Abstract
All organisms respond to a sudden increase in temperature by the so-called heat shock response. This response results in the induction of a subset of genes, designated heat shock genes coding for heat shock proteins, which allow the cell to cope with the stress regimen. Research carried out during the last 10 years with eubacteria has revealed that the heat shock genes of a given species fall into different classes (regulons), where each class is regulated by a different transcriptional regulator, which could be an alternative sigma factor, a transcriptional activator, or a transcriptional repressor. All regulons of a single species constitute the heat shock stimulon. In Bacillus subtilis, more than 200 genes representing over 7% of the transcriptionally active genes are induced at least 3-fold in response to a heat shock. This response becomes apparent within the first minute after exposure to heat stress, is transient, and is coordinated by at least 5 transcriptional regulator proteins, including 2 repressors, an alternate sigma-factor, and a 2-component signal transduction system. A detailed analysis of the regulation of all known heat shock genes has shown that they belong to at least 6 regulons that together comprise the B subtilis heat shock stimulon. Potential thermosensors are discussed in this article.
INTRODUCTION
When cells of bacteria or any other organism are subjected to a sudden temperature upshock, a group of genes is transiently induced, resulting in the synthesis of so-called heat shock proteins (Hsps). The primary structure of most of these Hsps appears to be highly conserved during evolution, suggesting that they exert similar functions in all organisms. Most Hsps belong to either of 2 classes, namely molecular chaperones and adenosine triphosphate (ATP)–dependent proteases. Whereas chaperones ensure that polypeptides will fold or assemble properly in the cell (Georgopoulos and Welch 1993), proteases will degrade those misfolded proteins, which are unable to refold into their native 3-dimensional structure (Gottesman 1996). In many cases, chaperones and proteases work together in the degradation of nonnative proteins (Gottesman et al 1997). The major problem caused for any cell by a sudden heat shock is the immediate appearance of denatured and misfolded proteins, often collectively designated as nonnative proteins, which tend to aggregate. This will be prevented by chaperones, which recognize and bind nonnative polypeptide chains in collaboration with ATP-dependent proteases that degrade those denatured proteins unable to adopt their native conformation.
All heat shock genes are expressed at ambient temperatures, most of them at a low level, and they are transiently induced after heat challenge. Induction of heat shock genes occurs primarily at the level of transcription, and extensive studies carried out during the past 10 years has shown that the heat shock genes of a single eubacterial species are organized into several regulons, constituting the heat shock stimulon. The Escherichia coli heat shock stimulon is composed of 2 regulons, the σ32 and the σE regulons, and the psp operon, which is under the positive control of PspF and σ54 (for a recent review, see Yura et al 2000). In both Streptomyces sp. and Bradyrhizobium japonicum, 3 regulons have been described so far (Narberhaus 1999; Servant and Mazodier 2001). In Bacillus subtilis, the genetic model organism of the gram-positive eubacteria, the heat shock stimulon comprises at least 6 classes of heat shock genes, where 2 classes are controlled by 2 transcriptional repressors, 1 class by an alternative sigma factor, a fourth class by a still unknown transcriptional activator, the fifth by a 2-component signal transduction system, and there are approximately 80 additional heat shock genes constituting Class VI where we do not yet know how they are regulated. All B subtilis heat shock genes are expressed at a low level at 37°C and transiently induced under heat shock conditions (sudden temperature increase to 48–50°C). Any regulation mechanism has to explain 3 facts: (1) the low level of transcription in the absence of heat stress, (2) the rapid increase in the amount of heat shock proteins that normally occurs within 2–3 minutes, and (3) the slow shut-off after about 10 minutes, even if the cells are further incubated at the high temperature. The purpose of this article will be to review our current understanding on the regulation of the 5 known classes of heat shock genes and to discuss the consequences of this fragmentation.
CLASS I HEAT SHOCK GENES: THE HRCA REGULON
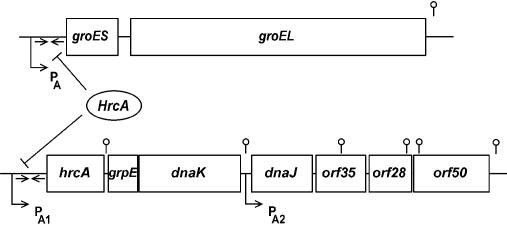
Class I heat shock genes consist of 2 operons only, the heptacistronic dnaK and the bicistronic groE operon (Li and Wong 1992; Schmidt et al 1992; Homuth et al 1997) shown in Figure 1. Both operons are preceded by a σA-type promoter (σA is the housekeeping sigma factor) and a perfect inverted repeat of 9 bp separated by a 9-bp spacer with the deoxyribonucleic acid (DNA) sequence TTAGCACTC-N9-GAGTGCTAA. In the case of the groE promoter, it was confirmed that sequences within the −10 and −35 regions of this promoter constitute the key elements recognized by the σA-containing ribonucleic acid (RNA) polymerase, when 1 invariant position in each region was mutated independently. These mutations could be suppressed by mutant forms of the σA factor, illustrating that the groE promoter is transcribed by the σA-containing RNA polymerase at both 37°C and 48°C (Yuan and Wong 1995a). The heptacistronic dnaK operon consists of the genes hrcA (coding for the transcriptional repressor of both operons, see below), followed by the genes grpE, dnaK, and dnaJ constituting the DnaK chaperone machine, and the 3 open reading frames orf35 (yqeT), orf28 (yqeU), and orf50 (yqeV). The deduced amino acid sequence of orf35 exhibits significant homology to the ribosomal protein L11 methyltransferase coded for by the prmA gene of E coli (Vanet et al 1993). Although the function of orf28 remains elusive, the deduced amino acid sequence of orf50 reveals homology to a lipoate synthase involved in lipoate biosynthesis. Transcriptional analyses of both operons have shown that whereas the groE operon is transcribed into a bicistronic transcript, expression of the dnaK operon starts from 2 promoters, one (PA1) precedes the whole operon, whereas the other (PA2) is located between dnaK and dnaJ (Fig 1). Both primary transcripts are subject to posttranscriptional regulation at a processing site located between hrcA and grpE, and degradation sites are present within the 3 distal genes (Homuth et al 1999). After heat stress, only the amount of the 2 transcripts initiated at PA1 is transiently enhanced (Homuth et al 1999).
Fig 1.
The HrcA regulon. This regulon consists of the 2 operons groE and dnaK. Both operons are expressed from σA-dependent promoters. Although the groE operon is transcribed into 1 bicistronic messenger ribonucleic acid the amount of which increases after heat shock, the heptacistronic dnaK operon is under the control of 2 promoters. Low level of transcription initiates at the 2 promoters PA1 and PA2 in the absence of heat stress, resulting in a full-length transcript and a second transcript encompassing the 4 distal genes. After a temperature upshift, only PA1 becomes activated. Both operons are under the negative control of the HrcA repressor binding to 2 inverted repeat structures indicated by the arrows. Hairpin structures indicate 1 processing site (between hrcA and grpE), 3 transcription termination sites (between dnaK and dnaJ, after orf50 and after groEL), and 3 potential degradation sites (within the 3 distal genes of the dnaK operon)
How are these 2 operons regulated in response to heat stress? The perfect inverted repeats located in both operons between the transcriptional and translational start sites suggested a function as a binding site for a regulatory protein. To prove this assumption, point mutations were introduced separately into the left, the right, and both arms, and these 3 mutated repeat sequences were used to replace the wild-type sequence preceding the dnaK operon. All 3 mutated sequences led to a high constitutive expression of the dnaK but not of the groE operon, already under nonheat shock conditions (Zuber and Schumann 1994). It was concluded from this result that this inverted repeat indeed serves as a cis-active binding site for a negative regulator. Meanwhile, the inverted repeat has been identified in an increasing number of bacterial species, and in almost all cases, it preceded either the dnaK or the groE operon. Because both operons code for molecular chaperones, the acronym CIRCE was coined, which stands for “controlling inverted repeat of chaperone expression.”
The gene coding for the predicted transcriptional repressor has been identified by 2 groups using different approaches. In 1 case, cells carrying a transcriptional fusion between the bgaB reporter gene encoding a heat-stable β-galactosidase and the controlling region of the groE operon were mutagenized, plated at low temperature, and screened for blue colonies. These experiments led to the detection of the first gene of the dnaK operon as the gene coding for the transcriptional repressor (Yuan and Wong 1995b). Following another strategy, construction and analysis of knockouts of the dnaK operon, it could be first shown that expression of the groE operon was enhanced significantly in an hrcA knockout, suggesting that either hrcA itself or a gene downstream of it codes for the repressor (Schulz et al 1995). When hrcA was completely deleted, this knockout caused a high constitutive expression of both class I operons in the absence of heat shock (Schulz and Schumann 1996). These 2 experiments clearly identified the first gene of the dnaK operon as the one coding for the repressor protein binding to the CIRCE element, and it was subsequently named hrcA (heat shock regulation at CIRCE; Roberts et al 1996; Schulz and Schumann 1996).
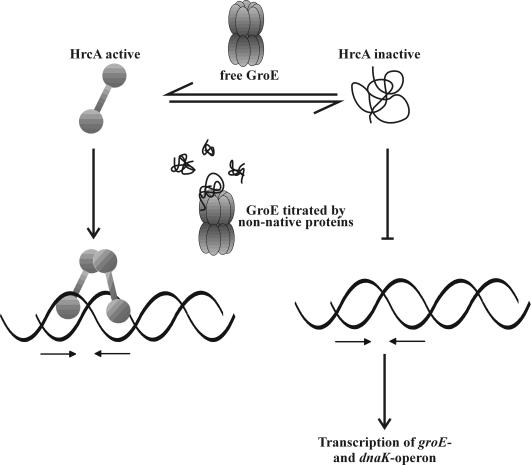
How is the activity of the repressor modulated after a heat shock? It has to be assumed that the repressor will dissociate immediately after heat challenge to allow for the rapid increase in transcription of the 2 operons. Furthermore, because the heat shock response is shut off 5 to 10 minutes later, the HrcA repressor must bind again to its 2 operators. Four lines of experimental evidence suggest that the activity of the repressor is modulated by the GroE chaperonin system (Mogk et al 1997). (1) When the hrcA gene was transferred to E coli, it could be transiently inactivated by a heat shock comparable with the situation in B subtilis, suggesting that all components required for its transient inactivation are present within E coli cells. When its activity was tested in different E coli groES and groEL temperature-sensitive mutants, the basal activity of a reporter enzyme under control of hrcA was significantly increased in the absence of a heat shock, the first hint that the GroE chaperonin system might be involved in modulating the activity of HrcA. (2) Depletion of the GroES and GroEL proteins in B subtilis resulted in a high constitutive expression of the genes of the dnaK operon at low temperature. Under these conditions, high amounts of seemingly inactive repressor protein are present within the cells. (3) Overproduction of just GroEL in B subtilis lowered both the basal level of dnaK operon expression and its expression after heat stress. (4) Purified HrcA repressor was almost unable to bind to its operator site as shown in a bandshift assay. Addition of purified GroEL significantly enhanced the amount of bound DNA. From all these results, we developed the following working hypothesis presented in Figure 2. The HrcA repressor is released from the ribosomes as an inactive protein (defined by its inability to bind to its operator). To become active, it has to interact with the GroEL chaperonin system, and active HrcA is able to bind to its operators. Upon dissociation from its DNA-binding sites, HrcA is again present in its inactive form and needs another interaction with the GroE system to become converted into its active form. Proteins that frequently need to interact with GroEL have been described recently in E coli (Houry et al 1999). In the absence of heat stress, sufficient amounts of free GroE proteins are available to keep most HrcA molecules in their active conformation. Immediately after exposure to heat stress, GroE is titrated by nonnative proteins arising as a result of the heat shock. As a consequence, de novo–synthesized HrcA proteins and repressor molecules dissociated from their operators will stay in their inactive form, allowing the RNA polymerase to initiate transcription at a higher rate. The more nonnative proteins are removed from the cytoplasm, the more the GroE chaperonins will be free to convert inactive HrcA into its active form, resulting in a gradual turn off of the HrcA regulon until the default state has been reached. An open question is whether titration of GroE immediately after a heat shock is sufficient to explain turn on of the HrcA regulon or whether an additional mechanism accounts for the rapid increase in transcription of the dnaK and groE operon. Such a mechanism could involve, for example, direct interaction of nonnative proteins with HrcA. Biochemical analyses of purified HrcA protein have been hampered by its strong tendency to aggregate. Using a genetic screen, a mutant form of the HrcA protein (HrcA114) with increased activity was isolated under conditions of decreased GroE function (Reischl et al 2002). DNase I–footprinting experiments exhibited full protection of the CIRCE element and neighboring nucleotides. Furthermore, an in vitro binding assay using affinity chromatography showed direct and specific interaction between HrcA114 and GroEL. All these data are in full agreement with our model, claiming that HrcA needs the GroE chaperonin system for activation. Open questions concern the domain structure of HrcA and the definition of its active and inactive form at the molecular level.
Fig 2.
The HrcA-GroE reaction cycle. Within the cells, the HrcA repressor is present in an active and an inactive form, where the latter is unable to bind to its operator, the CIRCE element symbolized by the 2 inverted arrows. The equilibrium between these 2 forms is influenced by the GroE chaperonin system. In the absence of heat stress, the GroE system converts most of the HrcA molecules into the active form. After a sudden temperature upshift, nonnative proteins titrate the GroE chaperonins, leading to an increase in the amount of inactive HrcA repressor, leading to enhanced transcription of the groE and dnaK operons
How widespread is the HrcA-CIRCE system among eubacteria? So far, the HrcA gene has been detected in 52 bacterial species and the CIRCE element more than 100 times (T. Wiegert and W. Schumann, in preparation). From all the published data, it can be concluded that this heat shock regulon is the most widespread among all those described to date.
CLASS II HEAT SHOCK GENES: THE σB REGULON
This class of heat shock genes is by far the largest, with 127 members (Price et al 2001). All these genes are under the positive control of the alternative sigma factor, σB, discovered in 1979 as the first alternate sigma factor in bacteria (Haldenwang and Losick 1979). These class II heat shock genes are not only induced by the classical heat shock regimen, such as the heat shock itself and ethanol, but also by salt; oxidation; desiccation or acid stress; and starvation for oxygen, glucose, or phosphate. Therefore, the σB regulon codes for general stress proteins (Hecker et al 1996) in contrast to specific heat shock proteins affected only by heat stress. Although the explicit role of most of the proteins in this regulon has not yet been elucidated, their coordinate induction in response to these different stress stimuli suggests that they serve a general and nonspecific protective function allowing adaptation of the cells to stress and starvation. How do these 127 proteins cope with the different stressful situations to allow survival of B subtilis cells and even growth? Based on their known or predicted function, these genes have been classified into 6 groups (Price 2002), with just a few examples mentioned here: (1) direct protection (proteases, catalases, thioredoxin, arsenate reductase), (2) modulation of σB-activity (antisigma and anti-antisigma factors, phosphatases; see below), (3) regulation downstream of σB (responses of SOS response and class II heat shock genes, members of the AraC, DeoR, and EbsC family), (4) influx and efflux (many permeases, antiporters, and symporters), (5) metabolism (glucosidase, dehydrogenases, and pyruvate oxidase), and (6) turnover (cysteine protease, ribonuclease R).
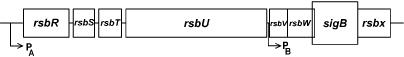
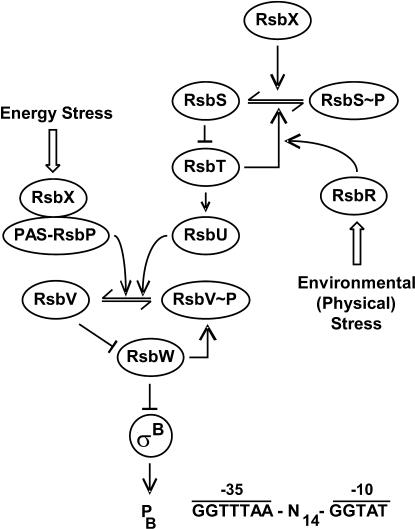
The regulation of class II heat shock genes occurs in a rather sophisticated manner and is only partly understood. An important regulatory mechanism effective in the modulation of σB activity is the presence of 2 “partner switching” modules. Each one consists of 3 partner proteins in which the particular binding decision is dictated by the phosphorylation status of one of the partners (see below). Such a mechanism was coined originally to account for the regulation of the sporulation-specific sigma factor σF (Alper et al 1994). As already mentioned, all these genes are under positive control by the σB sigma factor encoded by the seventh gene of an 8-gene operon (Fig 3). The whole operon is preceded by a σA-type promoter, ensuring the basal expression of all 8 genes (Wise and Price 1995). In addition, expression of the last 4 genes can become initiated at a σB-dependent promoter provided active σB is present, resulting in autoregulation of σB (Kalman et al 1990). In the absence of any stress factor (under physiological conditions), σB is sequestered by an antisigma factor encoded by the rsbW gene (for regulation of sigma B), thereby preventing its interaction with the RNA polymerase core enzyme (Fig 4). Besides binding to σB, the RsbW protein also possesses a serine kinase activity that is responsible for the phosphorylation of the RsbV anti-antisigma factor, thereby keeping this protein in an inactive state. If B subtilis cells are exposed to one of the stress factors mentioned above, the phosphate is removed from RsbV∼P by one of 2 phosphatases (RsbP, RsbU). Dephosphorylated RsbV attacks the σB-RsbW complex, and partner switching of RsbW from σB to RsbV leads to the release of σB. These 2 phosphatases respond to 2 classes of stress: (1) energy stress caused, eg, by carbon, oxygen, or phosphorous starvation or the addition of oxidative uncouplers to the growth medium leads to the activation of the energy-responsive phosphatase RsbP and (2) environmental stresses, including ethanol, heat, acid, and salt stress, activate the environmental-responsive phosphatase RsbU.
Fig 3.
The σB operon. The whole octacistronic sigB operon is transcribed at a basal level from the σA-type promoter PA, whereas under stress conditions σB greatly enhances transcription of the 4 distal genes at PB. The function of the different genes is described within the text
Fig 4.
Model of the signal transduction network leading to the activation of σB. Under physiological conditions, most σB molecules are sequestered by the antisigma factor RsbW, which also acts as a kinase to phosphorylate the anti-antisigma factor RsbV to keep it inactive. Energy stress leads to the dephosphorylation of the anti-antisigma factor RsbV by the RsbP phosphatase, whereas environmental (physical) stress activates the RsbU phosphatase. Dephosphorylated RsbV will attack the RsbW-σB complex, leading to the release of the sigma factor, which is now able to transcribe all the genes of the σB regulon. For further details, see text
Next, we have to explain how the 2 phosphatases become activated. The energy-responsive RsbP serine phosphatase is composed of at least 2 domains, a C-terminal PP2C phosphatase domain and an N-terminal Per-Arnt-Sim (PAS) domain (Fig 4). PAS domains have been shown to control either the interaction with other proteins or to sense changes in the energy level of cells in general (Taylor and Zhulin 1999). RsbP seems to be a flavoprotein, and it has been speculated that the redox state of the chromophore bound to RsbP might be involved in modulating the activity of the PP2C-phosphatase domain (Vijay et al 2000). Furthermore, it has been shown that this PAS domain is indeed essential for the energy stress response (mentioned in Price 2001). RsbP is complexed with RsbQ, a positive regulator, coding for an α/β–type hydrolase, but the exact function of RsbQ during signal transduction remains to be discovered (Brody et al 2001). It has been concluded that the RsbP phosphatase is synthesized in an inactive form and converted to an active enzyme by RsbQ. Therefore, RsbQ directly senses energy stress, or, alternatively, one or more as yet unknown proteins transmit a signal to RsbQ upon energy stress challenge. What molecule(s) is sensed by RsbQ? Because energy stress is often accompanied by a drop in the cytoplasmic ATP level and a subsequent increase in adenosine diphosphate (ADP), RsbQ could measure the amount of ADP molecules either directly or indirectly.
Activation of the environmental-responsive PP2C-type serine phosphatase RsbU is more complex, involving at least 10 proteins, of which only 4 are shown in Figure 4. In the absence of environmental stress, RsbS forms a complex with RsbT, thereby preventing its activation of RsbU. Environmental stress is sensed by RsbR (and most probably by 6 paralogs), which in turn interacts with RsbT to activate its latent kinase activity (Gaidenko et al 1999). Next, RsbT phosphorylates RsbS, thereby causing dissociation of the RsbT-RsbS complex, followed by binding of RsbT to RsbU, the second partner-switching module (Yang et al 1996). Return to the default state is ensured by the RsbX protein, another phosphatase specific for RsbS. Dephosphorylation of RsbS favors sequestering of RsbT by RsbS and thereby shuts off the signal transduction pathway. Although RsbR modulates the σB response after heat shock and salt stress (Akbar et al 1997), 6 RsbR paralogs have been identified (YkoB, YojH, YqhA, YteA, YetI, and YezB), displaying 50% sequence identity in their C-terminal halves, but the exact function of these proteins has yet to be elucidated. It is tempting to speculate that these 7 stress transmitters might sense different types of stresses, but overlapping activities are to be expected. It is also tempting to speculate whether one or more of the stress transmitters interact with the feedback phosphatase RsbX to activate or inactivate its enzymatic activity. In summary, activation of the energy-responsive RsbP phosphatase is regulated in a straightforward manner, whereas that of the environmental-responsive RsbU seems to involve several input regulators and a feedback loop. Activation of σB after imposition of environmental stress is always transient, reaching a maximum 10–40 minutes after the shift, but then σB activity decreases rapidly to the preshift level (Boylan et al 1993; Maul et al 1995; Voelker et al 1995). The RsbX phosphatase that specifically dephosphorylates RsbS∼P is part of the device ensuring the transient nature of the induction (Voelker et al 1997; Smirnova et al 1998).
How widespread is the σB network among eubacteria? The same 8-gene σB operon has been described in B licheniformis (Brody and Price 1998) and Listeria monocytogenes (Becker et al 1998; Wiedmann et al 1998). Smaller σB operons have been described in Staphylococcus aureus (Wu et al 1996; Kullik and Giachino 2002), Staphylococcus epidermis (Knobloch et al 2001), Mycobacterium tuberculosis (DeMaio et al 1996, 1997), and Streptomyces coelicolor (Kormanec 2000). Unfortunately, in the 2 latter species, the presumed σB orthologs have been called σF and σH, respectively.
CLASS III HEAT SHOCK GENES: THE CTSR REGULON
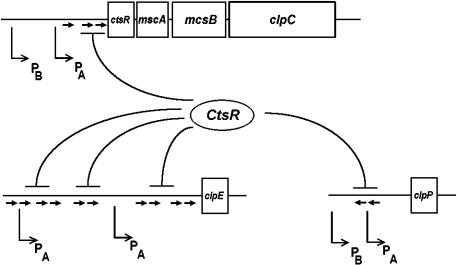
In contrast to the σB-regulon, the CtsR regulon coding for class III heat shock genes is composed of only 6 members, the tetracistronic clpC operon (Krüger et al 1996, 1997) and the 2 monocistronic clpP and clpE operons (Fig 5). Three of these genes encode Clp proteins: the negative regulator of this regulon, the CtsR repressor (for class III stress repressor), and the mcsA and mcsB (for modulator of CtsR repression), involved in regulating the activity of CtsR (see below). The genes clpC and clpE code for ATPase subunits, and clpP codes for a proteolytic subunit of the 2 ClpCP and potential ClpEP ATP-dependent proteases. All 3 operons are preceded by 2 promoters each, the clpC and clpP operons by an upstream σB- and a downstream σA-dependent promoter and clpE by 2 σA-type promoters (Fig 5). All σA-dependent promoters are under the negative control of the CtsR repressor, where the CtsR-binding sites usually overlap the transcriptional start site or the −35 and −10 regions of the promoter, suggesting that the repressor prevents binding of the RNA polymerase EσA holoenzyme to these promoters.
Fig 5.
The CtsR regulon. This regulon consists of 3 transcriptional units, the tetracistronic clpC operon and the 2 monocistronic clpP and clpE operons. All 3 operons are under the negative control of the CtsR repressor binding to 2 or more direct repeats indicated by the arrows
CtsR has been predicted to code for a repressor protein because it contains a classical helix-turn-helix DNA-binding motif. Inactivation of this gene led to a 10- to 20-fold increase in the expression of the clpC operon and the 2 monocistronic clpP and clpE genes (Krüger and Hecker 1998; Derré et al 1999a). The operator sequence, which is recognized by CtsR, has been determined as a highly conserved heptanucleotide direct repeat located upstream of all 3 transcriptional units: A/GGTCAAA NAN A/GGTCAAA (Derré et al 1999a, 1999b). In summary, the CtsR repressor regulates expression of 6 genes organized in 3 transcriptional units.
What is known about the mechanism of heat shock regulation of class III genes? CtsR is composed of at least 3 functional domains, a dimerization domain (within the first 24 amino acids), a helix-turn-helix domain (amino acids 64–67) responsible for DNA binding, and the central glycin-rich region (amino acids 64–67), which could be involved in heat sensing (Derré et al 2000). The 8 genes of the CtsR regulon are expressed at a low level at 37°C and are strongly derepressed after heat challenge. This regulatory mechanism seems to be based on maintaining a certain steady-state level of CtsR at 37°C, followed by a transient inactivation of the repressor upon exposure to heat stress. The steady-state level of CtsR is controlled by the ClpXP protease degrading superfluous molecules (Derré et al 2000). The 2 proteins encoded by the mcsA and mcsB genes act as modulators of the CtsR repressor. McsA reveals similarities to zinc finger proteins that function as DNA-binding proteins or participate in the mediation of protein-protein interactions. McsA may assist CtsR in adopting or stabilizing its active conformation (or both). McsB shares similarities with arginine kinases and is involved in inactivation of CtsR, most probably by phosphorylation, thereby targeting the repressor for ClpCP-mediated proteolysis (Krüger et al 2001). A database search revealed that the CtsR regulatory system is highly conserved among low G + C gram-positive bacteria, with CtsR-binding sites found mostly upstream from clp genes (Derré et al 1999a).
CLASS IV HEAT SHOCK GENE: THE HTPG OPERON
Only 1 class IV heat shock gene has been identified so far, the htpG gene. This gene, which is assumed to code for a molecular chaperone, is induced about 10-fold after a heat shock from 37°C to 48°C, both at the level of transcription and translation (Schulz et al 1997). htpG is dispensable for growth between 37°C and 48°C (Versteeg et al 1999), in agreement with data published for E coli htpG (Bardwell and Craig 1988). The regulatory site for htpG (GAAAGG) has been identified immediately downstream of its σA-dependent promoter (Versteeg et al 2003). Upon deletion of this sequence, the promoter lost heat inducibility. Fusion of the regulatory sequence to the lepA promoter, which is not subject to activation by heat stress, conferred heat inducibility. We concluded from all these results that htpG is under positive control by a transcriptional activator binding downstream of the −10 region (Versteeg et al 2003). Experiments are in progress to identify this transcriptional activator.
CLASS V HEAT SHOCK GENES: THE CSSRS REGULON
Class V heat shock genes have been described quite recently and consist of 2 members so far, htrA and htrB (Darmon et al 2002), encoding putative membrane-anchored proteases. Both genes are preceded by a −10 region of σA-type promoters but lack an obvious −35 region. Instead, the control regions have a 4-fold–repeated octameric consensus sequence (TTTTCATA) positioned close to the −35 regions (Noone et al 2001). Both genes are under the positive control of the CssRS 2-component signal transduction system (for control of secretion stress regulator and sensor), which responds not only to heat but also to secretion stress exerted, eg, by α-amylase overproduction (Darmon et al 2002). In both cases, it can be assumed that nonnative proteins present within or on the outer face of the cytoplasmic membrane will lead to the activation of the sensor kinase CssS.
The CssRS 2-component system is assumed to detect secretion stress by sensing the accumulation of misfolded proteins at the membrane–cell wall interface (Hyyryläinen et al 2001). In accordance with this assumption, the CssRS regulatory system does not respond to nonnative proteins within the cytosol because neither htrA nor htrB is induced by the addition of puromycin to the medium (Noone et al 2000; Darmon et al 2002). Because puromycin causes premature translation termination, we can assume that the truncated proteins synthesized under these conditions are degraded within the cytoplasm and not secreted to induce class V heat shock genes. It has been reported that the cssRS operon itself becomes heat inducible in an htrA knockout strain but not in a wild-type background. This finding can be explained by assuming that the level of nonnative proteins generated by heat stress in a wild-type background is sufficient to stimulate the expression of htrA and htrB but insufficient to stimulate expression of cssRS (Darmon et al 2002).
CLASS VI HEAT SHOCK GENES
Class VI comprises a group of genes whose expression is also responsive to stress, but the mechanism of induction is not affected by any one of the previously mentioned regulators. Therefore, these genes are controlled by one or more unknown mechanisms. Ten class VI heat shock genes have been reported previously, where 4 are arranged in monocistronic transcriptional units (ftsH, clpX, ykoZ, and sacB) (Gerth et al 1996; Deuerling et al 1997; Schumann et al 2002; Zuber et al 2001) and 6 in 2 bicistronic units (lonA-orfX, ahpC-ahpF, and nfrA-ywcH) (Riethdorf et al 1994; Antelmann et al 1996; Moch et al 2000). In a recent global transcriptional analysis using the DNA microarray technique, the group of J. D. Helmann has considerably extended class VI heat shock genes by another approximately 80 members (Helmann et al 2001), making this the second largest class. Class VI heat shock genes can be distinguished by 3 criteria: (1) the promoter-type; (2) the heat-induction factor; and most importantly (3) the stress factors besides heat that cause their induction, suggesting that more than 1 mechanism is responsible for their regulation.
CELLULAR THERMOMETERS AND THERMOSENSORS
The study of the induction mechanisms of heat shock genes has revealed 2 fundamentally different regulatory principles with different outcomes, direct and indirect heat sensing. Direct heat sensors are either RNA or protein molecules that change their conformation depending on the temperature, whereas indirect heat sensors identified so far are molecular chaperones involved in modulating the activity of a transcriptional regulator and titrated by nonnative proteins. Although expression of heat shock genes controlled by direct sensors continues at a high rate as long as the cells are exposed to the high temperature (these genes are not turned off), those regulated by indirect sensors are induced transiently, implying that the regulatory mechanism must ensure a return to the prestimulus state even if the cells are kept longer at the high temperature. To distinguish these 2 opposing mechanisms, I suggest designating direct sensors as thermosensors and indirect sensors, which involve several components, as cellular thermometers. With a few exceptions, heat shock genes are transiently induced, and these are under the control of cellular thermometers. Ribosomes were suggested first in 1990 to act as such a thermometer (VanBogelen and Neidhardt 1990), and in the following year, the DnaK chaperone machine of E coli cells was claimed to act as a thermometer (Craig and Gross 1991; McCarty and Walker 1991). Examples for heat shock genes being under the control of a thermosensor are the rpoH mRNA coding for σ32 (Morita et al 1999), the ROSE elements present at the beginning of the mRNA of some heat shock genes in Rhizobia (Nocker et al 2001), and the RheA transcriptional repressor protein (Servant et al 2001).
The best-studied example of indirect sensing is the modulation of the σ32 activity by the DnaK chaperone machine. In the absence of heat stress, the DnaK system will bind σ32 molecules and present them to an ATP-dependent protease such as FtsH (Herman et al 1995). Following a heat shock, the DnaK chaperone machine is titrated by nonnative proteins, allowing σ32 to escape sequestration and hydrolysis. The more nonnative proteins are removed by molecular chaperones and ATP-dependent proteases, the more σ32 molecules will be bound by the DnaK team and presented for proteolysis, resulting in a gradual turn off of the heat shock response. Thermosensing in the case of the transcripts involves secondary structures that sequester the Shine-Dalgarno sequence and the start codon, impairing binding of the 30S ribosomal subunit. Increasing temperatures will lead to the melting of the secondary structures, thereby enhancing translation of the transcript. In the case of the Rhe repressor protein, high temperatures induce a conformational change and subsequent dissociation from its operator. It has to be stressed again that direct-sensing mechanisms do not allow a turnoff of the affected heat shock genes.
What is the situation in B subtilis? Are we already able to define cellular thermometers and thermosensors? At least, we can postulate that there is more than 1 heat-sensing device. In the case of the HrcA regulon, it has been suggested that the GroE chaperonin system acts as a thermometer of heat stress (Mogk et al 1997). As outlined above, this system is responsible for modulating the activity of the HrcA repressor, senses nonnative proteins, and ensures autoregulation. Based on our current evidence, only HrcA and GroE are the components of the cellular thermometer of class I heat shock genes. The simplicity of this thermometer could be the reason for the widespread use of this device by more than 50 bacterial species.
In the case of the σB regulon, RsbR seems to be part of a complicated second cellular thermometer. This protein somehow senses a temperature increase and then interacts with the RsbT protein to stimulate its kinase activity. Return to the default state involves inactivation of RsbR by phosphorylation (the quick response?) and RsbX phosphatase, which demodifies RsbT (the slow response?). Because puromycin fails to induce the σB regulon (Mogk et al 1998), this observation strongly argues against nonnative proteins as inducers. Therefore, we have to assume that RsbR, present in an inactive state in the absence of heat stress, is able to sense some as yet unknown component by direct interaction to be converted into its active form. But it remains completely mystifying how RsbX becomes activated and how RsbR and RsbX might sense molecules different from nonnative proteins such as the ATP or ADP concentration within the cytoplasm. In vitro experiments should be able to answer this question.
Regulation of the CtsR regulon seems to be less complicated. Here, as in the case of HrcA, the activity of a repressor protein is modulated by heat stress. This modulation seems to involve the 2 proteins, McsA and McsB, where McsA is required for the stabilization of the CtsR repressor. Stabilization could involve chaperoning of CtsR into its active conformation or preventing its proteolytic degradation. McsB has been suggested to phosphorylate CtsR, thereby making it prone to degradation by ClpCP (Krüger et al 2001). If this picture is correct, McsB should be the heat sensor that leads to its activation, again by interaction with nonnative proteins? Here, too, it is largely unknown how the return to the default state is regulated. But it should involve a return of the McsB kinase from its active to its inactive conformation.
The cellular thermometer involved in the regulation of the htpG gene, the only member of class IV heat shock genes so far, is completely unknown. We can only state that nonnative proteins within the cytoplasm do not play any role. We concluded that the signal that is sensed by a still unknown protein must arise either within the membrane or outside the cell (Versteeg et al 2003). A similar conclusion has been reached for class V heat shock genes. Here, too, nonnative proteins within the cytoplasm are not sensed by the CssRS 2-component signal transduction system. Instead, it has been suggested that nonnative proteins either stuck within the membrane or accumulating on the outside are sensed by the CssR sensor kinase, leading to autophosphorylation followed by transfer of the phosphate group to the response regulator CssS, which in turn activates the gene htrA and htrB (Darmon et al 2002). Because both genes code for proteases, it is tempting to speculate that increased synthesis of these 2 proteases results in removal of the nonnative proteins and thereby turnoff of the expression of class V heat shock genes.
CONCLUSIONS AND PERSPECTIVES
Work carried out during the past 10 years has revealed that the B subtilis heat shock stimulon is by far the most complicated ever described in bacteria. It codes for more than 200 heat shock genes that are regulated by at least 6 mechanisms summarized in Table 1. Why has B subtilis developed such a sophisticated network of heat stress regulons? One answer that comes immediately to one's mind is that whereas all these regulons respond to heat stress, they might react to other stress factors in a specific manner. In favor of this assumption is the observation that class II genes are also induced by starvation of carbon and phosphate and by acid stress, to mention just a few, and that class V genes are also induced by secretion stress. B subtilis as a typical soil bacterium might be exposed to more stress regimen in its natural habitat as compared with the E coli residing in the gut, where most of the heat shock genes are members of just 1 class, the σ32 regulon (Yura et al 2000). Although, in the case of class I and class III, only heat has been identified as an inducing stress factor, it can be expected that additional stress factors specific for one of these 2 classes will be identified in future.
Table 1.
The heat shock stimulon of B subtilis
Is there a cross talk between these different regulons? A weak overlap involving class II and III has been reported. The clpC and clpP operons are both preceded, besides their major σA-type promoter, by a σB-type (see Fig 5). But clpC is poorly induced by oxygen and phosphate starvation (Krüger et al 1996), and in the case of clpP, transcription initiated at σB is very weak, accounting for only 25% of the total expression under stress conditions (Gerth et al 1998). To conclude, a cross talk between the different regulons does not seem to exist in B subtilis, and there are no experimental data in favor of a master regulator. All the regulons seem to be regulated completely independent of each other.
Work in progress deals with 2 important aspects: (1) identification of the transcriptional regulators of class IV and class VI heat shock genes and (2) a detailed analysis of the different cellular thermometers. There is no indication for thermosensors because all heat shock genes studied are turned off. The ultimate goal of the study of the heat shock response in B subtilis will be a complete and in-depth understanding of all the regulons, which should allow a convincing answer to the question “What is the biological significance of splitting the heat shock genes into so many different regulons?”
Acknowledgments
Financial support for this work was provided by the Deutsche Forschungsgemeinschaft and the Fonds der Deutschen Chemie.
REFERENCES
- Akbar S, Kang CM, Gaidenko TA, Price CW. Modulator protein RsbR regulates environmental signaling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell JCA, Craig EA. Ancient heat shock gene is dispensable. J Bacteriol. 1988;170:2977–2983. doi: 10.1128/jb.170.7.2977-2983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LA, Cetin MS, Hutkins RW, Benson AK. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;182:7083–7087. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan SA, Redfield AR, Brody MS, Price CW. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody MS, Price CW. Bacillus licheniformis sigB operon encoding the general stress transcription factor σB. Gene. 1998;212:111–118. doi: 10.1016/s0378-1119(98)00140-1. [DOI] [PubMed] [Google Scholar]
- Brody MS, Vijay K, Price CW. Catalytic function of an α/β hydrolase is required for energy stress activation of the σB transcription factor in Bacillus subtilis. J Bacteriol. 2001;183:6422–6428. doi: 10.1128/JB.183.21.6422-6428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Gross CA. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991;16:135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Darmon E, Noone D, Masson A, Bron S, Kuipers OP, Devine KM, Van Dijl JM. A novel class of heat and secretion stress-responsive genes is controlled by the autoregulated CssRS two-component system of Bacillus subtilis. J Bacteriol. 2002;184:5661–5671. doi: 10.1128/JB.184.20.5661-5671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaio J, Zhang Y, Ko C, Bishai WR. Mycobacterium tuberculosis sigF is part of a gene cluster with similarities to the Bacillus subtilis sigF and sigB operons. Tuber Lung Dis. 1997;78:3–12. doi: 10.1016/s0962-8479(97)90010-1. [DOI] [PubMed] [Google Scholar]
- DeMaio J, Zhang Y, Ko C, Young DB, Bishai WR. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derré I, Rapoport G, Devine K, Rose M, Msadek T. ClpE, a novel type of HSP100 ATPase, is part of the CtsR heat shock regulon of Bacillus subtilis. Mol Microbiol. 1999b;32:581–593. doi: 10.1046/j.1365-2958.1999.01374.x. [DOI] [PubMed] [Google Scholar]
- Derré I, Rapoport G, Msadek T. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol Microbiol. 1999a;31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- Derré I, Rapoport G, Msadek T. The CtsR regulator of stress response is active as a dimer and specifically degraded in vivo at 37°C. Mol Microbiol. 2000;38:335–347. doi: 10.1046/j.1365-2958.2000.02124.x. [DOI] [PubMed] [Google Scholar]
- Deuerling E, Mogk A, Richter C, Purucker M, Schumann W. The ftsH gene of Bacillus subtilis is involved in major cellular processes such as sporulation, stress adaptation and secretion. Mol Microbiol. 1997;23:921–933. doi: 10.1046/j.1365-2958.1997.2721636.x. [DOI] [PubMed] [Google Scholar]
- Gaidenko TA, Yang XF, Lee YM, Price CW. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the environmental stress signaling pathway of Bacillus subtilis. J Mol Biol. 1999;288:29–39. doi: 10.1006/jmbi.1999.2665. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gerth U, Krüger E, Derré I, Msadek T, Hecker M. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol Microbiol. 1998;28:787–802. doi: 10.1046/j.1365-2958.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- Gerth U, Wipat A, Harwood CR, Carter N, Emmerson PT, Hecker M. Sequence and transcriptional analysis of clpX, a class-III heat-shock gene of Bacillus subtilis. Gene. 1996;181:77–83. doi: 10.1016/s0378-1119(96)00467-2. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- Gottesman S, Wickner S, Maurizi MR. Protein quality control: triage by chaperones and proteases. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- Haldenwang WG, Losick R. A modified RNA polymerase transcribes a cloned gene under sporulation control in Bacillus subtilis. Nature. 1979;282:256–260. doi: 10.1038/282256a0. [DOI] [PubMed] [Google Scholar]
- Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- Helmann JD, Wu MF, Kobel PA, Gamo FJ, Wilson M, Morshedi MM, Navre M, Paddon C. Global transcriptional response of Bacillus subtilis to heat shock. J Bacteriol. 2001;183:7318–7328. doi: 10.1128/JB.183.24.7318-7328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C, Thevenet D, D'Ari R, Bouloc P. Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci U S A. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homuth G, Masuda S, Mogk A, Kobayashi Y, Schumann W. The dnaK operon of Bacillus subtilis is heptacistronic. J Bacteriol. 1997;179:1153–1164. doi: 10.1128/jb.179.4.1153-1164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homuth G, Mogk A, Schumann W. Post-transcriptional regulation of the Bacillus subtilis dnaK operon. Mol Microbiol. 1999;32:1183–1197. doi: 10.1046/j.1365-2958.1999.01428.x. [DOI] [PubMed] [Google Scholar]
- Houry WA, Frishman D, Eckerskorn C, Lottspeich F, Hartl FU. Identification of in vivo substrates of the chaperonin GroEL. Nature. 1999;402:147–154. doi: 10.1038/45977. [DOI] [PubMed] [Google Scholar]
- Hyyryläinen HL, Bolhuis A, and Darmon E. et al. 2001 A novel two-component regulatory system in Bacillus subtilis for the survival of severe secretion stress. Mol Microbiol. 41:1159–1172. [DOI] [PubMed] [Google Scholar]
- Kalman S, Duncan ML, Thomas SM, Price CW. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J Bacteriol. 1990;172:5575–5585. doi: 10.1128/jb.172.10.5575-5585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J, Bartscht K, Sabottke A, Rohde H, Fuecht H-H, Mack D. Biofilm formation of Staphylococcus epidermis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J Bacteriol. 2001;183:2624–2633. doi: 10.1128/JB.183.8.2624-2633.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormanec J, Sevcíková B, Halgasova N, Knirschová R, Rezuchova B. Identification and transcriptional characterization of the gene encoding the stress-response σ factor σH in Streptomyces coelicolor A3(2) FEMS Microbiol Lett. 2000;189:31–38. doi: 10.1111/j.1574-6968.2000.tb09202.x. [DOI] [PubMed] [Google Scholar]
- Krüger E, Hecker M. The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes. J Bacteriol. 1998;180:6681–6688. doi: 10.1128/jb.180.24.6681-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger E, Msadek T, Hecker M. Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon. Mol Microbiol. 1996;20:713–724. doi: 10.1111/j.1365-2958.1996.tb02511.x. [DOI] [PubMed] [Google Scholar]
- Krüger E, Msadek T, Ohlmeier S, Hecker M. The Bacillus subtilis clpC operon encodes DNA repair and competence proteins. Microbiology. 1997;143:1309–1316. doi: 10.1099/00221287-143-4-1309. [DOI] [PubMed] [Google Scholar]
- Krüger E, Zühlke D, Witt E, Ludwig H, Hecker M. Clp-mediated proteolysis in gram-positive bacteria is autoregulated by the stability of a repressor. EMBO J. 2001;20:852–863. doi: 10.1093/emboj/20.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullik I, Giachino P. The alternative sigma factor σB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 2002;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- Li M, Wong S-L. Cloning and characterization of the groESL operon from Bacillus subtilis. J Bacteriol. 1992;174:3981–3992. doi: 10.1128/jb.174.12.3981-3992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul B, Völker U, Riethdorf S, Engelmann S, Hecker M. σB-dependent regulation of gsiB in response to multiple stimuli in Bacillus subtilis. Mol Gen Genet. 1995;248:114–120. doi: 10.1007/BF02456620. [DOI] [PubMed] [Google Scholar]
- McCarty JS, Walker GC. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991;88:9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch C, Schrögel O, Allmansberger R. Transcription of the nfrA-ywcH operon from Bacillus subtilis is specifically induced in response to heat. J Bacteriol. 2000;182:4384–4393. doi: 10.1128/jb.182.16.4384-4393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Homuth G, Scholz C, Kim L, Schmid FX, Schumann W. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Völker A, Engelmann S, Hecker M, Schumann W, Völker U. Nonnative proteins induce expression of the Bacillus subtilis CIRCE regulon. J Bacteriol. 1998;180:2895–2900. doi: 10.1128/jb.180.11.2895-2900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita MT, Tanaka Y, Kodama TS, Kyogoku Y, Yanagi H, Yura T. Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Genes Dev. 1999;13:655–665. doi: 10.1101/gad.13.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F. Negative regulation of bacterial heat shock genes. Mol Microbiol. 1999;31:1–8. doi: 10.1046/j.1365-2958.1999.01166.x. [DOI] [PubMed] [Google Scholar]
- Nocker A, Hausherr T, Balsiger S, Krstulovic NP, Hennecke H, Narberhaus F. A mRNA-based thermosensor controls expression of rhizobial heat shock genes. Nucleic Acids Res. 2001;29:4800–4807. doi: 10.1093/nar/29.23.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone D, Howell A, Collery R, Devine KM. YkdA and YvtA, HtrA-like serine proteases in Bacillus subtilis, engage in negative autoregulation and reciprocal cross-regulation of ykdA and yvtA gene expression. J Bacteriol. 2001;183:654–663. doi: 10.1128/JB.183.2.654-663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone D, Howell A, Devine KM. Expression of ykdA, encoding a Bacillus subtilis homologue of HrcA, is heat shock inducible and negatively autoregulated. J Bacteriol. 2000;182:1592–1599. doi: 10.1128/jb.182.6.1592-1599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CW 2002 General stress response. In: Bacillus subtilis and its Closest Relatives: From Genes to Cells, ed Sonenshein AL, Hoch JA, Losick R. American Society for Microbiology, Washington, DC, 369–384. [Google Scholar]
- Price CW, Fawcett P, Cérémonie H, Su N, Murphy CK, Youngman P. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol Microbiol. 2001;41:757–774. doi: 10.1046/j.1365-2958.2001.02534.x. [DOI] [PubMed] [Google Scholar]
- Reischl S, Wiegert T, Schumann W. Isolation and analysis of mutant alleles of the Bacillus subtilis HrcA repressor with reduced dependency on GroE function. J Biol Chem. 2002;277:32659–32667. doi: 10.1074/jbc.M201372200. [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Völker U, Gerth U, Winkler A, Engelmann S, Hecker M. Cloning, nucleotide sequence, and expression of the Bacillus subtilis lon gene. J Bacteriol. 1994;176:6518–6527. doi: 10.1128/jb.176.21.6518-6527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Toochinda C, Avedissian M, Baldini RL, Gomes SL, Shapiro L. Identification of a Caulobacter crescentus operon encoding hrcA, involved in negatively regulating heat-inducible transcription, and the chaperone gene grpE. J Bacteriol. 1996;178:1829–1841. doi: 10.1128/jb.178.7.1829-1841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Schiesswohl M, Völker U, Hecker M, Schumann W. Cloning, sequencing, mapping, and transcriptional analysis of the groESL operon from Bacillus subtilis. J Bacteriol. 1992;174:3993–3999. doi: 10.1128/jb.174.12.3993-3999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A, Schumann W. hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat-shock genes. J Bacteriol. 1996;178:1088–1093. doi: 10.1128/jb.178.4.1088-1093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A, Schwab S, Versteeg S, Schumann W. The htpG gene of Bacillus subtilis belongs to class III heat shock genes and is under negative control. J Bacteriol. 1997;10:3103–3109. doi: 10.1128/jb.179.10.3103-3109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A, Tzschaschel B, Schumann W. Isolation and analysis of mutants of the dnaK operon of Bacillus subtilis. Mol Microbiol. 1995;15:421–429. doi: 10.1111/j.1365-2958.1995.tb02256.x. [DOI] [PubMed] [Google Scholar]
- Schumann W, Hecker M, and Msadek T 2002 Regulation and function of heat-inducible genes in Bacillus subtilis. In: Bacillus subtilis and its Closest Relatives: From Genes to Cells, ed Sonenshein AL, Hoch JA, Losick R. American Society for Microbiology, Washington, DC, 359–368. [Google Scholar]
- Servant P, Mazodier P. Negative regulation of the heat shock response in Streptomyces. Arch Microbiol. 2001;176:237–242. doi: 10.1007/s002030100321. [DOI] [PubMed] [Google Scholar]
- Smirnova N, Scott J, Voelker U, Haldenwang WG. Isolation and characterization of Bacillus subtilis sigB operon mutations that suppress the loss of the negative regulator RsbX. J Bacteriol. 1998;180:3671–3680. doi: 10.1128/jb.180.14.3671-3680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen RA, Neidhardt FC. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A. 1990;87:5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanet A, Plumbridge JA, Alix J-H. Cotranscription of two genes necessary for ribosomal protein L11 methylation (prmA) and pantothenate transport (panF) in Escherichia coli K-12. J Bacteriol. 1993;175:7178–7188. doi: 10.1128/jb.175.22.7178-7188.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg S, Escher A, Wende A, Wiegert T, Schumann W. Regulation of the Bacillus subtilis heat shock gene htpG is under positive control. J Bacteriol. 2003;185:466–474. doi: 10.1128/JB.185.2.466-474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg S, Mogk A, Schumann W. The Bacillus subtilis htpG gene is not involved in thermal stress management. Mol Gen Genet. 1999;261:582–588. doi: 10.1007/s004380051004. [DOI] [PubMed] [Google Scholar]
- Vijay K, Brody MS, Fredlund E, Price CW. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the σB transcription factor of Bacillus subtilis. Mol Microbiol. 2000;35:180–188. doi: 10.1046/j.1365-2958.2000.01697.x. [DOI] [PubMed] [Google Scholar]
- Voelker U, Luo T, Smirnova N, Haldenwang WG. Stress activation of Bacillus subtilis σB can occur in the absence of the σB negative regulator RsbX. J Bacteriol. 1997;179:1980–1984. doi: 10.1128/jb.179.6.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker U, Voelker A, Maul B, Hecker M, Dufour A, Haldenwang WG. Separate mechanisms activate σB of Bacillus subtilis in response to environmental and metabolic stresses. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M, Arvik TJ, Hurley RJ, Boor KJ. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AA, Price CW. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor σB in response to environmental signals. J Bacteriol. 1995;177:123–133. doi: 10.1128/jb.177.1.123-133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, de Lencastre H, Tomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Kang CM, Brody MS, Price CW. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]
- Yuan G, Wong S-L. Regulation of groE expression in Bacillus subtilis: the involvement of the σA-like promoter and the roles of the inverted repeat sequence (CIRCE) J Bacteriol. 1995a;177:5427–5433. doi: 10.1128/jb.177.19.5427-5433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Wong S-L. Isolation and characterization of Bacillus subtilis regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulating the expression of both groE and dnaK. J Bacteriol. 1995b;177:6462–6468. doi: 10.1128/jb.177.22.6462-6468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T, Kanemori M, and Morita M 2000 The heat shock response: regulation and function. In: Bacterial Stress Response, ed Storz G, Hengge-Aronis R. American Society for Microbiology, Washington, DC, 3–18. [Google Scholar]
- Zuber U, Drzewiecki K, Hecker M. Putative sigma factor SigI (YkoZ) of Bacillus subtilis is induced by heat shock. J Bacteriol. 2001;183:1472–1475. doi: 10.1128/JB.183.4.1472-1475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]