensitization and elevated blood eosinophils for whom daily ICS treatment conferred the most protection against symptoms and exacerbations. Given that young children have nearly 2-3 times the rate of emergency department visits and hospitalizations compared to older children,30,38 these results are clinically important and demonstrate the potential impact of phenotype-directed asthma care in this age group.
Although we were adequately powered for our primary analyses of best response, the proportion of children with a non-differential response (24%) was substantially greater than a previous study that found a differential response to Step 3 asthma therapy in >97% of older children.36 Because pulmonary function testing is challenging in young children, our composite outcome of asthma control included only two components, exacerbations and ACDs, which may explain this finding. We were not specifically powered for sub-analyses of non-differential responders and therefore it remains unclear whether those children have unique inflammatory profiles. However, the asthma control in those children throughout the study may also suggest that some children became candidates for step-down therapy despite initial qualification for controller medication.
Overall, children with a differential response in this study were most likely to respond best to daily ICS, consistent with other studies demonstrating efficacy of daily ICS in this age group overall12 and regardless of factors such as mAPI status.13,39 However, the overall probability of a best response to ICS was only 0.40 when non-differential responders are considered, highlighting the need for personalized medicine with the “right” therapies for the “right” patients.19 Indeed, many participants had a best response to daily LTRA or as-needed ICS. While we were unable to identify clear predictors of best response to these therapies, further study is warranted since these therapies are useful for many children. For example, a study of older children with mild persistent asthma noted similar efficacy between intermittent low-dose ICS and daily ICS treatment with regard to exacerbations.40 Other studies in preschool children demonstrate that pre-emptive high-dose ICS treatment (i.e., 1500-2000 mcg fluticasone equivalent) can reduce systemic corticosteroid requirements,29,41 although a recent systematic review was unable to firmly conclude equivalence between daily low dose and pre-emptive high dose ICS therapy due to the limited number of head-to-head comparisons.13 The double dummy design of the present study prevented a similar treatment strategy and is acknowledged as a potential limitation. Other studies in young children have also demonstrated improved asthma outcomes with LTRA with minimal adverse effects42-44 and greater tolerability.45
The INFANT study was not designed to study treatment group means for individual outcomes as a whole but rather to study responses at the individual patient level based on the composite outcome incorporating both the risk and impairment domains, with an emphasis on clinically accessible features and biomarkers, which have not been well studied in this age group.46 A secondary analysis of a previous study in mAPI positive children at high risk for asthma development16,47 found that episode-free days were increased with daily ICS versus placebo among boys and participants who were white, who had an emergency department visit or hospitalization for asthma within the past year, and who were more symptomatic at baseline.14 Systemic corticosteroid use and healthcare utilization and were also significantly reduced in children with aeroallergen sensitization in that study.14 Our primary predictor analysis was based on these prior observations14 as well as findings from older children.24,37,48,49 The fact that sex and previous exacerbations did not differentiate best response in the present study was surprising but may be due to differences in the baseline severity of the populations studied. However, a recent analysis of a birth cohort similarly found no association between sex and phenotype in young children at high risk for asthma development.50 Previous exacerbations may also have limited predictive potential given their self-reported nature and the lack of standardization for systemic corticosteroid administration in general practice.51 However, aeroallergen sensitization and blood eosinophils ≥300/μL were strong predictors of differential response and identified a phenotype of children at high risk for disease morbidity who benefit from treatment with daily ICS, although these medications are not without some risk. A previous study in preschool children demonstrated dose-dependent reductions in linear growth with daily ICS that may be worse in selected subpopulations, including children of lesser age and of lesser weight.17 In school-age children with long-term ICS exposure, these height reductions may also persist into the adult years.52 However, asthma exacerbations in children do carry a significant risk of hospitalization and in rare cases, death.53,54 Phenotype-directed daily ICS therapy is therefore beneficial in selected children, but may not be the optimal choice for other children with non-type 2 patterns of inflammation. Other biomarker analyses are needed to guide treatment selection in those children.
This study does have limitations. While overall adherence was quite good in children with evaluable data, adherence to study medications was self-reported on an electronic diary and it is unclear whether medication dose counters would have yielded different adherence estimates.55 This study also did not include a placebo washout phase between the treatment periods due to ethical concerns so carryover effects may have been present. Although we excluded data collected during the first 14 days of each treatment period from the calculation of ACDs, it is possible that carryover effects from daily ICS may be longer for children with less active disease. There may also be an impact of seasonal exacerbations that influences the selection of the best treatment. Although we adjusted for this in the overall population, it may still be a factor for assessing individual preference. We also measured biomarkers at the time of randomization and these may change over time and in association with treatment response. This is particularly true for specific IgE measures, since aeroallergen sensitization tends to develop with age and may not necessarily be present in young preschool children. Attrition and the number of participants with evaluable data, particularly among African Americans, is another consideration despite adequate power for the primary outcome analysis. Missing data from diary cards may also have resulted in underestimation or overestimation of ACDs and differential response.
In conclusion, daily low dose ICS is the most effective therapy for the majority of young children with asthma symptoms and recurrent wheezing episodes for whom Step 2 treatment with daily controller medication is warranted.2 However, phenotypic heterogeneity is abundant in this age group and is associated with differential responses to asthma medications. Readily available biomarkers of type 2 inflammation, namely aeroallergen sensitization and blood eosinophils, can also be used to identify a group of children for whom daily ICS treatment is beneficial. Other studies are needed to determine whether these findings would also apply to young children requiring higher treatment steps.
Supplementary Material
Figure 5.
The cumulative probability of an exacerbation requiring systemic corticosteroids for all participants with evaluable data (N = 230), stratified by (A) aeroallergen sensitization, (B) blood eosinophils ≥300/μL, and (C) combinations of aeroallergen sensitization and blood eosinophils.
CLINICAL IMPLICATIONS.
Although young children requiring Step 2 asthma treatment are phenotypically diverse, children with aeroallergen sensitization and elevated blood eosinophils respond best to daily inhaled corticosteroids (ICS), as opposed to leukotriene receptor antagonists or as-needed ICS.
CAPSULE SUMMARY.
Phenotyping with aeroallergen sensitization and blood eosinophils is useful for guiding treatment selection in young children requiring Step 2 asthma treatment and can identify children for whom treatment with daily ICS is best.
Acknowledgments
Funded by the National Heart, Lung and Blood Institute AsthmaNet INFANT ClinicalTrials.gov number, NCT01606306
LIST OF ABBREVIATIONS
- ACD
Asthma control days
- mAPI
Modified Asthma Predictive Index
- ECP
Eosinophil cationic protein
- ICS
Inhaled corticosteroid
- LTE4
Leukotriene E4
- LTRA
Leukotriene receptor antagonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anne M. Fitzpatrick, Emory University, Department of Pediatrics, Atlanta, GA
Daniel J. Jackson, University of Wisconsin School of Medicine and Public Health, Pediatrics Section of Allergy, Immunology and Rheumatology, Madison, WI
David T. Mauger, Penn State University, College of Medicine, Department of Public Health Sciences, Hershey, PA
Susan J. Boehmer, Penn State University, College of Medicine, Department of Public Health Sciences, Hershey, PA
Wanda Phipatanakul, Boston Children's Hospital, Division of Allergy/Immunology, Harvard Medical School, Boston, MA
William J. Sheehan, Boston Children's Hospital, Division of Allergy/Immunology, Harvard Medical School, Boston, MA
James N. Moy, Stroger Hospital of Cook County, Department of Pediatrics, Rush University Medical Center, Chicago, IL
Ian M. Paul, Penn State University, College of Medicine, Department of Pediatrics, Hershey, PA
Leonard B. Bacharier, Washington University in St. Louis School of Medicine and St. Louis Children's Hospital, Department of Pediatrics. St Louis, MO
Michael D. Cabana, University of California - San Francisco, Medicine, San Francisco, CA
Ronina Covar, The University of Pittsburgh Asthma Institute at UPMC/UPSOM, Pittsburgh, PA
Fernando Holguin, University of Wisconsin School of Medicine and Public Health, Pediatrics, Madison, WI
Robert F. Lemanske, Jr., University of Arizona, Arizona Respiratory Center, Tuscon, AZ
Fernando D. Martinez, Ann & Robert H Lurie Children's Hospital of Chicago, Chicago, IL
Jacqueline A. Pongracic, Washington University School of Medicine and St. Louis Children's Hospital, Department of Pediatrics. St Louis, MO
Avraham Beigelman, Boston Children's Hospital, Division of Allergy/Immunology, Harvard Medical School, Boston, MA
Sachin N. Baxi, University of California San Francisco Benioff Children's Hospital Oakland, Oakland, CA
Mindy Benson, Nemours Children's Health System, Jacksonville, FL
Kathryn Blake, Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children's Hospital, Cleveland, OH
James F. Chmiel, National Jewish Health, Pediatrics, Denver, CO
Cori L. Daines, University of Arizona, Arizona Respiratory Center, Tuscon, AZ
Michael O. Daines, University of Arizona, Arizona Respiratory Center, Tucson, AZ
Jonathan M. Gaffin, Boston Children's Hospital, Division of Respiratory Diseases, Harvard Medical School, Boston, MA
Deborah Ann Gentile, Allegheny General Hospital, Department of Pediatrics, Pittsburgh, PA
W. Adam Gower, Wake Forest School of Medicine, Winston-Salem, NC
Elliot Israel, Brigham & Women's Hospital, Harvard Medical School, Boston, MA
Harsha Vardhan Kumar, University of Illinois at Chicago, Chicago, IL
Jason E. Lang, Nemours Children's Hospital, University of Central Florida College of Medicine, Orlando, FL
Stephen C. Lazarus, University of California, San Francisco, Medicine, San Francisco, CA
John J. Lima, Nemours Children's Health System, Jacksonville, FL
Ngoc Ly, University of California - San Francisco, Airway Clinical Research Center, San Francisco, CA
Jyothi Marbin, University of California San Francisco Benioff Children's Hospital Oakland, Oakland CA
Wayne Morgan, University of Arizona, Arizona Respiratory Center, Tucson, AZ
Ross E. Myers, Rainbow Babies and Children's Hospital, Cleveland, OH
J. Tod Olin, National Jewish Health, Department of Pediatrics, Denver, CO
Stephen P. Peters, Wake Forest University School of Medicine, Winston-Salem, NC
Hengameh H. Raissy, University of New Mexico, Pediatrics / Pulmonary, Albuquerque, NM
Rachel G. Robison, Ann & Robert H Lurie Children's Hospital of Chicago, Chicago, IL
Kristie Ross, Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children's Hospital, Cleveland, OH
Christine A. Sorkness, University of Wisconsin-Madison, Madison, WI
Shannon M. Thyne, Olive View-UCLA Medical Center, Department of Pediatrics, David Geffen School of Medicine at UCLA, Los Angeles, CA
Stanley J. Szefler, Children's Hospital Colorado, The Breathing Institute, and University of Colorado School of Medicine, Aurora, CO
REFERENCES
- 1. [March 14, 2016];From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015 Available: http://www.ginasthma.org/.
- 2.National Asthma Education and Prevention Panel Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Szefler SJ. Advancing asthma care: the glass is only half full! The Journal of allergy and clinical immunology. 2011;128:485–94. doi: 10.1016/j.jaci.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy BD, Noel PJ, Freemer MM, et al. Future Research Directions in Asthma: An NHLBI Working Group Report. American journal of respiratory and critical care medicine. 2015 doi: 10.1164/rccm.201505-0963WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. The European respiratory journal. 2015;46:622–39. doi: 10.1183/13993003.00853-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depner M, Fuchs O, Genuneit J, et al. Clinical and epidemiologic phenotypes of childhood asthma. American journal of respiratory and critical care medicine. 2014;189:129–38. doi: 10.1164/rccm.201307-1198OC. [DOI] [PubMed] [Google Scholar]
- 7.Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63:974–80. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. The New England journal of medicine. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 9.Savenije OE, Granell R, Caudri D, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. The Journal of allergy and clinical immunology. 2011;127:1505–12. e14. doi: 10.1016/j.jaci.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland ER, Busse WW, National Heart L, Blood Institute's A. Designing clinical trials to address the needs of childhood and adult asthma: the National Heart, Lung, and Blood Institute's AsthmaNet. The Journal of allergy and clinical immunology. 2014;133:34–8. e1. doi: 10.1016/j.jaci.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szefler SJ, Chmiel JF, Fitzpatrick AM, et al. Asthma across the ages: knowledge gaps in childhood asthma. The Journal of allergy and clinical immunology. 2014;133:3–13. doi: 10.1016/j.jaci.2013.10.018. quiz 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123:e519–25. doi: 10.1542/peds.2008-2867. [DOI] [PubMed] [Google Scholar]
- 13.Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. Lancet. 2014;383:1593–604. doi: 10.1016/S0140-6736(14)60615-2. [DOI] [PubMed] [Google Scholar]
- 14.Bacharier LB, Guilbert TW, Zeiger RS, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. The Journal of allergy and clinical immunology. 2009;12382:1077–82. e1–5. doi: 10.1016/j.jaci.2008.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacharier LB, Phillips BR, Bloomberg GR, et al. Severe intermittent wheezing in preschool children: a distinct phenotype. The Journal of allergy and clinical immunology. 2007;119:604–10. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 16.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. The New England journal of medicine. 2006;354:1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 17.Guilbert TW, Mauger DT, Allen DB, et al. Growth of preschool children at high risk for asthma 2 years after discontinuation of fluticasone. The Journal of allergy and clinical immunology. 2011;128:956–63. e1–7. doi: 10.1016/j.jaci.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth. Cochrane Database Syst Rev. 2014;7:CD009878. doi: 10.1002/14651858.CD009878.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The White House Office of the Press Secretary [March 14, 2016];Fact Sheet: President Obama's Precision Medicine Initiative. 2015 Jan 30; Available: https://www.whitehouse.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative/.
- 20.Sheehan WJ, Mauger DT, Paul IM, et al. Acetaminophen versus Ibuprofen in Young Children with Mild Persistent Asthma. The New England journal of medicine. 2016;375:619–30. doi: 10.1056/NEJMoa1515990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strunk RC, Szefler SJ, Phillips BR, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. The Journal of allergy and clinical immunology. 2003;112:883–92. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong M, Liu AH, Harbeck R, Reisdorph R, Rabinovitch N, Reisdorph N. Leukotriene-E4 in human urine: Comparison of on-line purification and liquid chromatography-tandem mass spectrometry to affinity purification followed by enzyme immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3169–74. doi: 10.1016/j.jchromb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabinovitch N, Graber NJ, Chinchilli VM, et al. Urinary leukotriene E4/exhaled nitric oxide ratio and montelukast response in childhood asthma. The Journal of allergy and clinical immunology. 2010;126:545–51. e1–4. doi: 10.1016/j.jaci.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorkness CA, Lemanske RF, Jr., Mauger DT, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. The Journal of allergy and clinical immunology. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Allison PD, Christakis NA. Logit models for sets of ranked items. Sociological Methodol. 1994;24:199–228. [Google Scholar]
- 26.Rabinovitch N, Mauger DT, Reisdorph N, et al. Predictors of asthma control and lung function responsiveness to step 3 therapy in children with uncontrolled asthma. The Journal of allergy and clinical immunology. 2014;133:350–6. doi: 10.1016/j.jaci.2013.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. American journal of respiratory and critical care medicine. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 28.Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. The Journal of allergy and clinical immunology. 2004;114:1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Zeiger RS, Mauger D, Bacharier LB, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. The New England journal of medicine. 2011;365:1990–2001. doi: 10.1056/NEJMoa1104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 31.Karaca-Mandic P, Jena AB, Joyce GF, Goldman DP. Out-of-pocket medication costs and use of medications and health care services among children with asthma. Jama. 2012;307:1284–91. doi: 10.1001/jama.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5–34. doi: 10.1111/j.1398-9995.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen SE, Hurd SS, Lemanske RF, Jr., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46:1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan BF, Chartrand C, Ducharme FM. Intermittent versus daily inhaled corticosteroids for persistent asthma in children and adults. Cochrane Database Syst Rev. 2013;2:CD009611. doi: 10.1002/14651858.CD009611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong J, Haran C, Chauhan BF, Asher I. Intermittent inhaled corticosteroid therapy versus placebo for persistent asthma in children and adults. Cochrane Database Syst Rev. 2015;7:CD011032. doi: 10.1002/14651858.CD011032.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemanske RF, Jr., Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. The New England journal of medicine. 2010;362:975–85. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. The Journal of allergy and clinical immunology. 2005;115:233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Akinbami L, Centers for Disease Control and Prevention, National Center for Health Statistics The state of childhood asthma, United States, 1980-2005. Adv Data. 2006:1–24. [PubMed] [Google Scholar]
- 39.Papi A, Nicolini G, Baraldi E, et al. Regular vs prn nebulized treatment in wheeze preschool children. Allergy. 2009;64:1463–71. doi: 10.1111/j.1398-9995.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- 40.Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–7. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ducharme FM, Lemire C, Noya FJ, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. The New England journal of medicine. 2009;360:339–53. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 42.Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. American journal of respiratory and critical care medicine. 2005;171:315–22. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 43.Hakim F, Vilozni D, Adler A, Livnat G, Tal A, Bentur L. The effect of montelukast on bronchial hyperreactivity in preschool children. Chest. 2007;131:180–6. doi: 10.1378/chest.06-1402. [DOI] [PubMed] [Google Scholar]
- 44.Szefler SJ, Carlsson LG, Uryniak T, Baker JW. Budesonide inhalation suspension versus montelukast in children aged 2 to 4 years with mild persistent asthma. J Allergy Clin Immunol Pract. 2013;1:58–64. doi: 10.1016/j.jaip.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. The New England journal of medicine. 2011;364:1695–707. doi: 10.1056/NEJMoa1010846. [DOI] [PubMed] [Google Scholar]
- 46.Szefler SJ, Wenzel S, Brown R, et al. Asthma outcomes: biomarkers. The Journal of allergy and clinical immunology. 2012;129:S9–23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guilbert TW, Morgan WJ, Krawiec M, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Bacharier LB, Phillips BR, Zeiger RS, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. The Journal of allergy and clinical immunology. 2008;122:1127–35. e8. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knuffman JE, Sorkness CA, Lemanske RF, Jr., et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. The Journal of allergy and clinical immunology. 2009;123:411–6. doi: 10.1016/j.jaci.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garden FL, Simpson JM, Mellis CM, Marks GB, Investigators C. Change in the manifestations of asthma and asthma-related traits in childhood: a latent transition analysis. The European respiratory journal. 2016;47:499–509. doi: 10.1183/13993003.00284-2015. [DOI] [PubMed] [Google Scholar]
- 51.Fuhlbrigge A, Peden D, Apter AJ, et al. Asthma outcomes: exacerbations. The Journal of allergy and clinical immunology. 2012;129:S34–48. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. The New England journal of medicine. 2012;367:904–12. doi: 10.1056/NEJMoa1203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strid JM, Gammelager H, Johansen MB, Tonnesen E, Christiansen CF. Hospitalization rate and 30-day mortality among patients with status asthmaticus in Denmark: a 16-year nationwide population-based cohort study. Clin Epidemiol. 2013;5:345–55. doi: 10.2147/CLEP.S47679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Triasih R, Duke T, Robertson CF. Outcomes following admission to intensive care for asthma. Arch Dis Child. 2011;96:729–34. doi: 10.1136/adc.2010.205062. [DOI] [PubMed] [Google Scholar]
- 55.Krishnan JA, Bender BG, Wamboldt FS, et al. Adherence to inhaled corticosteroids: an ancillary study of the Childhood Asthma Management Program clinical trial. The Journal of allergy and clinical immunology. 2012;129:112–8. doi: 10.1016/j.jaci.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.